Abstract
This study investigated the combination of environmental enrichment (EE) with cocaine-cue extinction training on reacquisition of cocaine self-administration. Rats were trained under a second-order schedule for which responses were maintained by cocaine injections and cocaine-paired stimuli. During three weekly extinction sessions, saline was substituted for cocaine but cocaine-paired stimuli were presented. Rats received 4 h periods of EE at strategic time points during extinction training, or received NoEE. Additional control rats received EE or NoEE without extinction training. One week later, reacquisition of cocaine self-administration was evaluated for 15 sessions and then GluA1 expression, a cellular substrate for learning and memory, was measured in selected brain regions. EE provided both 24 h before and immediately after extinction training facilitated extinction learning and deterred reacquisition of cocaine self-administration for up to 13 sessions. Each intervention by itself (EE alone or extinction alone) was ineffective, as was EE scheduled at individual time points (EE 4 h or 24 h before, or EE immediately or 6 h after, each extinction training session). Under these conditions, rats rapidly reacquired baseline rates of cocaine self-administration. Cocaine self-administration alone decreased total GluA1 and/or pSer845GluA1 expression in basolateral amygdala and nucleus accumbens. Extinction training, with or without EE, opposed these changes and also increased total GluA1 in ventromedial prefrontal cortex and dorsal hippocampus. EE alone increased pSer845GluA1 and EE combined with extinction training decreased pSer845GluA1 in ventromedial prefrontal cortex. EE might be a useful adjunct to extinction therapy by enabling neuroplasticity that deters relapse to cocaine self-administration.
Keywords: cocaine, cocaine-cue extinction learning, environmental enrichment, GluA1 receptor, self-administration, reacquisition
Introduction
Addiction is thought to represent a pathological disruption of mechanisms associated with reward-related learning and memory (Hyman et al. 2006). Through associative learning, drug-paired stimuli (cues) can gain salience and exert a powerful influence over drug-seeking behavior. Even following abstinence, re-exposure to drug-paired cues can serve as a powerful trigger of relapse (Childress et al. 1999). Consequently, extinguishing the impact of cues paired with drug use is an especially important goal for effective treatment of drug addiction. Although cue-exposure (extinction) therapy appears to be minimally effective as a stand-alone treatment for drug addiction (Conklin and Tiffany 2002), preclinical research that employed cognitive-enhancing pharmacotherapy (e.g., d-cycloserine (DCS) or a glycine transporter-1 inhibitor) in conjunction with cocaine-cue extinction training has shown significant reductions in the reacquisition of cocaine self-administration in rats and monkeys (Nic Dhonnchadha et al. 2010; Achat-Mendes et al. 2012; Nic Dhonnchadha et al. 2012). An unexplored question is whether adjunct use of a cognitive-enhancing behavioral strategy with cocaine-cue extinction training is also efficacious in reducing relapse to cocaine self-administration.
Environmental enrichment (EE) improves learning and memory and produces neuroplasticity in the brains of animals and humans (van Praag et al. 2000; Sale et al. 2014). In preclinical studies, EE refers to conditions in which animals have access to social, physical and cognitive stimulation (van Praag et al. 2000). Most studies examining effects of EE in animals trained to self-administer cocaine used relatively long-term enriched housing conditions spanning several weeks (e.g., Chauvet et al. 2009; Thiel et al. 2009; Thiel et al. 2012). To be of practical use in therapeutic settings, however, EE would need to be relatively brief (i.e., on the order of a few hours). The potential efficacy of brief EE interventions is supported by studies showing activation of limbic and cortical sites and improvement of memory after as little as 1 hr of EE (Degroot et al. 2005; Ali et al. 2009). The main goal of the current study was to determine whether brief interventions of EE, provided in 4 h periods, could enhance cocaine-cue extinction learning and reduce subsequent reacquisition of cocaine self-administration in rats with cocaine self-administration histories.
We also measured changes in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) GluA1 at the conclusion of the self-administration reacquisition phase. There is now a sizable literature showing that AMPARs are the primary mediator for neuronal communication underlying basal and higher brain functions. Previous studies have established that the primary cellular means employed for synaptic plasticity, the cellular substrate for learning and memory, is alteration of AMPAR abundance at the postsynaptic domain (Malinow and Malenka 2002; Huganir and Nicoll 2013). Moreover, phosphorylation of AMPAR GluA1 subunits plays an important role in AMPAR trafficking and synaptic function (Lee et al. 2003; Man et al. 2007). Notably, GluA1 and phosphorylated GluA1 at serine 845 (pSer845GluA1) have been implicated previously in cocaine self-administration and cocaine-cue extinction learning (Nic Dhonnchadha et al. 2013; Pierce and Wolf 2013).
Materials and Methods
Subjects
Naïve male Wistar rats (Crl[WI]BR; Charles River Laboratories, USA) approximately 60 days old (276–300 g) upon arrival were housed in individual cages in a temperature- (21–23 °C) and light- (08:00 h on; 20:00 h off) controlled vivarium and had unlimited access to food and water. After 72 h of acclimation, 16–20 g of food was provided daily to maintain rats at approximately 90% of the growth-adjusted free-feeding body weight. Policies and procedures in the 8th edition of the NIH Guide for Care and Use of Laboratory Animals were followed. The Boston University Institutional Animal Care and Use Committee approved all research protocols.
Apparatus
Chambers used for cocaine self-administration and food pellet reinforcement were as previously described (Szalay et al. 2013). Environmental enrichment took place in a 76 × 46 × 74 cm powder-coated wire cage (Super Pet Inc., Walnut Creek, CA, USA) equipped with two running wheels, three levels of ramps and platforms, movable tunnel structures, numerous manipulable items and chew toys. Items were changed each session to maintain novelty. Commercial bedding covered the cage floor, and pieces of sweetened cereal were hidden in various locations throughout the cage to encourage foraging.
Catheter Surgery and Maintenance
Prior to surgery, rats were trained to lever press using a fixed-ratio (FR) 1 schedule of food presentation (45 mg food pellets; Bio-Serv, Frenchtown, NJ, USA). Catheters then were implanted in the right femoral vein as described previously (Szalay et al. 2013) and flushed daily with 30 IU/ml of heparinized 0.9% saline (SAGENT Pharmaceuticals, Schaumburg, IL, USA) and 67 mg/ml of Timentin (Glaxo-SmithKline, Research Triangle Park, NC, USA). Over weekends and holidays, a locking solution consisting of glycerol (Sigma-Aldrich, St. Louis, MO, USA) and 1000 U/ml heparin (3:1) filled the catheter dead space. The solution was removed and replaced with 3.0 IU/ml heparinized 0.9% saline prior to the start of the next behavioral session. Catheters were checked daily for leaks and tested periodically for patency by infusing 1.0 mg/0.1 ml of methohexital sodium (JHP Pharmaceuticals, Rochester, MI, USA), which induces rapid transient loss of muscle tone. Improperly functioning catheters were surgically removed and replaced by a catheter implanted in the left femoral vein.
Cocaine Self-Administration
Intravenous (i.v.) cocaine self-administration sessions began one week after catheterization surgery. Cocaine hydrochloride (NIDA, Bethesda, MD, USA) was dissolved in heparinized 0.9% saline (3.0 IU/ml) to a final concentration of 1.6 mg/ml. Rats were trained initially to self-administer 0.3 mg/kg i.v. injections of cocaine under a FR 1 schedule and then progressed to a FR 5 schedule. Each cocaine injection was paired with a 2-sec cue light. Once self-administration was well established, the schedule was changed to a second-order fixed-interval (FI), fixed-ratio schedule (FI 2 min [FR 5: S]). Under this schedule, every fifth press of the active lever (FR 5) produced the 2-sec cue light (S), and the first FR 5 response unit completed after the 2-min FI elapsed produced the 2-sec cue light paired with an i.v. injection of cocaine. Responses on the inactive lever (right or left, counterbalanced across subjects) were counted separately, but had no scheduled consequences. During 1 h sessions, rats could earn a maximum of 30 infusions. In addition, white noise (70-db) was presented as a cocaine-associated background cue for the duration of each session. Rats reached stable levels of cocaine self-administration under the second-order schedule within 25–35 sessions (Figure 1). During this phase of self-administration training, all rats also were exposed in weekly 15 min periods to the EE chamber and companion animals to ensure that all groups had the same handling history prior to the test phases and to minimize the potential unwanted influence of stress in rats receiving the EE treatment protocol described below.
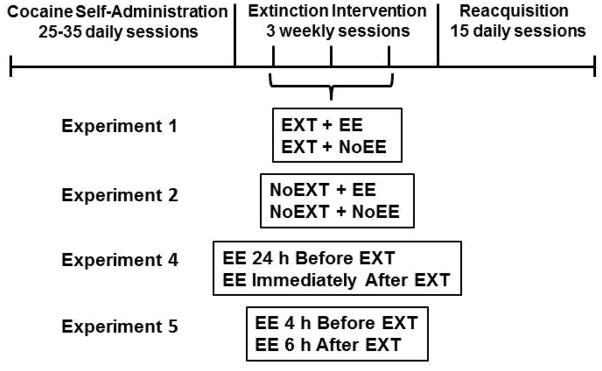
Figure 1.
Schematic showing the experimental timeline with various treatments administered once weekly during the 3-week intervention period. Abbreviations: EXT – extinction; EE – environmental enrichment; NoEXT – no extinction (abstinence); NoEE – no environmental enrichment.
Experiment 1: Evaluation of EE and Extinction Training
Once baseline self-administration performance was established, rats were randomly assigned to treatment for Experiment 1 (Figure 1) and received three weekly 1 h extinction sessions in conjunction with environmental enrichment (EXT + EE; n=19) or no environmental enrichment (EXT + NoEE; n=19). During extinction sessions, lever pressing was extinguished by substituting saline for cocaine while maintaining the presentation of cocaine-associated stimuli, i.e. background white noise and the response-contingent cue light, under the FI 2 min [FR 5: S] schedule. This spaced-extinction procedure is analogous to clinical treatment protocols utilizing weekly 1 h cue-extinction sessions (Hofmann et al. 2015). EE consisted of physical (exercise), social (presence of other rats) and cognitive (novel objects) components, as this combination is thought to provide the best outcome for improving learning and memory (van Praag et al. 2000). For EE, the same three to four rats were placed together during each enrichment period. The EE exposure time was a 4 h brief intervention occurring both 24 h before and immediately after each extinction session. These times were chosen based on previous findings showing that EE used 24 h before extinction training can activate limbic and cortical sites (Ali et al. 2009) and that EE used immediately after extinction training coincides with the time frame for memory consolidation, i.e., up to 4 h post-training (Inda et al. 2005). During the weekly extinction phase, rats remained in their individual home cages at all times except during the extinction sessions and periods of EE. One week following the final weekly extinction session, cocaine was again made available under the second-order schedule, and reacquisition of cocaine self-administration was evaluated for 15 daily sessions.
Experiment 2: Evaluation of Abstinence (No Extinction) Control Conditions
To determine specificity of EE combined with extinction training, Experiment 2 was conducted in two additional groups of rats randomly assigned to treatment and tested under abstinence control conditions following baseline cocaine self-administration training (Figure 1). During three weekly 1 h abstinence sessions, levers were retracted in the chambers, no cocaine-associated stimuli (cue light or white noise) were presented, and no infusions were delivered. Each group also received either environmental enrichment (NoEXT + EE; n=10) or no environmental enrichment (NoEXT + NoEE; n=15) as described above. The abstinence control sessions were followed by 15 cocaine self-administration reacquisition sessions as described above.
Experiment 3: Regional Changes in Total GluA1 and pSer845GluA1 Expression
To evaluate changes in total and pSer845 GluA1 expression, a subset of rats from each of the test groups in Experiments 1 and 2 was sacrificed by guillotine 24 h following the last reacquisition session (EXT + EE n=12; EXT + NoEE n=13; NoEXT + EE n=8; NoEXT + NoEE n=8). In addition, two yoked-saline groups were included for control purposes in the molecular analyses. Rats in the first saline group (n=8) were yoked to rats in the EXT + EE cocaine group and rats in the second saline group (n=6) were yoked to rats in the EXT + NoEE cocaine group. For this procedure, passive delivery of i.v. saline injections and cue light presentations in the yoked-rat was controlled by lever responding in the paired-rat undergoing cocaine self-administration and cocaine-cue extinction training under the second-order schedule. The saline group yoked to the EXT + EE cocaine group also received EE during the extinction phase, as described above. Selected brain areas were rapidly dissected using a coronal brain matrix (RBM-4000C, ASI Instruments, Warren, MI) using methods described previously (Heffner et al. 1980). The basolateral amygdala (BLA), ventromedial prefrontal cortex (vmPFC), nucleus accumbens (NAc), and dorsal hippocampus (DH) were dissected immediately on ice-cooled plates from the 1 mm slices, flash frozen in isopentane and stored at −80C. Western blot analysis was performed as previously described (Lin et al. 2011). The assay used antibodies against GluA1ct diluted at 1:1000 (Lin et al. 2011), pSer845GluA1 diluted at 1:500 (Millipore, Billerica, MA) and tubulin diluted at 1:1000 (Sigma, St. Louis, MO). Membranes were visualized using ECL (Amersham, Piscataway, NJ) and immunointensity was measured using Image J (http://rsbweb.nih.gov/ij/download.html) by a person blinded to the status of the animal. Samples from each behavioral group were processed at the same time, such that each western batch contained tissue from each experimental group. For GluA1 or pSer845GluA1 blots, a band at 110 kD was measured and quantified. All membranes were reprobed for tubulin to indicate protein loading.
Experiment 4: Evaluation of Individual EE Time Points
To compare the individual contribution of each EE exposure period to the combined effect found to be beneficial in Experiment 1, two additional groups of rats were tested in Experiment 4 (Figure 1). The overall experimental design was identical to that of Experiment 1 in that all rats were trained to self-administer 0.3 mg/kg cocaine under the FI 2 min [FR 5: S] schedule after which they received 3 weekly extinction training sessions followed by 15 daily cocaine self-administration reacquisition sessions. However, groups in Experiment 4 received only a single 4 h period of EE either 24 h before (n=8) or immediately after (n=10) each extinction session.
Experiment 5: Evaluation of Alternative EE Time Points
To evaluate the impact of EE scheduled at other time points in conjunction with cocaine-cue extinction training, two final groups of rats were exposed to EE outside the memory consolidation window, which is a period up to 4 h post-training (Inda et al. 2005). After cocaine self-administration baseline training, one group received EE 6 h after (n=10) and the other group received EE 4 h before (n=11) each of three weekly extinction-training sessions (Figure 1). The 6 h post-training time point was selected so that potential time-dependent effects for memory consolidation could be evaluated (Szalay et al. 2013), whereas the 4 h pre-training time point was selected so that the EE session (lasting 4 h) would be completed just before extinction training began. The 15 daily cocaine self-administration reacquisition sessions followed 1 week later.
Data Analysis
Baseline cocaine self-administration behavior was calculated for each subject as the mean of active lever responses, inactive lever responses and infusions earned during the last five sessions of stable cocaine self-administration behavior. Active lever responding during the weekly extinction and daily reacquisition sessions was expressed as percent of baseline due to ~5-fold differences in baseline response rates among individual rats within each test group. Statistical significance was determined by the appropriate one-way or two-way ANOVA, and the Dunnett t-test was used for multiple comparisons. The EXT + NoEE condition was used as the control group for behavioral measures in Experiments 1–2 and 4–5. This control group was plotted in each figure illustrating behavioral effects.
For the western blot analysis, GluA1 intensities first were normalized to their respective tubulin intensities in individual rats. Given no differences in normalized GluA1 between the two yoked-saline groups (total GluA1: t [12] = −1.88 – 0.44, p>0.05; pSer845GluA1: t [12] = 0.03 – 1.39, p>0.05), data were combined to form a single saline control group for each measure and brain region. Student t-tests compared the saline control to the cocaine control (NoEXT + NoEE) to determine the effects of cocaine self-administration alone on GluA1 expression. To determine how extinction, EE, and the combination of extinction and EE impacted GluA1 expression following reacquisition of cocaine self-administration, individual values were normalized to the cocaine control and compared by two-way ANOVA followed by Tukey tests for multiple comparisons in Experiment 3.
Results
Cocaine Self-Administration Baseline
Cocaine self-administration did not differ among the eight groups prior to initiating treatments (Table 1). One-way ANOVAs revealed that the mean number of active (p≤0.81) and inactive (p≤0.19) lever responses was not significantly different across groups. Inactive lever responses were < 25% of active lever responses for each group. The mean number of cocaine infusions also was not significantly different across groups (p≤0.72).
Table 1.
Mean (±SEM) number of responses and infusions per 1 h session during baseline self-administration prior to initiating treatments.
| Treatment Group | Active | Inactive | Infusions | |
|---|---|---|---|---|
| Experiment 1 | EXT + EE | 320.6 ± 34.4 | 50.6 ± 12.7 | 20.5 ± 0.9 |
| EXT + NoEE | 280.3 ± 18.7 | 31.7 ± 6.1 | 19.8 ± 0.5 | |
|
| ||||
| Experiment 2 | NoEXT + EE | 292.1 ± 46.5 | 31.0 ± 13.7 | 20.2 ± 0.9 |
| NoEXT + NoEE | 277.3 ± 32.8 | 36.7 ± 11.1 | 19.0 ± 0.9 | |
|
| ||||
| Experiment 4 | EE 24 h Before EXT | 320.6 ± 68.0 | 40.6 ± 18.6 | 19.5 ± 1.4 |
| EE Immediately After EXT | 284.6 ± 56.9 | 70.0 ± 20.7 | 20.1 ± 1.0 | |
|
| ||||
| Experiment 5 | EE 4 h Before EXT | 230.6 ± 30.3 | 39.4 ± 12.0 | 18.4 ± 0.8 |
| EE 6 h After EXT | 288.7 ± 42.2 | 18.0 ± 5.2 | 20.0 ± 0.7 | |
Experiment 1: Evaluation of EE and Extinction Training
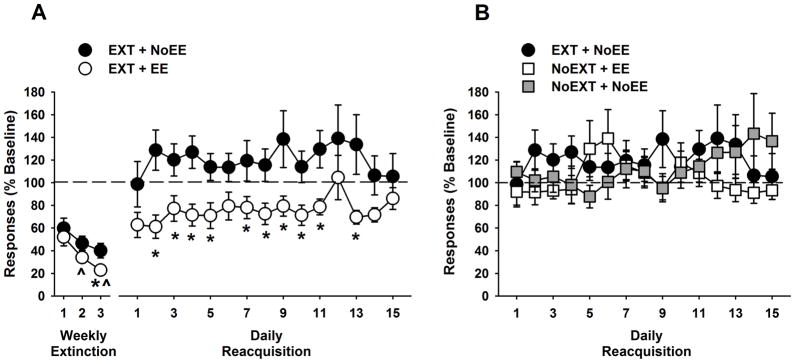
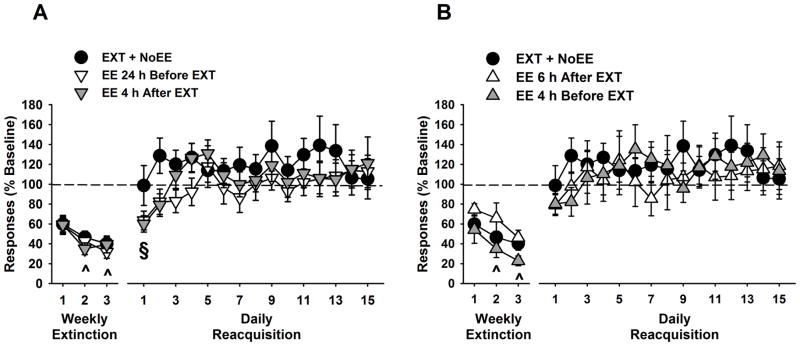
Responding declined over extinction sessions (F [2,72] = 13.7, p≤0.001), but was significantly lower (p≤0.04) in the EXT + EE group compared with the EXT + NoEE group during the third extinction session (Figure 2 A). During reacquisition sessions, treatment was a significant factor in the 2-way ANOVA (F [1,26] = 7.4, p≤0.01), and further testing showed that responding was significantly lower (p≤0.03) in the EXT + EE group compared with the EXT + NoEE group during reacquisition sessions 2–5, 7–11 and 13 (Figure 2 A). Cocaine intake during the reacquisition period varied by extinction treatment and session (F [14,364] = 1.9, p≤0.03; treatment x session interaction). The EXT + EE group received significantly fewer (p≤0.04) cocaine infusions compared with the EXT + NoEE group during reacquisition sessions 1–6 (14 vs. 20 infusions per session on average, respectively) and during reacquisition sessions 9–10 (17 vs. 21 infusions per session on average respectively).
Figure 2.
Effects of EE and NoEE in combination with EXT (A) or NoEXT (B) on responding during weekly extinction training sessions and daily cocaine self-administration reacquisition sessions. Data are the mean (± SEM) expressed as percent of baseline responses and shown relative to the EXT + NoEE control. ^ p<0.05 compared to extinction session 1 (main effect of session); * p<0.05 compared to the EXT + NoEE control group.
Experiment 2: Evaluation of Abstinence (No Extinction) Control Conditions
Under abstinence control conditions, EE provided without extinction training (NoEXT + EE) did not reduce reacquisition of cocaine self-administration (Figure 2 B) and no ANOVA factors were significant between treatments or over sessions (ps≥0.13). Rats reacquired baseline rates of cocaine self-administration during the 1st reacquisition session, and responding over the reacquisition period was comparable to no treatment (NoEXT + NoEE) or to extinction training without EE (EXT + NoEE). Cocaine infusions averaged 19 and 22 infusions per session in the NoEXT + EE and NoEXT + NoEE groups, respectively.
Experiment 3: Regional Changes in Total GluA1 and pSer845GluA1 Expression
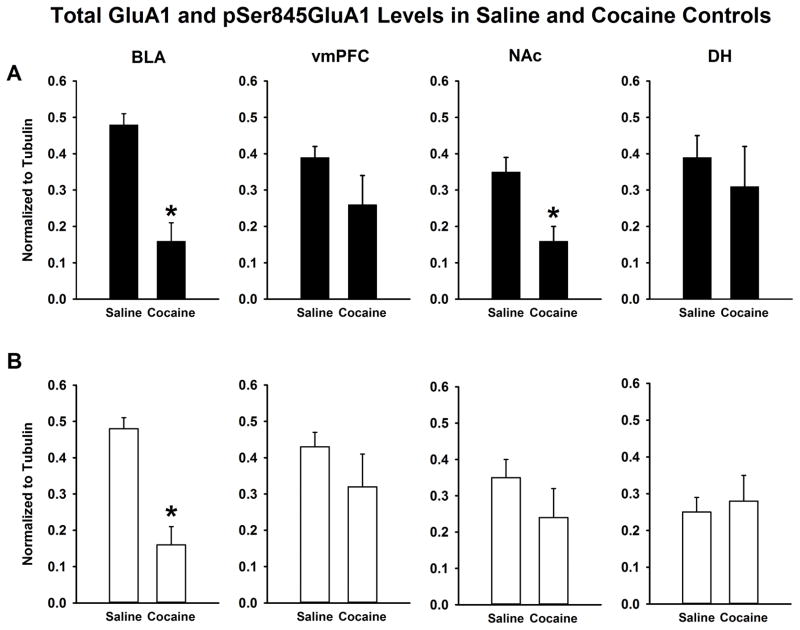
Cocaine self-administration alone reduced total GluA1 and pSer845GluA1 in a region-dependent manner (Figure 3). Compared to the yoked-saline control group that did not have a history of cocaine self-administration, the NoEXT + NoEE group with a history of cocaine self-administration had reduced expression of total GluA1 in BLA (t [20]= 5.60 p≤0.001) and NAc (t [20] = 3.32; p≤0.001), but not in vmPFC or DH. Expression of pSer845GluA1 in BLA also was lower in the NoEXT + NoEE cocaine group (t [20] = 3.90 p≤0.001), with no differences from the yoked-saline control group in vmPFC, NAc or DH.
Figure 3.
Total GluA1 (A) and pSer845GluA1 (B) protein expression in BLA, vmPFC, NAc and DH of the saline (yoked-saline) and cocaine (NoEXT + NoEE) control groups. Data are the mean (± SEM) values normalized to tubulin. * p<0.05 compared to the saline control.
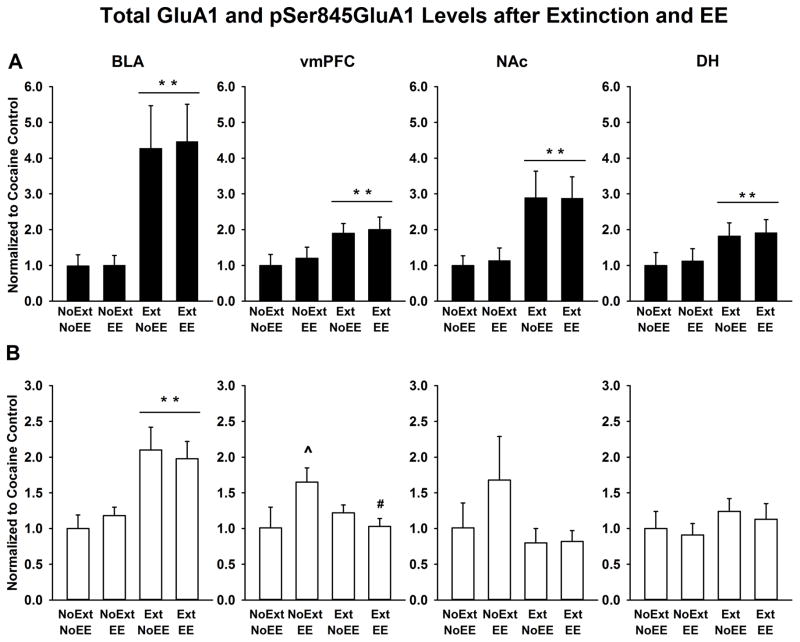
In animals with a history of cocaine self-administration, extinction, EE, and the combination of extinction and EE altered total GluA1 and pSer845GluA1 in a region-dependent manner as well (Figure 4). Several main effects of extinction training were revealed by 2-way ANOVAs. Compared to groups that did not receive extinction training (NoEXT + NoEE and NoEXT + EE), groups that received extinction training (EXT + NoEE and EXT + EE) showed increased total GluA1 in BLA (F [1,37] = 10.9, p≤0.002) and NAc (F [1,37] = 8.3, p≤0.006), and increased pSer845GluA1 in BLA (F [1,37] = 13.7, p ≤ 0.001), thus opposing the effects of cocaine self-administration alone. Extinction training with or without EE also increased total GluA1 in vmPFC (F [1,37] = 6.9, p≤0.01) and DH (F [1,37] = 4.8, p≤0.03) compared to no extinction training. EE had a specific influence only in vmPFC, for which an extinction x EE interaction was found (F [1,37] = 5.8, p≤0.02). Further testing showed that EE alone (NoEXT + EE) significantly (p≤0.02) increased pSer845GluA1 compared to the cocaine control (NoEXT + NoEE) and that EE combined with extinction training (EXT + EE) significantly (p≤0.02) reduced pSer845GluA1 compared to EE alone (NoEXT + EE). No individual molecular measure in any single brain region correlated with the behavioral measures obtained during the reacquisition phase.
Figure 4.
Total GluA1 (A) and pSer845GluA1 (B) protein expression in BLA, vmPFC, NAc and DH of the NoEXT + NoEE (cocaine control), NoEXT + EE (EE alone), EXT + NoEE (extinction alone), and EXT + EE (extinction combined with EE) groups. Data are the mean (± SEM) values normalized to the cocaine control. ** p<0.05 compared to NoEXT (main effect of EXT); ^ p<0.05 compared to the cocaine control; # p<0.05 compared to EE alone.
Experiment 4: Evaluation of Individual EE Time Points
As in the EXT + NoEE group, responses declined over extinction sessions in the groups receiving EE either 24 h before or immediately after extinction training (F [2,54] = 7.9, p≤0.001), but there were no differences between groups during any of the weekly sessions (Figure 5 A). During the reacquisition phase, groups receiving EE either 24 h before or immediately after extinction training did not show reduced reacquisition of cocaine self-administration compared to the EXT + NoEE group (Figure 5 A). All three groups reacquired baseline rates of cocaine self-administration during the 1st or 2nd reacquisition session. However, ANOVA revealed that session was a significant factor (F [14,392] = 2.4, p≤0.003), and across the three groups overall, there was less responding during the first reacquisition session compared with all other reacquisition sessions (p≤0.05). Cocaine intake during the reacquisition period also varied by session (F [14,392] = 4.2, p≤0.001), with overall fewer cocaine infusions during the first reacquisition session compared with all other reacquisition sessions (p≤0.05). Cocaine infusions averaged 19 infusions per session in the groups receiving EE either 24 h before or immediately after extinction sessions.
Figure 5.
Effects of EE provided 24 h before or immediately after each of three weekly extinction sessions (A) or provided 4 h before or 6 h after each of three weekly extinction sessions (B) on responding during weekly extinction training sessions and daily cocaine self-administration reacquisition sessions. Data are the mean (± SEM) expressed as percent of baseline responses and shown relative to the EXT + NoEE control. ^ p<0.05 compared to extinction session 1 (main effect of session). § p<0.05 compared to reacquisition sessions 2–15 (main effect of session).
Experiment 5: Evaluation of Alternative EE Time Points
In groups receiving EE either 4 h before or 6 h after each extinction training session, there was a similar decline in active lever responding relative to the EXT + NoEE group across weekly extinction sessions (F [2,64] = 10.9, p≤0.001), but there were no treatment differences during any of the weekly extinction sessions (Figure 5 B). Moreover, both EE groups in this experiment reacquired baseline rates of cocaine self-administration during the 1st reacquisition session, and responding over the reacquisition period did not differ from that of the EXT + NoEE group (Figure 5 B). Cocaine intake during the reacquisition period varied by session (F [14, 448] = 2.4, p≤0.003), with overall fewer cocaine infusions during the first reacquisition session compared with all other reacquisition sessions (p≤0.05). Cocaine infusions averaged 17 and 19 infusions per session in the groups receiving EE either 4 h before or 6 h after extinction sessions, respectively.
Discussion
The present findings show that brief periods of EE scheduled at suitable times in combination with cocaine-cue extinction sessions facilitated the rate of extinction learning and deterred subsequent reacquisition of cocaine self-administration. Each intervention by itself (EXT alone or EE alone), however, was ineffective in deterring reacquisition of cocaine self-administration. The outcome with EE alone is not surprising in light of evidence showing that long periods of EE provided during drug abstinence does not protect against reinstated cocaine-seeking behavior elicited by a cocaine priming injection (Chauvet et al. 2009; Thiel et al. 2009). Long periods of EE provided during drug abstinence, however, can reduce reinstated cocaine-seeking behavior elicited by cues or stress (Chauvet et al. 2009; Thiel et al. 2012), suggesting that EE alone may help lessen the impact of some relapse triggers. In the present study, strategic use of brief EE interventions scheduled both 24 h before and immediately after cocaine-cue extinction training significantly deterred reacquisition of cocaine self-administration. When one of these EE periods was eliminated, however, the protective effects of EE were lost. Furthermore, EE provided 4 h before or 6 h after extinction training, which are time points outside the memory consolidation window (Inda et al. 2005), also was ineffective in facilitating extinction learning or deterring reacquisition of cocaine self-administration. These findings suggest temporal specificity for the effects of EE and imply that time-dependent processes for neural activation (EE 24 h before extinction) and memory consolidation (EE immediately after extinction) combined may be critical to the beneficial effects of EE.
Our molecular analysis provides insights into the brain mechanisms that may underlie the effects of cocaine self-administration and of the effects of EE combined with extinction training for facilitating extinction learning and deterring reacquisition of cocaine self-administration. Our results show that a history of cocaine self-administration alone can reduce the expression of total GluA1 in NAc and the expression of total GluA1 and pSer845GluA1 in BLA. Others have reported a similar reduction in total GluA1 expression in NAc 24 h after the last cocaine self-administration session (Conrad et al. 2008), and the present study is the first to report reduced total and phosphorylated GluA1 expression in BLA 24 h after the last cocaine self-administration session. The functional significance of GluA1 reductions detected in close temporal proximity to the last cocaine self-administration session may relate to cocaine-induced impairments in learning and memory that are typically observed during early phases of cocaine abstinence in people (Potvin et al. 2014) and may be important for maintaining an addictive state (Volkow and Fowler 2000). In support of this view, loss of GluA1 can impair the ability of synapses to undergo long-term potentiation, which is an essential process for learning and memory (Malinow and Malenka 2002).
There is evidence that extinction training induces extensive synaptic reorganization, including up-regulation of AMPAR GluA1 (Sutton et al. 2003; Self et al. 2004). We found that 3 weekly sessions of cocaine-cue extinction training prior to the reacquisition test phase increased total GluA1 in BLA, vmPFC, NAc and DH, regardless of EE or NoEE exposure. However, others showed that when cocaine-cue extinction training was provided more frequently (e.g. daily for 10 or 22 sessions), total GluA1 was either unaltered (Zavala et al. 2007) or was elevated only in NAc (Ghasemzadeh et al. 2009). These findings support the use of weekly-spaced extinction training sessions (analogous to that used in some clinical situations; e.g. Hofmann et al. 2015) to optimize synaptic plasticity that opposes the effects of cocaine self-administration. Along these lines, GluA1 phosphorylation at Ser845 is crucial for AMPAR synaptic insertion and is correlated with induction of both long-term potentiation and long-term depression (Lee et al. 2000; Man et al. 2007). Thus, the increase in BLA pSer845GluA1 induced by extinction training may contribute to the consolidation of extinction memory (Peña et al. 2014), which presumably competed with the cocaine self-administration memory during the reacquisition phase of our study.
However, deterred reacquisition of cocaine self-administration in rats receiving extinction training combined with optimally scheduled EE cannot be accounted for solely by extinction-induced increases in total GluA1 and pSer845GluA1 expression in BLA and in total GluA1 expression in vmPFC, NAc, and DH. Rats receiving extinction training without EE had similar increases, but nonetheless rapidly reacquired baseline rates of cocaine self-administration. The effects of EE are complex, but relatively brief periods of EE exposure (e.g., for a total of 21 h over a 3-week period) can lead to significant synaptic reorganization throughout the cortex (Pinaud et al. 2001). As rats in the current study received the optimal EE protocol for a total of 24 h, it is reasonable to expect cortical changes in pSer845GluA1. Of relevance are findings in rats showing that facilitation of fear extinction learning requires plasticity in both the vmPFC and BLA (Akirav and Maroun 2007). In the present study, the combination of extinction training with EE altered pSer845GluA1 in both vmPFC and BLA, whereas extinction training without EE altered pSer845GluA1 only in BLA and EE without extinction training altered pSer845GluA1 only in vmPFC. It is not surprising, therefore, that no individual molecular measure in any single brain region correlated with the behavioral measures obtained during the reacquisition phase. The combined molecular changes in vmPFC and BLA may contribute to the facilitation of extinction learning as well as to the deterred reacquisition of cocaine self-administration observed in rats receiving extinction training combined with optimally scheduled EE. How the combined changes in pSer845GluA1 (decrease in vmPFC; increase in BLA) may contribute to these behavioral changes currently is not clear. Further research investigating AMPAR synaptic and surface expression, as well as its role in synaptic plasticity is needed to determine the functional significance of the observed changes in receptor abundance and phosphorylation. In this regard, an additional protein target to consider for understanding the role of AMPAR GluA1 in the combined effects of EE and extinction training is TrkB. TrkB is a receptor for brain derived neurotropic factor, an important regulator of AMPAR trafficking and synaptic plasticity (Li and Wolf 2011) and also implicated in cocaine self-administration, extinction learning and EE exposure (Franklin et al. 2006; Graham et al. 2007; Morrison and Ressler 2014). Also of importance may be the degree to which AMPAR changes occur on principle neurons or on GABA interneurons. For example, GluA1-containing AMPARs are located primarily on GABA interneurons in BLA to regulate excitability of BLA pyramidal neurons and control BLA output (McDonald 1996). Moreover, glutamatergic efferents from vmPFC synapse on GABA interneurons in BLA to regulate BLA function (Rosenkranz and Grace 2002).
In the present study, we evaluated cocaine self-administration and cocaine-cue extinction under a second-order schedule for which all sessions began with a cue-only component. During the initial cue-only component of the first extinction training session, rats received 2.1 ± 0.1 cue light presentations under the FR 5 contingency and alongside the background of cocaine-associated white noise. An anticipated injection of cocaine at the end of the initial cue-only component was withheld and immediately followed by extinction training. This aspect of our experimental design is notable because recently it has been emphasized that successful augmentation of extinction therapy in people relies on adherence to several key procedural steps, including pre-exposing subjects to cues before extinction therapy sessions begin (Hofmann et al. 2015). Exposure to cues prior to extinction training has been shown to facilitate the reduction of intrusive memories (Monfils et al. 2009; Clem and Huganir 2010). Using this type of strategy, a recent study showed that the cognitive-enhancing drug DCS significantly augmented alcohol-cue extinction learning and forestalled reacquisition of alcohol use in participants with Alcohol Use Disorder (MacKillop et al. 2015). Most clinical work that administered DCS before drug-cue exposure therapy lacked an episode of cue pre-exposure before the start of extinction training and found that extinction was not augmented and that relapse was not deterred (e.g., Price et al. 2009; Santa Ana et al. 2009; Watson et al. 2011; Hofmann et al. 2012; Price et al. 2013; Santa Ana et al. 2015). Furthermore, these studies also spaced exposure therapy sessions at too short or long an interval relative to the optimal 1-week period described by Hoffman colleagues (Hofmann et al. 2015). Thus, in the present study, we followed an approach similar to the optimized clinical procedure (cue pre-exposure; weekly extinction training sessions) to evaluate the effects of EE combined with extinction training for the deterrence of cocaine relapse.
In conclusion, this study provides new preclinical evidence that appropriately scheduled brief interventions with EE combined with cocaine-cue extinction training can facilitate extinction learning and deter subsequent reacquisition of cocaine self-administration. Changes in AMPAR GluA1 abundance and phosphorylation at Ser845 in BLA and vmPFC may contribute to these beneficial effects. The strategic use of EE as brief, rather than long-term, interventions is especially interesting from a clinical perspective. Whereas the particular composition of EE used in our research was selected for beneficial effects in rats, the conceptual basis of our EE protocol (physical exercise, social interaction, and cognitive stimulation) is documented to provide an enriching experience in humans (e.g., Miller et al. 2013) and might be readily translatable to clinical settings to augment the efficacy of drug-cue exposure therapy.
Acknowledgments
These studies were supported by NSF grant SMA-0835976 to the CELEST Science of Learning Center at Boston University and by NIH grants DA024315 (KMK) and MH079407 (HYM). We thank Iris Mile, Zachary Silber, Sharone Moverman, and Enjana Bylykbashi for expert technical assistance.
Footnotes
The authors declare no competing financial interests.
Authors Contribution
JMG, AL, BND performed the experiments and statistical analysis. JMG wrote the manuscript. KMK, RDS, and HYM were responsible for study concept and design, and provided important intellectual content in the writing of the report.
References
- Achat-Mendes C, Nic Dhonnchadha BÁ, Platt DM, Kantak KM, Spealman RD. Glycine transporter-1 inhibition preceding extinction training inhibits reacquisition of cocaine seeking. Neuropsychopharmacology. 2012;37:2837–2845. doi: 10.1038/npp.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AE, Wilson YM, Murphy M. A single exposure to an enriched environment stimulates the activation of discrete neuronal populations in the brain of the fos-tau-lacZ mouse. Neurobiol Learn Mem. 2009;92:381–390. doi: 10.1016/j.nlm.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Wolff MC, Nomikos GG. Acute exposure to a novel object during consolidation enhances cognition. Neuroreport. 2005;16:63–67. doi: 10.1097/00001756-200501190-00015. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Murphy JA, Myers TL, Clarke DB, Currie RW. Enriched environment during adolescence changes brain-derived neurotrophic factor and TrkB levels in the rat visual system but does not offer neuroprotection to retinal ganglion cells following axotomy. Brain Res. 2006;1095:1–11. doi: 10.1016/j.brainres.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009;1267:89–102. doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Hüweler R, MacKillop J, Kantak KM. Effects of D-cycloserine on craving to alcohol cues in problem drinkers: preliminary findings. Am J Drug Alcohol Abuse. 2012;38:101–107. doi: 10.3109/00952990.2011.600396. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Otto MW, Pollack MH, Smits JA. D-cycloserine augmentation of cognitive behavioral therapy for anxiety disorders: an update. Curr Psychiatry Rep. 2015;17:532. doi: 10.1007/s11920-014-0532-2. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Inda MC, Delgado-García JM, Carrión AM. Acquisition, consolidation, reconsolidation, and extinction of eyelid conditioning responses require de novo protein synthesis. J Neurosci. 2005;25:2070–2080. doi: 10.1523/jneurosci.4163-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/S0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Li X, Wolf ME. Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. Eur J Neurosci. 2011;34:190–198. doi: 10.1111/j.1460-9568.2011.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Hou Q, Jarzylo L, Amato S, Gilbert J, Shang F, Man HY. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J Neurochem. 2011;119:27–39. doi: 10.1111/j.1471-4159.2011.07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Localization of AMPA glutamate receptor subunits in subpopulations of non-pyramidal neurons in the rat basolateral amygdala. Neurosci Lett. 1996;208:175–178. doi: 10.1016/0304-3940(96)12585-4. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Few LR, Stojek MK, Murphy CM, Malutinok SF, Johnson FT, Hofmann SG, McGeary JE, Swift RM, Monti PM. D-cycloserine to enhance extinction of cue-elicited craving for alcohol: a translational approach. Transl Psychiatry. 2015;5:e544. doi: 10.1038/tp.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci. 2007;27:104, 3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Colella B, Mikulis D, Maller J, Green RE. Environmental enrichment may protect against hippocampal atrophy in the chronic stages of traumatic brain injury. Front Hum Neurosci. 2013;7:506. doi: 10.3389/fnhum.2013.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison FG, Ressler KJ. From the neurobiology of extinction to improved clinical treatments. Depress Anxiety. 2014;31:279–290. doi: 10.1002/da.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BÁ, Lin A, Leite-Morris KA, Kaplan GB, Man HY, Kantak KM. Alterations in expression and phosphorylation of GluA1 receptors following cocaine-cue extinction learning. Behav Brain Res. 2013;238:119–123. doi: 10.1016/j.bbr.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BÁ, Pinard E, Alberati D, Wettstein JG, Spealman RD, Kantak KM. Inhibiting glycine transporter-1 facilitates cocaine-cue extinction and attenuates reacquisition of cocaine-seeking behavior. Drug Alcohol Depend. 2012;122:119–126. doi: 10.1016/j.drugalcdep.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BÁ, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci. 2014;18:8, 327. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harb Perspect Med. 2013;3:a012021. doi: 10.1101/cshperspect.a012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Penner MR, Robertson HA, Currie RW. Upregulation of the immediate early gene arc in the brains of rats exposed to environmental enrichment: implications for molecular plasticity. Brain Res Mol Brain Res. 2001;91:50–56. doi: 10.1016/S0169-328X(01)00121-8. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. J Addict Med. 2014;8:368–376. doi: 10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Price KL, Baker NL, McRae-Clark AL, Saladin ME, Desantis SM, Santa Ana EJ, Brady KT. A randomized, placebo-controlled laboratory study of the effects of D-cycloserine on craving in cocaine-dependent individuals. Psychopharmacology (Berl) 2013;226:739–746. doi: 10.1007/s00213-011-2592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KL, McRae-Clark AL, Saladin ME, Maria MM, DeSantis SM, Back SE, Brady KT. D-cycloserine and cocaine cue reactivity: preliminary findings. Am J Drug Alcohol Abuse. 2009;35:434–438. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 1999;19:11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Berardi N, Maffei L. Environment and Brain Plasticity: Towards an Endogenous Pharmacotherapy. Physiol Rev. 2014;94:189–234. doi: 10.1152/physrev.00036.2012. [DOI] [PubMed] [Google Scholar]
- Santa Ana EJ, Prisciandaro JJ, Saladin ME, McRae-Clark AL, Shaftman SR, Nietert PJ, Brady KT. D-cycloserine combined with cue exposure therapy fails to attenuate subjective and physiological craving in cocaine dependence. Am J Addict. 2015;24:217–224. doi: 10.1111/ajad.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, Gonsai K, Hill KP, Carroll KM. D-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Szalay JJ, Jordan CJ, Kantak KM. Neural regulation of the time course for cocaine-cue extinction consolidation in rats. Eur J Neurosci. 2013;37:269–277. doi: 10.1111/ejn.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, Neisewander JL. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol. 2012;17:365–377. doi: 10.1111/j.1369-1600.2011.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Watson BJ, Wilson S, Griffin L, Kalk NJ, Taylor LG, Munafò MR, Lingford-Hughes AR, Nutt DJ. A pilot study of the effectiveness of D-cycloserine during cue-exposure therapy in abstinent alcohol-dependent subjects. Psychopharmacology (Berl) 2011;216:121–129. doi: 10.1007/s00213-011-2199-2. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145(2):438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]