Abstract
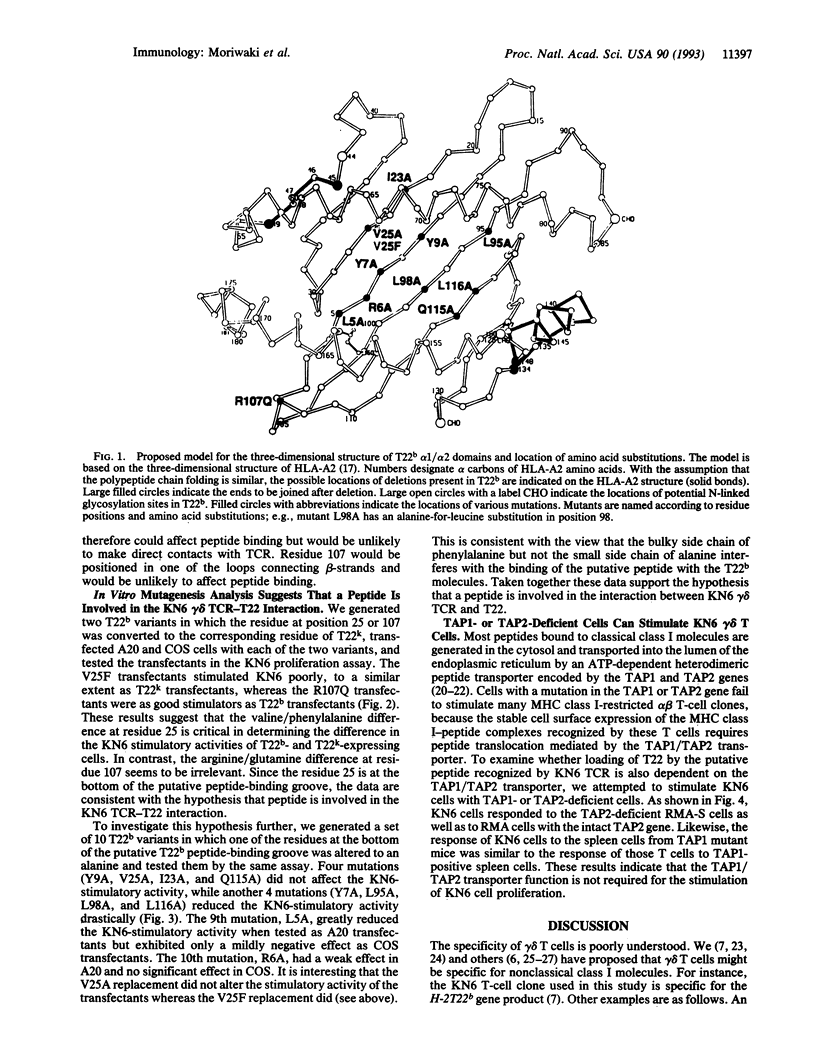
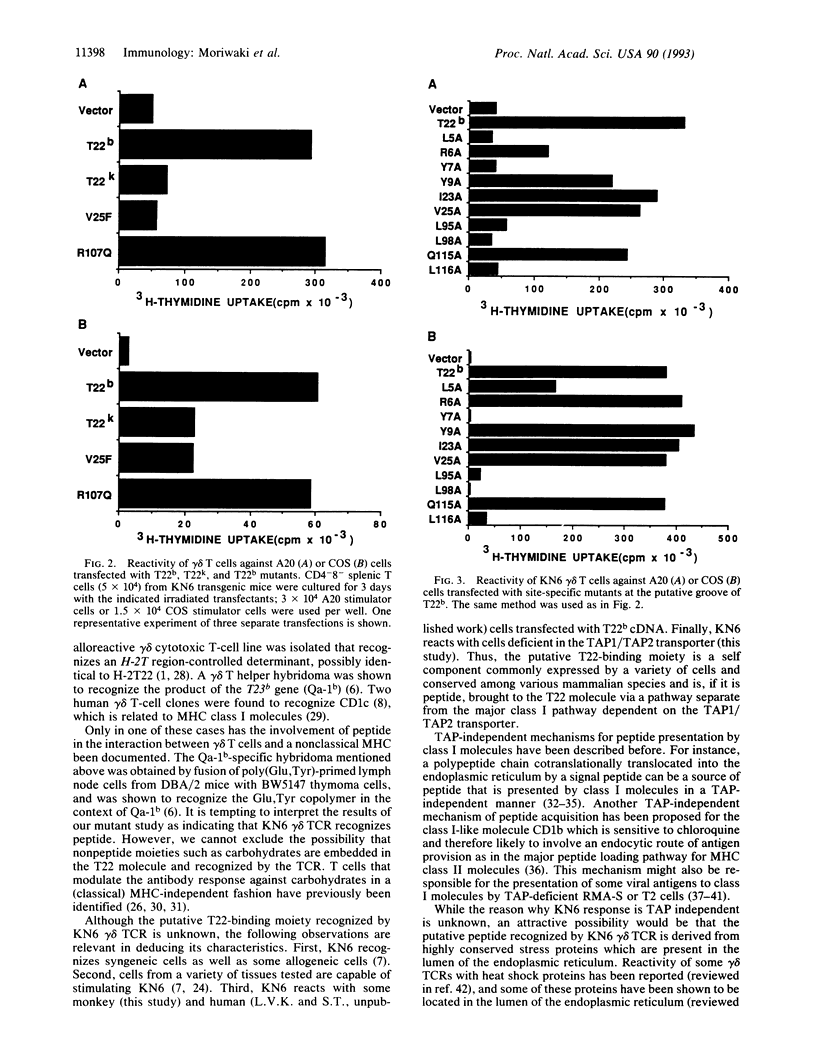
We have previously identified a self-reactive gamma delta T-cell clone (KN6) specific for the H-2T region gene product T22b. Now we have investigated by an in vitro mutagenesis analysis of the T22b gene the possibility that the interaction between the KN6 gamma delta T-cell receptor and T22b involves a peptide. The results demonstrate that mutations at the floor of the putative antigen-binding groove of T22b affect recognition by the gamma delta T-cell receptor. Furthermore, we have shown that KN6 cells react with cells that are deficient in the class I peptide transporter TAP1/TAP2. These results suggest that peptide is involved in the interaction of the KN6 T-cell receptor with T22 and that loading of T22 with the putative peptide is TAP1/TAP2-independent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich C. J., Waltrip R., Hermel E., Attaya M., Lindahl K. F., Monaco J. J., Forman J. T cell recognition of QA-1b antigens on cells lacking a functional Tap-2 transporter. J Immunol. 1992 Dec 15;149(12):3773–3777. [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Cron R. Q., Cotterman M., Houlden B. A., Matis L. A. Structure and specificity of T cell receptor gamma/delta on major histocompatibility complex antigen-specific CD3+, CD4-, CD8- T lymphocytes. J Exp Med. 1988 Nov 1;168(5):1899–1916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., Ito K., Krecko E. G., Itohara S., Kappes D., Ishida I., Kanagawa O., Janeway C. A., Murphy D. B., Tonegawa S. Recognition of a self major histocompatibility complex TL region product by gamma delta T-cell receptors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5928–5932. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Zinkernagel R. M. Immunodominant T cell epitope from signal sequence. Science. 1992 Aug 21;257(5073):1142–1142. doi: 10.1126/science.257.5073.1142. [DOI] [PubMed] [Google Scholar]
- Calabi F., Milstein C. A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature. 1986 Oct 9;323(6088):540–543. doi: 10.1038/323540a0. [DOI] [PubMed] [Google Scholar]
- Ciccone E., Viale O., Pende D., Malnati M., Battista Ferrara G., Barocci S., Moretta A., Moretta L. Specificity of human T lymphocytes expressing a gamma/delta T cell antigen receptor. Recognition of a polymorphic determinant of HLA class I molecules by a gamma/delta clone. Eur J Immunol. 1989 Jul;19(7):1267–1271. doi: 10.1002/eji.1830190718. [DOI] [PubMed] [Google Scholar]
- Esquivel F., Yewdell J., Bennink J. RMA/S cells present endogenously synthesized cytosolic proteins to class I-restricted cytotoxic T lymphocytes. J Exp Med. 1992 Jan 1;175(1):163–168. doi: 10.1084/jem.175.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hammond S. A., Bollinger R. C., Tobery T. W., Silliciano R. F. Transporter-independent processing of HIV-1 envelope protein for recognition by CD8+ T cells. Nature. 1993 Jul 8;364(6433):158–161. doi: 10.1038/364158a0. [DOI] [PubMed] [Google Scholar]
- Hemsley A., Arnheim N., Toney M. D., Cortopassi G., Galas D. J. A simple method for site-directed mutagenesis using the polymerase chain reaction. Nucleic Acids Res. 1989 Aug 25;17(16):6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. A., Michel H., Sakaguchi K., Shabanowitz J., Appella E., Hunt D. F., Engelhard V. H. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992 Mar 6;255(5049):1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- Hermel E., Grigorenko E., Lindahl K. F. Expression of medial class I histocompatibility antigens on RMA-S mutant cells. Int Immunol. 1991 Apr;3(4):407–412. doi: 10.1093/intimm/3.4.407. [DOI] [PubMed] [Google Scholar]
- Hosken N. A., Bevan M. J. An endogenous antigenic peptide bypasses the class I antigen presentation defect in RMA-S. J Exp Med. 1992 Mar 1;175(3):719–729. doi: 10.1084/jem.175.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden B. A., Matis L. A., Cron R. Q., Widacki S. M., Brown G. D., Pampeno C., Meruelo D., Bluestone J. A. A TCR gamma delta cell recognizing a novel TL-encoded gene product. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):45–55. doi: 10.1101/sqb.1989.054.01.006. [DOI] [PubMed] [Google Scholar]
- Ishida I., Verbeek S., Bonneville M., Itohara S., Berns A., Tonegawa S. T-cell receptor gamma delta and gamma transgenic mice suggest a role of a gamma gene silencer in the generation of alpha beta T cells. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3067–3071. doi: 10.1073/pnas.87.8.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Van Kaer L., Bonneville M., Hsu S., Murphy D. B., Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse gamma delta T cell receptor. Cell. 1990 Aug 10;62(3):549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Lam V., DeMars R., Chen B. P., Hank J. A., Kovats S., Fisch P., Sondel P. M. Human T cell receptor-gamma delta-expressing T-cell lines recognize MHC-controlled elements on autologous EBV-LCL that are not HLA-A, -B, -C, -DR, -DQ, or -DP. J Immunol. 1990 Jul 1;145(1):36–45. [PubMed] [Google Scholar]
- Letvin N. L., Benacerraf B., Germain R. N. B-lymphocyte responses to trinitrophenyl-conjugated Ficoll: requirement for T lymphocytes and Ia-bearing adherent cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5113–5117. doi: 10.1073/pnas.78.8.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985 Dec 1;162(6):1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Nakanishi N., Rogers B. L., Haser W. G., Shitara K., Yoshida H., Takagaki Y., Augustin A. A., Tonegawa S. Expression of the T-cell receptor gamma-chain gene products on the surface of peripheral T cells and T-cell blasts generated by allogeneic mixed lymphocyte reaction. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6536–6540. doi: 10.1073/pnas.84.18.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis L. A., Fry A. M., Cron R. Q., Cotterman M. M., Dick R. F., Bluestone J. A. Structure and specificity of a class II MHC alloreactive gamma delta T cell receptor heterodimer. Science. 1989 Aug 18;245(4919):746–749. doi: 10.1126/science.2528206. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Momburg F., Hämmerling G. J. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993 Aug 6;261(5122):769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Brenner M. B., Greenstein J. L., Balk S. P., Terhorst C., Bleicher P. A. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989 Oct 5;341(6241):447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Morita C. T., Brenner M. B. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992 Dec 10;360(6404):593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Shapiro M. E., Kasper D. L., Zaleznik D. F., Spriggs S., Onderdonk A. B., Finberg R. W. Cellular control of abscess formation: role of T cells in the regulation of abscesses formed in response to Bacteroides fragilis. J Immunol. 1986 Jul 1;137(1):341–346. [PubMed] [Google Scholar]
- Shepherd J. C., Schumacher T. N., Ashton-Rickardt P. G., Imaeda S., Ploegh H. L., Janeway C. A., Jr, Tonegawa S. TAP1-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell. 1993 Aug 13;74(3):577–584. doi: 10.1016/0092-8674(93)80058-m. [DOI] [PubMed] [Google Scholar]
- Sijts A. J., De Bruijn M. L., Nieland J. D., Kast W. M., Melief C. J. Cytotoxic T lymphocytes against the antigen-processing-defective RMA-S tumor cell line. Eur J Immunol. 1992 Jun;22(6):1639–1642. doi: 10.1002/eji.1830220644. [DOI] [PubMed] [Google Scholar]
- Spits H., Paliard X., De Vries J. E. Antigen-specific, but not natural killer, activity of T cell receptor-gamma delta cytotoxic T lymphocyte clones involves secretion of N alpha-benzyloxycarbonyl-L-lysine thiobenzyl ester serine esterase and influx of Ca2+ ions. J Immunol. 1989 Sep 1;143(5):1506–1511. [PubMed] [Google Scholar]
- Spits H., Paliard X., Engelhard V. H., de Vries J. E. Cytotoxic activity and lymphokine production of T cell receptor (TCR)-alpha beta+ and TCR-gamma delta+ cytotoxic T lymphocyte (CTL) clones recognizing HLA-A2 and HLA-A2 mutants. Recognition of TCR-gamma delta+ CTL clones is affected by mutations at positions 152 and 156. J Immunol. 1990 Jun 1;144(11):4156–4162. [PubMed] [Google Scholar]
- Strominger J. L. The gamma delta T cell receptor and class Ib MHC-related proteins: enigmatic molecules of immune recognition. Cell. 1989 Jun 16;57(6):895–898. doi: 10.1016/0092-8674(89)90326-7. [DOI] [PubMed] [Google Scholar]
- Templeton D., Eckhart W. N-terminal amino acid sequences of the polyoma middle-size T antigen are important for protein kinase activity and cell transformation. Mol Cell Biol. 1984 May;4(5):817–821. doi: 10.1128/mcb.4.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Berns A., Bonneville M., Farr A., Ishida I., Ito K., Itohara S., Janeway C. A., Jr, Kanagawa O., Katsuki M. Diversity, development, ligands, and probable functions of gamma delta T cells. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):31–44. doi: 10.1101/sqb.1989.054.01.005. [DOI] [PubMed] [Google Scholar]
- Van Kaer L., Ashton-Rickardt P. G., Ploegh H. L., Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992 Dec 24;71(7):1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Van Kaer L., Wu M., Ichikawa Y., Ito K., Bonneville M., Ostrand-Rosenberg S., Murphy D. B., Tonegawa S. Recognition of MHC TL gene products by gamma delta T cells. Immunol Rev. 1991 Apr;120:89–115. doi: 10.1111/j.1600-065x.1991.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Vidović D., Roglić M., McKune K., Guerder S., MacKay C., Dembić Z. Qa-1 restricted recognition of foreign antigen by a gamma delta T-cell hybridoma. Nature. 1989 Aug 24;340(6235):646–650. doi: 10.1038/340646a0. [DOI] [PubMed] [Google Scholar]
- Wei M. L., Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992 Apr 2;356(6368):443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- Wolf P. R., Cook R. G. The TL region gene 37 encodes a Qa-1 antigen. J Exp Med. 1990 Dec 1;172(6):1795–1804. doi: 10.1084/jem.172.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Früh K., Chambers J., Waters J. B., Wu L., Spies T., Peterson P. A. Major histocompatibility complex (MHC)-encoded HAM2 is necessary for antigenic peptide loading onto class I MHC molecules. J Biol Chem. 1992 Jun 15;267(17):11669–11672. [PubMed] [Google Scholar]
- Zhou X., Glas R., Liu T., Ljunggren H. G., Jondal M. Antigen processing mutant T2 cells present viral antigen restricted through H-2Kb. Eur J Immunol. 1993 Aug;23(8):1802–1808. doi: 10.1002/eji.1830230811. [DOI] [PubMed] [Google Scholar]
- Zhou X., Glas R., Momburg F., Hämmerling G. J., Jondal M., Ljunggren H. G. TAP2-defective RMA-S cells present Sendai virus antigen to cytotoxic T lymphocytes. Eur J Immunol. 1993 Aug;23(8):1796–1801. doi: 10.1002/eji.1830230810. [DOI] [PubMed] [Google Scholar]



