Abstract
Rationale
Enhanced activation of the mineralocorticoid receptors (MR) in cardiovascular tissues increases oxidative stress, maladaptive immune responses and inflammation with associated functional vascular abnormalities. We previously demonstrated that consumption of a Western Diet (WD) for 16 weeks results in aortic stiffening, and that these abnormalities were prevented by systemic MR blockade in female mice. However, the cell specific role of endothelial MR (ECMR) in these maladaptive vascular effects has not been explored.
Objective
We hypothesized that specific deletion of the ECMR would prevent WD-induced increases in endothelial sodium channel (ENaC) activation, reductions in bioavailable nitric oxide (NO), increased vascular remodeling and associated increases in vascular stiffness in females.
Methods and Results
Four week-old female ECMR knockout and wild type mice were fed either mouse chow or WD for 16 weeks. WD feeding resulted in aortic stiffness and endothelial dysfunction as determined in vivo by pulse wave velocity (PWV) and ex vivo by atomic force microscopy, and wire and pressure myography. The WD-induced aortic stiffness was associated with enhanced ENaC activation, attenuated endothelial NO synthase (eNOS) activation, increased oxidative stress, a pro-inflammatory immune response and fibrosis. Conversely, cell specific ECMR deficiency prevented WD-induced aortic fibrosis and stiffness in conjunction with reductions in ENaC activation, oxidative stress and macrophage pro-inflammatory polarization, restoration of eNOS activation.
Conclusions
Increased ECMR signaling associated with consumption of a WD plays a key role in endothelial ENaC activation, reduced NO production, oxidative stress, and inflammation that lead to aortic remodeling and stiffness in female mice.
Keywords: Vascular stiffness, endothelial cell mineralocorticoid receptor, inflammation, endothelial dysfunction, nitric oxide, stiffness
INTRODUCTION
It is well accepted that obesity is associated with increased arterial stiffness, which is a prognosticator for increased cardiovascular disease (CVD).1-3 In this context, data from the Framingham Heart Study including an analysis of 2232 participants support that arterial stiffness is an independent predictor of CVD morbidity and mortality in the general population, hypertensive patients, the elderly, and patients with end-stage renal disease.4 Obesity is promoted by consumption of a Western Diet (WD) high in fat and refined carbohydrates.2, 3 There is accumulating evidence that plasma aldosterone levels are higher in overweight and hypertensive women and that the elevated plasma aldosterone is positively associated with cardiac and vascular dysfunction in females but not in males.5-8 Mineralocorticoid excess and enhanced mineralocorticoid receptor (MR) activation promote oxidative stress, inflammation, endothelial dysfunction, arterial remodeling as well as fibrosis.3, 9
We recently reported that consumption of a WD contributed to both impairments in cardiac diastolic relaxation and aortic stiffening in young female mice, abnormalities that were prevented by MR antagonism.3, 9 One fundamental understanding is that MR mediates the WD-mediated attenuation of endothelial nitric oxide synthase (eNOS) activity and the increase in reactive oxygen species (ROS) production that mediates the destruction of nitric oxide (NO) leading to reduced bioavailable NO in the development cardiac and vascular stiffness.3 There is emerging evidence that enhanced MR activation increases activation of serum-and glucocorticoid-regulated kinase 1(SGK1)10, 11 and epithelial Na+ channel (ENaC) expression on the endothelial cell (EC) surface. This, in turn, leads to reduction of NO production and bioavailability which, in turn, increases cortical stiffness of the cytoskeleton.12, 13 Thus, cell specific endothelial MR signaling may play an important role in the pathogenesis of cardiovascular stiffness. We hypothesized that consumption of a WD would promote endothelial cell MR (ECMR) mediated aortic stiffness via increases in endothelial ENaC expression, reductions in eNOS activity, enhanced oxidative stress, maladaptive inflammation and subsequent vascular remodeling. The corollary to this hypothesis was that specific ECMR knockout (KO) (ECMR−/−) mice, driven by a VE-cadherin promoter, would prevent WD-induced aortic pathophysiological changes and associated aortic stiffness.
METHODS
Animals and treatments
ECMR−/− mice were generated by crossing MRf/f mice with VE-Cad-Cre+ mice as previously described.14 MRf/f Cad-Cre− littermates were used as controls. In these mice, Exon 5 and Exon 6 of the MR gene are flanked by loxP sites via homologous recombination (MRf/f), as previously described.14, 15 All procedures were approved in advance by the Institutional Animal Care and Use Committee of the University of Missouri and the Harry S. Truman VA Research Center and mice were cared for according to NIH guidelines. Groups of four-week-old female mice were fed a WD consisting of high fat (46%) and a high carbohydrate component as constituted with sucrose (17.5%) and high fructose corn syrup (17.5%) and water with or without amiloride (1mg/kg/d) for 16 weeks.16 Parallel groups of age-matched female controls (ECMR+/+) were fed regular mouse chow (CD) for the same period of time.
Aortic stiffness by pulse wave velocity (PWV) in vivo
Doppler ultrasound (Indus Mouse Doppler System, Webster, TX) was performed on mice according to a previously established protocol to evaluate pulse wave velocity (PWV).3
Atomic force microscopy (AFM) imaging and force measurement
ECs were isolated using anti-PECAM-1 antibody-conjugated Dynabeads as previously described.17 To evaluate the stiffness of the endothelium in aortic preparations, a 2×2 mm segment of the thoracic aorta was obtained from mice following the 16-week experimental period. The aorta was opened longitudinally and the adventitial surface of each explant was fastened to a glass cover slip using Cell Tak allowing enface access by the AFM to the EC surface. Stiffness of the EC surface was measured by AFM. The stiffness of EC within intact aortic explants from mice and primary cultured ECs was measured using a nano-indentation protocol with AFM according to previously described procedures.3, 18
Ex vivo aortic activity and flow-induced dilation
Aortic mechanical activity was measured by wire myography as previously described.3 Mesenteric resistance arteries were isolated and cannulated onto glass micropipettes, pressurized at 70 mmHg without flow, and warmed to 37°C in commercial pressure myograph chambers (Living Systems Instrumentation, Burlington, VT, USA) as previously described.3, 19, 20
Chromatin immunoprecipitation (ChIP) and quantitative RT-PCR
ChIP analysis and qPCR were carried out as previously described.3, 21
Western blot and slot blot
Protein expression was measured by western blot as previously described.3 For slot blot, 5 μg proteins were added in the well of a slot bolt apparatus (Hoefer Inc, Holliston, MA). Nitrocellulose was removed and blocked with 5% dry milk in tris-buffered saline and tween 20 and subsequently was incubated overnight at 4°C with blocking buffer containing antibodies to 3-nitrotyrosine (3-NT) (Millipore, Billerica, MA).
Aortic remodeling and fibrosis
Aortic remolding was evaluated by immunostaining and transmission electron microscopy as previously described.3, 15
Statistical analysis
Histologic data were collected by genotype- and treatment-blinded investigators. Data are reported as means ± SEM. Differences in outcomes were determined using one- or two-way ANOVA and paired t tests and were considered significant when p< 0.05. All statistical analyses were performed using Sigma Plot (version 12) software (Systat Software).
RESULTS
ECMR−/− prevents aortic and endothelium stiffness
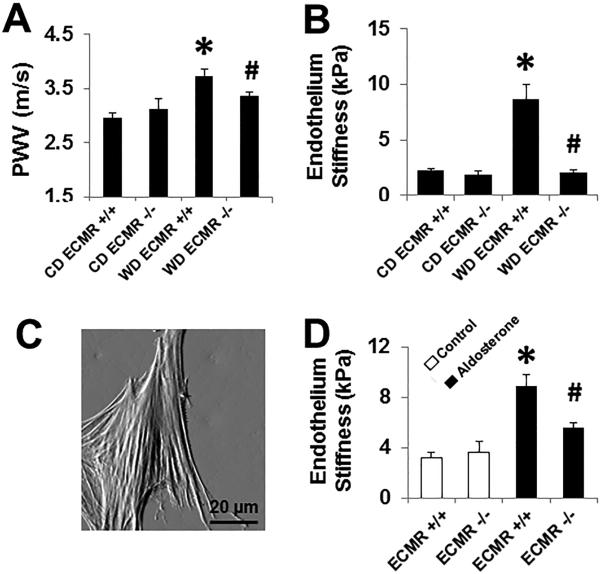
As previously reported, tthe MR gene is specifically and completely recombined in ECs from Cre+ mice and MR mRNA is significantly reduced in primary ECs cultured from mouse lungs and hearts, but not in leukocytes from ECMR KO mice.14 Consumption of a WD for 16 weeks by ECMR+/+ mice induced increases in whole body fat mass, a more than six fold increase in peri-reproductive fat mass and insulin resistance compared to CD-fed mice.15 There were no significant difference in WD-induced changes in body composition or insulin sensitivity in ECMR−/− vs ECMR+/+ mice.15 Also, there were also no significant differences in lean body weight or mean arterial pressures (MAP) between any of the groups.15 WD induced increases in in vivo PWV and ex vivo endothelium stiffness in ECMR+/+ and these effects were prevented in WD ECMR−/− (Fig. 1A and 1B). Both measures were unaffected by CD feeding for both ECMR−/− and ECMR+/+ mice (Fig. 1A). To verify the effects observed on stiffness were aldosterone-MR dependent, we isolated and cultured endothelial cells by anti-PECAM-1 antibody-conjugated Dynabeads and then exposed them to aldosterone (10−8M). ECMR−/− significantly inhibited aldosterone-induced an increased in ECMR+/+ EC stiffness in vitro (Fig. 1C). These data are consistent with improved endothelium and aortic stiffness in WD ECMR−/− mice.
Figure 1. WD induced EC stiffness and aortic relaxation dysfunction is prevented in ECMR−/− female mice.
(A) Aortic pulse wave velocity (PWV) measured in vivo after 16 week feeding trial on WD. Values are mean± SE; n=6 to 10 per group. (B) The ex vivo measurement of endothelium stiffness by using atomic force microscopy. n=4-5 per group. (C) A representative deflection image in EC shows clear stress fibers in the cytoskeleton. ECMR−/− prevented aldosterone treatment (10−8 M) - induced cultured EC stiffness in vitro. Values are mean±SE; n=6 per group. *P<0.05 compared with CD ECMR+/+; # P<0.05 compared with WD ECMR+/+.
ECMR modulates ENaC expression
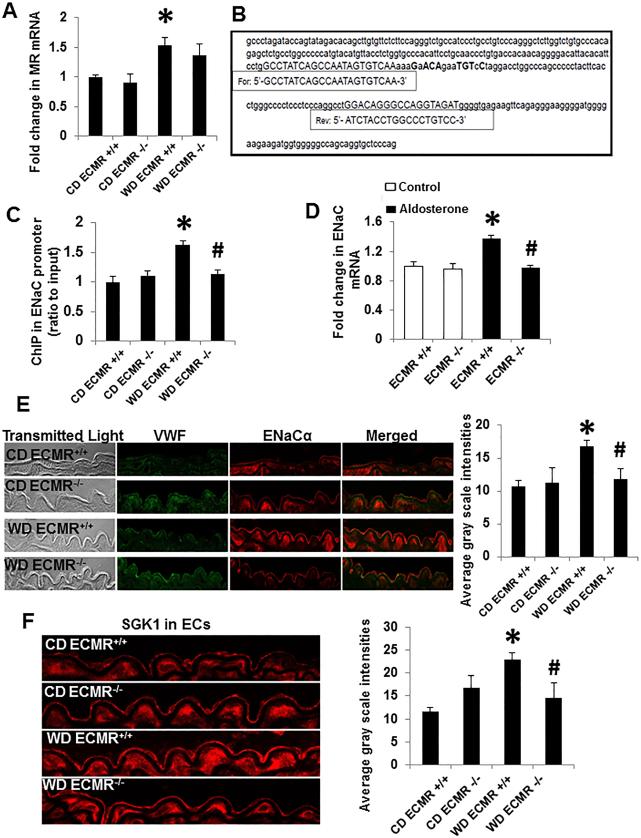
Recent data suggest endothelial ENaC is tightly regulated by MR signaling and also promoted by increased serum sodium thereby promoting cell swelling, stiffness and impaired relaxation. In this context, WD feeding in ECMR+/+ mice was associated with a significant increase in expression of MR (Fig. 2A) and its binding to the ENaC promoter, which contains at least 6 of 8 conserved nucleotides of the consensus sequence (NGNACAnnnTGTNCN) (Fig. 2B and 2C).22 WD indirectly induced an increase in ENaC promoter activity (Fig. 2C) and ENaC expression with up-regulation of SGK1 in ECs of ECMR+/+ mice (Fig. 2 and Online Fig. IA and IB). However, these effects were attenuated in ECMR−/− female mice (Fig. 2). In primary culture EC, aldosterone (10−8 M)-increased the expression of ENaC in the cultured ECMR+/+ ECs that was prevented in ECMR−/− cells (Fig. 2D)
Figure 2. ECMR mediates ENaC expression in WD-induced aortic relaxation dysfunction. WD ECMR+/+ increased MR expression.
(A), which bind the hormone response element (nGnACAnnnTGTnCn) on the site of ENaC promoter (B) and prompted an increase in ENaC expression (C). ECMR−/− prevented WD (C) and Aldosterone (D)-induced expression of ENaC in vivo and in vitro, respectively. Representative images immunostaining for ENaC (E) and SGK1 (F) in ECs with corresponding measures of average gray scale intensities. Scale bar = 50 μm. n=4 to 5 per group. *P<0.01 compared with CD ECMR+/+; # P<0.05 compared with WD ECMR+/+.
ENaC antagonist prevents endothelial dysfunction
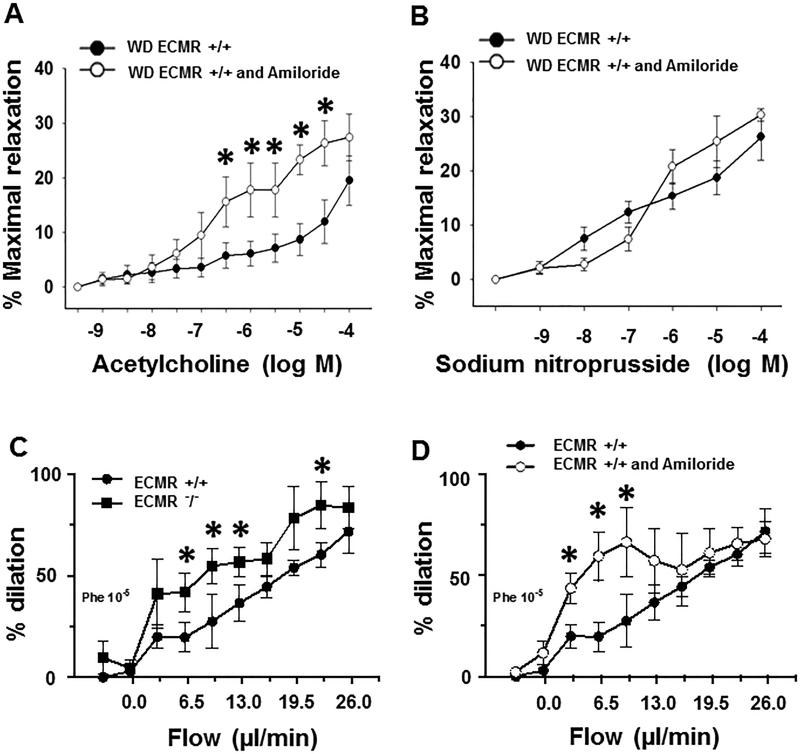
In order to further investigate ENaC in the development of EC stiffness, amiloride (1mg/kg/d), an antagonist for ENaC, was administered in the WD ECMR+/+ female mice for 16 weeks. Amiloride treatment increased endothelium vasodilatory responses to acetylcholine (Fig. 3A) in aorta, but not to sodium nitroprusside (Fig. 3B); suggesting that amiloride could improve endothelium-dependent relaxation in aorta but not endothelium independent responses. Furthermore, ECMR−/− (Fig. 3C) and amiloride (Fig. 3D), respectively improved flow-induced mesenteric artery dilation, suggesting that both ECMR and ENaC participate in promoting endothelial dysfunction in ECMR+/+ mice.
Figure 3. In vivo amiloride administration ameliorated WD-impaired aortic relaxation and flow-induced mesenteric artery dilation in ECMR+/+ female mice.
Vasodilator responses of isolated aortic rings to the endothelium-dependent dilators, acetylcholine (A) and to the endothelium-independent vasodilator, sodium nitroprusside (B). ECMR−/− (C) and amiloride (D) improved flow-induced mesenteric artery dilation.n=4 to 5 per group. *P<0.05 compared with WD ECMR+/+ or ECMR+/+.
ECMR signaling promotes impaired aortic relaxation dysfunction through attenuation of eNOS activation
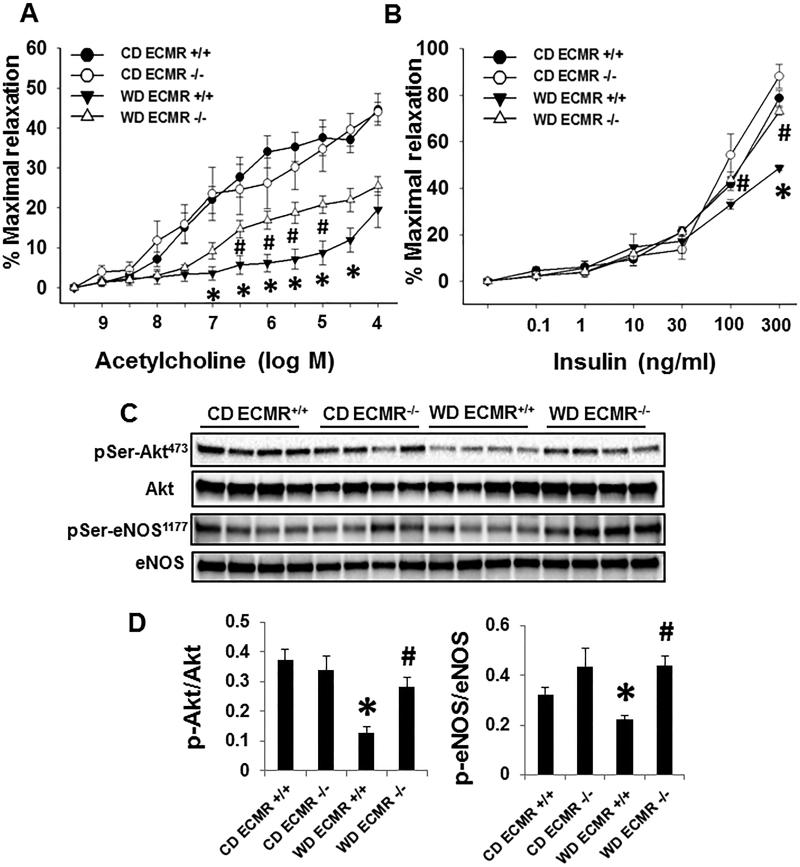
Endothelium-dependent vasodilatory responses to both acetylcholine (Fig. 4A) and insulin (4B) were attenuated and this decrease occurred in concert with reduced of p-protein kinase B (Akt)/p-eNOS signaling in WD ECMR+/+ compared with CD ECMR+/+ (Fig. 4A and 4D), these adverse effects were not present in the WD ECMR−/− vasculature (Fig. 4). Meanwhile, acetylcholine-induced bioavailable NO was greater in aortic explants of ECMR−/− ex vivo compared with ECMR+/+ (Online Fig. II). Thus, ECMR mediated impairment of aortic relaxation with WD consumption is driven by a reduction in activation of eNOS and bioavailable NO.
Figure 4. ECMR−/− prevents WD-impaired eNOS activation and endothelial dependent aortic relaxation.
Vasodilator responses of isolated aortic rings to the endothelium-dependent dilators, acetylcholine (A) and insulin (B). (C) The expression and activation of Akt and eNOS were performed with immunoblotting. (B) Quantitative analysis of protein expression in p-Akt and p-eNOS. n=4 to 5 per group. *P<0.05 compared with CD ECMR+/+; # P<0.05 compared with WD ECMR+/+.
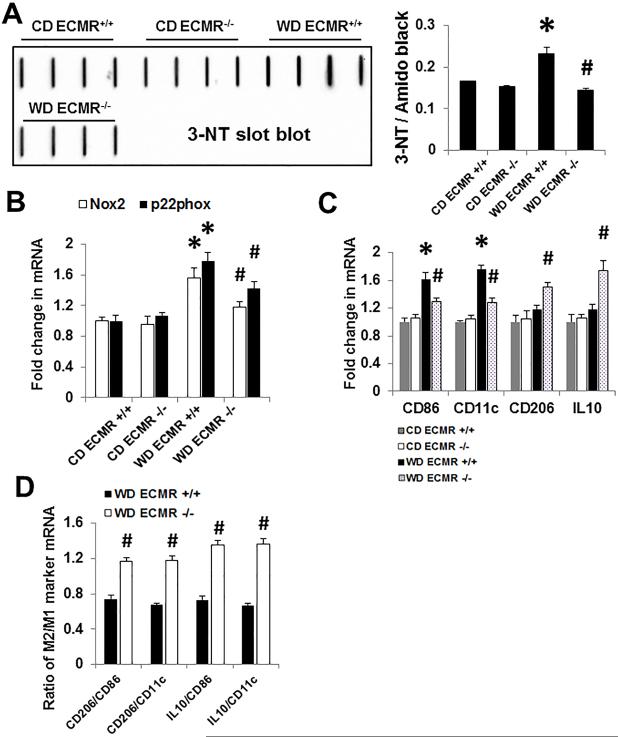
ECMR signaling mediates oxidative stress, maladaptive pro-inflammatory cytokine generation, and macrophage M1/M2 polarization
Vascular nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) generated superoxide anions react with NO to generate peroxynitrite, thereby reducing bioavailable NO. In wild type mice WD consumption promoted aortic oxidant stress as indicated by increases in NADPH oxidase subunits Nox2, Nox4, and p22phox as well as 3-NT, a marker for peroxynitrite formation (Fig. 5A, 5B and Online Fig. IIIA). The attendant increase in oxidant stress was associated with increases in aortic tissue expression of the M1 macrophage markers, CD86 and CD11c (Fig. 5). ECMR−/− deletion prevented WD-induced increases in M1 macrophage polarization. Furthermore, vasculature from ECMR−/− mice also displayed increased M2 marker expression (CD206 and IL10) and an increased M2/M1 marker gene expression ratio suggesting a shift in polarization to a M2 phenotype in the ECMR −/− (Fig. 5).
Figure 5. WD-induced aortic oxidative stress and maladaptive pro-inflammatory cytokines are ameliorated in ECMR−/− female mice.
(A) Western blot for 3-NT, a marker of oxidant stress from accumulation of oxidant peroxynitrite (ONOO−). (B) mRNA expression of Nox2 and p22phox in aortic tissues. (C) Expression of M1/M2 macrophage marker CD86, CD11c, CD206, and IL10. (D) WD ECMR−/− increased M2 macrophage marker IL10 and CD206 mRNA expression in WD fed mice as measured by real-time PCR. n=4 per group. *P<0.01 compared with CD ECMR+/+; # P<0.05 compared with WD ECMR+/+.
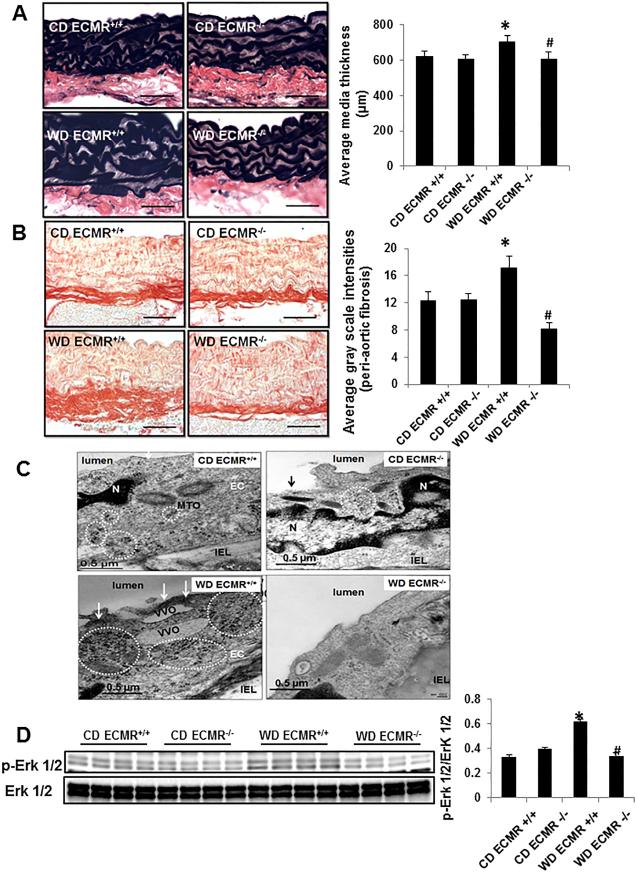
Cell specific ECMR signaling mediates WD-induced aortic fibrosis/remodeling
Oxidant stress and inflammation contribute to maladaptive tissue remodeling and in the current study we observed that WD ECMR+/+ exhibited increased aortic thickness and fibrosis, findings prevented in WD ECMR−/− (Fig. 6A and 6B). Transmission electron emission analysis revealed western diet-induced increases in thickened electron dense plasmalemma and free ribosomes, which was largely corrected in WD ECMR−/− (Fig. 6C). Consistent with the aortic remodeling, WD also enhanced expression of pextracellular signal regulated kinase 1/2 (Erk 1/2) signaling (Fig. 6D), fibroblast growth factor 23 (FGF23) (Online Fig. IIIB), osteopontin (Online Fig. IIIC) and promotes elastin fiber breakdown (Online Fig. IV) in aorta. However, ECMR−/− prevented these abnormalities (Fig.6).
Figure 6. WD-induced aortic remodeling and ultrastructural abnormalities are prevented in in ECMR−/− female mice.
Representative micrographs show medial wall thickening staining with Verhoeff-Van Gieson (A) and periaortic fibrosis staining with picrosirius red staining (B). (C) ECMR−/− prevents WD-induced increases in thickened electron dense plasmalemma and free ribosomes, which contributed to EC stiffness. MTO= microtuble organizing center; IEL = internal elastic lamina; VVO=vesiculovacuolar organelles. Magnification X 10,000; bar = 0.5 μm. (D) ECMR−/− prevents WD-induced upregulation of p-Erk 1/2. n=4-6 per group. *P<0.05 compared with CD ECMR+/+; # P<0.05 compared with WD ECMR+/+.
DISCUSSION
The present investigation demonstrates that consumption of a WD for 16 weeks in female mice results in aortic stiffness, indicated by increases in PWV, impairment of aortic relaxation, and endothelial stiffness. Aortic stiffness was associated with enhanced membrane ENaC, oxidative stress, attenuated eNOS activity, classical macrophage polarization and inflammation. We previously observed that WD was associated with impaired diastolic relaxation at an earlier age in female mice than in male mice6 and systemic MR receptor blockade with spironolactone prevented the development of cardiac and vascular stiffness in females consuming a WD.3, 9 Here, we further demonstrate that EC-specific deletion of the MR prevents the development of WD-induced aortic stiffness in female mice. In this regard, WD-promotion of endothelial membrane ENaC expression, eNOS inactivity, oxidative stress, inflammation, M1/2 macrophage polarization, and associated aortic fibrosis was also prevented in ECMR−/− female mice. To the best of our knowledge, this is the first study to examine the impact of EC specific deletion of the MR on aortic stiffness and endothelial function in females. Moreover, this is the first endeavor to investigate the impact of EC specific MR deletion on vascular stiffness associated with consumption of a highly translational WD.
Mechanical stiffening of EC cortex in concert with impairment of eNOS activation characterizes the stiff endothelial cell syndrome (SECS).23 One study indicated that aldosterone and MR activation contribute to SECS by increasing membrane ENaC and mechanical stiffening of the EC cortex in concert with impaired generation of NO.24 Further, spironolactone prevents SECS manifestation and improves endothelial function.12 Additionally, studies conducted in mice with Liddle syndrome, a disease characterized by impaired ubiquinization of endothelial membrane ENaC have also demonstrated the critical role of this sodium channel in regulation of endothelial function and blood pressure.25 Our study further highlights the role of ECMR signaling in increasing membrane ENaC localization to promote aortic stiffness and decrease endothelium-dependent relaxation in a dietary model of vascular disease. The current data supports the notion that both ECMR and ENaC activation integratively promote stiffness and reduce eNOS activity, NO bioavailability, and endothelium mediated relaxation.26
It is noteworthy that the magnitude of dilatory responses to acetylcholine that we observed in the thoracic aorta of 20 week old female ECMR+/+ and ECMR−/− are similar to those observed in some previous studies,27-29 yet lower than those reported in other studies, despite the fact that most previous investigations have examined aortic vasoreactivity only in male mice.30, 31 In this regard, murine vascular function measurements are known to be influenced by a variety of genetic, physiological and experimental factors including genetic background, age, vascular collection time relative to the time of mouse sacrifice, circadian cycle and the segment of the vessel tested. For example, one study reported that endothelium-dependent and -independent vasodilation in response to carbachol and nitroprusside differ in magnitude between thoracic and abdominal aorta, as well as in arteries from other vascular beds.32 Other studies also found the maximum response to acetylcholine is less than 60% and are thus similar to our results in control wild type mice with C57BL16J and C57BI/6N mice background.28, 29, 33-37 Furthermore, current data are consistent with our previous study in 20 week old female C57Bl/6J mice using the same technique.3
Normally, the EC is protected by a well-developed glycocalyx and membrane expression of ENaC is maintained in an attenuated state of activity.38 Thus, the access of Na+ into the EC is limited, eNOS activation is optimized and vasodilation is maintained.38 In states of obesity and activated reninangiotensin aldosterone system, there is evidence that increased ENaC membrane abundance, together with a damaged glycocalyx, facilitate Na+ entry into EC and triggers the polymerization of G-actin to F-actin.38 Under these conditions, normal caveolar function and eNOS activation are compromised and the endothelial plasma membrane and immediate sub-membrane cytoskeletal compartment ‘stiffens’.13 In this study, SGK1 was increased with consumption of a WD and this was prevented with ECMR deletion. This is important as SGK1 inhibits ubiquinization by up-regulation of E3 ubiquitin ligase (Nedd4-2), and thus increases membrane insertion of this sodium channel.39 Therefore, these data suggest that enhancement of ECMR, SGK1 and ENaC coordinately contribute to aortic stiffness in female mice fed a WD.
One of the important new observations is that cell specific ECMR activation also mediates WD-induced NADPH oxidase activity as evidenced by increases in p22phox, Nox2, and Nox4. The resultant oxidative stress and inflammation responses are well known to play key roles in the development of aortic stiffness.40 To this point, increases in free radicals and inflammation directly suppress eNOS activation and reduce NO bioavailability.41, 42 Additionally, increases in vascular ROS result in destruction of generated NO,3 which normally exerts a negative feedback to suppress ENaC activity by unclear signaling pathways.43, 44 One study has shown that H2O2 increased ENaC activity by both activation of phosphatidylinositide 3-kinases and inactivation of phosphatase and tensin homolog in renal tubular epithelium.44 Both IL6 and tumor necrosis factor activate EnaC and stimulate sodium uptake, in part, by activation of the Erk 1/2 signal pathway.45, 46
MR activation may also promote EC injury by increasing inflammatory cytokines such as vascular cell adhesion molecule 1 and intercellular adhesion molecule 1, which attract and promote immune cell adhesion to and transmigration through the endothelial barrier and to the artery wall from the bloodstream.30 Our previous study showed that WD induces an increase in CD11b in aorta, which represents a total macrophage cell marker.3 Interestingly, current data indicates that ECMR signaling also mediates WD-induced macrophage M1/M2 polarization as this was prevented in the ECMR−/− mice. M1/M2 polarization as indicated by both increases in M1 markers CD86 and CD11c and decreases in M2 markers CD206 and IL10 have been posited to be involved in the pathogenesis of tissue fibrosis and stiffness.3, 9, 47 The increase in M1/M2 polarization with consumption of a WD is consistent with previous results in mice showing that deletion of MRs in macrophages- resulted in reduction in M1 phenotype and mRNA levels for markers of vascular inflammation and fibrosis.48, 49 These observed pathophysiological changes leading to aortic fibrosis and remodeling were associated with increases in p-Erk 1/2, FGF 23, osteopontin, broken elastin fibers, and ultrastructural abnormalities were also induced by consumption of a WD. To this point, Erk1/2 is a family of serine/threonine protein kinases activated as an early response to a variety of cytokines, growth factors, and regulate transcription factor activation, ultimately contributing to vascular cell differentiation, proliferation, and vascular remolding.50 In the present study, phospho-Erk1/2 protein levels were significantly increased in mice fed a WD and this abnormality was prevent in ECMR KO mice, suggesting a role for Erk 1/2 in ECMR mediated aortic remodeling. Thus, activation of ECMR in the setting of obesity resulting in increased ROS, inflammation response, and macrophage M1/M2 polarization could contribute to aortic remodeling and fibrosis that is characteristic of EC dysfunction and vascular stiffness in females with obesity and CVD.
Collectively, results of this investigation suggest a pivotal role of ECMR activation in development of aortic stiffness and endothelial dysfunction. These vascular changes are associated with increases in endothelial membrane ENaC, reduced eNOS activity, oxidative stress and maladaptive immune responses in the aorta of female mice fed a WD. These preclinical highly translational data fill a gap in our knowledge of the role of cell specific MR signaling in promotion of vascular stiffness. Further investigation of the precise role of MR signaling in other cells, (including vascular smooth muscle cells and macrophages) in promotion of vascular stiffness in conjunction with dietary factors is an important area of future investigation.
Supplementary Material
Novelty and Significance.
What Is Known?
● Arterial stiffness is increased to a greater extent in women during obesity and diabetes than men.
● Consumption of a Western diet (WD) high in saturated fat and refined sugar increases plasma aldosterone and enhances vascular mineralocorticoid receptor (MR) activation.
● Vascular MR contributes to vascular stiffness by enhancing endothelial dysfunction and upregulating oxidase stress, inflammation, and fibrosis.
What New Information Does This Article Contribute?
● Activation of endothelial MR signaling is associated with consumption of a WD.
● Enhanced ECMR activation and resultant increases in ENaC activation lead to a reduction in NO production and bioavailability and associated vascular stiffness.
● ECMR mediated-promotion of oxidative stress, M1 macrophage polarization, leads to further decreases in NO bioavailability and associated aortic fibrosis and stiffness in females consuming a WD.
In comparison with male mice fed a WD, pulse wave velocity (PWV) is elevated earlier in female mice. This abnormality is prevented by MR antagonist. Here we show that ECMR mediated activation of ENaC on endothelial cells (EC) leads to reduction of NO production and bioavailability. We found that WD consumption prompts ECMR to bind the hormone response element on the site of ENaC promoter and increases ENaC expression in ECs. This ECMR-mediated response was associated with increased aortic vascular and EC stiffness. WD increased aortic remolding with an increase in macrophage M1 markers CD 86 and CD11c, and this inflammatory immune response was prevented by ECMR deletion in WD-fed female mice. These findings suggest that increased ECMR signaling plays a key role in ENaC activation, reduced NO bioavailability, and macrophage recruitment that lead to aortic stiffness in females.
ACKNOWLEDGMENTS
The authors would like to thank Brenda Hunter for her editorial assistance.
SOURCES OF FUNDING
This research was supported by NIH (R01 HL73101, R01 HL107910) to JRS, the Veterans Affairs Merit System (0018) to JRS, NIH (HL095590) to IZJ, AHA (EIA18290005) to IZJ, the Department of Medicine Research Council to V.G. DeMarco and 2015DMRC to GJ.
Nonstandard Abbreviations and Acronyms
- CVD
cardiovascular disease
- WD
Western Diet
- MR
mineralocorticoid receptor
- eNOS
endothelial nitric oxide synthase
- ROS
reactive oxygen species
- NO
nitric oxide
- ENaC
epithelial Na+ channel
- SGK1
serum-and glucocorticoid-regulated kinase 1
- EC
endothelial cell
- ECMR
endothelial cell MR
- ECMR−/−
ECMR knockout
- PWV
pulse wave velocity
- AFM
atomic force microscopy
- 3-NT
3-nitrotyrosine
- NADPH
oxidase nicotinamide adenine dinucleotide phosphate-oxidase
- SECS
stiff endothelial cell syndrome
- Erk 1/2
extracellular signal regulated kinase 1/2
- Akt
protein kinase B
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–10. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen KS, Blanch N, Keogh JB, Clifton PM. Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35:243–52. doi: 10.1161/ATVBAHA.114.304798. [DOI] [PubMed] [Google Scholar]
- 3.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-Dose Mineralocorticoid Receptor Blockade Prevents Western Diet-Induced Arterial Stiffening in Female Mice. Hypertension. 2015;66:99–107. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–54. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 6.Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology. 2013;154:3632–42. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43:957–62. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 8.Mihailidou AS, Ashton AW. Cardiac effects of aldosterone: does gender matter? Steroids. 2014;91:32–7. doi: 10.1016/j.steroids.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Bostick B, Habibi J, DeMarco VG, Jia G, Domeier TL, Lambert MD, Aroor AR, Nistala R, Bender SB, Garro M, Hayden MR, Ma L, Manrique C, Sowers JR. Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. American journal of physiology Heart and circulatory physiology. 2015;308:H1126–35. doi: 10.1152/ajpheart.00898.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi X, Liu S, Shi H, Yang M, Qi Y, Wang J, Du J. Serum-glucocorticoid regulated kinase 1 regulates macrophage recruitment and activation contributing to monocrotaline-induced pulmonary arterial hypertension. Cardiovasc Toxicol. 2014;14:368–78. doi: 10.1007/s12012-014-9260-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Zheng J, Miao Y, Wang Y, Cui W, Guo J, Qiu S, Han Y, Jia L, Li H, Cheng J, Du J. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32:1675–86. doi: 10.1161/ATVBAHA.112.248732. [DOI] [PubMed] [Google Scholar]
- 12.Druppel V, Kusche-Vihrog K, Grossmann C, Gekle M, Kasprzak B, Brand E, Pavenstadt H, Oberleithner H, Kliche K. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013;27:3652–9. doi: 10.1096/fj.13-228312. [DOI] [PubMed] [Google Scholar]
- 13.Kusche-Vihrog K, Callies C, Fels J, Oberleithner H. The epithelial sodium channel (ENaC): Mediator of the aldosterone response in the vascular endothelium? Steroids. 2010;75:544–9. doi: 10.1016/j.steroids.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Barrett Mueller K, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, Hill MA, Jaffe IZ. Endothelial Mineralocorticoid Receptors Differentially Contribute to Coronary and Mesenteric Vascular Function Without Modulating Blood Pressure. Hypertension. 2015;66:988–97. doi: 10.1161/HYPERTENSIONAHA.115.06172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, Barrett Mueller K, Jaffe IZ, Sowers JR. Endothelial Mineralocorticoid Receptor Deletion Prevents Diet-Induced Cardiac Diastolic Dysfunction in Females. Hypertension. 2015;66:1159–67. doi: 10.1161/HYPERTENSIONAHA.115.06015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bostick B, Habibi J, Ma L, Aroor A, Rehmer N, Hayden MR, Sowers JR. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism. 2014;63:1000–11. doi: 10.1016/j.metabol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Wang Q, Wu Y, Moriasi C, Liu Z, Dai X, Wang Q, Liu W, Yuan ZY, Zou MH. Endothelial cell-specific liver kinase B1 deletion causes endothelial dysfunction and hypertension in mice in vivo. Circulation. 2014;129:1428–39. doi: 10.1161/CIRCULATIONAHA.113.004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trache A, Trzeciakowski JP, Gardiner L, Sun Z, Muthuchamy M, Guo M, Yuan SY, Meininger GA. Histamine effects on endothelial cell fibronectin interaction studied by atomic force microscopy. Biophysical journal. 2005;89:2888–98. doi: 10.1529/biophysj.104.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez-Perez FI, Schenewerk AL, Coffman KL, Foote C, Ji T, Rivera RM, Martinez-Lemus LA. Effects of the use of assisted reproductive technologies and an obesogenic environment on resistance artery function and diabetes biomarkers in mice offspring. PloS one. 2014;9:e112651. doi: 10.1371/journal.pone.0112651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Lemus LA. Persistent agonist-induced vasoconstriction is not required for angiotensin II to mediate inward remodeling of isolated arterioles with myogenic tone. J Vasc Res. 2008;45:211–21. doi: 10.1159/000112513. [DOI] [PubMed] [Google Scholar]
- 21.Begum G, Stevens A, Smith EB, Connor K, Challis JR, Bloomfield F, White A. Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1694–703. doi: 10.1096/fj.11-198762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziera T, Irlbacher H, Fromm A, Latouche C, Krug SM, Fromm M, Jaisser F, Borden SA. Cnksr3 is a direct mineralocorticoid receptor target gene and plays a key role in the regulation of the epithelial sodium channel. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3936–46. doi: 10.1096/fj.09-134759. [DOI] [PubMed] [Google Scholar]
- 23.Lang F. Stiff endothelial cell syndrome in vascular inflammation and mineralocorticoid excess. Hypertension. 2011;57:146–7. doi: 10.1161/HYPERTENSIONAHA.110.164558. [DOI] [PubMed] [Google Scholar]
- 24.Korte S, Strater AS, Druppel V, Oberleithner H, Jeggle P, Grossmann C, Fobker M, Nofer JR, Brand E, Kusche-Vihrog K. Feedforward activation of endothelial ENaC by high sodium. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:4015–25. doi: 10.1096/fj.14-250282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, Jaisser F, Kusche-Vihrog K. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension. 2013;61:1053–9. doi: 10.1161/HYPERTENSIONAHA.111.199455. [DOI] [PubMed] [Google Scholar]
- 26.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. The Journal of physiology. 2005;568:357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pojoga LH, Yao TM, Opsasnick LA, Garza AE, Reslan OM, Adler GK, Williams GH, Khalil RA. Dissociation of hyperglycemia from altered vascular contraction and relaxation mechanisms in caveolin-1 null mice. The Journal of pharmacology and experimental therapeutics. 2014;348:260–70. doi: 10.1124/jpet.113.209189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pojoga LH, Adamova Z, Kumar A, Stennett AK, Romero JR, Adler GK, Williams GH, Khalil RA. Sensitivity of NOS-dependent vascular relaxation pathway to mineralocorticoid receptor blockade in caveolin-1-deficient mice. American journal of physiology Heart and circulatory physiology. 2010;298:H1776–88. doi: 10.1152/ajpheart.00661.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, Williams GH, Khalil RA. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. American journal of physiology Heart and circulatory physiology. 2008;294:H1258–65. doi: 10.1152/ajpheart.01014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rickard AJ, Morgan J, Chrissobolis S, Miller AA, Sobey CG, Young MJ. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension. 2014;63:1033–40. doi: 10.1161/HYPERTENSIONAHA.113.01803. [DOI] [PubMed] [Google Scholar]
- 31.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nature medicine. 2000;6:1362–7. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 32.Kleinbongard P, Schleiger A, Heusch G. Characterization of vasomotor responses in different vascular territories of C57BL/6J mice. Experimental biology and medicine. 2013;238:1180–91. doi: 10.1177/1535370213502621. [DOI] [PubMed] [Google Scholar]
- 33.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nature medicine. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 34.Rinne P, Nordlund W, Heinonen I, Penttinen AM, Saraste A, Ruohonen ST, Makela S, Vahatalo L, Kaipio K, Cai M, Hruby VJ, Ruohonen S, Savontaus E. alpha-Melanocyte-stimulating hormone regulates vascular NO availability and protects against endothelial dysfunction. Cardiovascular research. 2013;97:360–8. doi: 10.1093/cvr/cvs335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egecioglu E, Andersson IJ, Bollano E, Palsdottir V, Gabrielsson BG, Kopchick JJ, Skott O, Bie P, Isgaard J, Bohlooly YM, Bergstrom G, Wickman A. Growth hormone receptor deficiency in mice results in reduced systolic blood pressure and plasma renin, increased aortic eNOS expression, and altered cardiovascular structure and function. American journal of physiology Endocrinology and metabolism. 2007;292:E1418–25. doi: 10.1152/ajpendo.00335.2006. [DOI] [PubMed] [Google Scholar]
- 36.Patel D, Kandhi S, Kelly M, Neo BH, Wolin MS. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1alpha. American journal of physiology Lung cellular and molecular physiology. 2014;306:L383–91. doi: 10.1152/ajplung.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toque HA, Nunes KP, Rojas M, Bhatta A, Yao L, Xu Z, Romero MJ, Webb RC, Caldwell RB, Caldwell RW. Arginase 1 mediates increased blood pressure and contributes to vascular endothelial dysfunction in deoxycorticosterone acetate-salt hypertension. Frontiers in immunology. 2013;4:219. doi: 10.3389/fimmu.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng Y, Waters M, Andrews A, Honarmandi P, Ebong EE, Rizzo V, Tarbell JM. Fluid shear stress induces the clustering of heparan sulfate via mobility of glypican-1 in lipid rafts. American journal of physiology Heart and circulatory physiology. 2013;305:H811–20. doi: 10.1152/ajpheart.00764.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. The Journal of clinical investigation. 2013;123:657–65. doi: 10.1172/JCI61110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, English M, Segal MS, Christou DD. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci (Lond) 2013;125:513–20. doi: 10.1042/CS20130200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med. 2002;346:1999–2001. doi: 10.1056/NEJMe020054. [DOI] [PubMed] [Google Scholar]
- 42.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Overnutrition, mTOR signaling, and cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1198–R1206. doi: 10.1152/ajpregu.00262.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. American journal of physiology Cell physiology. 2013;304:C102–11. doi: 10.1152/ajpcell.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Chen S, Liu H, Zhang B, Zhao Y, Ma K, Zhao D, Wang Q, Ma H, Zhang Z. Hydrogen sulfide prevents hydrogen peroxide-induced activation of epithelial sodium channel through a PTEN/PI(3,4,5)P3 dependent pathway. PloS one. 2013;8:e64304. doi: 10.1371/journal.pone.0064304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li K, Guo D, Zhu H, Hering-Smith KS, Hamm LL, Ouyang J, Dong Y. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am J Physiol Regul Integr Comp Physiol. 2010;299:R590–5. doi: 10.1152/ajpregu.00207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiPetrillo K, Coutermarsh B, Soucy N, Hwa J, Gesek F. Tumor necrosis factor induces sodium retention in diabetic rats through sequential effects on distal tubule cells. Kidney Int. 2004;65:1676–83. doi: 10.1111/j.1523-1755.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 47.Sharma N, Lu Y, Zhou G, Liao X, Kapil P, Anand P, Mahabeleshwar GH, Stamler JS, Jain MK. Myeloid Kruppel-like factor 4 deficiency augments atherogenesis in ApoE−/− mice--brief report. Arterioscler Thromb Vasc Biol. 2012;32:2836–8. doi: 10.1161/ATVBAHA.112.300471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bienvenu LA, Morgan J, Rickard AJ, Tesch GH, Cranston GA, Fletcher EK, Delbridge LM, Young MJ. Macrophage mineralocorticoid receptor signaling plays a key role in aldosterone-independent cardiac fibrosis. Endocrinology. 2012;153:3416–25. doi: 10.1210/en.2011-2098. [DOI] [PubMed] [Google Scholar]
- 49.Frieler RA, Meng H, Duan SZ, Berger S, Schutz G, He Y, Xi G, Wang MM, Mortensen RM. Myeloid-specific deletion of the mineralocorticoid receptor reduces infarct volume and alters inflammation during cerebral ischemia. Stroke. 2011;42:179–85. doi: 10.1161/STROKEAHA.110.598441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–76. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.