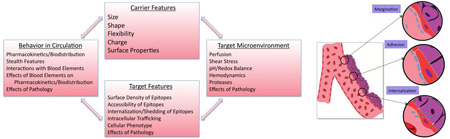
Abstract
Particles capable of homing and adhering to specific vascular biomarkers have potential as fundamental tools in drug delivery for mediation of a wide variety of pathologies, including inflammation, thrombosis, and pulmonary disorders. The presentation of affinity ligands on the surface of a particle provides a means of targeting the particle to sites of therapeutic interest, but a host of other factors come into play in determining the targeting capacity of the particle. This review presents a summary of several key considerations in nano- and microparticle design that modulate targeted delivery without directly altering epitope-specific affinity. Namely, we describe the effect of factors in definition of the base carrier (including shape, size, and flexibility) on the capacity of carriers to access, adhere to, and integrate in target biological milieus. Furthermore, we present a summary of fundamental dynamics of carrier behavior in circulation, taking into account interactions with cells in circulation and the role of hemodynamics in mediating the direction of carriers to target sites. Finally, we note non-affinity aspects to uptake and intracellular trafficking of carriers in target cells. In total, recent findings presented here may offer an opportunity to capitalize on mitigating factors in the behavior of ligand-targeted carriers in order to optimize targeting.
Graphical Abstract
1. Introduction
The vascular system is both the route to and the intended site for therapeutic intervention via drug delivery in many diseases. Blood components and endothelial cells lining the luminal surface of blood vessels represent preferred targets for pharmacotherapy of ischemia, inflammation, bleeding and thrombotic disorders, stroke, pulmonary diseases, and neurological diseases, among others. Devising carriers that optimize delivery of drugs in the vascular system may improve management of these prevalent conditions with high morbidity and mortality [1–6].
Carriers designed for this goal (including liposomes, polymeric and non-polymeric particles, protein conjugates and dendrimers, etc.) may accumulate at the desired site either relatively non-specifically (e.g., by mechanical or charge-mediated retention) or via specific interaction provided by ligands with affinity to molecules typical to or enriched in target tissues. Ligand presentation allows these carriers to specifically bind to endothelial surface determinants or, for instance, components of blood clots (e.g. platelets and fibrin) and blood cells. The latter - red blood cells, white blood cells, platelets - may serve as either target or a secondary carrier for drug delivery. “Active targeting” using ligands (e.g., antibodies and their derivatives, peptides, aptamers, etc.,) in theory offers more controlled delivery. It also enables guided sub-cellular addressing of drugs via anchoring to specific cellular determinants providing internalization via appropriate pathways [7–11].
However, many characteristics of a drug carrier and its microenvironment in the vascular system modulate its circulation and distribution and its interactions with target and non-target counterparts. As a result, these factors must be taken into account in the course of design and application of a targeted drug delivery system [12–15]. The goal of this review is to briefly analyze how factors pertaining to vascular physiology and parameters of carrier design other than affinity modulate vascular targeting and drug delivery with nanocarriers and microcarriers.
2. Modulation of pharmacokinetics and targeting by carrier geometry
Two parameters defining carrier geometry, size and shape, profoundly modulate every aspect of behavior in the body, including access to delivery routes, clearance route and rate, specific and non-specific accumulation in target and non-target sites, binding, uptake and intracellular trafficking, and ultimately effects of the drug cargo.
2.1. Carrier Geometry and Blood Clearance
One of the most important and extensively studied parameters modulating carrier behavior in the bloodstream is size. The sizes of typical carrier particles can range from below ten nanometers to a few microns. Dendrimers, micelles, gold nanoparticles, and iron oxide nanoparticles often manifest diameters below 50nm [16,17], while polymeric spheres, liposomes, and nano-shells are hundreds of nanometers in diameter [18,19]. Polymeric, lipid, and silica-based microspheres and microemulsions have diameters up to a few microns [20,21]. Moreover, carrier shape can also vary from spherical (e.g. lipid-based beads) to spheroidal, cylindrical, or discoidal particles [22–24], virus-templated particles [25], and nanopolypods [26] (Figure 1a).
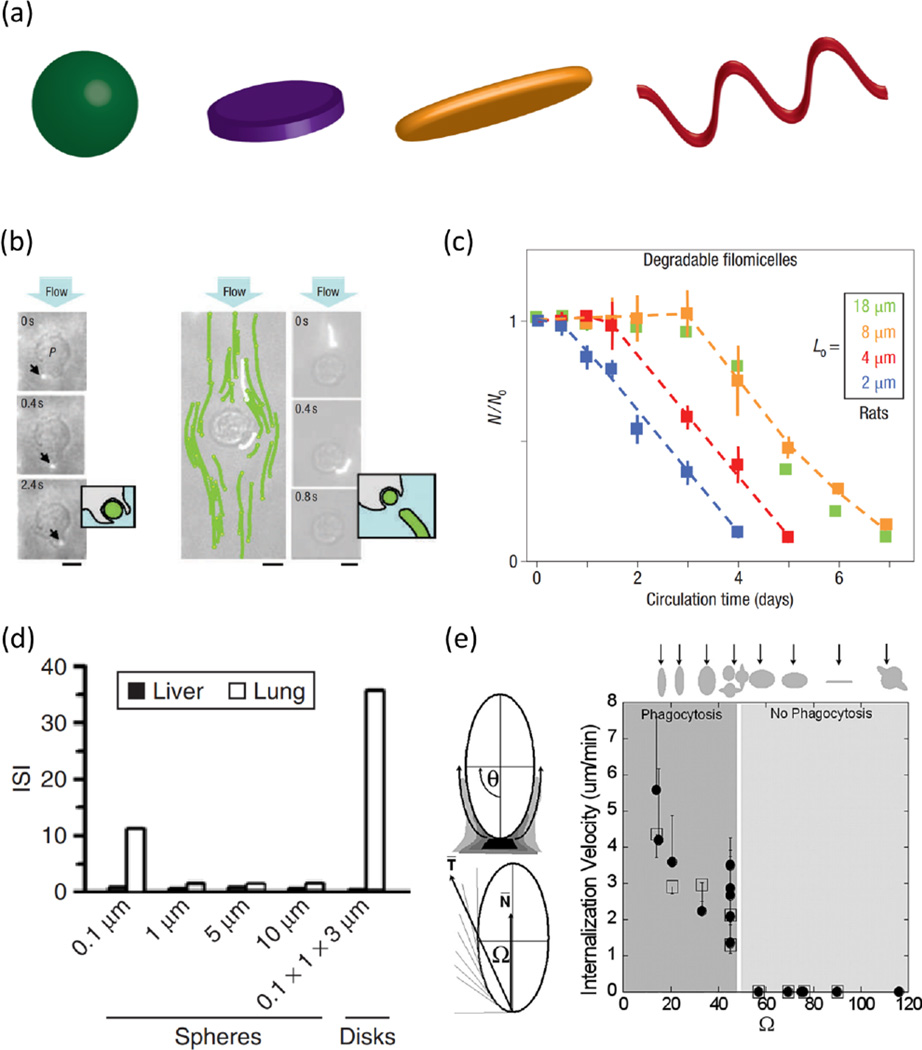
Fig. 1.
Particle shape influences and controls their biological fate and function. (a) Particles commonly investigated and utilized in vascular targeting applications include spheres, disks, rods and worm-like filomicelles. (b) Under flow conditions, longer cylindrical filomicelles align with flow and avoid association with immobilized phagocytes whereas spherical vesicles are internalized. Scale bar = 5 microns. (c) Filomicelles of longer initial lengths circulate for longer times than their shorter counterparts in rats. (d) The immunospecifity index (IS), which is the ratio of % ID/g of vascular/lung targeted to non-targeted control carriers, in the liver (black bars) and lungs (white bars) for differently sized spherical and disk shaped particles. Disk-shaped particles have high specificity for lung targeting than any of their spherical counterparts. (e) Summary of a systematic investigation that determined the roles of both particle shape and point of contact between macrophage and particle in their phagocytosis/internalization speed. (b) and (c) Adapted by permission from [43]. (d) Adapted by permission from [45] (e) Adapted from [186], Copyright (2006) National Academy of Sciences, U.S.A.
Generally, intravascular injection is needed for carriers in the size range of 50–300nm. Smaller particles may access other administration routes (e.g. pulmonary), though the effectiveness of the vascular route is generally unrivaled. After administration, size is a key parameter modulating the pharmacokinetics of carriers in the vasculature. Particles smaller than 10nm undergo renal filtration [27,28] and extravasation in tissues [29]. Drug carriers bigger than approximately 20nm are eliminated from circulation predominantly by the reticulo-endothelial system (RES, including liver, spleen and lymph nodes) [30,31].
Ultra-short carbon nanotubes, quantum dots, gold nanoparticles, dendrimers and other smaller particles spread into various organs by passing through tight endothelial junctions (10–20 nm diameter) and can be easily excreted through the glomeruli of the kidneys [29,32]. Larger particles have the advantage of carrying higher payloads when used as targeted vascular carriers.
Overall, for longer circulation after intravascular injection, carriers should escape recognition and sequestration by the RES (including accumulation in bone marrow, red pulp, lymph nodes, and phagocytic cells in the sinusoids of the liver). In order to increase circulating half-life, sub-micron carriers can be coated with excipient polymers (e.g. poly(ethylene) glycol, PEG) [33–35], but size and shape also have an impact on rate and mechanism of clearance.
Deposition of spherical silica beads in non-RES organs was shown to reduce monotonically with diameter between 700nm and 3µm [36], while 3–4µm plastic microspheres tend to accumulate permanently in the spleen [37]. Moreover, while engulfment by RES phagocytic cells happens for particles as big as 4–5µm [38,39], generally, particles larger than ~500nm are prone to mechanical entrapment in capillaries [40]. For instance, polystyrene particles larger than 5–7µm mainly trap in the alveolar capillaries of the lungs [40], while particles bigger than 10µm are retained in the liver, RES and lungs [41].
Generally, rigid spherical particles of diameter between 100nm and 200nm in diameter manifest longer circulation times because they are large enough to avoid sequestration in the liver and small enough to escape splenic filtration. For non-spherical particles, at least one dimension should be kept >100nm to avoid accumulation in the liver. To avoid entrapment in the sinusoids of the spleen, at least two dimensions must be maintained <200nm [42]. For long-circulating non-spherical particles, the effects of particle shape and size are thus closely related, where the geometry of non-spherical or flexible particles can significantly prolong the circulation time. In rodents, long worm-shaped PEG-polyethylethylene filomicelles manifest prolonged circulation time, avoid macrophage internalization, and favor accumulation in tumors [43,44] (Figure 1b, 1c), and disk-shaped polystyrene particles have lengthened circulation half-lives relative to analogous spherical particles [45], with work comparing silica spheres and discs finding lesser accumulation in the liver for discoidal particles [36]. Similarly for hydrogel discs, Merkel et al. demonstrated prolonged circulation for particles with diameter comparable to RBCs, as compared to smaller particles of identical composition [46]. As discussed later in this review, carrier mechanical flexibility can generally confound trends relating clearance time to particle size, where softer particles generally exhibit longer circulation times related to altered hemodynamic behavior [43, 47] and interactions with phagocytic cells [48,49].
2.2. Carrier Geometry and Target Site Accessibility
Variation in particle geometry can dramatically influence deposition at sites of therapeutic interest. Carrier enlargement generally negatively impacts permeation through biological barriers to poorly accessible intravascular targets, including endothelial target epitopes localized in the intercellular junctions, in invaginations of the plasmalemma, or masked by the glycocalyx. Importantly, pathological factors may alter accessibility of targets. For example, shedding of endothelial glycocalyx and widening of intercellular junctions (both typical of inflammation) may augment targeting [50,51].
Similarly, particles with a characteristic size less than a few hundred nanometers can pass through fenestrations of the tumor endothelial barrier due to enhanced permeability of the tumor vasculature [52,53]. Carriers with size between 100 and 200 nm are generally favorable for delivery using enhanced permeability and retention in tumors [54,55], though the literature data vary; For example, animal studies of gold nanoparticles with diameter from 15 to 70nm reported the highest tumor uptake for 50 nm particles [56]. One caveat of studies of the role of size in target accessibility is that real size and shape may change dramatically after injection due to agglomeration and adsorption of biomolecules.
Thrombi represent a special type of vascular target with limited and variable permeability to carriers. Cross-linked fibrin and platelet agglomerates impede diffusion into relatively mature clots for even smaller nanocarriers (<20nm). However, the interior of nascent growing thrombi is fairly accessible even for big carriers like red blood cells [57–59]. This difference provides a basis for short-term thromboprophylaxis using RBC carriers. Further, combined local features including clot porosity, mural localization and hemodynamics are being explored for design of polymeric carriers targeted to growing clots, as in [60] where shear-responsive PLGA aggregates enabled targeting to forming arterial thrombi.
2.3. Carrier Geometry and Modulation of Affinity Interactions
Carrier geometry can also modulate affinity targeting by affecting efficacy and specificity of ligand-mediated binding to target determinants. For example, a study comparing endothelial targeting of polystyrene spheres with sizes of 0.1–10µm coated with antibody directed to endothelial surface determinant ICAM-1 showed that, while overall uptake in the target organ (lungs) increased with size, targeting specificity decreased with enlargement due to elevated non-specific retention of large untargeted carriers [45]. Similarly, specific targeting to the pulmonary vasculature increased with enlargement of antibody-drug conjugates from <50nm to ~300–400 nm diameter (likely due to higher avidity of particles), but further increasing size of conjugates resulted in enhanced uptake of non-targeted carriers, thereby reducing specificity [15].
Accessibility of anchoring molecules generally diminishes with carrier enlargement, which may be especially important for carriers requiring multivalent binding for effective retention at the target. For example, determinants located in caveolae, invaginations in the endothelial plasmalemma with neck diameter <50 nm, are accessible to antibodies but not to micron-sized carriers [61,62]. In a relevant study, 100 nm polystyrene spheres coated with antibodies to different epitopes on endothelial surface adhesion molecule PECAM-1 localized at the intercellular endothelial contacts. Carriers coated with antibody to the epitope most proximal to the plasmalemma failed to bind to the cells, presumably due to lack of the access, since the antibody itself showed excellent binding [61].
Some studies have shown that antibody-coated elongated nanocarriers, including elliptic rigid discs and flexible PEG-polyethylene or poly(ethylethylene)-poly(ethyleneoxide) filomicelles, bind to endothelium more effectively and with higher specificity than their spherical counterparts [45,63] (Figure 1d). In vivo, elliptical discoid polystyrene particles targeted against ICAM-1 have exhibited higher targeting specificity than similar spherical particles [45]. Model polystyrene nano-rods (aspect ratio ~3) coated with ICAM antibody showed 2–3-fold higher binding to endothelial cells in vitro [64]. Nanorods coated with transferrin-receptor monoclonal antibody exhibited 10-fold higher binding to brain endothelium [64]. Finally, ellipsoidal polystyrene rods targeted to VCAM-1 have exhibited stronger adhesion in atherosclerotic plaques than analogous spheres [65].
It should be noted that while the above trends concerning modulation of targeting by carrier geometry are evolving, significant variability in delivery parameters has been observed for ligand-targeted carriers from different labs. Furthermore, an increasingly wide range of particle shapes has become available for biocompatible nanomaterials capable of drug delivery, with hydrogel capsules achieving cubic [66–68], discoidal [69,70], and hemispherical geometries [71] and hydrogel hemispheres and discs showing promise for uptake in macrophages, endothelial cells, and cancer cells [71,72]. Keeping in mind that carrier geometry affects practically every aspect of targeting – access, routing, binding, and uptake - more systematic, unified, and thorough studies of the role of geometry in targeting are necessary to guide carrier improvement and provide the data for computational analysis of this important subject.
3. Modulation of vascular targeting by carrier mechanical properties
As may be inferred from the discussion of carrier geometry above, the ability to change size or shape can be immensely important for the targeting properties of vascular nanocarriers. Advances in the characterization of individual particle mechanical properties (e.g. AFM and optical trap methods currently being applied to cells) have allowed identification of promising carriers exploiting a range of flexibilities [73–76].
Carrier platforms allowing sufficient flexibility to respond dynamically to mechanical influences of the milieu include liposomes, some dendrimers and polymer particles, and hydrogels. Hydrogels are particularly interesting as carriers with designed mechanical properties. Among many versatile properties available to hydrogel nanoparticles in biomedical applications, nanogels are capable of tunable viscoelasticity. Carrier flexibility can be controlled by chemical structure (e.g. enhanced cholesterol content reduces fluidity of liposomal membranes and polymer backbone properties can control nanogel deformability) and specifics of the assembly of elements in multi-molecular structures (e.g. cross-linking, inter-molecular arrangement, layering) [77].
Generally, mechanical properties are a critical consideration in the design of biocompatible materials. Carrier flexibility modulates binding and retention as well as circulation time and stability [78–80]. As reviewed previously, mechanical stimulation influences cellular physiological processes regulating cell-nanocarrier interactions including adhesion, cytoskeletal remodeling and uptake [81]. Likewise, tuning the mechanical properties of nanoparticles can affect interactions with target and off-target cells.
3.1. Circulation of Mechanically Flexible Carriers
Carrier flexibility influences circulation in the vasculature. Rigid elongated particles (rods, disks, tubes) larger than a few hundred nanometers quickly become entrapped in the microvasculature. However, carriers with sufficient flexibility to reversibly change shape in response to hydrodynamic factors and vessel narrowing may be able to pass through the microcirculation.
The design of flexible carriers often emulates natural objects exerting desirable features in the body, including bacterial and viral particles and blood elements, including red blood cells. RBCs represent a natural model for targeting behavior enabled by elasticity, possessing the capability to repeatedly squeeze through capillaries of 1–2 micron lumen diameter (despite a ~7 micron RBC diameter) while avoiding clearance [82]. In line with this notion, flexible hydrogel particles have been shown to have extended in vivo circulation times [46, 83]. Particles that mimic some mechanical attributes of RBCs, including size, shape, elastic modulus, ability to deform under flow, and oxygen-carrying capacity, have been fabricated by layer-by-layer self-assembly of BSA and polyallylamine hydrochloride [84]. Varying the extent of cross-linking in hydrogel particles has yielded mechanical flexibility similar to that of RBCs [85], resulting in prolonged circulation of particles close in diameter to RBCs [46].
There is a positive correlation between the enhanced permeability and retention effect and circulation time, and adjustment of flexibility of nanoparticles is a valid means of accessing that correlation. Mechanical retention of this sort (as discussed above in terms of particle size) may also be better engaged by mechanically deformable particles [86].
Elongated flexible carriers have unique behaviors due to the combined effects of geometry and flexibility. Filomicelles are a distinctive subset of micelles made of amphiphilic block copolymers that assemble in flexible flow-responsive filaments [43,87]. Filomicelles are able to persist in blood for extended periods of time by taking advantage of both their worm-like shape and cell-like flexibility. As noted in the previous section, PEG-polyethylethylene filomicelles are able to align with blood flow (Figure 1b) and avoid immune system clearance, enabling them to persist in circulation for days, which is up to 10 times longer than their spherical counterparts [43]. Pristine PEG-polyethylethylene filomicelles exhibit little to no cellular binding, an attractive feature for a long-circulating carrier [88].
3.2. Carrier Flexibility and Penetration in Porous/Microstructured Environments
Highly solvated nanogels display both liquid and solid-like behavior, deforming in the presence of flow in such a manner as to permit movement through extracellular matrix and between densely packed cells. Recent studies show that flexible gel particles with minimal cross-linking translocate through barrier materials with pores tenfold smaller than nanogel diameter under pressures resembling those experienced during renal filtration (Figure 2a). Flexible NIPAm particles of ~1100nm and ~850nm diameter passed through pores of 100nm diameter at levels commensurate with those observed for 88nm polystyrene beads [89,90] (Figure 2b). Alternative nanogels of 116nm diameter also passed through 10nm pores where 88nm polystyrene beads did not [89,91]. Earlier work indicated that nanogels with minimal cross-linking can experience a 15fold reduction in volume under compression [90].
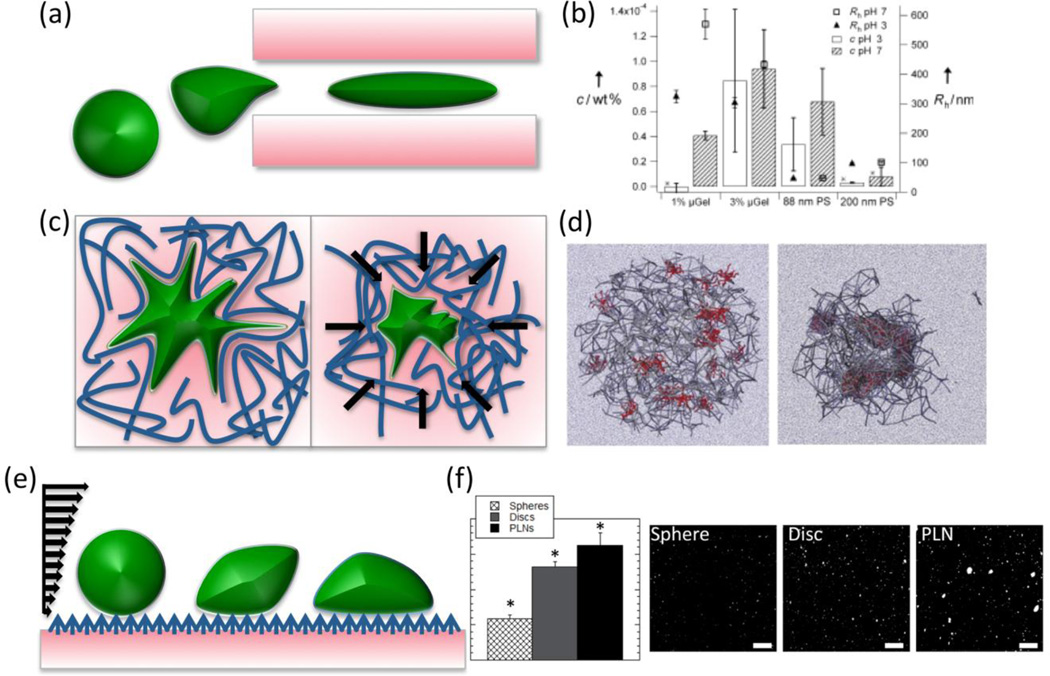
Fig. 2.
Modulation of target access, interactions with target microenvironment, and affinity via carrier flexibility. (a) Highly flexible nanocarriers are capable of translocation through pores smaller than their hydrodynamic diameter, as depicted in (b), where hydrogel particles of greater than 400nm diameter translate through 100nm pores in quantities (left axis) similar to those observed for 80nm rigid particles [89]. High-plasticity nanoparticles have exhibited a capacity for integrating in porous environments and, in the case of fibrin networks, participating in contraction of the environment microstructure (c), as observed in molecular dynamics simulations of fibrin-targeted hydrogel particles in fibrin networks in (d) [92]. Carriers more capable of deforming under shear may present greater adherent surface area and flatten out to avoid dynamically dislodging from the target surface (e), as exemplified in the fluorescence data in (f) comparing binding of rigid spheres, rigid discs, and flexible discs in a microfluidic cell under shear [93]. (b) Reproduced with permission from [89]. (d) Reprinted by permission from Macmillan Publishers Ltd: Nature Materials [92], copyright 2014. (f) Reprinted with permission from [93]. Copyright 2014 American Chemical Society.
In another application of particle flexibility, a recent study demonstrated that highly deformable particles can specifically incorporate in the porous fibrin network in clots. There, NIPAm microgels binding to fibrin were shown to fundamentally alter fibrin clot microstructure by participating in clot contraction. As demonstrated in vitro with clot contraction assays and in silico with dynamic simulations of fibrin networks, flexible microgels permitted contraction of fibrin networks, whereas rigid spheres with the same fibrin-targeting capacity did not [92] (Figure 2c, Figure 2d).
These studies indicate that flexible particles can be designed to accommodate environmental features and processes requiring a certain size (e.g. kidney filtration, extravasation, and endocytosis) even while the carrier dimensions exceed that size limit. In theory, such particles can carry large amounts of cargo while fitting into small spaces.
3.3. Carrier Flexibility and Targeting
Carrier flexibility is likely to contribute to affinity targeting. Carriers made from soft PAH-BSA multilayers have been shown to manifest increased targeting [93] (Figure 2f). First, flexible carriers may be able to flatten on the target surface under shear. This phenomenon both reduces the drag force of perfusion that leads to detachment and enhances binding via spreading over and engaging a higher number of binding sites. Second, lateral movement of ligand molecules in flexible carriers favors congruency of ligand interaction with multiple binding sites and their clusters [94] (Figure 2e). These advantages of more flexible carriers are beginning to be reported in literature where flexible ligand-presenting carriers outperform rigid counterparts of the same shape and identical coating in terms of binding and binding strength under shear [95].
In addition, as noted above, flexible particles circulate for a longer time and have a better chance of reaching their targets than rigid particles. A recent study compared targeting to endothelial surface determinant ICAM-1 in vitro and in vivo with soft (~10 kPa elastic modulus) and hard (~3000 kPa) PEG diacrylate hydrogel nanoparticles. Softer particles showed prolonged circulation and enhanced binding in the lungs in vivo and avoided non-specific cellular uptake and binding to a variety of cells in vitro (e.g. tumor, endothelial, and macrophage cells) [83]. Mechanistically, in comparison to rigid counterparts, flexible polyacrylamide-BSA particles have proven to be greater than 5-fold less likely to be internalized by immune cells in vitro [96]. It has been shown that softer PAAm nanoparticles avoided phagocytosis and exhibited enhanced macropinocytosis in macrophages [96,97]. These results support the notion that elasticity provides targeting advantages in vivo.
While cross-linking density provides a direct approach to varying the stiffness of polymeric carriers, including nanogels [77,98], it should be noted that addition of cross-linkers can also alter size, shape, and surface chemistry of nanoparticles. Therefore, careful consideration of the totality of nanoparticle properties is necessary prior to drawing conclusions about the relationship between nanoparticle targeting and nanoparticle mechanics.
4. Role of blood elements in vascular targeting
Blood elements, including biomolecules and circulating cells, can modulate carrier targeting in many ways (Figure 3). Both cellular and molecular elements in blood can affect particle clearance and modulate carrier interactions with the endothelium. Additional work has explored use of the properties of blood cells (RBCs, leukocytes, and platelets) to explicitly target carriers to sites of therapeutic relevance.
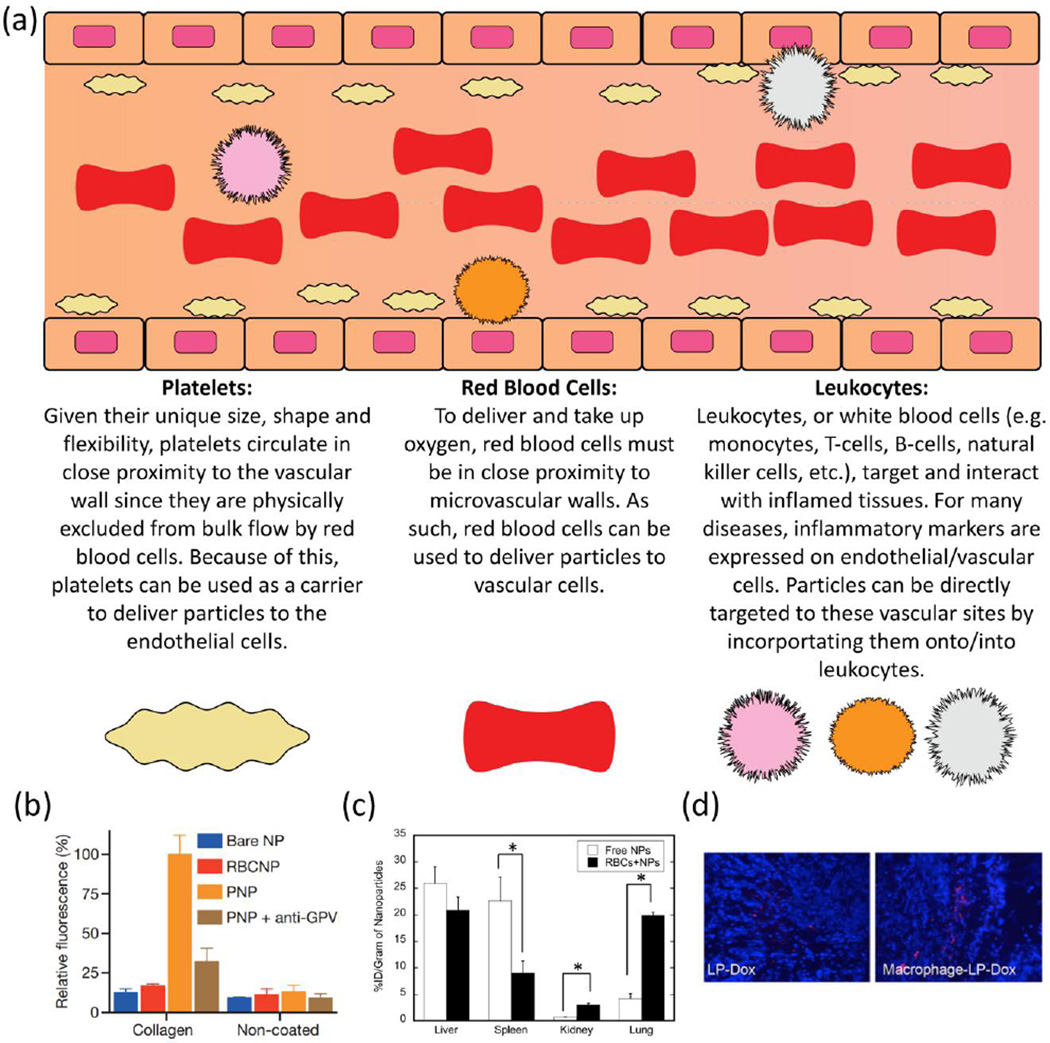
Fig. 3.
Schematic of the three main cellular components of blood: erythrocytes (red blood cells), thrombocytes (platelets) and leukocytes (e.g. monocytes, T-cells, B-cells, natural killer cells, etc.). Each of these cell types possess unique cellular functions that can be leveraged to target nanoparticles to vascular/endothelial cells (a). Data presented in (b) show that polymeric particles may be designed for specific adhesion to collagen surfaces [138]. In (c), nanoparticles adhered to red blood cells show greater adhesion to lung endothelium and lesser sequestration in the spleen [121]. In (d), fluorescent doxorubicin-loaded liposomes appear in tumors in greater quantities after first being loaded into macrophages [129]. (b) Reprinted by permission from Macmillan Ltd.: Nature [138], copyright 2015. (c) Adapted with permission from [121]. Copyright 2013 American Chemical Society. (d) Reprinted from [129], Copyright (2012) with permission from Elsevier.
Primarily, carriers interact with the full spectrum of biomolecules in plasma. The range of plasma molecules, the kinetics of the interactions, and the consequences of the interactions depend on the carrier surface properties and pathophysiological context. Coating carriers with PEG and other masking or stealth compounds helps to delay plasma adsorption on the carrier surface, yet this process likely starts immediately after injection [33–35]. This may lead to unintended inactivation and activation of plasma molecules and cascades, acceleration of carrier aggregation, and targeting to unexpected binding sites. For example, adsorption of lipoproteins and albumin stimulates carrier uptake by vascular cells via receptors for these proteins, whereas fixation of complement and immunoglobulin directs particles to defense cells via C3b and Fc-receptors.
4.1. Indirect Effects of Cellular Blood Components on Vascular Targeting
Cellular blood components greatly influence targeted nanocarrier behavior in the vasculature [99]. For example, the glycocalyx layer, extending hundreds of nanometers from the endothelium into the vessel lumen, interferes with the interaction of circulating blood components with the endothelial plasmalemma [100]. However, under high shear conditions, RBCs are known to push particles to the marginal glycocalyx-protected flow layer, enhancing interaction with endothelial cells [101, 102]. Indeed, it has long been known that the hematocrit, the concentration of RBCs, modulates interactions of targeted particles with the vessel wall, as exemplified by an early series of studies examining targeting of antibody-carrying RBCs to collagen-coated surfaces imitating blood vessels with denuded endothelium [103]. When antibody-carrying RBCs were perfused in these collagen-coated channels, addition of naive RBCs to a perfusion buffer enhanced binding of targeted RBCs to the collagen target [104]. Other work has recapitulated this paradigm with ligand-targeted nanoparticles, showing that naive RBCs stimulate binding of targeted nanoparticles to target molecules in model vessels simulating pathologically altered endothelium [101,105–107]. Computational and intravital microscopy work indicates a role for size-dependent sequestration of particles near the vessel wall in the RBC-mediated enhancement of interactions with the endothelial surface [101].
Furthermore, computational studies simulated interactions between nanocarriers and endothelium in large and small vessels in the presence and absence of RBCs [108,109]. Two factors, margination of particles by RBCs and an increase in particle dispersion coefficient via rotation and tumbling of RBCs under shear, act together to increase the carrier concentration in the cell-free-layer at the vessel wall. In smaller vessels, similar simulations show that carrier accumulation is enhanced further, likely due to the larger role volume exclusion plays when RBCs may be forced to physically contact the vessel wall [109].
For the remainder of this discussion, we note that the term “margination” refers to particle distribution in the vessel lumen towards the vessel wall, not to adhesion or binding to the wall. There is a correlation between margination and binding, even for non-targeted particles [110], but it is not necessarily the case that more effective margination of a particle will enhance binding; for example, if there is a repulsion between the particle and vessel wall due to electric charge [111]. Binding beyond margination is mostly controlled by factors pertaining to particle avidity, so the details of binding as a result of margination are beyond the scope of this review.
The margination of particles in blood cell suspensions is enhanced by increased carrier size. Smaller particles show a reduction in margination to the cell-free-layer as they tend to flow along with blood, accessing the spaces between blood cells. In vitro work by Liu et al. found that blood enhances both 210nm and 2µm polystyrene particle binding in microcirculation. The binding density increased threefold more for the 2µm particles compared to the 210nm particles [112]. Eniola-Adefeso et al. examined the effect of particle size (500nm to 10µm) on margination propensity in blood and observed an increase in this phenomenon with increasing particle size [101,105]. In vivo, better margination in mouse microvasculature was observed for 1µm PEG-coated polystyrene particles compared to 200nm particles [102].
Spherical particles exhibit slightly better margination than ellipsoidal carriers, but ellipsoidal particles are more favorable for adhesion due to slower rotational dynamics, as determined computationally and experimentally in microfluidic model vessels [113,114]. Margination probability also depends on the hematocrit in simulations [113]. Extensive particle margination was observed at high hematocrit, which is favorable for drug delivery to tumors because the tumor microvasculature tends to concentrate blood cells [115].
4.2. Interactions with Red Blood Cells to Augment Vascular Targeting
Blood cells themselves, especially RBCs, represent attractive “super-carriers” for drug delivery. RBCs spend the majority of their lifetime in blood circulation, and are natural carriers that (i) take up oxygen in the lungs, (ii) circulate throughout the body’s vasculature while carrying oxygen, and (iii) deliver oxygen to all tissues in the body while remaining in blood circulation. These RBC biological functions are a direct result of unique physical properties, especially flexibility and high surface area to volume ratio, which permits passage through blood vessels that are smaller in diameter than the cell itself. These features have been explored for decades as the basis for loading RBCs with drugs to improve delivery and effects in the bloodstream [116]. Drugs can be encapsulated in isolated RBC prior to re-infusion, or coupled to RBC surface [82,117].
To facilitate oxygen transfer, RBCs must be in close proximity to the microvascular walls [82]. As such, since RBCs spend a significant amount of their lifetime interacting with the endothelium, RBC-based delivery of nanoparticles to endothelium is an interesting and promising approach. Methods of attachment of particles to cells and specific applications of these cell-mediated delivery systems have been reviewed extensively elsewhere [118–120]. In model studies in vitro and in vivo, 200nm diameter polystyrene nanoparticles were loaded onto mouse RBCs ex vivo and injected intravenously into mice. For time points ranging from 30 minutes to 24 hours, lung accumulation of red blood cell-adsorbed nanoparticles was increased 7-fold compared to identical non-adsorbed nanoparticles (Figure 3c. The most dramatic effects of lung targeting were seen at shorter time points, as lung accumulation was transient and shown to sharply decrease for red blood cell-adsorbed nanoparticles at 24 hours. Other benefits of this delivery system included increased blood persistence of nanoparticles and also further enhanced lung targeting, both at short and long time points, when nanoparticles were modified with lung targeting antibodies [121]. Likewise, it has been reported that polystyrene particles of various sizes attached to rat RBCs circulate longer (over 10 hours) than those not attached [122]. It is tempting to hope that these initial observations will be translated to medically useful drug delivery approaches, warranting studies of the mechanisms of carrier loading on RBCs and transfer to endothelium and, perhaps, other targets.
4.3. Interactions with Leukocytes to Augment Vascular Targeting
Leukocytes (e.g. monocytes, T-cells, B-cells, etc.) have also been used as vehicles to enhance the delivery of particles to the vasculature. Typically, carrier-leukocyte systems are designed to take advantage of the innate targeting and barrier penetration abilities of leukocytes [119]. Leukocytes are circulatory immune cells with the natural ability to target pathological tissues (e.g. sites of infection and inflammation) [123,124] and in theory can be used to facilitate drug delivery to inflamed tissues in many disorders [125]. Of note, leukocytes transmigrate through the endothelial barrier into tissues, including through the blood brain barrier and into the brain, which are otherwise not accessible to drug carriers.
For example, loading of drug carriers in monocytes/macrophages has been explored in animals for delivery of: (i) micron-sized antiviral drug-loaded particles to reduce HIV replication in the brain [126]; (ii) sub-50nm polystyrene particles to breast cancer metastases in the brain [127]; and (iii) catalase loaded polyethyleneimine-PEG nanoparticles to reduce inflammation [128]. Macrophage-mediated delivery systems for doxorubicin-bearing liposomes have also been devised and displayed anti-tumor effects [129] (Figure 3d). Most recently, it was shown that monocytes can be used to target delivery of micron sized polyelectrolyte particles to locally inflamed lung and skin tissue following antibody-mediated attachment of the particles to monocytes. In this case, monocytes showed ~2-fold preference for inflamed over normal lung tissue. This approach also augmented delivery of monocyte-bound drugs to pathological sites in a local skin inflammation model [130].
4.4. Interactions with Platelets for Targeting and Enhancement of Thrombosis
Nano- and microparticles capable of mimicking and complementing the behavior of platelets have received growing attention in recent years. Enhancing interactions between nanocarriers and platelets represents a promising means of targeting sites of vascular injury contributing to ongoing thrombosis, either to modulate that process directly or for purposes of drug delivery. The range of synthetic particles recapitulating or taking advantage of platelet behavior has been addressed elsewhere [131]. This subsection provides a condensed summary of recent work with an emphasis on directed interactions with platelets for vascular targeting.
A basic approach to enhancement of platelet-carrier interactions employs fibrinogen-derived RGD sequences. Particles coated with these peptides bind to the platelet fibrinogen receptor, GPIIb/IIIa [132–135]. Natural homing of platelets to sites of clotting facilitates targeting of such nanoparticles, which in turn support platelet aggregation by cross-linking fibrinogen receptors. Particles binding GPIIb/IIIa have a stabilizing effect on hemostatic clots and provide an anti-hemorrhagic effect at sites of vascular injury. In an early example of employing fibrinogen mimetic peptides to participate in platelet aggregation, Coller et al. functionalized RBCs to preferentially bind to activated platelets [132]. Targeting to activated platelets has also been reported with synthetic carriers, including liposomes [136] and PLGA nanoparticles [135,137], presenting fibrinogen-based sequences. Recently, another approach involving coating of nanoparticles with platelet membranes was shown to enhance the binding of these particles to collagen coated surfaces, as compared to bare nanoparticles and control RBC-membrane coated nanoparticles (Figure 3b) [138]. PAH-BSA particles have also been designed to adhere directly to platelets by presenting vWF fragments targeted to platelet GPIbα [95]. Most recently, particles engaging in both platelet aggregation (via RGD peptides) and adhesion (via collagen and vWF binding) have been demonstrated [93,139].
Non-affinity factors profoundly modulate interaction of carriers targeted to platelets and vascular injuries. Enhancement of interaction with platelets and platelet-like behavior has been achieved through modulation of particle size and shape, with larger and more oblate particles exhibiting more localization towards the vessel wall and improved participation in aggregation and adhesion [93,95,140]. Individual particle mechanical properties have additional effects on platelet-like behavior of vascular carriers. Lipid membrane fluidity and thus flexibility of GPIbα liposomes was shown to enhance adhesion to vWF [141]. Albumin-based particles developed by Mitragotri’s group have combined the effects of platelet-mimetic geometry and flexibility (as well as surface chemistry) to enhance association with platelet adhesion relative to analogous polystyrene spheres [93,95].
As addressed above, particle flexibility has also been used to mimic the role of platelets in clot contraction. Brown et al. employed microgels with no cross-linking to recapitulate the compatibility of platelets with clot microstructure [92]. Essentially, it was demonstrated that only fibrin-binding particles with minimized cross-linking could incorporate in contracting clots. Rigid polystyrene particles and even hydrogel particles with small amounts of additional cross-linking interfered with clot microstructure, while “ultrasoft” microgels successfully participated in clot contraction alongside platelets [92].
In general, loading carriers onto cells that naturally home in on pathological tissues may evolve into a new strategy and, perhaps, help to reduce immunological side effects of using exogenous proteins or antibodies for particle targeting. Of note, the specific characteristics of this approach, including percent of dose taken up by the “transporting” cells and that delivered to the target tissue, remain to be determined. On the other hand, these cells are defense agents and their effects (not mediated by or perhaps countering that of the drug cargo) at pathological sites may not necessarily be desirable.
5. Regulation of delivery and targeting by hemodynamic factors
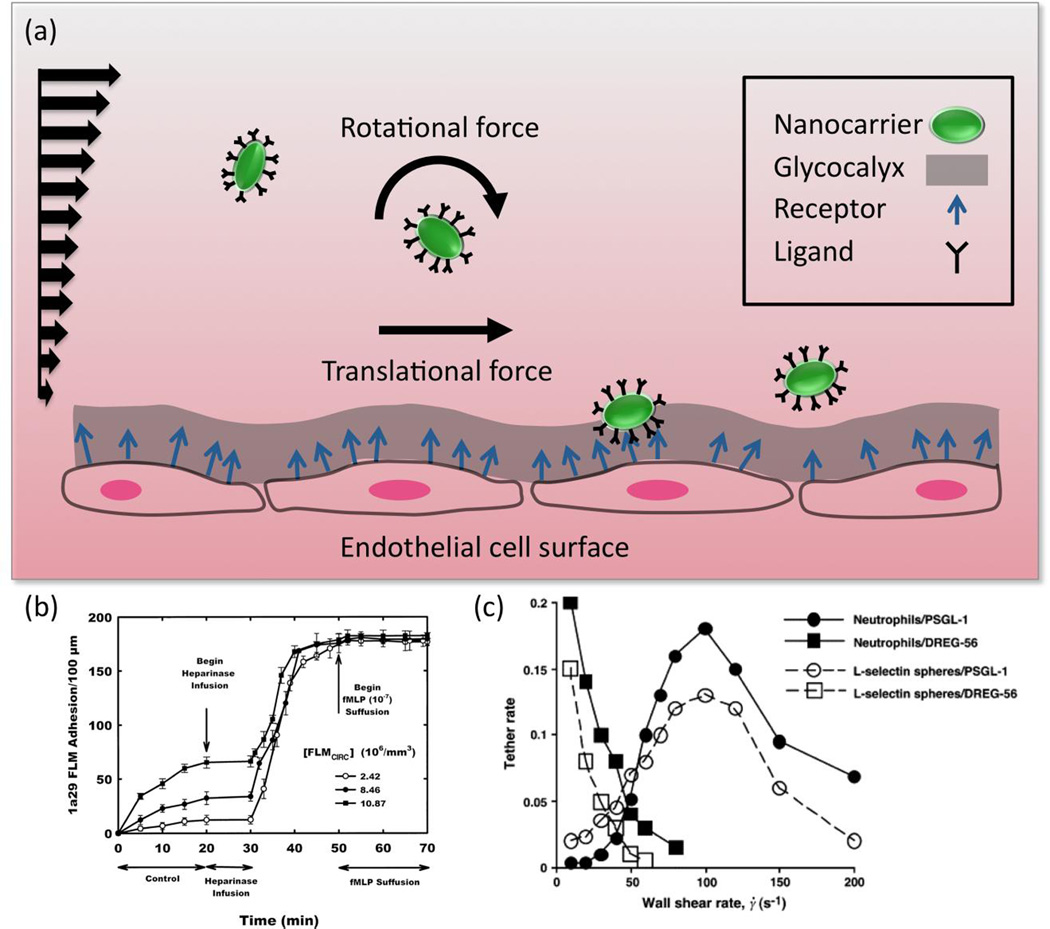
Expanding on aspects of the above discussions of geometry and mechanical properties, hemodynamic factors play an important role in delivering nanocarriers to the vessel wall so that binding interactions, uptake, and drug delivery can occur. Among the most important factors regulating carrier motion in blood flow are carrier geometry, the role of the glycocalyx, and the exposure to fluid mechanical forces. A broad-spectrum computational protocol devised for calculating binding between functionalized nanocarriers and the endothelial cell surface has considered rotational as well as translational nanocarrier movement under flow [99] (Figure 4). With carrier shape restricted to spherical objects, changing particle size alone only moderately enhances binding due to the entropy loss associated with bound receptors [142]. Extension of this work into effects of cell membrane features (e.g., degree of membrane excess and membrane elasticity) and nanocarrier shape (e.g., ellipsoidal carriers) is revealing additional non-affinity related determinants of targeting efficiency mediated through both the carrier behavior in flow and mechanical effects of flow at the endothelial cell-carrier binding interface.
Fig. 4.
Depiction of flow-induced nanocarrier translational and rotational (tumbling) motion induced by fluid forces, which will be dependent on nanocarrier size and shape. The wavy, deformable endothelial cell layer surface and presence of the glycocalyx are included as additional factors regulating binding (a). For instance, as determined in [51], shedding of the glycocalyx via heparinase infusion significantly enhances the binding of ICAM-targeted 100nm diameter fluorescent polystyrene spheres (1a29 FLMs) in postcapillary venules (b). As depicted in [167], there is a non-linear relationship between wall shear and binding of 3µm L-selectin targeted polystyrene microspheres to a complementary surface (L-selectin spheres/PSGL-1) (c). A threshold shear rate, implicated in inducing tumbling of the spheres near the binding surface, resulted in optimal binding. (b) Reproduced with permission from [51]. (c) Reprinted from [167] with permission from Elsevier.
5.1. Interaction Between Hemodynamic Factors and Nanocarrier Size
The influence of hydrodynamic factors on carrier behavior and targeting depends on carrier size. In vitro flow chamber binding experiments using targeted spherical polystyrene particles of diameter 100nm to 10 µm coated with antibodies to the endothelial surface determinant E-Selectin showed that specific binding to endothelium increased as diameter increased from 500nm to 10µm at a shear rate of 200 s−1. However at higher shear (e.g. 1500 s−1), spheres of 5µm and 10µm diameter were less likely to adhere to E-Selectin than smaller spheres [101].
As noted in section 4, RBCs segregate away from the vessel wall in vessels between 10 and 300µm in diameter, leading to changes in viscosity and local hematocrit as characterized by both the Fåhraeus and Fåhraeus-Lindqvist effects [143]. As a result, particles in vascular flow can demonstrate “margination,” dispersing towards the wall into the cell-free plasma layer [101,102,105,108,109,112–114,144].
To exploit this effect for targeted carrier based drug delivery, theoretical studies have suggested that a diameter of ~200nm or smaller provides optimal margination, while diameters greater than 200nm result in less efficient margination (as determined by the time a particle takes to migrate towards the vessel wall) [111]. However, shear flow experiments have shown that particles of diameter larger than 200nm undergo effective margination driven by gravitational force [110]. These studies performed with a parallel plate flow chamber indicated that polystyrene nanocarriers larger than 200nm undergo the greatest margination due to sedimentation in horizontal capillaries and the greatest lateral drift in vertical capillaries with downward flow. This motion toward the vessel wall increased the probability of binding to the endothelium [110].
It is worth mentioning that the model presented in reference [110] works in terms of volume concentration, rather than number of particles, assuming nanospheres are fed into blood at a higher numerical concentration than microspheres in concluding that the quantity of marginating particles scales with the volume concentration for particles larger than 200nm. Namdee et al. [105] found that increasing the numerical concentration of nanospheres in blood flow does not necessarily result in a linear increase in adhesion to endothelial cells. While a five-fold increase in concentration resulted in a five-fold increase in adhesion for 2µm and 5µm particles, a five-fold increase in concentration produced an approximately twofold increase in adhesion for 200nm and 500nm particles. Decuzzi et al. have also predicted a positive correlation between carrier density and propensity to migrate towards the vessel wall [111]. Thompson et al. have experimentally addressed the problem of density in margination, comparing polystyrene, silica, and titania particles in an endothelialized flow cell and finding greater margination for more dense particles [107].
5.2. Effect of Nanocarrier Shape on Hemodynamic Behavior
Nanocarrier shape has significant influence on nanocarrier motion in blood flow [145]. Particles that are non-spherical can migrate laterally in flow [146]. Moreover, it has been shown that the lateral drifting velocity is directly related to aspect ratio [147,148] for non-spherical particles. Discoid particles circulate longer than spherical particles [45], and flexible filomicelles can be present in the circulation up to ten times as long as spherical particles of similar volume [43,149] (Figure 1b, 1c). This is due to their becoming aligned with the flow, enabling avoidance of collisions with blood cells that contributes to margination. One adverse consequence of this is that carrier-wall interactions can be reduced: in vivo targeting of anti-ICAM coated poly(ethylethylene)-poly(ethyleneoxide) filomicelles showed lower absolute targeting than spheres (although the ratio between targeted and non-targeted counterparts was similar for filomicelles and spheres) [63].
However, the probability of a nanorod carrier binding under modest shear stress has been computationally modeled to be three-fold higher than that of a sphere, indicating that shape manipulation can enhance nanocarrier-wall interactions [150]. Further support for this concept is that targeted polystyrene disks have been shown in experiments to have greater targeting specificity than spheres (targeted to non-targeted carrier ratio = 35.7) [45]. A physical explanation for these phenomena is provided through mathematical modeling, which reveals that the nonsymmetrical rolling and tumbling motions that rod-shaped particles experience near the vessel wall causes them to have greater likelihood of adhering to endothelium than do spherical particles under the same flow conditions [151]. For discoid particles, mathematical models and in-vitro experiments have predicted lateral drift [152] and more avid adherence to vascular walls under flow [99,153].
Further modeling studies have demonstrated the possible advantages of other non-spherical shapes for particles binding to endothelial cells in shear flow. For instance, oblate particles have greater adherence to surfaces under flow [153], and elongated particles having a diameter < 200nm can marginate optimally and thereby have increased interactions with the endothelium [111].
In vitro, microfluidic devices are particularly useful for studying binding of different nanocarrier shapes under flow [154], especially given that geometric features relevant to the vasculature in different organs [155] and significant vascular segments or tissues [60,156] can be incorporated into their design. Fluidics experiments have demonstrated margination of discoid silica particles to be greater than that of spherical particles, without, however, a method distinguishing margination from binding or adhesion [140]. Micron-sized particles have been studied using straight and bifurcated channels to examine the role of particle shape (e.g., aspect ratio, flatness) in influencing targeting with flow. Studies of binding of targeted discshaped polystyrene particles in a bifurcated system indicated discs have 5-fold greater binding than targeted spheres [157]. Additional work has found that selectin-targeted ellipsoidal polystyrene rods have greater adherence to endothelial cells compared to identically targeted spheres under flow, with higher aspect ratio increasing the quantity of bound particles [114]. Particle shape affects binding more for large particles and for particles transiting bifurcations. Of note, higher numbers of rod-shaped particles and disk-like structures adhere in model fluidics systems compared to spherical objects [114,157], in agreement with in vivo studies [45,65].
5.3. Carrier Interactions with the Glycocalyx Under Flow
The glycocalyx is a gel-like layer which extends ~500nm from the in vivo luminal surface of endothelial and blood cells (among other cell types). A brush-like structure, formed by strongly negatively charged carbohydrate chains of glycoproteins, the glycocalyx affects numerous cardiovascular physiological and pathological processes, including sensing of flow by endothelium and separating blood cells from the endothelial plasmalemma proper.
The glycocalyx has a significant role in modulating nanocarrier binding to endothelial cells by acting as an energy barrier (see Figure 4). Mechanical and biochemical properties of the glycocalyx as well as its interactions with erythrocytes and leukocytes [158] have been probed experimentally [159]. Generally, the strong negative charge of the glycocalyx represents a mechanism for non-specific (i.e., bypassing ligand-mediated targeting) binding of cationic carriers in the vasculature. Furthermore, the glycocalyx regulates interaction of biotherapeutic proteins targeted to endothelial and blood cells with their physiological counterparts in plasma [160,161].
The in vivo binding of 100 nm diameter polystyrene microspheres coated with ICAM-1 antibody has been shown to increase as much as 500-fold following chemical degradation of the glycocalyx (Figure 4b) [51]. The glycocalyx’s mechanical behavior is demonstrably viscoelastic, and its responses to fluid shear and cellular motion have been used to predict the bending rigidity of its core proteins to be in the range of 700 pN·nm2 [162]. This stiffness translates into an energy barrier that acts to reduce the binding between nanocarriers and the endothelial cell surface [163].
Of note, biologically relevant glycocalyx layer that can be seen in vivo is very difficult to reproduce in cell culture, especially in static cells lacking hemodynamic stimulation. The magnitude by which glycocalyx thickness and stiffness are altered in vitro compared to in vivo is unknown, so the effect it bears on nanocarrier binding in vivo cannot be extrapolated from results of cell culture-based studies.
5.4. Effect of Flow Dependent Forces on Carrier Targeting
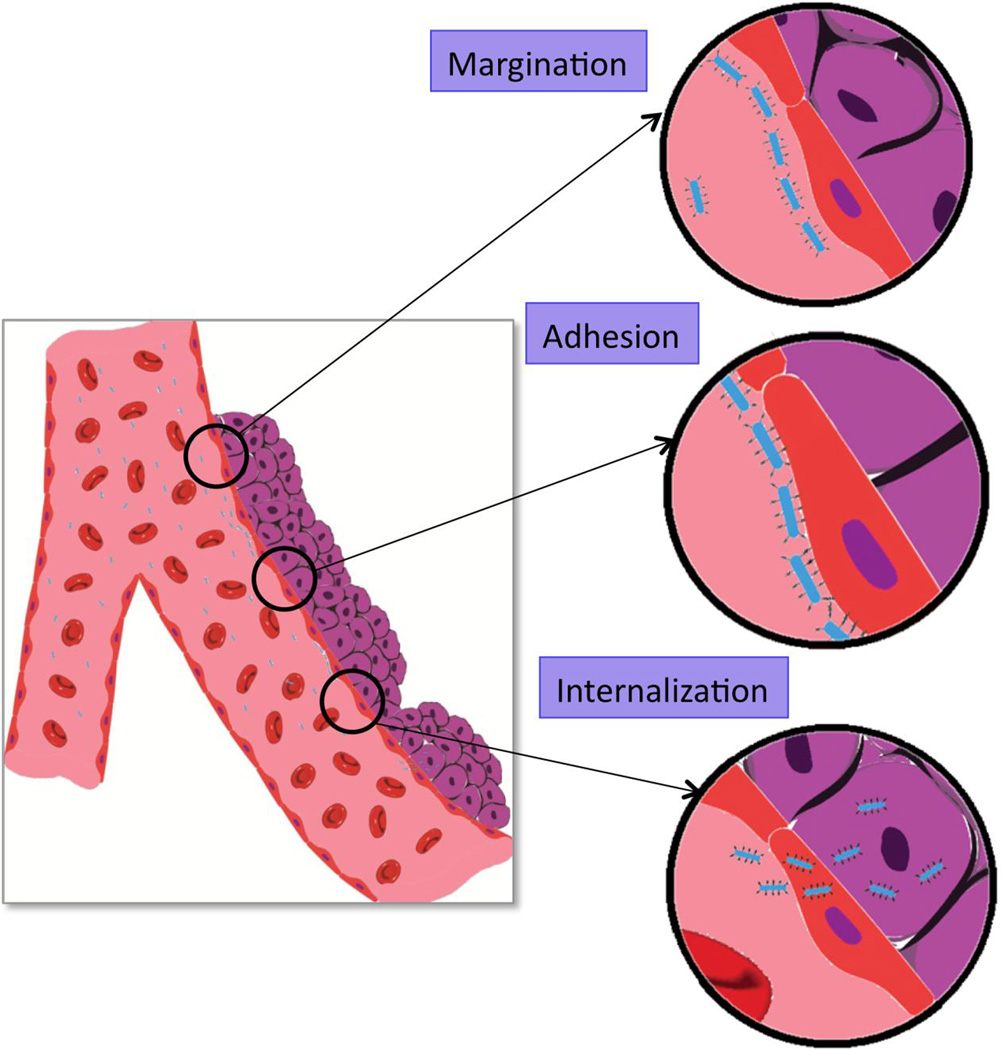
Nanocarrier circulation in the bloodstream is dependent to a large extent on local flow conditions. As depicted for rod-shaped carriers in Figure 5, margination, a process dependent on flow conditions, can represent a first step in vascular delivery. Flow dependent forces that generate nanocarrier rotational and translational motion regulate adhesion. Shear flow introduces both torque and drag forces, which can influence nanocarrier orientation, deformation, and transport within blood flow. Through the mechanical effects of torque and drag, blood flow modulates the interaction between nanocarriers and cell surfaces. For example, targeted 1 µm spherical polystyrene carriers displayed a few typical patterns of behavior in binding to endothelium in vitro, depending on the targeting conditions: 1) they move with the flow without showing any sign of binding interactions with cells; 2) they roll continuously over cells; 3) they begin by rolling followed by binding firmly to cells; 4) or they initially roll and subsequently detach and continue traveling along the cell surface [164]. These motions indicate interplay between binding interactions and the motion induced by fluid forces.
Fig. 5.
Carrier delivery process in microcirculation, including margination, adhesion, and internalization. An elongated carrier is shown as an example.
Though available studies modeling carrier behavior in buffer do not fully reflect conditions in the bloodstream, previous work with polystyrene carriers in buffer has shown that binding is inversely proportional to shear stress [165,166]. Computational analysis has shown that the influence of shear stress on nanocarrier binding diminishes once a threshold level of antibody surface density is surpassed [99]. In experimental observations, flow actually enhances adhesion between targeted small particles (or leukocytes) and the endothelial cell surface, as well as cellular uptake of particles after binding events occur. This phenomenon is counter-intuitive: one should anticipate that increases in flow would favor the displacement of particles in the downstream direction. However, the initial tethering and continuous rolling of small particles (or cells) requires some threshold flow shear stress (Figure 4c). This seems to be in violation of the idea that the dissociation rate increases exponentially with increasing applied force. Flow enhancement of adhesion has two stages: initial tethering followed by rolling after attachment. Flow enhanced tethering may result from convective transport which increases the collision frequency between binding molecules (e.g., receptor and ligand) [167]. Flow-enhanced rolling is typically explained by “catch-slip” kinetics in which the lifetime of receptor-ligand bonds is prolonged by application of tensile force acting on the cells [168] or as a result of changes in molecular structures as a result of the applied shear stress [169]. In either case, these are fluid mediated forces.
The underlying physics and mechanisms by which flow enhances, rather than destabilizing, binding is still a topic of discussion. The effect of drag force on binding following margination depends on many factors, including particle size, shape, avidity, and endothelial topography. As result, a particle can exhibit high margination but have low binding, for example, due to inadequate ligand density. Various theoretical models have been developed to elucidate deposition mechanisms under flow. Numerical methods developed in recent years provide a rigorous way to study the full transportation and adhesion dynamics of arbitrarily shaped nanoparticles. At the micron scale, Liu et al. estimated PLGA nanoparticle binding affinity with endothelial cells [99]; King et al. studied multiparticle adhesion dynamics for polystyrene beads and applied their findings to leukocyte rolling [170,171]. Fogelson et al. coupled ligand-receptor binding with platelet aggregation [172]. Shipley et al. [173] and Modok et al. [174] modeled delivery of spherical NPs to tumors. Liu et al. [175] and Zhang et al. [176] studied the deposition of NPs in the lungs. It is also important to predict the loss of NPs in the up-stream and the NPs distribution under a specific vascular environment [177]. However, there are not many attempts in literature to link molecular and cellular scale particle adhesion dynamics to tissue and organ scale transport and distribution.
6. Non-Affinity Modulation of Intracellular Uptake of Nanocarriers
In many instances, the intended site of drug action is inside the target cell. Achieving optimal sub-cellular addressing is one of the most important and challenging problems in the field of drug delivery. This extremely complicated process, mediated by intricate interactions between the carrier and the cell, is greatly influenced by features of the carrier, the cell, and their milieu.
6.1. Carrier Geometry and Intracellular Uptake
More than a century ago, Metchnikoff noted that phagocytosis is modulated by particle size. In macrophages, IgG-opsonized micron sized particles are taken up and transported to lysosomes more quickly than 100nm counterparts [178]. For spherical polystyrene particles in macrophages and B16 tumor cells, size has been shown to affect both rate (with protracted internalization for larger particles) and mechanism of internalization [179,180]. Optimal size for uptake depends on factors including the receptor, endocytic pathway, cell type, and functional status of the target cell. For example, the optimal size range for uptake of particles by endothelial cells is about an order of magnitude smaller than for uptake by macrophages [181,182]. Likewise, mathematical models have predicted a critical particle radius (as a function of ligand and receptor density) below which internalization is not energetically favorable for spherical beads [183]. Decuzzi et al. [184] have extended such models to investigate the effects of different surface physicochemical properties and non-spherical shapes [185].
By some accounts, shape is a more important determinant of carrier uptake than size. For example, the difference in the phagocytosis rate by macrophages is more pronounced for elongated vs spherical IgG polystyrene particles than for large vs. small ones [186]. It has been predicted [187] and verified in vitro [186,188] that elongated particles can effectively deliver drugs to various types of cells. One reason for this is that elongated objects can elude phagocytosis [189] (Figure 1b). Specifically, macrophages efficiently internalize opsonized disk-shaped particles contacting the cell by the rounded end, but cannot completely internalize disk-shaped particles contacting the cell by the flat face [186]. Mitragotri et al. documented internalization within three minutes for the former case, but observed spreading without internalization for the latter [186] (Figure 1e). By contrast, Desimone et al. reported that high aspect ratio rod-like triblock copolymer hydrogel particles (d=150nm, h=450nm) internalize about 4 times faster than more symmetric cylindrical particles (d=200nm, h=200nm) [188], while Caruso et al. found that internalization of hollow ellipsoid hydrogel capsules of similar aspect ratio was slowed relative to spherical counterparts, with lysosomal compartmentalization not being affected by aspect ratio [190]. In extreme cases, high aspect ratio carriers (aspect ratio close to 10, i.e., needle-shaped particles) can breach the membrane of cells to deliver cargo into the cytoplasm [64].
A theoretical study by Decuzzi and Ferrari [65] found similar results for receptor-mediated endocytosis of non-spherical particles. Yang et al. [191] came to the same conclusion using dissipative particle dynamics simulations to investigate the translocation of nanometer-scale spheres, ellipsoids, rods, discs and pushpinlike particles across a lipid bilayer. There, it was reported that anisotropy and initial orientation of the particle are crucial to the interactions between the particle and the lipid bilayer. In further work, elongated polystyrene carriers coated with phagocytosis-promoting ligands were able to avoid phagocytosis by macrophages in cell culture [189].
As with carrier size, cell type can change the relationship between carrier geometry and intracellular delivery. For instance, in HeLa cells, Gratton et al. found that elongated PEG hydrogel particles internalize better than spherical counterparts with identical volume [188]. In endothelial cells, the opposite trend has been observed. In one prototype study of vascular targeting to endothelium, ICAM targeted polystyrene disks entered endothelial cells more slowly than spherical carriers of similar size, whereas the pace of traffic through the vesicular compartments was controlled by size; smaller particles reached the lysosomes faster, regardless of shape [45]. In a follow up study, co-delivery of different shapes or sizes of polystyrene carrier was investigated for preferential perinuclear distribution. Larger particles (either spheres or rods) were more likely to localize in this region. In the same study, rods were far less likely than spheres of the same volume to accumulate in perinuclear regions [182].
Large particles coated with antibodies or other ligands may be excluded from the natural internalization pathways normally serving those ligands. Since internalization starts with anchoring to the targeting determinant on the cell, target accessibility for carriers of a given size and shape is a key parameter. For example, some anchoring molecules that could provide favorable pathways for internalization, such as caveolar molecules, are poorly accessible for particles bigger than 50–100nm [192]. On the other hand, in some cases multivalent ligand-coated particles induce endocytosis via binding to determinants that do not normally favor uptake of the ligand. For example, PECAM-1 antibodies bind to endothelial cells but do not accumulate significantly in intracellular compartments [16,42], while the multivalent binding of nanocarriers coated with PECAM-1 antibody leads to intracellular uptake mediated by the pathway known as CAM-endocytosis, distinct from clathrin-mediated or caveolar endocytosis, phagocytosis, and pinocytosis [17,42].
Large particles, especially non-spherical ones, require excessive wrapping by plasmalemma, which may exceed the capacity of a given cell type, as demonstrated in computational work addressing geometry and size with model membranes [193]. In this context, a smooth particle requires less wrapping than a polymorphous “particle” with the same effective diameter, making endocytosis easier with the former. For example, endothelial cells internalize PECAM-targeted polymorphous protein conjugates with size <500 nm [178], but internalize spherical particles with diameter of up to a few microns coated with the same antibody [194]. Similar results have been observed when comparing polymethacrylate hydrogel cubes to spheres for non-targeted uptake in HeLa cells [195].
6.2. Cell Phenotype and Microenvironment as Factors in Carrier Internalization
The functional status of cells modulates endocytosis. Conditions that up-regulate expression and turnover of the targeting determinant in the plasmalemma usually facilitate endocytosis. For example, activated endothelium internalizes ICAM-targeted polystyrene nanocarriers faster than quiescent endothelium in vitro and in vivo [196]. Most likely, this is due to the fact that the cell surface density of ICAM is elevated in cytokine-challenged cells. But additional and/or alternative mechanisms may involve stimulation of vesicular turnover involved in cytokine signaling.
The hydrodynamic factors regulating endocytosis of targeted carriers have just recently started to emerge as an important consideration in vascular drug delivery. Despite the fact that endothelial cells in vivo are exposed to flow, the majority of studies on cellular uptake and trafficking of nanocarriers have employed static cell lines. A few studies have attempted to define the role of flow in endothelial uptake of nanoparticles using flow chambers and microfluidics, mostly dealing with non-targeted particles, though with a variety of particle compositions and sizes, including microparticles derived from activated cells, semiconductor quantum dots, and silica particles [197,198].
The rheological regulation of intracellular delivery represents an intriguing area of bioengineering and biomedicine. Blood flow alters the adhesive interactions between carriers and endothelium [99]. Flow-driven rolling on the endothelial surface may assist carriers in engaging PECAM-1, thereby increasing the strength of endocytic signaling [199]. Alternatively, rotational motion due to the flow-derived torque applied to PECAM-1-anchored carriers may further mechanically stimulate endothelial cells and enhance signaling internalization. On the other hand, flow is known to modulate many parameters of endothelial functional status, some of which may be involved in endocytic processes directly or indirectly. In fact, shear stress governs endothelial processes including cytoskeletal remodeling, gene expression, ion transport, and endocytosis [199–202]. It has long been recognized that internalization of extracellular fluid and macromolecules (such as LDL) into endothelial cells is stimulated by flow [199, 203]. Stimulatory effects of flow have been observed in endothelial pinocytosis [196,199,204], clathrin-dependent endocytosis [194,205], and CAM-dependent endocytosis [44, 199]. It is conceivable that flow stimulates endocytosis via a generalized mechanism, such as enhancement of the rate of plasmalemma vesicle maturation or dynamic changes of the cytoskeleton.
Recent studies in flow chambers revealed opposite effects of chronic vs. acute flow on endothelial uptake of targeted particles. Prolonged exposure to flow led to partial, yet significant, inhibition of endocytosis of polystyrene nanocarriers targeted to ICAM and PECAM [196, 199]. These results correlated with in vivo data showing less effective internalization of anti-ICAM nanocarriers in arterioles relative to capillaries, i.e., vascular areas in which endothelial cells do and do not adapt to flow, respectively [196]. This effect is attributed to organization of the actin cytoskeleton into stress fibers in the course of endothelial adaptation to flow, which impedes actin involvement in the endocytosis [196,199]. In contrast, acute shear stress at physiological levels typical of veins accelerated endothelial endocytosis of PECAM-targeted carriers, likely via signaling mechanisms including caveolae [199]. Such a stimulatory effect may happen in reperfusion and in physiological hyper-perfusion in exertion.
7. Conclusion
Targeted delivery of drug carriers in the vasculature is an important biomedical goal. It is safe to postulate that affinity features of the drug delivery system, controlling specific recognition of molecular targets and anchoring on the target surface, is the main factor of the carrier design that governs targeting. Nonetheless, targeting itself is a complicated outcome of the interplay of characteristics of the carrier (including features of the targeting ligands, their surface density, spatial freedom, ability to engage in multivalent interactions with clusters of anchoring molecules, etc.), and the target (surface density, clustering and accessibility of anchoring molecules). Furthermore, other factors in carrier design, target, and target microenvironment exert important, and in some instances decisive, influences over targeting. For example, if the binding site becomes inaccessible under given circumstances, no matter how avidly a carrier would bind given access, no targeting can occur.
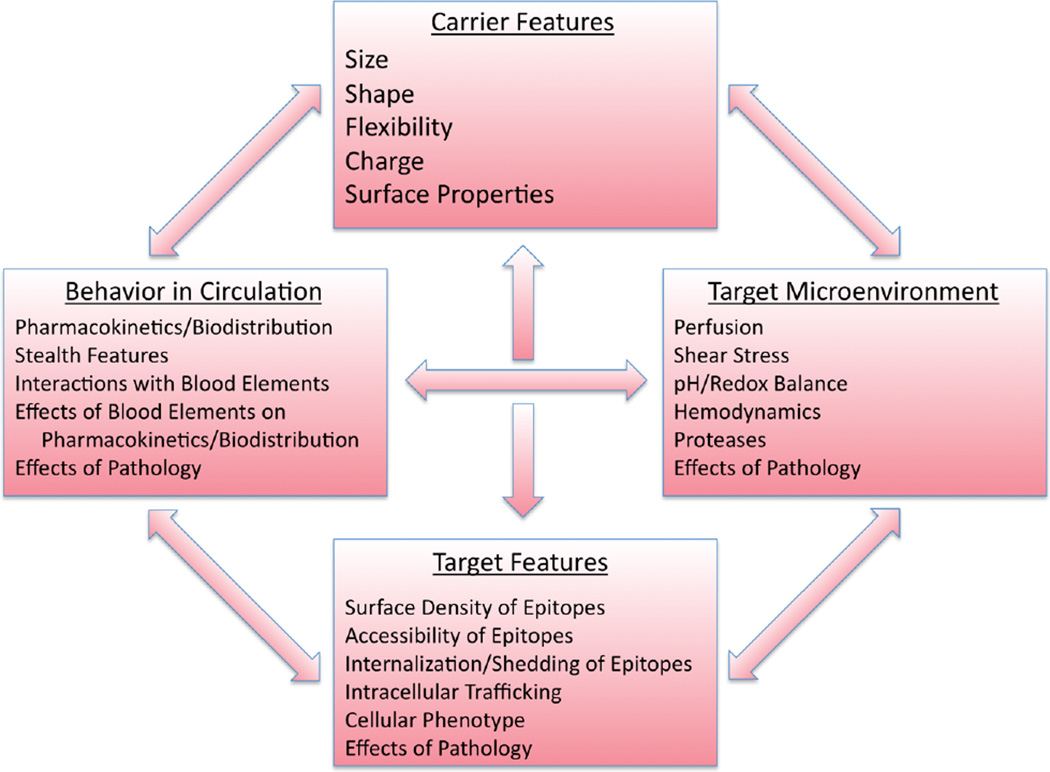
In this article, we have attempted a systematic analysis of several key non-affinity influences on targeting of drug carriers. We acknowledge a depth of additional considerations in the design of nanomaterials for targeted drug delivery applications, including a wealth of possibilities for materials design, but leave a deeper consideration of the materials science of biomedical nanocarriers and microcarriers to other sources [42,81]. Although inevitably oversimplified, our analysis may convey an impression of the complexity of drug delivery via vehicles at the nanometer and micron length scales. Indeed, as Figure 6 illustrates, numerous factors of systemic and local biological features interplay with carrier design characteristics, individually and collectively, creating the intertwined matrix of cues that either favor or impede targeting. The goal is to employ the factors that we can rationally modulate to achieve the former outcome. Our ability to control the biological factors is, mildly put, limited (such factors include choosing the route for infusion and modulation of perfusion, e.g., using hyperthermia and vasoactive agents). However, understanding of the influence of non-controllable biological factors will guide iterative re-engineering of carriers to optimize targeting. It is tempting to believe that continuing accumulation of experimental data and empirical paradigms in this domain will eventually support design of theoretical models and approaches that will accelerate this optimization process.
Fig. 6.
Summary of key interacting non-affinity factors in targeting behavior. Noteworthy biological factors defining the target, the target microenvironment, and broader physiology impact the in vivo disposition of targeted carriers and inform the design of carrier characteristics.
Therefore, non-affinity characteristics of both the delivery system and biological factors govern targeted drug delivery and its effects. This realization puts yet more emphasis on the necessity to pursue drug delivery in a multidisciplinary fashion, combining approaches and ideas coming from both materials and biomedical science and engineering. It also dictates use of more adequate model systems for investigation of vascular targeting, including endothelial cell culture systems that account for effects of flow, specifics of cellular phenotype, and cell microenvironment.
Acknowledgments
This work was supported by NIH R01 HL087036 and NIH R01 HL125462 for JWM and VRM, NIH R01 EB006818 and NIH U01 EB16027 for DME, NSF CBET-1113040, NSF CBET-1067502, DMS-1516236, and NIH EB015105 for YL, and the Duncan and Suzanne Mellichamp Chair and Fellowship for ACA and SM.
Abbreviations
- RES
reticulo-endothelial system
- PEG
poly(ethylene) glycol
- RBC
red blood cell
- ICAM
intercellular adhesion molecule
- NIPAm
n-isopropylacrylamide
- AFM
atomic force microscopy
- GPIIb/IIIa
glycoprotein IIb/IIIa, integrin αIIbβ3
- PLGA
poly(lactic-co-glycolic acid)
- vWF
von Willebrand Factor
- GPIbα
glycoprotein Ib, alpha subunit
- RGD
arginylglycylaspartic acid
- IgG
Immunoglobulin G
- PECAM
platelet endothelial cell adhesion molecule
- VCAM-1
vascular cell adhesion molecule 1
- PAAm
polyallylamine
- PAH
poly(allylamine hydrochloride)
- BSA
bovine serum albumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hood E, Simone E, Wattamwar P, Dziubla T, Muzykantov V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine. 2011;6(7):1257–1272. doi: 10.2217/nnm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner JS, Greineder C, Shuvaev V, Muzykantov V. Endothelial nanomedicine for the treatment of pulmonary disease. Expert Opin Drug Deliv. 2015;12(2):239–261. doi: 10.1517/17425247.2015.961418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myerson JW, Brenner JS, Greineder CF, Muzykantov VR. Systems approaches to design of targeted therapeutic delivery. Wiley Interdiscip Rev Syst Biol Med. 2015 May 6; doi: 10.1002/wsbm.1304. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard M, Zern BJ, Anselmo AC, Shuvaev VV, Mitragotri S, Muzykantov V. Vascular targeting of nanocarriers: perplexing aspects of the seemingly straightforward paradigm. ACS Nano. 2014;8(5):4100–4132. doi: 10.1021/nn500136z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des. 2005;11(18):2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 7.Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery. Mol Membr Biol. 2010;27(7):312–327. doi: 10.3109/09687688.2010.522117. [DOI] [PubMed] [Google Scholar]
- 8.Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Targeted drug delivery and penetration into solid tumors. Med Res Rev. 2012;32(5):1078–1091. doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- 9.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25(4):667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 10.Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC. Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets. 2004;8(4):335–353. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- 11.Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53(19):6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 12.Shuvaev VV, Christofidou-Solomidou M, Scherpereel A, Simone E, Arguiri E, Tliba S, Pick J, Kennel S, Albelda SM, Muzykantov VR. Factors modulating the delivery and effect of enzymatic cargo conjugated with antibodies targeted to the pulmonary endothelium. J Control Release. 2007;118(2):235–244. doi: 10.1016/j.jconrel.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 14.Calderon AJ, Baig M, Pichette B, Muzykantov V, Muro S, Eckmann DM. Effect of Glycocalyx on Drug Delivery Carriers Targeted to Endothelial Cells. Int J Transp Phenom. 2011;12(1):63–75. [PMC free article] [PubMed] [Google Scholar]
- 15.Shuvaev VV, Tliba S, Pick J, Arguiri E, Christofidou-Solomidou M, Albelda SM, Muzykantov VR. Modulation of endothelial targeting by size of antibody-antioxidant enzyme conjugates. J Control Release. 2011;149(3):236–241. doi: 10.1016/j.jconrel.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 17.Duncan R. Polymer conjugates as anticancer nanomedicines. Nature Reviews Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 18.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences. 2003;100(23):13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MM, Decuzzi P, Tour JM, Robertson F, Ferrari M. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature nanotechnology. 2008;3(3):151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 21.Slowing II, Vivero-Escoto JL, Wu C, Lin VSY. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Advanced drug delivery reviews. 2008;60(11):1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Hashimoto M, Dang TT, Hoare T, Kohane DS, Whitesides GM, Langer R, Anderson DG. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow- focusing device for controlled drug delivery. Small. 2009;5(13):1575–1581. doi: 10.1002/smll.200801855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. Journal of the American Chemical Society. 2005;127(28):10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 24.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro-and nanoscale drug delivery carriers. Journal of Controlled Release. 2007;121(1):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinmetz NF, Shah SN, Barclay JE, Rallapalli G, Lomonossoff GP, Evans DJ. Virus- Templated Silica Nanoparticles. Small. 2009;5(7):813–816. doi: 10.1002/smll.200801348. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Tilley RD. Shape- Controlled Growth of Platinum Nanoparticles. Small. 2007;3(9):1508–1512. doi: 10.1002/smll.200700135. [DOI] [PubMed] [Google Scholar]
- 27.Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2007;293(4):L823–L842. doi: 10.1152/ajplung.00436.2006. [DOI] [PubMed] [Google Scholar]
- 28.Oh P, Borgström P, Witkiewicz H, Li Y, Borgström BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotech. 2007;25(3):327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nature biotechnology. 2007;25(10):1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leucuta SE. Systemic and biophase bioavailability and pharmacokinetics of nanoparticulate drug delivery systems. Curr Drug Deliv. 2013;10(2):208–240. doi: 10.2174/1567201811310020007. [DOI] [PubMed] [Google Scholar]
- 31.Fraley R, Papahadjopoulos D. Liposomes: the development of a new carrier system for introducing nucleic acid into plant and animal cells. Curr Top Microbiol Immunol. 1982;96:171–191. doi: 10.1007/978-3-642-68315-2_11. [DOI] [PubMed] [Google Scholar]
- 32.De Jong WH, Hagensb WI, Krystekc P, Burgera MC, Sipsb AJAM, Geertsmad RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Moghimi S, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Progress in lipid research. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 34.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International journal of pharmaceutics. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. The FASEB Journal. 2005;19(3):311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 36.Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, Ferrari M. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010;141(3):320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Chen LT. Microcirculation of the spleen: and open or closed circulation? Science. 1978;201(4351):157–159. doi: 10.1126/science.663644. [DOI] [PubMed] [Google Scholar]
- 38.Litzinger DC, Buiting AMJ, Rooijen NV, Huang L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1994;1190(1):99–107. doi: 10.1016/0005-2736(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 39.Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: experiments and quantitative models. Journal of cell science. 2006;119(9):1903–1913. doi: 10.1242/jcs.02876. [DOI] [PubMed] [Google Scholar]
- 40.Slack JD, Kanke M, Simmons GH, DeLuca PP. Acute hemodynamic effects and blood pool kinetics of polystyrene microspheres following intravenous administration. Journal of pharmaceutical sciences. 1981;70(6):660–664. doi: 10.1002/jps.2600700621. [DOI] [PubMed] [Google Scholar]
- 41.Illum L, Davis S. The targeting of drugs parenterally by use of microspheres. PDA Journal of Pharmaceutical Science and Technology. 1982;36(6):242–248. [PubMed] [Google Scholar]
- 42.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 43.Geng Y, Dalhaimer P, Cai S, Tsal R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nano. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Tan J, Thomas A, Ou-Yang D, Muzykantov VR. The shape of things to come: importance of design in nanotechnology for drug delivery. Therapeutic delivery. 2012;3(2):181–194. doi: 10.4155/tde.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1 targeted carriers. Mol Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merkel TJ, Chen K, Jones SW, Pandya AA, Tian S, Napier ME, Zamboni WE, DeSimone JM. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J Control Release. 2012;162(1):37–44. doi: 10.1016/j.jconrel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai S, Vijayan K, Cheng D, Lima E, Discher D. Micelles of different morphologies - advantages of worm-like filomicelles of peo-pcl in paclitaxel delivery. Pharm Res. 2007;24:2099–2109. doi: 10.1007/s11095-007-9335-z. [DOI] [PubMed] [Google Scholar]
- 48.Liu W, Zhou X, Mao Z, Yu D, Wang B, Gao C. Uptake of hydrogel particles with different stiffness and its influence on HepG2 cell functions. Soft Matter. 2012;8:9235–9245. [Google Scholar]
- 49.Zelikin AN, Breheney K, Robert R, Tjipto E, Wark K. Cytotoxicity and internalization of polymer hydrogel capsules by mammalian cells. Biomacromolecules. 2010;11(8):2123–2129. doi: 10.1021/bm100500v. [DOI] [PubMed] [Google Scholar]
- 50.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286(5):H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 51.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283(4):H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 52.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer research. 1986;46(12 Part 1):6387–6392. [PubMed] [Google Scholar]
- 53.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacological reviews. 1999;51(4):691–744. [PubMed] [Google Scholar]
- 54.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release. 2012;164(2):138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 55.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nature materials. 2009;8(1):15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano letters. 2007;7(6):1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 57.Gersh KC, Zaitsev S, Muzykantov V, Cines DB, Weisel JW. The spatial dynamics of fibrin clot dissolution catalyzed by erythrocyte-bound vs. free fibrinolytics. J Thromb Haemost. 2010;8(5):1066–1074. doi: 10.1111/j.1538-7836.2010.03802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaitsev S, Spitzer D, Murciano JC, Ding BS, Tliba S, Kowalska MA, Bdeir K, Kuo A, Stepanova V, Atkinson JP, Poncz M, Cines DB, Muzykantov VR. Targeting of a mutant plasminogen activator to circulating red blood cells for prophylactic fibrinolysis. J Pharmacol Exp Ther. 2010;332(3):1022–1031. doi: 10.1124/jpet.109.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaitsev S, Danielyan K, Murciano JC, Ganguly K, Krasik T, Taylor RP, Pincus S, Jones S, Cines DB, Muzykantov VR. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108(6):1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, Feldman CL, Wagner DD, Ingber DE. Shear-Activated Nanotherapeutics for Drug Targeting to Obstructed Blood Vessels. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 61.Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J Control Release. 2008;130(3):226–233. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chrastina A, Valadon P, Massey KA, Schnitzer JE. Lung vascular targeting using antibody to aminopeptidase P: CT-SPECT imaging, biodistribution and pharmacokinetic analysis. J Vasc Res. 2010;47(6):531–543. doi: 10.1159/000313880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shuvaev VV, Ilies MA, Simone E, Zaitsev S, Kim Y, Cai S, Mahmud A, Dziubla T, Muro S, Discher DE, Muzykantov VR. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano. 2011;5:6991–6999. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc Natl Acad Sci U S A. 2013;110:10753–10758. doi: 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Namdee K, Thompson AJ, Golinski A, Mocherla S, Bouis D, Eniola-Adefeso O. In vivo evaluation of vascular-targeted spheroidal microparticles for imaging and drug delivery application in atherosclerosis. Atherosclerosis. 2014;237(1):279–286. doi: 10.1016/j.atherosclerosis.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 66.Shchepelina O, Kozlovskaya V, Kharlampieva E, Mao W, Alexeev A, Tsukruk VV. Anisotropic micro- and nano-capsules. Macromol Rapid Commun. 2010;31:2041. doi: 10.1002/marc.201000490. [DOI] [PubMed] [Google Scholar]
- 67.Yashchenok A, Parakhonskiy B, Donatan S, Kohler D, Skirtach A, Möhwald H. Polyelectrolyte multilayer microcapsules templated on spherical, elliptical and square calcium carbonate particles. J Mater Chem B. 2013;1:1223–1228. doi: 10.1039/c2tb00416j. [DOI] [PubMed] [Google Scholar]
- 68.Kozlovskaya V, Wang Y, Higgins W, Chen J, Chen Y, Kharlampieva E. pH-triggered shape response of cubical ultrathin hydrogel capsules. Soft Matter. 2012;8:9828–9839. [Google Scholar]
- 69.Kozlovskaya V, Baggett J, Godin B, Liu X, Kharlampieva E. Hydrogen-bonded multilayers of silk fibroin: from coatings to cell-mimicking shaped microcontainers. ACS Macro Lett. 2012;1:384–387. doi: 10.1021/mz200118f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.She S, Li Q, Shan B, Tong W, Gao C. Fabrication of red-blood-cell-like polyelectrolyte microcapsules and their deformation and recovery behavior through a microcapillary. Adv Mater. 2013;25:5814. doi: 10.1002/adma.201302875. [DOI] [PubMed] [Google Scholar]
- 71.Kozlovskaya V, Alexander JF, Wang Y, Kuncewicz T, Liu X, Godin B, Kharlampieva E. Internalization of red blood cell-mimicking hydrogel capsules with pH-triggered shape responses. ACS Nano. 2014;8(6):5725–5737. doi: 10.1021/nn500512x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Kozlovskaya V, Goins A, Campos-Gomez J, Saeed M, Kharlampieva E. Biocompatible shaped particles from dried multilayer polymer capsules. Biomacromolecules. 2013;14(11):3830–3841. doi: 10.1021/bm4008666. [DOI] [PubMed] [Google Scholar]
- 73.Mahaffy RE, Shih CK, MacKintosh FK, Käs J. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys Rev Lett. 2000;85:880–883. doi: 10.1103/PhysRevLett.85.880. [DOI] [PubMed] [Google Scholar]
- 74.Lam WA, Chaudhuri O, Crow A, Webster KD, Li TD, Kita A, Huang J, Fletcher DA. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10(1):61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H, Liu KK. Optical tweezers for single cells. JR Soc Interface. 2008;5(24):671–690. doi: 10.1098/rsif.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo J, Xia G, Guo D. Mechanical properties of nanoparticles: basics and applications. J Phys D: Appl Phys. 2014;47:013001. [Google Scholar]
- 77.Eckmann DM, Composto RJ, Tsourkas A, Muzykantov VR. Nanogel carrier design for targeted drug delivery. J Mater Chem B Mater Biol Med. 2014;2(46):8085–8097. doi: 10.1039/C4TB01141D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan YY, Du JZ, Song WJ, Wang F, Yang XZ, Xiong MH, Wang J. Biocompatible and functionalizable polyphosphate nanogel with a branched structure. J Mater Chem. 2012;22:9322–9329. [Google Scholar]
- 79.Urakami H, Hentschel J, Seetho K, Zeng H, Chawla K, Guan Z. Surfactant-free synthesis of biodegradable, biocompatible, and stimuli-responsive cationic nanogel particles. Biomacromolecules. 2013;14(10):3682–3688. doi: 10.1021/bm401039r. [DOI] [PubMed] [Google Scholar]
- 80.Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, Luft JC, Wu H, Zamboni WC, Wang AZ, Bear JE, DeSimone JM. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci U S A. 2011;108(2):586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nature Materials. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Deliv. 2010;7(4):403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anselmo AC, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, Mitragotri S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis and targeting. ACS Nano. 2015;9(3):3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 84.Doshi N, Zahra AS, Bhaskar S, Lahann J, Mitragotri S. Red blood cell-mimicking synthetic biomaterial particles. Proceedings of the National Academy of Sciences. 2009;106(51):21495–21499. doi: 10.1073/pnas.0907127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fung YC. Biomechanics: Mechanical properties of living tissues. 1993 [Google Scholar]
- 86.Euliss LE, DuPont JA, Gratton S, DeSimone J. Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev. 2006;35:1095–1104. doi: 10.1039/b600913c. [DOI] [PubMed] [Google Scholar]
- 87.Simone EA, Dziubla TD, Colon-Gonzalez F, Discher DE, Muzykantov VR. Effect of polymer amphiphilicity on loading of a therapeutic enzyme into protective filamentous and spherical polymer nanocarriers. Biomacromolecules. 2007;8(12):3914–3921. doi: 10.1021/bm700888h. [DOI] [PubMed] [Google Scholar]
- 88.Dalhaimer P, Engler AJ, Parthasarathy R, Discher DE. Targeted worm micelles. Biomacromolecules. 2004;5(5):1714–1719. doi: 10.1021/bm049884v. [DOI] [PubMed] [Google Scholar]
- 89.Hendrickson GR, Lyon LA. Microgel translocation through pores under confinement. Angewandte Chemie Int Ed. 2010;49(12):2193–2197. doi: 10.1002/anie.200906606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iyer AS, Lyon LA. Self-healing colloidal crystals. Angewandte Chemie Int Ed. 2009;48(25):4562–4566. doi: 10.1002/anie.200901670. [DOI] [PubMed] [Google Scholar]
- 91.Blackburn WH, Lyon LA. Size-controlled synthesis of monodisperse core/shell nanogels. Colloid and Polymer Science. 2007;286(5):563–569. doi: 10.1007/s00396-007-1805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown AC, Stabenfeldt SE, Ahn B, Hannan RT, Dhada KS, Herman ES, Stefanelli V, Guzzetta N, Alexeev A, Lam WA, Lyon LA, Barker TH. Ultrasoft microgels displaying emergent platelet-like behaviours. Nature Materials. 2014;13:1108–1114. doi: 10.1038/nmat4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL, Chen M, Squires TM, Sen Gupta A, Mitragotri S. Platelet-like nanoparticles: Mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS nano. 2014;8:11243–11253. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gunawan RC, Auguste DT. The role of antibody synergy and membrane fluidity in the vascular targeting of immunoliposomes. Biomaterials. 2010;31(5):900–907. doi: 10.1016/j.biomaterials.2009.09.107. [DOI] [PubMed] [Google Scholar]
- 95.Doshi N, Orje JN, Molins B, Smith JW, Mitragotri S, Ruggeri Z. Platelet mimetic particles for targeting thrombi in flowing blood. Adv Mater. 2012;24(28):3864–3869. doi: 10.1002/adma.201200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beningo KA, Wang Yl. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. Journal of cell science. 2002;115(4):849–856. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
- 97.Banquy X, Suarez F, Argaw A, Rabanel JM, Grutter P, Bouchard JF, Hildgen P, Giasson S. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. Soft Matter. 2009;20(5):3984–3991. [Google Scholar]
- 98.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment- Implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Weller GER, Zern B, Ayyaswamy PS, Eckmann DM, Muzykantov DMVR, Radhakrishnan R. Computational model for nanocarrier binding to endothelium validated using in vivo in vitro atomic force microscopy experiments. Proc Natl Acad Sci USA. 2010;107:16530–16535. doi: 10.1073/pnas.1006611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol. 1990;258(3 Pt 2):H647–H654. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- 101.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31:1392–1402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Lee TR, Choi M, Kopacz AM, Yun SY, Liu WK, Decuzzi P. On the near-wall accumulation of injectable particles in the microcirculation: smaller is not better. Scientific Reports. 2013;3:2079. doi: 10.1038/srep02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Samokhin GP, Smirnov MD, Muzykantov VR, Domogatsky SP, Smirnov VN. Red blood cell targeting to collagen-coated surfaces. FEBS Lett. 1983;154(2):257–261. doi: 10.1016/0014-5793(83)80160-4. [DOI] [PubMed] [Google Scholar]
- 104.Samokhin GP, Smirnov MD, Muzykantov VR, Domogatsky SP, Smirnov VN. Effect of flow rate and blood cellular elements on the efficiency of red blood cell to collagen-coated surfaces. J Appl Biochem. 1984;6(1–2):70–75. [PubMed] [Google Scholar]
- 105.Namdee K, Thompson AJ, Charoenphol P, Adefeso OE. Margination propensity of vascular-targeted spheres from blood flow in a microfluidic model of human microvessels. Langmuir. 2013;29(8):2530–2535. doi: 10.1021/la304746p. [DOI] [PubMed] [Google Scholar]
- 106.Migliorini C, Qian Y, Chen H, Brown EB, Jain RK, Munn LL. Red blood cells augment leukocyte rolling in a virtual blood vessel. Biophys J. 2002;83(4):1834–1841. doi: 10.1016/S0006-3495(02)73948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thompson AJ, Eniola-Adefeso O. Dense nanoparticles exhibit enhanced vascular wall targeting over neutrally buoyant nanoparticles in human blood flow. Acta Biomateriala. 2015;21:99–108. doi: 10.1016/j.actbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Johnson PC, Popel AS. Effects of erythrocyte deformability and aggregation on the cell free layer and apparent viscosity of microscopic blood flows. Microvasc Res. 2009;77(3):265–272. doi: 10.1016/j.mvr.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan J, Thomas A, Liu Y. Influence of red blood cells on nanoparticle targeted delivery in microcirculation. Soft Matter. 2011;8(6):1934–1946. doi: 10.1039/C2SM06391C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gentile F, Curcio A, Indolfi C, Ferrari M, Decuzzi P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J Nanobiotechnology. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng. 2005;33:179–190. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 112.Thomas A, Tan J, Liu Y. Characterization of nanoparticle delivery in microcirculation using a microfluidic device. Microvascular Research. 2014;94(0):17–27. doi: 10.1016/j.mvr.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muller K, Fedosov DA, Gompper G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Scientific Reports. 2014;4:4871. doi: 10.1038/srep04871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thompson AJ, Mastria EM, Eniola-Adefeso O. The margination propensity of ellipsoidal micro/nanoparticles to the endothelium in human blood flow. Biomaterials. 2013;34(23):5863–5871. doi: 10.1016/j.biomaterials.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 115.Sevick EM, Jain RK. Viscous resistance to blood-flow in solid tumors - effect of hematocrit on Intratumor blood viscosity. Cancer Research. 1989;49(13):3513–3519. [PubMed] [Google Scholar]
- 116.Smirnov VN, Domogatsky SP, Dolgov VV, Hvatov VB, Klibanov AL, Koteliansky VE, Muzykantov VR, Repin VS, Samokhin GP, Shekhonin BV. Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall. Proc Nat Acad Sci USA. 1986;83(17):6603–6607. doi: 10.1073/pnas.83.17.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rossi L, Serafini S, Pierigé F, Antonelli A, Cerasi A, Fraternale A, Chiarantini L, Magnani M. Erythrocyte-based drug delivery. Expert Opinion on Drug Delivery. 2005;2(2):311–322. doi: 10.1517/17425247.2.2.311. [DOI] [PubMed] [Google Scholar]
- 118.Anselmo AC, Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J Control Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8(4):415–433. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stephan MT, Irvine DJ. Enhancing cell therapies from the outside in: Cell surface engineering using synthetic nanomaterials. Nano Today. 2011;6(3):309–325. doi: 10.1016/j.nantod.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anselmo AC, Gupta V, Zern BJ, Pan D, Zakrewsky M, Muzykantov V, Mitragotri S. Delivering Nanoparticles to Lungs while Avoiding Liver and Spleen through Adsorption on Red Blood Cells. ACS Nano. 2013;7(12):11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chambers E, Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. Journal of Controlled Release. 2004;100(1):111–119. doi: 10.1016/j.jconrel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 123.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4(6):432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 124.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104(8):2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 126.Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman HE. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009;183(1):661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi MR, Bardhan R, Stanton-Maxey KJ, Badve S, Nakshatri H, Stantz KM, Cao N, Halas NJ, Clare SE. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol. 2012;3(1–6):47–54. doi: 10.1007/s12645-012-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brynskikh AM, Zhao Y, Mosley RL, Li S, Boska MD, Klyachko NL, Kabanov AV, Gendelman HE, Batrakova EV. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine (Lond) 2010;5(3):379–396. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, Lee JS, Lee JS, Park HJ, Song SY, Jeong SY, Choi EK. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33(16):4195–4203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 130.Anselmo AC, Gilbert JB, Kumar S, Gupta V, Cohen RE, Rubner MF, Mitragotri S. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: a generalized strategy to deliver drugs to treat inflammation. Journal of Controlled Release. 2015;199:29–36. doi: 10.1016/j.jconrel.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 131.Modery-Pawlowski CL, Tian LL, Pan V, McCrae KR, Mitragotri S, Sen Gupta A. Approaches to Synthetic Platelet Analogs. Biomaterials. 2013;34(2):526–541. doi: 10.1016/j.biomaterials.2012.09.074. [DOI] [PubMed] [Google Scholar]
- 132.Coller BS, Springer KT, Beer JH, Mohandas N, Scudder LE, Norton KJ, West SM. Thromboerythrocytes. In vitro studies of a potential autologous, semi-artificial alternative to platelet transfusions. J Clin Invest. 1992;89(2):546–555. doi: 10.1172/JCI115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Okamura Y, Fujie T, Maruyama H, Handa M, Ikeda Y, Takeoka S. Prolonged hemostatic ability of polyethylene glycol-modified polymerized albumin particles carrying fibrinogen gamma-chain dodecapeptide. Transfusion. 2007;47(7):1254–1262. doi: 10.1111/j.1537-2995.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 134.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med. 2009;1(11):11–22. doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okamura Y, Takeoka S, Eto K, Maekawa I, Fujie T, Maruyama H, Ikeda Y, Handa M, Development of fibrinogen gamma-chain peptide-coated. adenosine diphosphate-encapsulated liposomes as a synthetic platelet substitute. J Thromb Haemost. 2009;7(3):470–477. doi: 10.1111/j.1538-7836.2008.03269.x. [DOI] [PubMed] [Google Scholar]
- 136.Nishiya T, Kainoh M, Murata M, Handa M, Ikeda Y. Platelet interactions with liposomes carrying recombinant platelet membrane glycoproteins or fibrinogen: approach to platelet substitutes. Artif Cells Blood Substit Immobil Biotechnol. 2001;29(6):453–464. doi: 10.1081/bio-100108550. [DOI] [PubMed] [Google Scholar]
- 137.Lashof-Sullivan MM, Shoffstall E, Atkins KT, Keane N, Bir C, VandeVord P, Lavik EB. Intravenously administered nanoparticles increase survival following blast trauma. Proc Nat Acad Soc USA. 2014;111(28):10293–10298. doi: 10.1073/pnas.1406979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hu CMJ, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015 doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ravikumar M, Modery CL, Wong TL, Sen Gupta A. Peptide-decorated liposomes promote arrest and aggregation of activated platelets under flow on vascular injury relevant protein surfaces in vitro. Biomacromolecules. 2012;13(5):1495–1502. doi: 10.1021/bm300192t. [DOI] [PubMed] [Google Scholar]
- 140.Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM, Liu X, Ferrari M, Decuzzi P. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. Journal of Biomechanics. 2008;41:2312–2318. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 141.Takeoka S, Teramura Y, Okamura Y, Tsuchida E, Handa M, Ikeda Y. Rolling properties of rGPIbalpha-conjugated phospholipid vesicles with different membrane flexibilities on vWf surface under flow conditions. Biochem Biophys Res Commun. 2002;296(3):765–770. doi: 10.1016/s0006-291x(02)00934-8. [DOI] [PubMed] [Google Scholar]
- 142.Liu J, Agrawal NJ, Calderon AJ, Ayyaswamy PS, Eckmann DM, Radhakrishnan R. Multivalent binding of nanocarrier to endothelial cells under shear flow. Biophys J. 2011;101(2):319–326. doi: 10.1016/j.bpj.2011.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ayyaswamy PS. In: Fluid Mechanics. Kundu PK, Cohen IM, editors. Maryland Heights, Missouri: Academic Press; 2008. pp. 765–840. [Google Scholar]
- 144.Uijttewaal WS, Nijhof EJ, Bronkhorst PJ, Den Hartog E, Heethaar RM. Near-wall excess of platelets induced by lateral migration of erythrocytes in flowing blood. Am J Physiol Heart Circ Physiol. 1993;264:H1239–H1244. doi: 10.1152/ajpheart.1993.264.4.H1239. [DOI] [PubMed] [Google Scholar]
- 145.Daum N, Tscheka C, Neumeyer A, Schneider M. Novel approaches for drug delivery systems in nanomedicine: effects of particle design and shape. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(1):52–65. doi: 10.1002/wnan.165. [DOI] [PubMed] [Google Scholar]
- 146.Swaminathan TN, Mukundakrishnan K, Hu HH. Sedimentation of an ellipsoid inside an infinitely long tube at low and intermediate Reynolds numbers. J Fluid Mech. 2006;551:357–385. [Google Scholar]
- 147.Gavze E, Shapiro M. Motion of inertial spheroidal particles in a shear flow near a solid wall with special application to aerosol transport in microgravity. Journal of Fluid Mechanics. 1998;371:59–79. [Google Scholar]
- 148.Kojić M, Filipović N, Stojanović B, Kojić N. Computer Modeling in Bioengineering: Theoretical background, examples and software. John Wiley & Sons; 2008. [Google Scholar]
- 149.Cai S, Vijayan K, Cheng D, Lima E, Discher D. Micelles of different morphologies - advantages of worm-like filomicelles of peo-pcl in paclitaxel delivery. Pharm Res. 2007;24:2099–2109. doi: 10.1007/s11095-007-9335-z. [DOI] [PubMed] [Google Scholar]
- 150.Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid Nanofluidics. 2013;14(1–2):77–87. doi: 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shah S, Liu Y, Hu W, Gao J. Modeling particle shape-dependent dynamics in nanomedicine. J Nanosci Nanotechnol. 2011;11:919–928. doi: 10.1166/jnn.2011.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee SY, Ferrari M, Decuzzi P. Design of bio-mimetic particles with enhanced vascular interaction. Journal of biomechanics. 2009;42(12):1885–1890. doi: 10.1016/j.jbiomech.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 153.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 154.Björnmalm M, Yan Y, Caruso F. Engineering and evaluating drug delivery particles in microfluidic devices. Journal of Controlled Release. 2014;190:139–149. doi: 10.1016/j.jconrel.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 155.Bhise NS, Ribas J, Manoharan V, Zhang YS, Polini A, Massa S, Dokmeci MR, Khademhosseini A. Organ-on-a-chip platforms for studying drug delivery systems. Journal of Controlled Release. 2014;190:82–93. doi: 10.1016/j.jconrel.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Prabhakarpandian B, Shen MC, Nichols JB, Mills IR, Sidoryk-Wegrzynowicz M, Aschner M, Pant K. SyM-BBB: a microfluidic blood brain barrier model. Lab on a Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J Control Release. 2010;146:196–200. doi: 10.1016/j.jconrel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 159.Potter DR, Damiano ER. The hydrodynamically relavant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 160.Ganguly K, Murciano JC, Westrick R, Leferovich J, Cines DB, Muzykantov VR. The glycocalyx protects erythrocyte-bound tissue-type plasminogen activator from enzymatic inhibition. J Pharmacol Exp Ther. 2007;321(1):158–164. doi: 10.1124/jpet.106.114405. [DOI] [PubMed] [Google Scholar]
- 161.Greineder CF, Chacko AM, Zaytsev S, Zern BJ, Carnemolla R, Hood ED, Han J, Ding BS, Esmon CT, Muzykantov VR. Vascular immunotargeting to endothelial determinant ICAM-1 enables optimal partnering of recombinant scFv-thrombomodulin fusion with endogenous cofactor. PLoS One. 2013;8(11):e80110. doi: 10.1371/journal.pone.0080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weinbaum S, Zhang X, Han Y, Vink H, Cowin C. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Agrawal NJ, Radhakrishnan R. The role of glycocalyx in nanocarrier-cell adhesion investigated using a thermodynamics model and monte carlo simulations. J Phys Chem C. 2007;111:15848–15856. doi: 10.1021/jp074514x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Calderon AJ, Muzykantov V, Muro S, Eckmann DM. Flow dynamics, binding and detachment of spherical carriers targeted to ICAM-1 on endothelial cells. Biorheology. 2009;46:323–341. doi: 10.3233/BIR-2009-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blackwell JE, Dagia NM, Dickerson JB, Berg EL, Goetz DJ. Ligand coated nanosphere adhesion to E- and P-selectin under static and flow conditions. Ann Biomed Eng. 2001;29:523–533. doi: 10.1114/1.1376697. [DOI] [PubMed] [Google Scholar]
- 166.Lin A, Sabnis A, Kona S, Nattama S, Patel H, Dong JF, Nguyen KT. Shear-regulated uptake of nanoparticles by endothelial cells and development of endothelial-targeting nanoparticles. Journal of Biomedical Materials Research Part A. 2010;93A:833–842. doi: 10.1002/jbm.a.32592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C. Transport governs flow-enhanced cell tethering through l-selectin at threshold shear. Biophys J. 2007;92:330–342. doi: 10.1529/biophysj.106.090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 169.Lou J, Yago T, Klopocki AG, Mehta P, Chen W, Zarnitsyna VI, Bovin NV, Zhu C, McEver RP. Flow-enhanced adhesion regulated by a selectin interdomain hinge. J Cell Biol. 2006;174:1107–1117. doi: 10.1083/jcb.200606056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.King MR, Hammer DA. Multiparticle adhesive dynamics: Hydrodynamic recruitment of rolling leukocytes. Proc Natl Acad Sci USA. 2001;98(26):14919–14924. doi: 10.1073/pnas.261272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.King MR, Rodgers SD, Hammer DA. Hydrodynamic collisions suppress fluctuations in the rolling velocity of adhesive blood cells. Langmuir. 2001;17(14):4139–4143. [Google Scholar]
- 172.Fogelson AL, Guy RD. Immersed-boundary-type models of intravascular platelet aggregation. Computer Methods in Applied Mechanics and Engineering. 2008;197(25–28):2087–2104. [Google Scholar]
- 173.Shipley RJ, Chapman SJ. Multiscale modelling of fluid and drug transport in vascular tumours. Bulletin of Mathematical Biology. 2010;72(6):1464–1491. doi: 10.1007/s11538-010-9504-9. [DOI] [PubMed] [Google Scholar]
- 174.Modok S, Scott R, Alderden RA, Hall MD, Mellor HR, Bohic S, Roose T, Hambley TW, Callaghan R. Transport kinetics of four-coordinate and six-coordinate platinum compounds in the multicell layer tumour model. British Journal of Cancer. 2007;97(2):194–200. doi: 10.1038/sj.bjc.6603854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Y, So RMC, Zhang CH. Modeling the bifurcating flow in a human lung airway. Journal of Biomechanics. 2002;35(4):465–473. doi: 10.1016/s0021-9290(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 176.Zhang Z, Kleinstreuer C. Airflow structures and nano-particle deposition in a human upper airway model. Journal of Computational Physics. 2004;198(1):178–210. [Google Scholar]
- 177.Sohrabi S, Zheng J, Finol EA, Liu Y. Numerical simulation of particle transport and deposition in the pulmonary vasculature. Journal of biomechanical engineering. 2014;136(12):121010. doi: 10.1115/1.4028800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99(3):912–922. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]
- 179.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH. Size of IgG-opsonized particles determines macrophage response during internalization. Experimental cell research. 1998;242(1):265–273. doi: 10.1006/excr.1998.4110. [DOI] [PubMed] [Google Scholar]
- 181.Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Curr Vasc Pharmacol. 2004;2(3):281–299. doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- 182.Kolhar P, Mitragotri S. Polymer Microparticles exhibit size and shape dependent accumulation around the nucleus after endocytosis. Advanced Functional Materials. 2012;22(18):3759–3764. [Google Scholar]
- 183.Gao H, Shi W, Freund LB. Mechanics of receptor-mediated endocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9469–9474. doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Decuzzi P, Ferrari M. The role of specific and non-specific interactions in receptor-mediated endocytosis of nanoparticles. Biomaterials. 2007;28(18):2915–2922. doi: 10.1016/j.biomaterials.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 185.Decuzzi P, Ferrari M. Design maps for nanoparticles targeting the diseased microvasculature. Biomaterials. 2008;29(3):377–384. doi: 10.1016/j.biomaterials.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 186.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Decuzzi P, Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophysical journal. 2008;94(10):3790–3797. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proceedings of the National Academy of Sciences. 2008;105(33):11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26(1):244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shimoni O, Yan Y, Wang Y, Caruso F. Shape-dependent cellular processing of polyelectrolyte capsules. ACS Nano. 2013;7(1):522–530. doi: 10.1021/nn3046117. [DOI] [PubMed] [Google Scholar]
- 191.Yang K, Ma Y-Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nature nanotechnology. 2010;5(8):579–583. doi: 10.1038/nnano.2010.141. [DOI] [PubMed] [Google Scholar]
- 192.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3(5):311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 193.Dasgupta S, Auth T, Gompper G. Shape and orientation matter for the cellular uptake of nonspherical particles. Nano Letters. 2014;14(2):687–693. doi: 10.1021/nl403949h. [DOI] [PubMed] [Google Scholar]
- 194.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 195.Xue B, Kozlovskaya V, Liu F, Chen J, Williams JF, Campos-Gomez J, Saeed M, Kharlampieva E. Intracellular degradable hydrogel cubes and spheres for anti-cancer drug delivery. ACS Applied Mat and Interface. 2015;7(24):13633–13644. doi: 10.1021/acsami.5b03360. [DOI] [PubMed] [Google Scholar]
- 196.Bhowmick T, Berk E, Cui X, Muzykantov VR, Muro S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. Journal of Controlled Release. 2012;157(3):485–492. doi: 10.1016/j.jconrel.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Samuel SP, Jain N, O’Dowd F, Paul T, Kashanin D, Gerard VA, Gun’ko YK, Prina-Mello A, Volkov Y. Multifactorial determinants that govern nanoparticle uptake by human endothelial cells under flow. Int J Nanomedicine. 2012;7:2943–2956. doi: 10.2147/IJN.S30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Terrisse AD, Puech N, Allart S, Gourdy P, Xuereb JM, Payrastre B, Sié P. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J Thromb Haemost. 2010;8(12):2810–2819. doi: 10.1111/j.1538-7836.2010.04088.x. [DOI] [PubMed] [Google Scholar]
- 199.Han J, Zern BJ, Shuvaev VV, Davies PF, Muro S, Muzykantov V. Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1. ACS Nano. 2012;6(10):8824–8836. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Helmke BP. Molecular control of cytoskeletal mechanics by hemodynamic forces. Physiology. 2005;20:43–53. doi: 10.1152/physiol.00040.2004. [DOI] [PubMed] [Google Scholar]
- 201.Cybulsky MI, Marsden PA. Effect of disturbed blood flow on endothelial cell gene expression: a role for changes in RNA processing. Arterioscler Thromb Vasc Biol. 2014;34(9):1806–1808. doi: 10.1161/ATVBAHA.114.304099. [DOI] [PubMed] [Google Scholar]
- 202.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87(2):320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Traoré M, Sun RJ, Fawzi-Grancher S, Dumas D, Qing X, Santus R, Stoltz JF, Muller S. Kinetics of the endocytotic pathway of low density lipoprotein (LDL) in human endothelial cells line under shear stress: an in vitro confocal microscopy study. Clin Hemorheol Microcirc. 2005;33:243–251. [PubMed] [Google Scholar]
- 204.Davies PF, Dewey CF, Jr, Bussolari SR, Gordon EJ, Gimbrone MA., Jr Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984;73(4):1121–1129. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): A strategy for vascular immunotargeting of drugs. Proc Nat Acad Sci. 1999;96:2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]