Abstract
Busulfan (Bu)/cyclophosphamide (Cy) is a standard conditioning platform for allogeneic transplantation. We developed a strategy separating the Cy into two pre/post-transplantation doses (PTCy), providing myeloablative conditioning and single-agent graft-versus-host disease (GVHD) prophylaxis. We investigated the impact of Bu route on treatment-related toxicity for 131 consecutive adult patients. Busulfan was administered in four daily divided doses either orally (n = 72) or intravenously (n = 59) with pharmacokinetics on the first-dose and as necessary on subsequent doses to achieve a target area-under-the-concentration-curve (AUC) of 800–1400 µmol*min/L per dose. BuCy/PTCy with pharmacokinetics is well-tolerated with low treatment-related toxicity. Hepatic veno-occlusive disease incidence was 6% with two fatal events. Bu administration route in the context of BuCy/PTCy did not statistically impact hepatotoxicity, GVHD, relapse, disease-free survival, or overall survival. The BuCy/PTCy platform has a low incidence of treatment-related toxicity, including hepatotoxicity, in hematologic malignancies when using pharmacokinetics for a target AUC of 800–1400 µmol*min/L, irrespective of Bu administration route.
Keywords: Busulfan, post-transplantation cyclophosphamide, route, toxicity
Introduction
The aim of the conditioning regimen in allogeneic blood or marrow transplantation (alloBMT) is to provide tumor cytoreduction as well as sufficient immunosuppression to prevent host rejection of the donor graft. High-dose busulfan (Bu) combined with cyclophosphamide (Cy) in the BuCy conditioning regimen represents the first example of a chemotherapy-based myeloablative strategy [1–3]. Since its development, tens of thousands of patients worldwide have received this conditioning regimen followed by the transplantation of either allogeneic bone marrow or peripheral blood stem cells [1,2]. Originally, Bu was given orally at 1 mg/kg every 6 h for 4 days followed by Cy at 200 mg/kg over 4 days (BuCy4) [1]. This regimen was modified in the late 1980s with the aim of improving tolerability by reducing the Cy dosage to 120 mg/kg over 2 days (BuCy2) [3,4].
Several studies suggested a relationship between Bu exposure and outcomes. Higher Bu exposures were found to be associated with an increased risk of liver toxicity, especially hepatic veno-occlusive disease (VOD), and possibly graft-versus-host disease (GVHD), while lower Bu exposures were associated with higher risk of graft rejection and relapse [5–10]. Therapeutic drug monitoring (TDM) and intravenous (i.v.) Bu were introduced in the hope of achieving more consistent drug exposure [11]. However, although i.v. Bu results in more consistent drug levels, at least three-fold inter-patient variability in exposure has been demonstrated. Thus, TDM and individually adjusted dosing are commonly employed for i.v. Bu [12]. Overall, the advancements of pharmacokinetic (PK) monitoring and i.v. Bu have facilitated the exploration of other Bu-based conditioning strategies, including new dosing schedules and effective combination with other agents [13–16].
We have reported that modifying the original BuCy4 by spacing the Cy dosing into two pre- and two post-transplantation Cy (PTCy) doses of 50 mg/kg/day (BuCy/PTCy) is an effective approach to GVHD prophylaxis [17]. With increasing utilization of the BuCy/PTCy conditioning platform with either oral or i.v. Bu, it is important to characterize the relationship between Bu PK and transplantation outcomes, especially as relates to different routes of Bu administration. Therefore, we performed a retrospective review of 131 consecutive patients treated with BuCy/PTCy for whom we had Bu PK data. We focused on hepatotoxicity and secondarily on the impact of route of Bu administration on survival endpoints.
Materials and methods
Study design and patients
This was a single-center, retrospective analysis of a database of 143 consecutive adult patients with advanced hematologic malignancies treated at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins with myeloablative conditioning and HLA-matched-related or -unrelated bone marrow allografts using the BuCy/PTCy transplantation platform on an IRB-approved protocol (NCT00134017) between June 2004 and October 2009 [17]. Eligibility criteria for enrollment included age <66 years old; the availability of a donor matched at HLA-A, -B, -Cw, -DRB1, and -DQB1; high-risk hematologic malignancy; and adequate end-organ function [17]. Outcomes for 117 of these patients were previously reported and an additional 26 patients were enrolled on the protocol after data analysis was closed for the original publication [17]. Of these 143 patients, only the 131 patients (92%) with available Bu PK were included for analysis; 72 received oral Bu and 59 received i.v. Bu. The reasons for unavailable Bu PK in 12 cases were that Bu PK was not performed in nine cases (all i.v.) and records were missing in three cases (one oral, two i.v.). The first nine patients treated with i.v. Bu on the protocol did not receive Bu PK; because of adverse events that could possibly be mitigated by measuring PK, all subsequent patients received PK.
Regimen and supportive care
Patients were admitted in the early afternoon and the conditioning regimen began in the evening which consisted of Bu for 16 consecutive doses over approximately 96 h; Bu was scheduled every 6 h for the first six doses at a minimum then every 5 h for the remaining doses. i.v. Bu was administered over 2 h. Cyclophosphamide was given at 50 mg/kg/day i.v. for two consecutive days. A full 24-h period was mandated between the final dosage of cyclophosphamide and the infusion of the stem cell product.
Busulfan was started at an initial dosage of 1 mg/kg/dose orally or 0.8 mg/kg/dose i.v. with doses adjusted based on measured PK (see Busulfan therapeutic monitoring). The choice of either oral or i.v. Bu depended on the availability of repeat PK at the reference laboratory and physician preference. Oral Bu was the default choice and was recommended to be utilized for those admitted on Sunday–Wednesday, but i.v. Bu was preferentially utilized for Thursday admissions as PKs were not available for two consecutive days (weekend PKs were not routinely available during this time period; but could be performed by special request). All other aspects of care and eligibility were identical, regardless of the route of Bu administration or whether patients received HLA-matched-related or -unrelated allografts. All allografts were T-cell-replete bone marrow at a targeted collection of ~4 × 108 nucleated cells/kg and infused at least 24 h after the last dosage of pre-transplantation Cy. Due to this requirement, it was permissible for Bu to be given every 5 h starting with dose number seven to accommodate for afternoon patient admission with evening Bu start time and mandated wash-out period between Cy and stem cells. GVHD prophylaxis consisted solely of PTCy 50 mg/kg/day administered on days +3 and +4. Mesna (80% of Cy dosage) was administered in four divided doses with each dose of Cy. Drug dosing was based on ideal body weight. All patients were treated as inpatients for the duration of the conditioning regimen and until initial count recovery. The conditioning regimen and supportive care were provided as previously described [17], with no specific VOD prophylaxis administered.
Busulfan therapeutic monitoring
Evening PK analysis was performed for each patient, picked up by the outside reference laboratory in the early morning on the following day, and reported in the afternoon to a BMT Clinical Pharmacy Specialist who performed calculations. Briefly, blood samples were drawn with the first-dose of Bu. For oral Bu, samples were obtained prior to administration and at 72, 232, 328, and 360 min (trough level) post-administration. For i.v. Bu, samples were obtained prior to infusion and at 120, 180, 240, 300, and 360 min (trough level) post-infusion. The next Bu dose was not administered until the 360-min blood sample had been obtained. Samples were drawn from a central venous catheter via a lumen not being used for Bu after a 20 mL normal saline flush and a 5 mL blood withdrawal and discard. Bu samples were stored on ice and analyzed by high-pressure liquid chromatography/gas chromatography by Quest Diagnostics®. Repeat PKs when necessary were performed in the same manner.
A fitted polynomial equation was used to calculate the area-under-the-concentration-curve (AUC) for oral Bu as previously described [18]. i.v. Bu AUC was calculated using a one-compartment model with first-order elimination and fit with WinNonLin Professional 5.2.1© (Pharsight, St. Louis, MO). Doses were adjusted to achieve a target AUC exposure of 800–1400 µmol*min/L [5,6,19]. This target Bu AUC was chosen based on original studies by Grochow and colleagues as well as our historical experience [5,6].
When the first-dose AUC was within the target range, no repeat PK analysis was performed. If a patient’s first-dose AUC was outside the target range, the dosage was adjusted on the 5th dose and repeat PK analysis was performed as needed. Further dosage adjustments were made on the 10th and 14th doses as necessary to achieve the target AUC. When adjustments were made they were typically a 15–35% decrease or up to a 20% increase from the prior dosage. Total Bu exposure, described by an estimated total cumulative AUC, was retrospectively calculated as previously published by Bartelink and colleagues [20], but was not used for clinical decision-making in this study population.
Endpoints and definitions
Primary graft failure was defined as failure to achieve a sustained absolute neutrophil count of ≥500/µL in the absence of persistent or relapsed disease. Toxicities evaluated included elevation of total bilirubin (T Bili), aspartate aminotransferase (AST), or alanine aminotransferase (ALT), as well as the occurrence of VOD or acute GVHD. In order to best isolate effects of the BuCy/PTCy transplantation platform itself (and not, for example, GVHD), T Bili, AST, and ALT toxicity grades were based on the maximum laboratory value up to day +20 and were graded according to the Common Terminology Criteria for Adverse Events, version 4 (toxicities were graded retrospectively using the normal ranges of laboratory values at the time of laboratory draw). VOD was assessed using both the Baltimore and McDonald criteria [19,21]. Acute GVHD was graded according to the Keystone Criteria [22]. An event for disease-free survival (DFS) was defined as relapsed or persistent disease or death from any cause.
Statistical analysis
The primary objective was to describe toxicity of the regimen with a focus on hepatotoxicity. Proportions were compared with Chi-square tests and reported with exact binomial 95% confidence intervals. Continuous outcomes were compared with the Wilcoxon rank-sum test. The Cochran-Armitage trend test was used to evaluate the impact of the route of Bu administration over time. The Cochran-Mantel-Haenszel test was used to assess associations of route of administration with toxicities, adjusting for type of transplant. Cochran-Mantel-Haenszel odds ratios were calculated for stratified analyses, adjusting for transplant type. The Breslow-Day test for homogeneity of odds ratios was used to confirm assumptions underlying stratified analyses. Overall survival (OS) and DFS probabilities were estimated using the Kaplan-Meier method [23] and comparisons were made using the log-rank statistic (Mantel) or the Cox proportional hazards regression model [24,25]. Stratification was used to adjust Cox regressions for transplant type. The cumulative incidence of non-relapse mortality (NRM) was estimated with the proportional subdistribution hazard regression model for competing risks [26]. A stratified Fine-Gray model was used to assess the effect of route of administration on NRM, adjusted for type of transplant [27]. Relapse was a competing risk for NRM and vice versa. Median follow-up was calculated as the 50%-point of the censoring function. Data analysis was locked 7 January 2014.
Results
Patient characteristics
Patient, disease, and transplantation characteristics are summarized in Table I by route of Bu administration. A total of 72 patients (55%) received oral Bu, and 59 patients (45%) received i.v. Bu. The median age at the time of transplantation was 49 years (range 20–66). A diagnosis of acute myeloid leukemia, myelodysplastic syndrome, or chronic myelomonocytic leukemia was the transplantation indication for 65% of patients. Utilizing previously published standard definitions [28], 34% (44/131) of patients had active disease at the time of transplantation and 28% (37/131) had minimal residual disease. The retrospectively calculated median HCT-CI score was 2 (range 0–8) with 47% of patients having a score of ≥3 [29]. Allogeneic BMT was performed using an HLA-matched-related donor allograft in 72 patients (55%) and an HLA-matched-unrelated donor (MUD) allograft in 59 patients (45%). No patient had received a prior allogeneic transplant.
Table I.
Patient, disease, and transplantation characteristics by route of busulfan administration*.
| Oral busulfan n = 72 |
i.v. busulfan n = 59 |
Combined n = 131 |
|
|---|---|---|---|
| Age in years | 43 50 55 (49 ± 10) | 34 49 56 (45 ± 13) | 40 49 56 (47 ±11) |
| Sex | |||
| Female | 46% (33) | 53% (31) | 49% (64) |
| Male | 54% (39) | 47% (28) | 51% (67) |
| Diagnosis | |||
| AML/MDS/CMML | 61% (44) | 69% (41) | 65% (85) |
| ALL | 8% (6) | 10% (6) | 9% (12) |
| HL | 10% (7) | 3% (2) | 7% (9) |
| NHL | 8% (6) | 2% (1) | 5% (7) |
| CLL | 3% (2) | 3% (2) | 3% (4) |
| MM | 4% (3) | 2% (1) | 3% (4) |
| CML | 6% (4) | 10% (6) | 8% (10) |
| Disease status at AlloBMT | |||
| CR without MRD | 32% (23) | 46% (27) | 38% (50) |
| MRD | 25% (18) | 32% (19) | 28% (37) |
| Active disease | 43% (31) | 22% (13) | 34% (44) |
| Relatedness | |||
| Unrelated | 14% (10) | 83% (49) | 45% (59) |
| Related | 86% (62) | 17% (10) | 55% (72) |
| HCT-CI | 1 3 3 (2 ±2) | 1 2 4 (2 ± 2) | 1 2 4 (2 ± 2) |
| Transplantation year | |||
| 2004–2005 | 25% (18) | 14% (8) | 20% (26) |
| 2006 | 35% (25) | 22% (13) | 29% (38) |
| 2007 | 35% (25) | 20% (12) | 28% (37) |
| 2008–2009 | 6% (4) | 44% (26) | 23% (30) |
| First-dose AUC | 890 1066 1224 | 755 860 1066 | 823 993 1169 |
| (1082 ±269) | (938 ±259) | (1017 ± 273) |
a b c represent the lower quartile a, the median b, and the upper quartile c for continuous variables. x ± s represents the mean ± the standard deviation. Numbers after percentages are frequencies.
i.v., intravenous; AUC, area-under-the-concentration-curve; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CMML, chronic myelomonocytic leukemia; ALL, acute lymphoblastic leukemia; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; CML, chronic myelogenous leukemia; alloBMT, allogeneic bone marrow transplantation; CR, morphologic complete remission; MRD, minimal residual disease; HCT-CI, hematopoietic cell transplantation comorbidity index.
Significant differences between the oral and i.v. Bu groups were more HLA-matched-related patients received oral Bu (p < 0.0001), more oral Bu patients had active disease at the time of alloBMT (p = 0.04), and oral Bu was used more often in the initial years of the protocol (p < 0.0001) (Supplementary Figure I, available online).
Busulfan pharmacokinetics
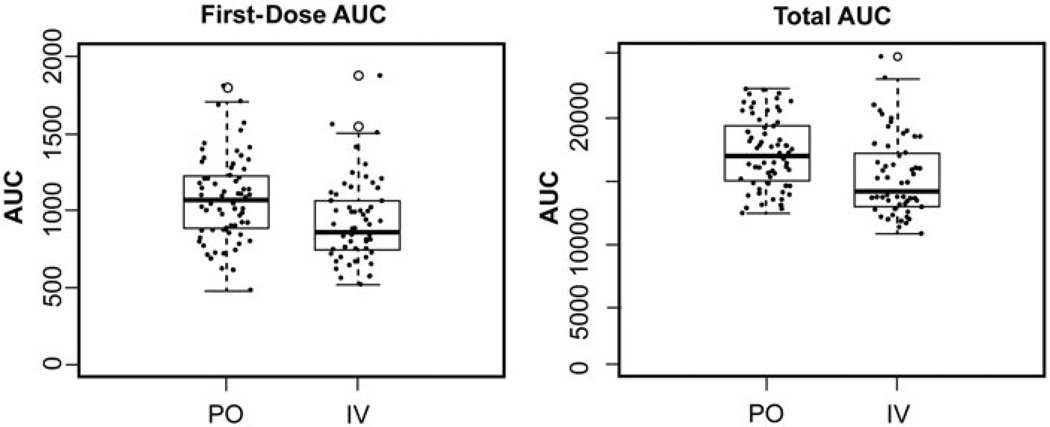
Median first-dose AUCs for oral and i.v. Bu were 1066 and 860 µmol*min/L, respectively (Fig. 1, p = 0.001). First-dose AUCs were within the pre-defined therapeutic range (800–1400 µmol*min/L AUC/dose) in 75% of patients, irrespective of whether they received oral Bu (54/72, 75%) or i.v. Bu (44/59, 75%). These patients underwent no further monitoring or dosage adjustment. In the 25% of patients who did require a dosage change, 50% (9/18) had a dose decrease in the oral group and 27% (4/15) had a dose decrease in the i.v. group; 50% (9/18) required a dose increase in the oral group and 73% (11/15) had a dose increase in the i.v. group. Repeat PKs were recommended after a dosage change, and a third set of PKs were needed on the 10th dose in less than 5% of patients overall. Dosing strategies based on total cumulative AUC were not used for clinical decision making; however, the retrospectively calculated median total cumulative AUCs [20] for oral and i.v. Bu were 17 001 µmol*min/L and 14286 µmol*min/L, respectively (Fig. 1, p = 0.0001).
Figure 1.
Busulfan exposure by route of busulfan administration. In these box-and-whisker plots, the thick horizontal line represents the median area-under-the-concentration-curve (AUC), and the thin black lines represent the lower and upper quartiles. The whiskers demonstrate 1.5× the interquartile range, and outliers beyond the whiskers are individually plotted as open circles. The raw data are overlaid (solid dots) on this plot. The left graph represents oral (PO) and intravenous (i.v.) busulfan separately plotted versus first-dose AUC (goal range of 800–1400 µmol*min/L). The right graph demonstrates PO and i.v. busulfan separately plotted versus calculated total cumulative AUC (goal range of 12 800–22 400 µmol*min/L). The median first-dose and total cumulative AUC were significantly higher in the PO busulfan group (first-dose AUC, p = 0.001; total cumulative AUC, p = 0.0001).
Regimen-related toxicities and GVHD
The therapeutic window of Bu is narrow; both supra- and subtherapeutic exposures (expressed as AUC) have been associated with negative outcomes [5,7,8,31]. Two of our 131 patients (1.5%) had primary graft failure. The first-dose AUCs of these two patients were 1017 and 978(µmol*min/L and thus within the targeted range.
We focused on hepatotoxicity measured as T Bili elevation (≥grade 2), transaminase elevation (AST or ALT ≥ grade 2), or VOD as well as acute GVHD (grades II–IV or III-IV) [Table II]. Grade ≥ 2 elevations in T Bili, AST, and ALT occurred in 25% (33/131), 20% (26/131), and 27% (35/131) of patients, respectively. Grade III elevations in T Bili, AST, and ALT occurred in 5.3%, 7.6%, and 13% of patients, respectively. Grade IV elevations in T Bili, AST, and ALT occurred in 0.8%, 0%, and 0.8%, respectively. Stratified analyses, adjusting for HLA-matched-related and -unrelated alloBMT, were used given the possible confounding between route of administration and transplant type. There were no detectable associations between the route of Bu administration and elevation of T Bili [oral Bu as reference; OR 1.22 (95% CI 0.38–3.93); p = 0.74], AST [OR 0.87 (95% CI 0.26–2.94); p = 0.82], or ALT [OR 1.87 (95% CI 0.61–5.76); p = 0.29] [Table II].
Table II.
Regimen-related toxicities by route of busulfan administration*.
| Oral busulfan n = 72 |
i.v. busulfan n = 59 |
Combined n = 131 |
|
|---|---|---|---|
| Total bilirubin elevation grade† | |||
| 0 | 43% (31) | 58% (34) | 50% (65) |
| 1 | 25% (18) | 25% (15) | 25% (33) |
| 2 | 25% (18) | 12% (7) | 19% (25) |
| 3 | 6% (4) | 5% (3) | 5% (7) |
| 4 | 1% (1) | 0% (0) | 1% (1) |
| AST elevation grade† | |||
| 0 | 43% (31) | 14% (8) | 30% (39) |
| 1 | 42% (30) | 61% (36) | 50% (66) |
| 2 | 8% (6) | 17% (10) | 12% (16) |
| 3 | 7% (5) | 8% (5) | 8% (10) |
| 4 | 0% (0) | 0% (0) | 0% (0) |
| ALT elevation grade† | |||
| 0 | 25% (18) | 14% (8) | 20% (26) |
| 1 | 57% (41) | 49% (29) | 53% (70) |
| 2 | 7% (5) | 20% (12) | 13% (17) |
| 3 | 11% (8) | 15% (9) | 13% (17) |
| 4 | 0% (0) | 2% (1) | 1% (1) |
| VOD‡ | |||
| No | 90% (65) | 98% (58) | 94% (123) |
| Yes | 10% (7) | 2% (1) | 6% (8) |
| VOD grade# | |||
| None | 90% (65) | 98% (58) | 94% (123) |
| Mild | 3% (2) | 2% (1) | 2% (3) |
| Moderate | 4% (3) | 0% (0) | 2% (3) |
| Severe | 3% (2) | 0% (0) | 2% (2) |
| Acute GVHD§ | |||
| None or Grade I | 60% (43) | 41% (24) | 51% (67) |
| Grade II | 26% (19) | 37% (22) | 31% (41) |
| Grade III– IV | 14% (10) | 22% (13) | 18% (23) |
Given the non-randomized study design, changes in clinical practice over time, and differences in the donor relatedness for each group, significance testing by route of administration was not performed (see text for comparisons after stratifying by donor relatedness).
Through the first 20 days post-transplant. Graded by the Common Terminology Criteria for Adverse Events, version 4.0.
Diagnosed per the Baltimore Criteria [19].
Graded per the McDonald Criteria [21].
Graded per the Keystone Criteria [22].
AST, aspartate aminotransferase; ALT, alanine aminotransferase; VOD, veno-occlusive disease; GVHD, graft-versus-host disease.
In this patient cohort, hepatic VOD developed in eight patients (6.1%). VOD severity, based on the McDonald Criteria [21], was mild in three patients, moderate in three patients, and severe (fatal) in two patients (1.5%). All patients were treated with supportive care and/or diuretics only. The median first-dose AUC for patients with VOD was 1256 µmol*min/L (range: 750–1504) as compared with 993 µmol*min/L (range: 520–1875) for those without VOD; however, only one patient with VOD had a first-dose AUC above the target range. For those developing VOD, the median calculated total cumulative AUC was 20 090 µmol*min/L (range: 12 000–23 046) versus 15 888 µmol*min/L (range: 10 830–24 739) for those without VOD. Of the eight patients who developed VOD, seven received oral Bu (risk: oral 10% vs. i.v. 2%), and both deaths attributed to VOD were in the oral Bu group (due to the low frequency of VOD, statistical testing was not performed). Interestingly, of the nine cases on the clinical protocol who did not have Bu PK performed (see Materials and methods: Study design and patients) and hence were not included in our current study (all received i.v. Bu), there were two cases of VOD (2/9) with one fatal event.
Grade II–IV acute GVHD occurred in 64 patients (49%) of whom 41 (31%) had grade II only and 23 (18%) had grade III–IV acute GVHD. Route of Bu administration was not significantly associated with increased risk of either grade II–IV [oral as reference; OR 2.2 (95% CI 0.83–5.81); p = 0.11] or grade III–IV [OR 1.72 (95% CI 0.52–5.63); p = 0.34] acute GVHD, stratifying based on donor type (HLA-matched-related vs. -unrelated allografts).
No impact of the route of Bu administration on relapse, DFS, or OS
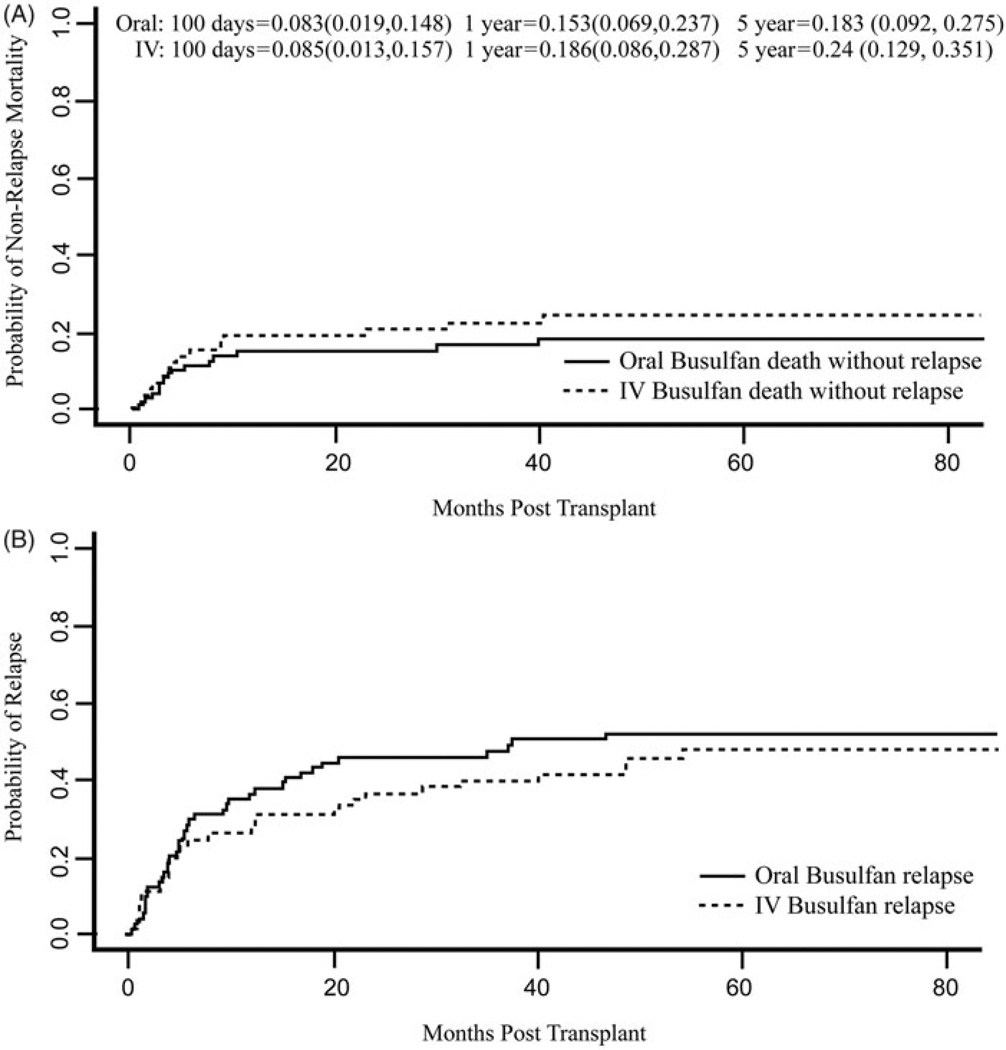
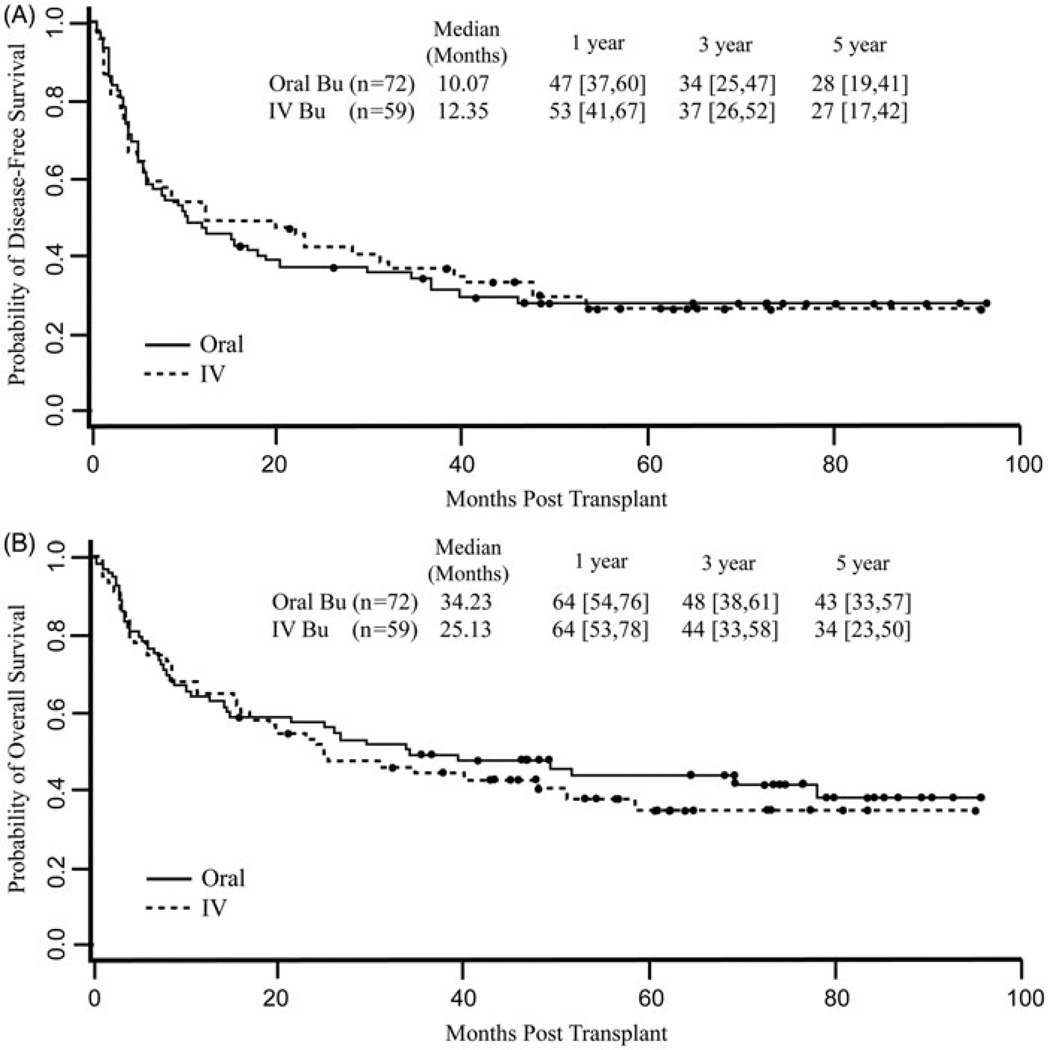
The median follow-up was 6.2 years (range 1.3–8.0) for those receiving oral Bu and 5.1 years (range 1.8–8.0) for those receiving i.v. Bu. The oral Bu cohort had significantly longer follow-up (p = 0.049) (Supplementary Figure I, available online). The cumulative incidence of NRM for the entire cohort was 8.4% (95% CI 3.6–13%) at 100 days, 17% (95% CI 10–23%) at 1 year, and 19% (95% CI 12–26%) at 3 years. i.v. Bu was associated with a higher risk of NRM, adjusted for HLA-matched-related vs. -unrelated donor alloBMT [i.v. Bu subdistribution hazard ratio (SDHR) 2.58 (95% CI 1.02–6.5, p = 0.045)], although the increased risk seemed to be a result of a few late deaths not obviously related to the conditioning regimen [Fig. 2]. The cumulative incidence of relapse for all patients was 34% (95% CI 26–42%) at 1 year and 45% (95% CI 37–54%) at 3 years. Relapse was not significantly different for the two routes of Bu administration [i.v. Bu SDHR 0.63 (95% CI 0.29–1.4, p = 0.26)] [Fig. 2]. The estimated DFS and OS for all patients at 3 years after transplantation were 36% (95% CI 28–45%) and 46% (95% CI 38–56%), respectively. Route of Bu administration did not significantly affect DFS [i.v. Bu hazard ratio (HR) 1.1, 95% CI 0.59–2.08; p = 0.76] or OS [i.v. Bu HR 1.24, 95% CI 0.63–2.42; p = 0.53], even after stratifying for donor relatedness [Fig. 3].
Figure 2.
Non-relapse mortality and relapse by route of busulfan administration. Intravenous (i.v.) busulfan is associated with a slightly increased risk of non-relapse mortality [i.v. busulfan subdistribution hazard ratio 2.58 (95% CI 1.02, 6.5), p = 0.045]. No statistically significant difference in relapse was noted by route of busulfan administration [i.v. busulfan subdistribution hazard ratio 0.63 (95% CI 0.29, 1.4), p = 0.258].
Figure 3.
Disease-free survival (DFS) and overall survival (OS) by route of busulfan administration. Survival outcomes were similar for patients receiving either intravenous (i.v.) or oral busulfan [DFS i.v. busulfan HR 1.1 (95% CI 0.59, 2.08), p = 0.76; OS i.v. busulfan HR 1.24 (95% CI 0.63, 2.42), p = 0.534].
Discussion
The use of PK-guided monitoring to decrease toxicity of high-dose Bu conditioning without compromising its anti-tumor efficacy is a critical step towards improving the outcomes of transplantation. In this study using routine TDM of Bu for the BuCy/PTCy platform, we found a low incidence of severe hepatotoxicity and an acceptable NRM of 8.4% at 100 days. Furthermore, the route of Bu administration in the context of pre- and post-transplantation Cy was not statistically related to any toxicity or survival outcome, with the exception of a higher NRM with i.v. Bu.
The historical incidence of VOD with BuCy4 is approximately 25% when Cy is administered over four consecutive days at 50 mg/kg with oral Bu [19]. Consistent with other reports of myeloablative Bu conditioning using TDM routinely [30–32], the overall incidence of hepatotoxicity in our patients was low with VOD occurring in only 6.1% with a lethality of 1.5%. Although a recent prospective study showed a higher risk of VOD (5%) after i.v. Bu-based conditioning than after total body irradiation (TBI)-based conditioning, only 56% of Bu-treated patients received TDM [32]. Indeed, a recent European retrospective study showed similar risk of VOD after i.v. BuCy (5.9%) or Cy/TBI (4.7%) conditioning [33]. In addition to routine PK monitoring, the overall low incidence of VOD and treatment-related toxicity seen with the BuCy/PTCy regimen [17] may be the result of the spacing of Cy dosing into two pre-transplant and two post-transplant doses; split dosing of Cy possibly allows sufficient time for depleted intrahepatic glutathione to recover, thereby limiting Cy toxicity when compared with BuCy4 [34,35]. A direct comparison of patients treated with BuCy with or without PTCy would be necessary to assess whether such split dosing is indeed associated with less VOD and other toxicity; however, all patients at our institution receiving BuCy (or any myeloablative) conditioning for alloBMT for the past decade have also received PTCy, thus making a direct retrospective comparison impossible.
A major theoretical advantage of i.v. Bu is the potentially decreased need for Bu PK monitoring to reduce toxicity compared with the oral formulation [36,37], even though retrospective data thus far have not supported this assertion [38,39]. In our study, we found that the need for dose adjustment was similar regardless of the route of administration (25% for either oral or i.v.), although patients receiving oral Bu were more likely to have a higher first-dose AUC than patients receiving i.v. Bu. Calculated total cumulative Bu exposure was significantly higher for oral Bu despite the median total cumulative AUCs of both routes being within the desired range. Andersson and colleagues originally recommended an i.v. Bu starting dose of 0.8 mg/kg based on their oral Bu bioavailability data in comparison to i.v. [40]. A study by Le´ger and colleagues compared exposure equivalence between i.v. and oral Bu in adults and found oral Bu exposure to be significantly higher; however, when body weight normalization was utilized, bioequivalent AUCs between the routes were evident [41]. The AUC differences in our data despite similar clinical decision making with the results (25% of patients in each group required at least one dosage adjustment) may in part be related to slight variation in AUC computational technique.
Overall, our data support the hypothesis that routine TDM with traditionally prescribed Bu via either route of administration for the BuCy/PTCy regimen is feasible, safe, and effective. From a logistical perspective, we had rapid turnaround time from an outside reference laboratory which has been described by others as well [42]; however, the implementation of this may prove difficult to replicate. Our limited experience of i.v. Bu without TDM, wherein two of nine patients developed VOD, supports the practice of many centers (including Johns Hopkins) which perform PK monitoring on i.v. Bu administration [30]. However, a larger comparative study would be needed to more rigorously confirm the necessity of TDM with i.v. Bu. In addition, the safety and efficacy of PTCy with daily i.v. Bu using TDM has recently been demonstrated [28], although that study combined Bu with fludarabine; whether such findings would apply to i.v. Bu given daily in combination with Cy conditioning remains to be demonstrated. Lastly, the use of TDM to target a more narrow dosing window could theoretically reduce toxicity and standardize treatment exposure, but whether such a strategy would result in any detectable improvement in outcomes to justify this intensive monitoring would require determination in a prospective study.
Previous retrospective data are conflicting with regard to whether differences exist in VOD or survival outcomes with i.v. vs. oral Bu [33,36,43–47]. Kashyap and colleagues retrospectively compared the outcomes of 90 patients who received the BuCy2 regimen; although Bu TDM was not performed for either group, i.v. compared with oral Bu administration was the strongest predictor of improved NRM and reduced VOD [36]. In a retrospective analysis of 1230 patients from the Center for International Blood and Marrow Transplant Research comparing Cy/TBI with BuCy, patients receiving i.v. BuCy had significantly improved survival compared with patients receiving Cy/TBI, while the benefit of oral BuCy compared with Cy/TBI did not reach statistical significance; however, PK data were not collected [47]. Another study of 135 patients evaluating oral vs. i.v. Bu without TDM in the BuCy2 platform showed no differences in VOD or 100-day mortality, although significantly more patients in the i.v. group received allografts from MUDs [38]. Kato and colleagues investigated oral Bu with TDM vs. i.v. without TDM in a retrospective cohort of 460 pediatric patients and showed neither a survival advantage nor a reduced VOD rate for i.v. Bu [39]. In our patients, the frequency of VOD may indeed be reduced after i.v. Bu compared with oral Bu (1 case vs. 7 cases, respectively), but due to the low frequency of VOD overall, statistical testing was deemed inappropriate. Furthermore, significantly more patients in our oral Bu group had active disease at the time of transplantation which also may have contributed to the higher VOD rate [19].
Even though in this study we observed nearly equivalent outcomes across all survival endpoints between patients treated with oral or i.v. Bu, a disproportionate percentage of patients receiving MUD allografts were given i.v. Bu [40]. While this imbalance could potentially have biased the results towards worse outcomes for the i.v. Bu group, outcomes for patients receiving MUD allografts for alloBMT using BuCy/PTCy were similar to those patients receiving HLA-matched-related allografts [17,28,48]. In order to control this confounder as much as possible, we utilized stratified-analyses to adjust for HLA-matched-related vs. -unrelated allograft-associated outcome differences. The only difference we observed between the oral and i.v. Bu groups using this stratified analysis was higher NRM in the i.v. Bu group. This elevated NRM seemed related to an excess of deaths from GVHD in the i.v. Bu arm including a few late deaths that separated the survival curves (Supplementary Table I). Although patients receiving i.v. Bu were disproportionately composed of MUD alloBMT patients and we have previously published that patients receiving MUDs compared with HLA-matched-related allografts have a higher incidence of acute and chronic GVHD after treatment with BuCy/PTCy [48,49], surprisingly the percentage of fatal GVHD events in this cohort of MUD alloBMT patients was not higher than in the HLA-matched-related cohort (Supplementary Table I). Whether i.v. Bu is actually associated with higher risk for NRM or whether this is more likely a type I statistical error is unknown. Randomized studies are required for any definitive conclusions on the overall equivalency between the oral and i.v. formulations of Bu [30]. Moreover, any differences in outcomes between patients receiving oral vs. i.v. Bu with routine PKs would need to be weighed against the relative costs of each approach and would make an intriguing pharmacoeconomic analysis.
In conclusion, our findings indicate that using a divided dosing schedule of Bu with TDM in combination with pre-and post-transplantation Cy results in low risks of toxicity and NRM in patients with advanced hematologic malignancies regardless of the route of Bu administration. The safety and toxicity profiles of the BuCy/PTCy transplantation platform are favorable compared with those seen using other myeloablative strategies and challenge the view that Cy should be eliminated as a component of myeloablative conditioning and replaced with other agents, such as fludarabine [14,50,51]. Additional prospective studies of the BuCy regimen, particularly for the BuCy/PTCy platform, compared with conditioning regimens that incorporate nucleoside analogs are warranted and should help to further define myeloablative conditioning that can provide optimal clinical outcomes.
Supplementary Material
Acknowledgments
This work was supported by a PO1 grant from the National Cancer Institute of the National Institutes of Health [CA 015396].
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
Supplementary material available online Supplementary Figure S1 and Supplementary Table 1
References
- 1.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 2.Lu C, Braine HG, Kaizer H, et al. Preliminary results of high-dose busulfan and cyclophosphamide with syngeneic or autologous bone marrow rescue. Cancer Treat Rep. 1984;68:711–717. [PubMed] [Google Scholar]
- 3.Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70:1382–1388. [PubMed] [Google Scholar]
- 4.Copelan EA, Grever MR, Kapoor N, et al. Marrow transplantation following busulfan and cyclophosphamide for chronic myelogenous leukaemia in accelerated or blastic phase. Br J Haematol. 1989;71:487–491. doi: 10.1111/j.1365-2141.1989.tb06307.x. [DOI] [PubMed] [Google Scholar]
- 5.Grochow LB, Jones RJ, Brundrett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25:55–61. doi: 10.1007/BF00694339. [DOI] [PubMed] [Google Scholar]
- 6.Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993;20:18–25. quiz 26. [PubMed] [Google Scholar]
- 7.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- 8.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17:225–230. [PubMed] [Google Scholar]
- 9.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89:3055–3060. [PubMed] [Google Scholar]
- 10.Andersson BS, Thall PF, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 11.Bhagwatwar HP, Phadungpojna S, Chow DS, et al. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996;37:401–408. doi: 10.1007/s002800050404. [DOI] [PubMed] [Google Scholar]
- 12.Russell JA, Kangarloo SB. Therapeutic drug monitoring of busulfan in transplantation. Curr Pharm Des. 2008;14:1936–1949. doi: 10.2174/138161208785061382. [DOI] [PubMed] [Google Scholar]
- 13.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 14.Bornhauser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102:820–826. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 15.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 16.Andersson BS, Valdez BC, de Lima M, et al. Clofarabine ± fludarabine with once daily i.v busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011;17:893–900. doi: 10.1016/j.bbmt.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson MT, Lombardi L, Clarke W. Clinical consequences of analytical variance and calculation strategy in oral busulfan pharmacokinetics. Clin Chim Acta. 2011;412:2316–2321. doi: 10.1016/j.cca.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Bartelink IH, Bredius RG, Belitser SV, et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:231–241. doi: 10.1016/j.bbmt.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 21.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 23.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–480. [Google Scholar]
- 24.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 25.Cox DR. Regression models and life-tables (with discussion) J Roy Statist Soc (B) 1972;34:187–220. [Google Scholar]
- 26.Gray RJ. Some diagnostic methods for Cox regression models through hazard smoothing. Biometrics. 1990;46:93–102. [PubMed] [Google Scholar]
- 27.Zhou BLA, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics. 2011;67:661–670. doi: 10.1111/j.1541-0420.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCune JS, Holmberg LA. Busulfan in hematopoietic stem cell transplant setting. Expert Opin Drug Metab Toxicol. 2009;5:957–969. doi: 10.1517/17425250903107764. [DOI] [PubMed] [Google Scholar]
- 31.Perkins JB, Kim J, Anasetti C, et al. Maximally tolerated busulfan systemic exposure in combination with fludarabine as conditioning before allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1099–1107. doi: 10.1016/j.bbmt.2011.12.584. [DOI] [PubMed] [Google Scholar]
- 32.Bredeson C, Lerademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122:3871–3878. doi: 10.1182/blood-2013-08-519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagler A, Rocha V, Labopin M, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen - a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31:3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 34.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 35.Hassan M, Ljungman P, Ringden O, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25:915–924. doi: 10.1038/sj.bmt.1702377. [DOI] [PubMed] [Google Scholar]
- 36.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 37.Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:523–536. doi: 10.1016/j.bbmt.2008.12.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobecks RM, Rybicki L, Yurch M, et al. Intravenous compared with oral busulfan as preparation for allogeneic hematopoietic progenitor cell transplantation for AML and MDS. Bone Marrow Transplant. 2012;47:633–638. doi: 10.1038/bmt.2011.167. [DOI] [PubMed] [Google Scholar]
- 39.Kato M, Takahashi Y, Tomizawa D, et al. Comparison of intravenous with oral busulfan in allogeneic hematopoietic stem cell transplantation with myeloablative conditioning regimens for pediatric acute leukemia. Biol Blood Marrow Transplant. 2013;19:1690–1694. doi: 10.1016/j.bbmt.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Andersson BS, Madden T, Tran HT, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6:548–554. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- 41.Léger F, Nguyen L, Puozzo C. Exposure equivalence between i.v (0.8 mg/kg) and oral (1 mg/kg) busulfan in adult patients. Eur J Clin Pharmacol. 2009;65:903–911. doi: 10.1007/s00228-009-0652-5. [DOI] [PubMed] [Google Scholar]
- 42.Buffery PJ, Allen KM, Chin PK, et al. Thirteen years’ experience of pharmacokinetic monitoring and dosing of busulfan: can the strategy be improved? Ther Drug Monit. 2014;36:86–92. doi: 10.1097/FTD.0b013e31829dc940. [DOI] [PubMed] [Google Scholar]
- 43.Kim SE, Lee JH, Choi SJ, et al. Morbidity and non-relapse mortality after allogeneic bone marrow transplantation in adult leukemia patients conditioned with busulfan plus cyclophosphamide: a retrospective comparison of oral versus intravenous busulfan. Haematologica. 2005;90:285–286. [PubMed] [Google Scholar]
- 44.Lee JH, Choi SJ, Kim SE, et al. Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan+ cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol. 2005;84:321–330. doi: 10.1007/s00277-004-0982-4. [DOI] [PubMed] [Google Scholar]
- 45.Pidala J, Kim J, Anasetti C, et al. Pharmacokinetic targeting of intravenous busulfan reduces conditioning regimen related toxicity following allogeneic hematopoi-etic cell transplantation for acute myelogenous leukemia. J Hematol Oncol. 2010;3:36. doi: 10.1186/1756-8722-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veal GJ, Nguyen L, Paci A, et al. Busulfan pharmacokinetics following intravenous and oral dosing regimens in children receiving high-dose myeloablative chemotherapy for high-risk neuroblastoma as part of the HR-NBL-1/SIOPEN trial. Eur J Cancer. 2012;48:3063–3072. doi: 10.1016/j.ejca.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanakry CG, Tsai HL, Bolanos-Meade J, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124:3817–3827. doi: 10.1182/blood-2014-07-587477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JH, Joo YD, Kim H, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31:701–709. doi: 10.1200/JCO.2011.40.2362. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Zhai X, Song Z, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol. 2013;6:15. doi: 10.1186/1756-8722-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.