Abstract
Background and study aims: Despite the clinical advantages of colorectal endoscopic submucosal dissection (ESD), an effective training system, especially for Western endoscopists, has been challenging to establish. Herein, we propose a novel training program using ex vivo animal models and evaluate the learning curve of colorectal ESD trainees without gastric ESD experience.
Patients and methods: A total of 80 colorectal lesions were prospectively collected and removed by two novice operators. Before human ESD procedures, they received ESD training using an ex vivo porcine “proximal colon” model, which simulates a lumen with many folds and flexions. To assess the validity of our training system, the self-completion and en bloc R0 resection rates, the operation time, and prevalence of complications were compared between the first and latter period. Moreover the factors associated with prolonged operation time were evaluated.
Results: The overall rates of self-completion and en bloc R0 resection were 98 % (78/80) and 100 % (80/80), respectively. The operation time during the first period was significantly longer than that during the latter period (86 ± 50 minutes vs. 60 ± 36 minutes, P = 0.01). Regarding complications, only two cases of perforations and delayed hemorrhage were observed during the first period; however, all of the complications were successfully managed endoscopically. The presence of fibrosis was identified as a significant independent predictor of a prolonged operation time during the first period (coefficient, 5.90; 95 %CI, 2.36 – 9.44, P = 0.002).
Conclusions: Our trainees achieved high rates of self-completion and R0 resection without severe complications even during the first 20 cases, suggesting that our training programs using ex vivo animal models are useful for trainees without gastric ESD experience.
Study registration: UMIN000013566
Introduction
Although colorectal cancer (CRC) is one of the most common causes of cancer-related death in the world, the incidence and mortality rates associated with CRC have gradually decreased because of improved access to screening and standard treatment 1. Colorectal adenoma has been established as a premalignant lesion, and the endoscopic removal of such lesions can reportedly reduce the incidence of CRC by as much as 90 % 2. The management of adenomas and intraepithelial carcinoma with a low risk of lymph node metastasis usually consists of a classic polypectomy and/or endoscopic submucosal resection (EMR). When carcinoma is treated endoscopically, a precise histopathological examination of the excised specimen is essential; thus, an en bloc resection is fundamentally needed. Therefore, lesions with a diameter of less than 2 cm should be treated using EMR 3.
Recently, non-polypoid colorectal neoplasms that do not exhibit a macroscopic protruding appearance have been documented not only in Japan 4 5, but also in Western countries 6. These lesions are characterized by lateral extensions along the luminal wall with a low vertical axis; such tumors with a diameter of > 10 mm are called laterally spreading tumors (LSTs) 4. LSTs are usually no more likely to exhibit high grade dysplasia than conventional protruded adenomas, despite their larger size 7. However, a proportion (e. g. those with a large nodule and/or central depression) display high grade dysplasia and submucosal invasion, to some extent 5. As piecemeal resection is reportedly associated with an increased risk of the local recurrence of carcinoma 8, colorectal endoscopic submucosal dissection (ESD) has been indicated for the treatment of these lesions as well as other gastrointestinal tract lesions, such as those occurring in the stomach or esophagus 9 10.
ESD was developed in Japan in the late 1990 s as a minimally invasive technique for the endoscopic removal of early gastric cancers 11, and its use has now spread worldwide. The main advantages of ESD are a higher rate of complete resection, decreased local recurrence, no limitations because of lesion size, and a superior histopathological assessment of cancer invasion in the specimen 8 12. With the recent development of ESD-associated tools and devices and well-designed training procedures, gastric and esophageal ESD have been established as standard techniques. Nevertheless, colorectal ESD remains technically difficult because of the difficulty in maneuvering the endoscope in the lumen, which contains many folds and flexions, and the thin intestinal walls, which can be easily perforated 13. Thus, colorectal ESD remains a challenging procedure, even for Japanese experts. Moreover, a long learning curve in colorectal ESD training has also contributed to the low penetration of this technique worldwide, in addition to a relatively higher risk of severe complications (e. g. bleeding and perforation) 8 14.
Previously, effective colorectal ESD training programs have been developed by ourselves and others for trainees with an adequate experience performing gastric ESD 15 16 17 18 19. However, colorectal ESD training is still challenging, especially for Western endoscopists, given the lack of training opportunities in patients with early gastric cancers, which are assumed to be relatively safer and easier to resect, because of the lower incidence of gastric cancer 20. Therefore, we conducted this prospective clinical trial to investigate whether our training programs using ex vivo animal models could enable novice operators without experience performing gastric ESD to become proficient at performing colorectal ESD.
Materials and methods
Patients
Between April 2014 and January 2015, 182 patients who required treatment for colorectal neoplasms using ESD at the NTT Medical Center, Tokyo, were prospectively enrolled in this study. The inclusion criteria were the presence of lesions larger than 20 mm for which submucosal invasion was estimated to be absent or superficial based on white light and magnification chromoendoscopy as well as a negative no-lifting sign 17. In addition to patients who did not provide informed consent, 11 patients with recurrent and residual mucosal lesions were also excluded. Finally, 77 patients with 80 lesions participated in this study. The sample size was estimated based on the results of a previous prospective study 16. All of the lesions were removed by two novice ESD operators, and the medical records of 80 consecutive ESD cases were prospectively collected. After the histopathological analyses, additional surgical resection was performed because of an increased risk of lymph node metastasis, when the following findings were identified: (1) vertical margin (+), (2) submucosal lymph and/or vessel invasion (+), (3) submucosal invasion more than 1000 μm, and (4) a poorly differentiated component. Written informed consent was obtained from each of the enrolled patients. This study was approved by the ethics committee of the NTT Medical Center, Tokyo, and was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials as UMIN000013566 at December 31, 2014.
ESD trainees
Two trainees (Y. M. and T. T.), who had not performed gastric ESD, participated in this study. Before starting our colorectal ESD training program, the trainees were required to have skillfully performed more than 1000 colonoscopies. In addition, proficient EMR techniques and the ability to diagnosis target lesions precisely during magnifying endoscopy were also necessary. For the next step, trainees were asked to learn ESD using a step-by-step program, as previously described 17. In the first step, trainees were taught what kinds of colorectal tumors are suitable for ESD; the trainees needed to know whether an ESD would be easy or difficult to complete for a given lesion. The second and third steps were the observation of ESD procedures performed by an expert followed by assistance with the procedure itself. Experience with at least 10 cases of colorectal ESD was required to learn the most suitable ESD strategies. Our two trainees participated as assistants in about 20 colorectal ESD procedures in addition to gastric/esophageal ESD procedures. Importantly, all of the steps were performed under expert supervision. After meeting these prerequisites, as confirmed by a single experienced endoscopist (K. O.), each trainee began to learn colorectal ESD using a newly developed “proximal colon” porcine model before performing human ESD procedures.
ESD procedure
In the present study, for the safe performance of colorectal ESD, rectal lesions were selected as the initial three cases for each trainee. ESD was performed under conscious sedation with flunitrazepam and buprenorphine. We optionally selected an upper gastrointestinal endoscope (GIF-Q260J; Olympus Optical, Tokyo, Japan) to treat lesions mainly located at the rectum/sigmoid colon, or a lower gastrointestinal scope (PCF-Q260JI; Olympus Optical) for those at the descending colon to cecum. In some cases, we used a balloon overtube to reduce paradoxical movements of the endoscope and to protect areas in the colon that are especially vulnerable to injury, such as the ascending/transverse colon 21. After the injection of sodium hyaluronate solutions, circumferential incision and submucosal dissections were performed using a Dual knife (KD650Q; Olympus Optical), without marking. Hemostasis for procedural bleeding was attempted using the knife that was in use or hemostatic forceps (Coagrasper; Olympus Optical). Visible exposed vessels were coagulated at the end of the procedure to prevent delayed bleeding. In all of the cases, we used a high frequency generator unit (ICC200 or VIO300D; Erbe Elektromedizin, Tübingen, Germany). The operation time was defined as the period between the identification of the target lesion and the completion of the above procedures. When a trainee could not complete ESD because of an inability to continue the procedure as judged by the expert supervisor (e. g. perforation, uncontrolled bleeding, or severe fibrosis), the expert took over the operation.
Animal models
Our simulator consists of a porcine lower gastrointestinal package suspended in a square box. Except for the rectum, porcine colon is unsuitable for ESD training because of its thin luminal wall. Therefore, we placed the porcine colorectum upside down and used the rectum as the location of the proximal target lesions ( Fig.1). Next, demarcated marks were made using a Dual knife, and the ESD procedure was performed in a manner similar to that used for a human procedure using an upper gastrointestinal endoscope (Fig. 2). All of the ESD procedures were performed under expert man-to-man instruction, with technical advice as to how to resect the target lesions. The animal training model was completed once an expert deemed that the trainee had obtained the required ESD-specific techniques.
Fig. 1.
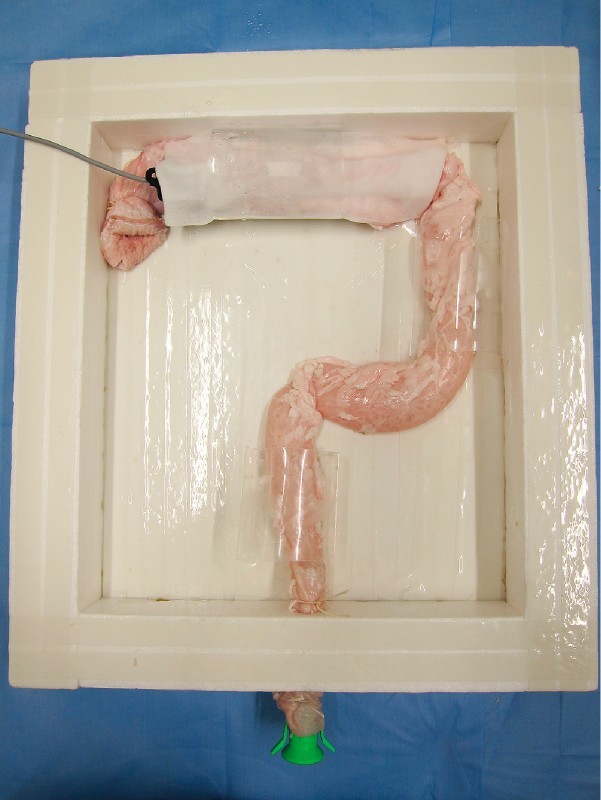
Ex vivo porcine “proximal colon” model. The porcine colorectum was placed upside down and anchored inside a square box, so as to simulate the human colon.
Fig. 2.
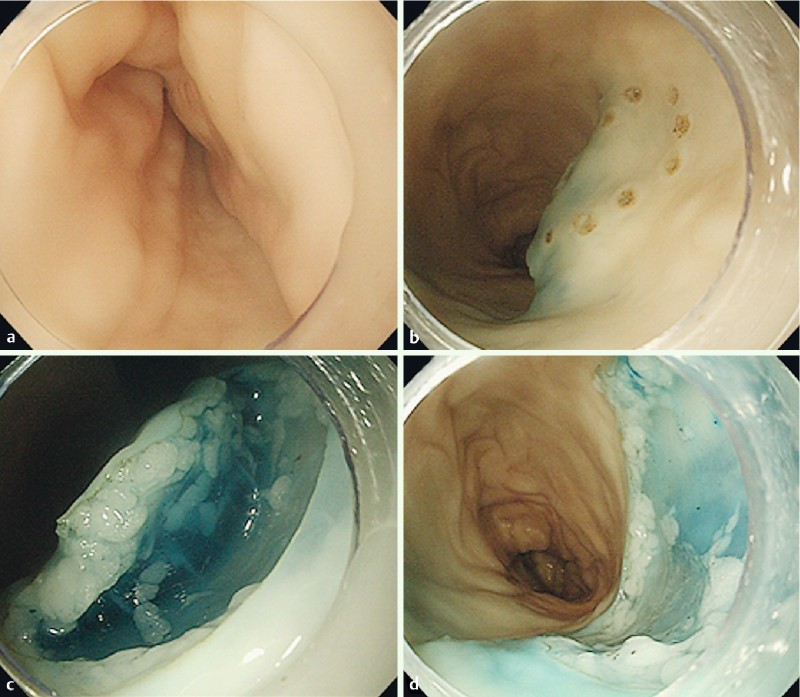
ESD training using ex vivo porcine model. An upper gastrointestinal scope (GIF-Q240; Olympus Optical) was inserted into the reconstructed proximal porcine colon (a). After the target lesion was demarcated, sodium hyaluronate solutions were injected into the submucosal layer around the lesion (b). After the circumferential incision outside of the marks, the submucosal dissections were performed (c), and the target lesion was completely resected (d). All of the procedures were performed using a Dual knife (KD650Q; Olympus Optical).
Outcomes and data analysis
The primary outcome was the self-completion rate. The secondary outcomes were the en bloc R0 resection rate, the operation time, and the prevalence of complications. An R0 resection was defined as a complete resection that showed negative lateral and vertical margins when examined pathologically.
The 40 lesions resected by each of the two trainees were divided into two groups: the first 20 cases (first period) and the latter 20 cases (latter period). To assess the validity of our colorectal ESD training system, the operation time, the rates of self-completion and en bloc R0 resection, and the prevalence of complications were compared between the two period groups. The learning curve for colorectal ESD was evaluated as the change in the operation time per unit area of the specimen (min/cm2). To determine whether clinical factors (e. g. age, sex, tumor location, morphological, and pathological characteristics) affect the difficulty of colorectal ESD, those associated with a prolonged operation time were evaluated.
Statistical analysis
Continuous data are shown as the mean ± SD. The differences in the values of the clinical parameters between the first and second sets of 20 lesions for each of the trainees were analyzed using the Fisher exact test apart from age, tumor size, and operation time, which were analyzed using the Student’s t test. To reveal factors associated with a prolonged operation time, univariate and multivariate linear regression analyses were performed. Unless otherwise specified, P values of < 0.05 were considered to denote statistical significance. All of the analyses were performed using SPSS, ver. 11.0 (SPSS Inc., Chicago, IL, United States).
Results
Training using animal models
A total of 12 target lesions located in proximal porcine colon were resected using ESD techniques by each of the two trainees. The operation time per unit area of the specimen for the latter six cases was significantly lower than that for the first six cases (9.6 ± 6.0 min/cm2 vs. 3.6 ± 1.4 min/cm2, P = 0.04). No perforations occurred during the ESD procedures. Thus, they were regarded as having acquired colorectal ESD-specific techniques, such as circumferential incision and submucosal dissection.
Clinicopathological characteristics of resected lesions
The clinicopathological characteristics of the resected lesions are shown in Table 1. No significant differences in tumor size or pathological characteristics were observed between the first and latter periods for each trainee. For trainee A, the number of cases with fibrosis and the number of cases with a central depression were significantly higher during the latter period (10 % vs. 55 %, P = 0.006; and 0 % vs. 35 %, P = 0.008, respectively). The lesions resected during the latter period were predominantly located in the proximal colon (Fig. 3).
Table 1. Clinicopathological characteristics of resected colorectal neoplasms.
| Total | Trainee A | Trainee B | |||||
| First period | Latter period | P value | First period | Latter period | P value | ||
| Number of lesions | 80 | 20 | 20 | 20 | 20 | ||
| Tumor size, mm | 32 ± 12 | 30 ± 11 | 33 ± 14 | 0.41 | 43 ± 15 | 39 ± 11 | 0.32 |
| Macroscopic type | |||||||
| LST-G | 49 | 8 | 12 | 11 | 18 | ||
| LST-NG | 25 | 10 | 8 | 6 | 1 | ||
| Protruded | 6 | 2 | 0 | 3 | 1 | ||
| Location of the lesions | 0.34 | 0.70 | |||||
| Colon | 67 | 16 | 19 | 15 | 17 | ||
| Rectum | 13 | 4 | 1 | 5 | 3 | ||
| Fibrosis | 19 | 2 | 11 | 0.006 | 5 | 1 | 0.18 |
| Large nodule | 16 | 3 | 3 | 0.99 | 7 | 3 | 0.27 |
| Central depression | 9 | 0 | 7 | 0.008 | 2 | 0 | 0.49 |
| Pathological characteristics | |||||||
| Adenocarcinoma | 25 | 6 | 6 | 0.99 | 9 | 4 | 0.11 |
| Lymph and/or vascular invasion | 4 | 2 | 0 | 0.24 | 2 | 0 | 0.24 |
| Submucosal invasion | 15 | 3 | 4 | 0.99 | 7 | 1 | 0.04 |
LST-G, laterally spreading tumor, granular type; LST-NG, laterally spreading tumor, nongranular type.Continuous data are shown as the mean ± SD. P values were analyzed using the Fisher exact test, or the Student’s t test for tumor size.
Fig. 3.
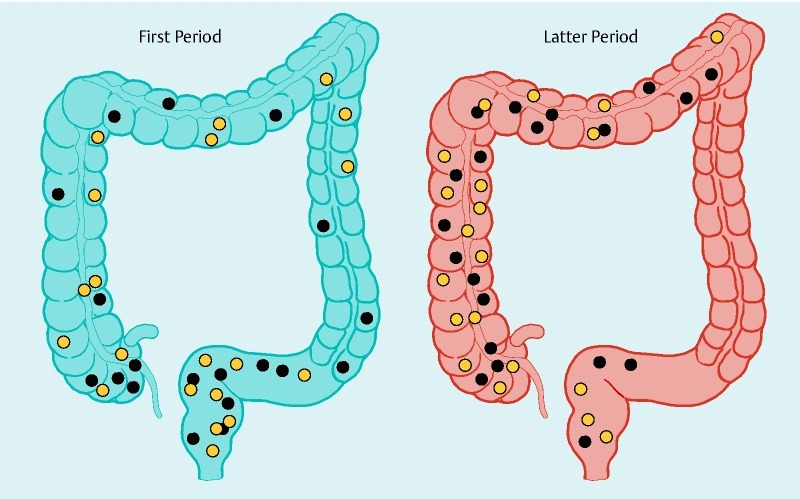
Locations of the resected lesions during the first and latter periods. The locations of the resected lesions are shown as black (trainee A) and yellow (trainee B) circles.
Outcomes of colorectal ESD
As summarized in Table 2, the overall rates of self-completion and en bloc R0 resection were 98 % (78/80) and 100 % (80/80), respectively. One of the lesions for which self-completion was not achieved was an adenocarcinoma with submucosal invasion located in the sigmoid colon, while the other was an intramucosal adenocarcinoma located in the rectum. These lesions presented with severe fibrosis, and perforations occurred during the submucosal dissection, requiring the supervising expert to take control. The mean procedure time was 73 ± 45 minutes. The operation time during the first period was significantly longer than that during the latter period (86 ± 50 minutes vs. 60 ± 36 minutes, P = 0.01). Regarding complications, two cases with perforations (3 %) during the procedure and two cases with delayed hemorrhage (3 %) were observed during the first period; however, all of the complications were successfully managed endoscopically. None of the cases experienced delayed perforations. As a result, a total of six patients required an additional surgical resection because of advanced pathological findings; the remaining patients achieved good clinical outcomes (93 %).
Table 2. Outcomes of ESD according to the first and latter 20 sets.
| Total | Trainee A | Trainee B | |||||
| First period | Latter period | P value | First period | Latter period | P value | ||
| Number of lesions | 80 | 20 | 20 | 20 | 20 | ||
| Total operation time, min | 73 ± 45 | 73 ± 49 | 66 ± 41 | 0.63 | 100 ± 48 | 55 ± 29 | 0.001 |
| Self-completion rate, n (%) | 78 (98) | 20 (100) | 20 (100) | n.a. | 18 (90) | 20 (100) | 0.24 |
| En bloc R0 resection, n (%) | 80 (100) | 20 (100) | 20 (100) | n.a. | 20 (100) | 20 (100) | n.a. |
| Complications | |||||||
| Delayed bleeding, n (%) | 2 (3) | 0 (0) | 0 (0) | n.a. | 2 (10) | 0 (0) | 0.24 |
| Perforation, n (%) | 2 (3) | 0 (0) | 0 (0) | n.a. | 2 (10) | 0 (0) | 0.24 |
| Delayed perforation, n (%) | 0 (0) | 0 (0) | 0 (0) | n.a. | 0 (0) | 0 (0) | n.a. |
Continuous data are shown as the mean ± SD.
When the results for each trainee were compared between the first and latter periods, only one of the trainees exhibited a significant reduction in the operation time (trainee A: 73 ± 49 minutes vs. 66 ± 41 minutes, P = 0.63; trainee B: 100 ± 48 minutes vs. 55 ± 29 minutes, P = 0.001). This outcome may have been partly due to the increase in the number of cases with fibrosis and/or a central depression during the latter period for trainee A. To correct for differences in tumor size, proficiency was evaluated as the operation time per unit area of the specimen (min/cm2). The learning curve for each trainee demonstrated a change in proficiency over time (Fig. 4).
Fig. 4.
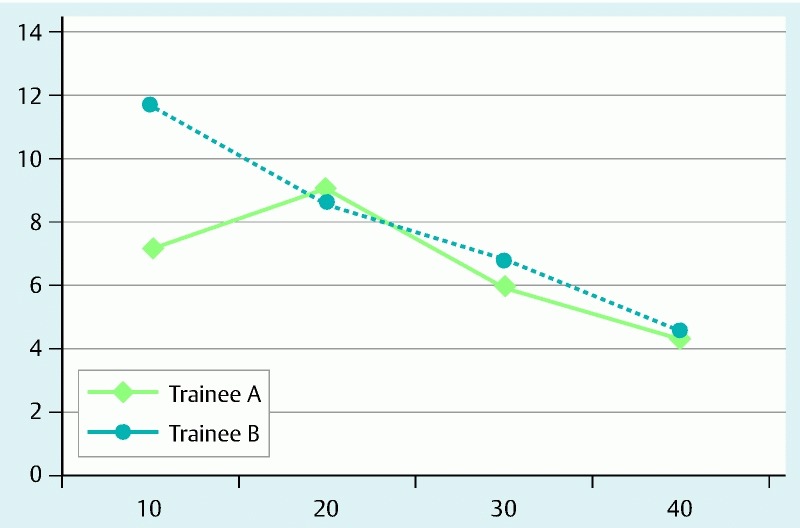
Learning curves for colorectal ESD as evaluated according to operation time per unit area of specimen (min/cm2).
Factors associated with prolonged operation time
The results of univariate and multivariate linear regression analyses are summarized in Table 3. When risk factors for a prolonged operation time were evaluated among the first 40 cases, the presence of fibrosis was identified as a significant independent risk factor (coefficient, 5.90; 95 %CI, 2.36 – 9.44, P = 0.002). On the other hand, when risk factors were evaluated among the latter 40 cases, no clinicopathological characteristics were identified as significant risk factors. In the present study, the tumor location was not identified as a significant risk factor associated with a prolonged operation time.
Table 3. Factors associated with prolonged operation time.
| Prolonged operation time | ||||
| Univariate, Coefficient (95 %CI) | P value | Multivariate, Coefficient (95 %CI) | P value | |
| First period | ||||
| Age, years | – 0.08 ( – 0.19 to 0.03) | 0.15 | ||
| Male sex | 0.04 ( – 3.34 to 3.43) | 0.98 | ||
| Rectal lesion | – 0.41 ( – 4.27 to 3.46) | 0.83 | ||
| Fibrosis | 5.90 (2.36 to 9.44) | 0.002 | 5.90 (2.36 to 9.44) | 0.002 |
| Large nodule | 0.48 ( – 3.25 to 4.20) | 0.80 | ||
| Central depression | – 0.53 ( – 7.94 to 6.88) | 0.89 | ||
| Presence of adenocarcinoma | – 1.96 ( – 5.23 to 1.32) | 0.23 | ||
| Submucosal invasion | 1.38 ( – 2.33 to 5.08) | 0.46 | ||
| Latter period | ||||
| Age, years | – 0.05 ( – 1.23 to 0.03) | 0.20 | ||
| Male sex | 1.56 ( – 0.27 to 3.39) | 0.09 | ||
| Rectal lesion | – 0.91 ( – 4.33 to 2.52) | 0.60 | ||
| Fibrosis | 0.68 ( – 1.34 to 2.70) | 0.50 | ||
| Large nodule | – 0.79 ( – 3.31 to 1.74) | 0.53 | ||
| Central depression | 1.48 ( – 0.85 to 3.82) | 0.21 | ||
| Presence of adenocarcinoma | 0.75 ( – 1.33 to 2.83) | 0.47 | ||
| Submucosal invasion | 1.76 ( – 0.92 to 4.43) | 0.19 | ||
CI, confidence interval; P values were analyzed using univariate and multivariate linear regression analyses.
Discussion
Because of the technical difficulty and the high risk of severe complications, colorectal ESD is usually not attempted unless the endoscopist has experience performing gastric ESD. Tanaka et al. proposed that competence performing ESD should be acquired in the stomach before advancing to more difficult lesions, such as those in the colon 22. However, our results indicated that an appropriate training program enabled trainees to perform safe and proficient colorectal ESD, regardless of their gastric ESD experience. This is the first report to investigate prospectively the efficacy of a training program using an ex vivo porcine proximal colon model and the learning curve for colorectal ESD in trainees without experience performing gastric ESD.
In the present study, we developed a novel ex vivo colorectal animal model, instead of providing initial ESD training in patients with gastric lesions. Gotoda et al. developed a training program for ESD using porcine models and suggested that a rapid increase in the learning curve for ESD could be obtained within a relatively short period of time using their model 11. When trainees are ready to acquire colorectal ESD techniques but do not yet have experience performing gastric ESD, training using an animal model appears to be essential. Although Hon et al. developed a porcine rectal model for the acquisition of colorectal ESD techniques, the difficulty in maneuvering the endoscope was unlike that required in a real human colon 23. Our newly developed “proximal colon” model more accurately simulates the paradoxical movements of the endoscope and colorectal luminal folds and flexions, thus, trainees are able to learn how to maneuver the endoscope. In addition, compared with in vivo animal models, our model is more cost-effective. Importantly, our ex vivo porcine model can help novice operators to learn ESD-specific techniques (e. g. circumferential incision and submucosal dissection skills), which can be difficult to acquire in daily practice. However, this model is not suitable for learning how to control active bleeding and/or to compensate for respiratory movements. Therefore, prior intensive training in advanced techniques for endoscopic hemostasis is also necessary. ESD training in animal models without an expert’s supervision is reportedly less effective for beginners hoping to acquire this technique 24. Furthermore, Draganov et al. reported that observation by an expert while performing ESD can significantly reduce removal times and can contribute to the acquisition of ESD skills over a short period of time 25. These results highlight the importance of supervision by an expert, even during animal model training.
Learning curves for colorectal ESD have been evaluated in several Japanese studies. Hotta et al. found that significant improvements in the en bloc resection rate (85 %) and operation time were observed after 40 procedures 15. Sakamoto et al. reported that at least 30 colorectal ESDs are required to achieve a sufficient en bloc resection rate (92 %) 18. Importantly, although they achieved a sufficient en bloc resection rate without the occurrence of severe complications, the trainees who participated in these training programs had experience performing gastric ESD. Thus, the immediate application of this program at Western institutions is difficult. Unlike Japanese studies, Iacopini et al. conducted a prospective study to evaluate the efficacy of a gastric animal model for colorectal ESD trainees without gastric ESD experience 16. They demonstrated the feasibility and safety of the procedure, with a competency threshold set at 20 rectal ESD procedures, but not at 20 colonic ESD procedures. Recently, Shiga et al. also conducted a study investigating the learning curve for colorectal ESD in trainees with some gastric ESD experience and reported that the en bloc R0 resection rate was 75 % 19. They also revealed that a colonic location was closely associated with a prolonged operation time. These results may have been affected by differences in the strategies and techniques used for gastric and colorectal ESD. On the other hand, a higher en bloc R0 resection rate (98 %) was achieved in the present study, despite the predominantly colonic locations of the resected lesions. Importantly, good clinical outcomes were achieved even for the first 20 cases, suggesting the possible contribution of our novel ex vivo “proximal colon” animal model, although these differences may have been partly associated with the heterogeneity of the lesions and/or the type of ESD devices that were used. In this study, all of the procedures during the latter period were completed by the trainees, indicating that 20 cases may be sufficient to proficiently perform colorectal ESD. However, we should keep in mind that all of the procedures were performed under the supervision of an expert. Additional studies are needed to confirm this speculation.
Expert centers have reported higher en bloc resection rates (87 – 93 %) and lower perforation rates (5 – 10 %) in large colorectal series 26 27 28. In the present study, each trainee performed ESD at various locations with sufficient levels of submucosal dissection and hemostasis skill, enabling good clinical outcomes to be achieved, even among the first 20 cases. Although this result may be due to our training program, daily practice, during which trainees acquire the skills needed to maintain a stable field during endoscopic treatment, is also important. Maintaining a stable operative field during an ESD procedure is quite difficult because the operator must control the scope and maintain a stable field using one hand while using the other hand to handle the devices. This technique cannot be mastered in one day. At our institution, endoscopists who hope to perform colorectal ESD must meet the following criteria: a high skill level in non-loop insertion colonoscopy technique completed, the ability to perform an exact diagnosis and biopsy of colorectal neoplasms identified using magnified endoscopy, proficiency in conventional and/or piecemeal EMR 17. Most importantly, they must constantly maintain a stable endoscopic field using one hand during all of the procedures. To maintain the operative view, the operator must use only the left hand to make up/down and right/left directional manipulations simultaneously. Use of the right hand for right/left directional manipulations is strictly prohibited at our endoscopy center because the ability to perform up to four different simultaneous manipulations, such as (1) scope insertion and withdrawal, (2) scope rotation, (3) endo-device insertion and withdrawal, and (4) directional manipulations, is needed during ESD procedures. We believe that these prerequisites are closely associated with qualified colorectal ESD procedures.
According to the results of a linear regression analysis, the presence of fibrosis was identified as a significant predictor of a prolonged operation time, although most of the fibrosis identified in the present study was mild. Severe fibrosis has been reportedly identified as a risk factor for complications (e. g. perforations and delayed bleeding) and can interfere with en bloc resections, even when performed by ESD experts 29. Actually, the two cases with perforations in the present study presented submucosal fibrosis. A higher rate of severe fibrosis has been observed for large protruding tumors 29. Although the accumulation of experience may decrease the rate of difficult cases and perforations, such cases should be avoided during the introductory stages of performing colorectal ESD.
Although our training programs may be useful for learning colorectal ESD techniques, several flaws must be resolved before they can be successfully applied in Western counties. At present, only a few highly qualified experts in ESD exist; thus, performing ESD under direct expert supervision is not feasible in most Western countries. Consequently, trainees must visit a high-volume ESD center, requiring a significant financial and time commitment, even though close observation by experts while performing ESD can enhance ESD training. Furthermore, performing hands-on training using our “proximal colon model” under an expert’s supervision is also strongly recommended. In addition, laparoscopic surgery for early CRC is more established as a standard technique, despite the economical advantage and minimal invasiveness of ESD 30. A close collaboration between Western and Asian countries will be important to improve colorectal ESD techniques in Western countries and to spread these techniques worldwide.
In conclusion, our trainees achieved relatively high self-completion and R0 resection rates without the occurrence of any severe complications, suggesting that our training programs are useful for trainees without experience performing gastric ESD. Ex vivo animal models, which were constructed to simulate the paradoxical endoscopic movements and a lumen with many folds and flexions, may be useful for the acquisition of colorectal ESD-specific techniques. The presence of fibrosis was identified as a significant independent predictor of a prolonged operation time; thus, cases with fibrosis should be avoided during the introductory stages of performing colorectal ESD. To achieve safe and proficient colorectal ESD, prior intensive learning and expert supervision are also necessary.
Footnotes
Competing interests: None
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Winawer S J, Zauber A G, Ho M N. et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. New Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe T, Itabashi M, Shimada Y. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29. doi: 10.1007/s10147-011-0315-2. [DOI] [PubMed] [Google Scholar]
- 4.Kudo S, Kashida H, Nakajima T. et al. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg. 1997;21:694–701. doi: 10.1007/s002689900293. [DOI] [PubMed] [Google Scholar]
- 5.Sakai E, Ohata K, Chiba H. et al. Methylation epigenotypes and genetic features in colorectal laterally spreading tumors. Int J Cancer. 2014;135:1586–1595. doi: 10.1002/ijc.28814. [DOI] [PubMed] [Google Scholar]
- 6.Soetikno R, Friedland S, Kaltenbach T. et al. Nonpolypoid (flat and depressed) colorectal neoplasms. Gastroenterology. 2006;130:566–576. doi: 10.1053/j.gastro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien M J, Winawer S J, Zauber A G. et al. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenterol Hepatol. 2004;2:905–911. doi: 10.1016/s1542-3565(04)00392-1. [DOI] [PubMed] [Google Scholar]
- 8.Terasaki M, Tanaka S, Oka S. et al. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol. 2012;27:734–740. doi: 10.1111/j.1440-1746.2011.06977.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujishiro M, Yahagi N, Kakushima N. et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678–683. doi: 10.1016/j.cgh.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Ohata K, Nonaka K, Minato Y. et al. Endoscopic submucosal dissection for large colorectal tumor in a Japanese general hospital. J Oncol. 2013;2013:218670. doi: 10.1155/2013/218670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotoda T, Yamamoto H, Soetikno R M. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Liao C, Tan A. et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751–757. doi: 10.1055/s-0029-1215053. [DOI] [PubMed] [Google Scholar]
- 13.Chiba H, Ohata K, Ohno A. et al. Perforation with retroperitoneal emphysema after endoscopic submucosal dissection for a rectal carcinoid tumor. Endoscopy. 2010;42:E85–E86. doi: 10.1055/s-0029-1243873. [DOI] [PubMed] [Google Scholar]
- 14.Probst A, Golger D, Anthuber M. et al. Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy. 2012;44:660–667. doi: 10.1055/s-0032-1309403. [DOI] [PubMed] [Google Scholar]
- 15.Hotta K, Oyama T, Shinohara T. et al. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010;22:302–306. doi: 10.1111/j.1443-1661.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- 16.Iacopini F, Bella A, Costamagna G. et al. Stepwise training in rectal and colonic endoscopic submucosal dissection with differentiated learning curves. Gastrointest Endosc. 2012;76:1188–1196. doi: 10.1016/j.gie.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Ohata K, Ito T, Chiba H. et al. Effective training system in colorectal endoscopic submucosal dissection. Dig Endosc. 2012;24:84–89. doi: 10.1111/j.1443-1661.2012.01272.x. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto T, Sato C, Makazu M. et al. Short-term outcomes of colorectal endoscopic submucosal dissection performed by trainees. Digestion. 2014;89:37–42. doi: 10.1159/000356215. [DOI] [PubMed] [Google Scholar]
- 19.Shiga H, Endo K, Kuroha M. et al. Endoscopic submucosal dissection for colorectal neoplasia during the clinical learning curve. Surg Endosc. 2014;28:2120–2128. doi: 10.1007/s00464-014-3443-8. [DOI] [PubMed] [Google Scholar]
- 20.Fock K M. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250–260. doi: 10.1111/apt.12814. [DOI] [PubMed] [Google Scholar]
- 21.Ohya T, Ohata K, Sumiyama K. et al. Balloon overtube-guided colorectal endoscopic submucosal dissection. World J Gastroenterol. 2009;15:6086–6090. doi: 10.3748/wjg.15.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka S, Oka S, Kaneko I. et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100–107. doi: 10.1016/j.gie.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Hon S S, Ng S S, Lee J F. et al. In vitro porcine training model for colonic endoscopic submucosal dissection: an inexpensive and safe way to acquire a complex endoscopic technique. Surg Endosc. 2010;24:2439–2443. doi: 10.1007/s00464-010-0982-5. [DOI] [PubMed] [Google Scholar]
- 24.Parra-Blanco A, Arnau M R, Nicolas-Perez D. et al. Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol. 2010;16:2895–2900. doi: 10.3748/wjg.v16.i23.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Draganov P V, Chang M, Coman R M. et al. Role of observation of live cases done by Japanese experts in the acquisition of ESD skills by a western endoscopist. World J Gastroenterol. 2014;20:4675–4680. doi: 10.3748/wjg.v20.i16.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee E J, Lee J B, Lee S H. et al. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220–2230. doi: 10.1007/s00464-012-2164-0. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama H, Isomoto H, Yamaguchi N. et al. Endoscopic submucosal dissection for laterally spreading tumours of the colorectum in 200 consecutive cases. Surg Endosc. 2010;24:2881–2887. doi: 10.1007/s00464-010-1071-5. [DOI] [PubMed] [Google Scholar]
- 28.Saito Y, Uraoka T, Yamaguchi Y. et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Lee S P, Kim J H, Sung I K. et al. Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: Pathologic review of 173 cases. J Gastroenterol Hepatol. 2015;30:872–878. doi: 10.1111/jgh.12886. [DOI] [PubMed] [Google Scholar]
- 30.Conlin A, Kaltenbach T, Kusano C. et al. Endoscopic resection of gastrointestinal lesions: advancement in the application of endoscopic submucosal dissection. J Gastroenterol Hepatol. 2010;25:1348–1357. doi: 10.1111/j.1440-1746.2010.06402.x. [DOI] [PubMed] [Google Scholar]


