Abstract
Collagen is the most abundant protein in animals. Its overproduction is associated with fibrosis and cancer metastasis. The stability of collagen relies on post-translational modifications, the most prevalent being the hydroxylation of collagen strands by collagen prolyl 4-hydroxylases (CP4Hs). Catalysis by CP4Hs enlists an iron cofactor to convert proline residues to 4 hydroxyproline residues, which are essential for the conformational stability of mature collagen. Ethyl 3,4-dihydroxybenzoate (EDHB) is commonly used as a “P4H” inhibitor in cells, but suffers from low potency, poor selectivity, and off-target effects that cause iron deficiency. Dicarboxylates of 2,2′-bipyridine are among the most potent known CP4H inhibitors but suffer from a high affinity for free iron. A screen of biheteroaryl compounds revealed that replacing one pyridyl group with a thiazole moiety retains potency and enhances selectivity. A diester of 2 (5-carboxythiazol-2-yl)pyridine-5-carboxylic acid is bioavailable to human cells and inhibits collagen biosynthesis at concentrations that neither cause general toxicity nor disrupt iron homeostasis. These data anoint a potent and selective probe for CP4H and a potential lead for the development of a new class of antifibrotic and antimetastatic agents.
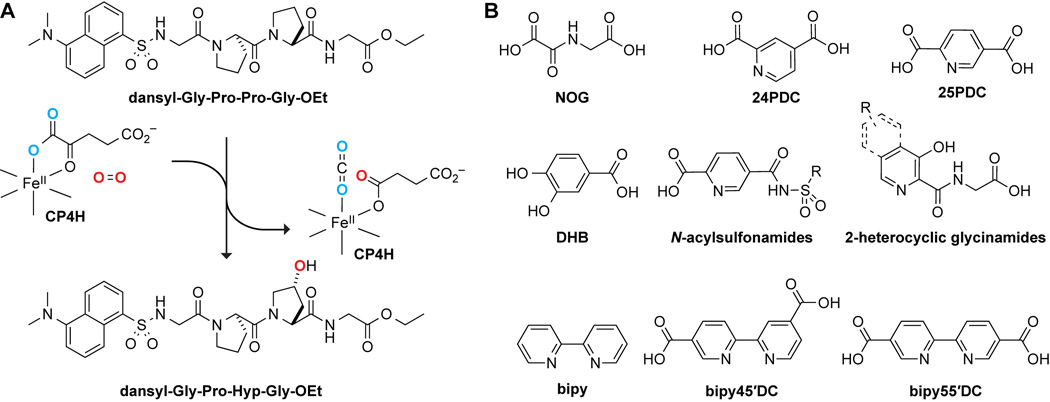
Collagen is the principal component of bone, connective tissues, and the extracellular matrix in animals.1 The overproduction of collagen is associated with a variety of diseases, including fibrotic diseases2 and cancers.3–7 The stability of collagen relies on posttranslational modifications that occur throughout the secretory pathway.8 By far the most prevalent of these modifications is the hydroxylation of collagen strands by collagen prolyl 4-hydroxylases (CP4Hs), which are Fe(II)- and α-ketoglutarate (AKG)-dependent dioxygenases (FAKGDs) located in the lumen of the endoplasmic reticulum.9 Catalysis by CP4Hs converts (2S)-proline (Pro) residues in protocollagen strands into (2S,4R)-4-hydroxyproline (Hyp) residues (Figure 1A), which are essential for the conformational stability of mature collagen triple helices.10 Importantly, CP4Hs are validated targets for treating both fibrotic diseases11 and metastatic breast cancer.6
Figure 1.
Reaction catalyzed by collagen prolyl 4-hydroxylase (CP4H) and its inhibition. (A) In an Fe(II)- and AKG-dependent reaction, CP4Hs catalyze the hydroxylation of Pro residues in collagenous peptides to form Hyp residues. (B) Examples of known human CP4H inhibitors.
Like all enzymes of the FAKGD superfamily, catalysis by CP4Hs requires Fe(II) and the cosubstrates AKG and dioxygen.12–14 The Fe(II) is bound by a conserved His-X-Asp/Glu…Xn…His motif, and AKG chelates to enzyme-bound Fe(II) using its C-1 carboxylate and C-2 keto groups, while the C-5 carboxylate group forms hydrogen bonds and engages in Coulombic interactions with a basic residue (typically, arginine or lysine). All FAKGDs are believed to effect catalysis through a similar two-stage mechanism in which AKG is first decarboxylated oxidatively to generate a highly reactive Fe(IV)=O species (ferryl ion), which effects the hydroxylation of a hydrocarbon via a radical rebound process.12–14
In vertebrates, CP4Hs exist as α2β2 tetramers. In these tetramers, the α-subunit contains the catalytic and substrate-binding domains, and the β-subunit is protein disulfide isomerase, which is a multifunctional protein that is responsible for maintaining the α-subunit in a soluble and active conformation.9 Three isoforms of the α-subunit, α(I), α(II), and α(III), have been identified in humans.9 All α-subunit isoforms form tetramers with the β-subunit, which we refer to herein as the CP4H1, CP4H2, and CP4H3 holoenzymes. CP4H1 is the most prevalent of the isoforms, and has been characterized most extensively. Whereas the structure of the tetrameric complex is unknown, those of the individual domains of the α(I)-subunit have provided insight into the interaction of CP4Hs with the protocollagen substrate, as well as the dimerization of the α(I)-subunits to facilitate formation of the tetramer.15–18
The development of CP4H inhibitors has been of interest since the mid-1970s. Like many FAKGDs, human CP4Hs are inhibited by AKG mimics,19 such as N-oxalyl glycine (NOG), pyridine-2,4 dicarboxylic acid (24PDC), pyridine-2,5-dicarboxylic acid (25PDC), and 3,4-dihydroxybenzoic acid (DHB),20 as well as by simple metal chelators, such as 2,2′-bipyridine (bipy) (Figure 1B). These compounds suffer from low potency in cellular assays, insufficient selectivity for CP4H, and intolerable cytotoxicity.21,22 Still, the ethyl ester of DHB (that is, EDHB) is often used as a cellular “P4H” inhibitor,23,24 even though DHB is not selective for CP4H compared to other FAKGDs, requires high dosing, and leads to an iron-deficient phenotype.24
The most potent inhibitors of human CP4Hs identified to date are bipyridinedicarboxylic acids (bipyDCs) (Figure 1B). Two bipyDCs have high potency: 2,2′-bipyridine-4,5′-dicarboxylic acid (bipy45′DC)25 and 2,2′-bipyridine-5,5′-dicarboxylic acid (bipy55′DC).26 Both of these bipyDCs inhibit human CP4H competitively with respect to AKG and bind selectively to human CP4H1 compared to PHD2, another human P4H.25
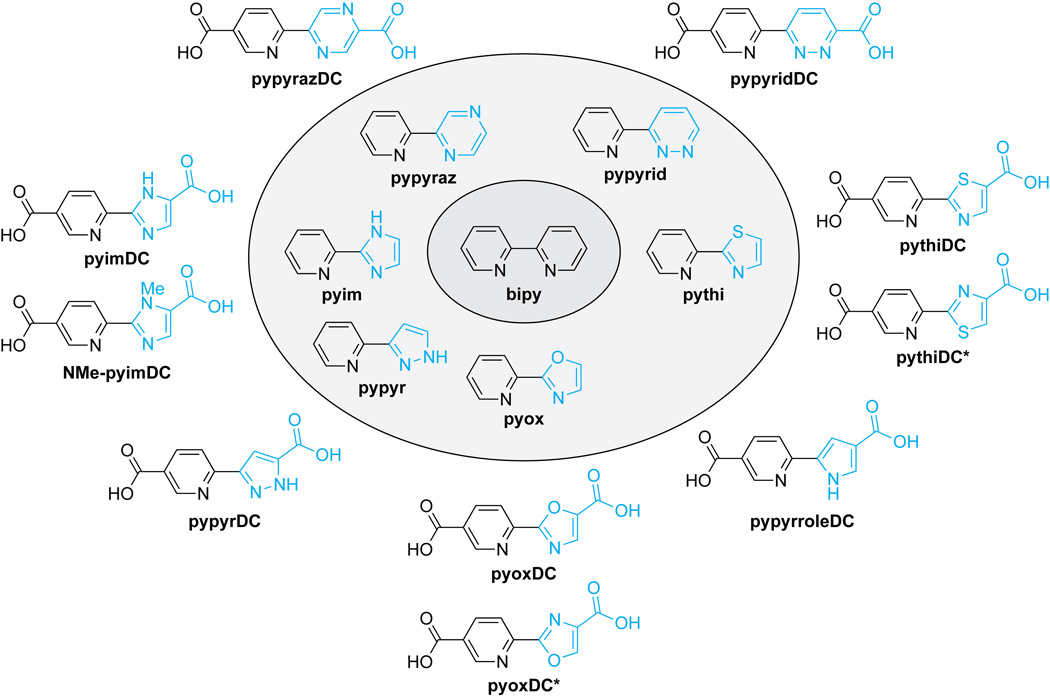
An intrinsic property of bipyDCs limits their utility in a biological context. Like bipy itself, bipyDCs form tight complexes with free iron,25 which is the dominant metal in life.27 We hypothesized that replacing the bipy core with another biheteroaryl scaffold could lead to compounds that retain the potency and selectivity of bipy45′DC and bipy55′DC, but not their high affinity for free iron. Accordingly, we synthesized bipy analogs that retain one pyridyl group but replace the second with six distinct 5- or 6-membered nitrogen-containing heterocycles: pyrazine, pyridazine, oxazole, pyrazole, imidazole, pyrrole, and thiazole (Figure 2). We find that an asymmetric scaffold containing a thiazolyl group has especially desirable attributes as an inhibitor of human CP4H.
Figure 2.
Biheteroaryl compounds used in this study. Novel moieties are rendered in cyan, and similar compounds are grouped together.
RESULTS AND DISCUSSION
Biheteroaryl Compounds
To begin our analysis, we prepared a library of six biheteroaryl compounds (Figure 2) and evaluated them as iron chelators in vitro. The library was assembled either from commercial vendors (pypyr and pyim) or by synthesis using palladium-catalyzed cross-coupling reactions (pypyraz, pypyrid, pyox, and pythi). To evaluate the iron affinity of these compounds in vitro, we performed titration experiments to determine the half-maximal concentration required to form a complex with 20 µM Fe(II) at pH 7.0. The ensuing Fe20-EC50 parameter provides a metric for iron affinity.25 With the exception of pyox, all members of the library were observed to form distinct colored Fe(ligand)32+ complexes under these conditions (see: Supporting Information).
The Fe20-EC50 values of the biheteroaryl compounds varied by more than two orders of magnitude (Figure S1; Table 1). All, however, were significantly greater than that of bipy or bipyDCs.25 The Fe20-EC50 value appeared to rely on both the ring size and pKa of the conjugate acid of the alternative heterocycle, as scaffolds with 5-membered rings were substantially weaker chelators than were those with 6 membered rings and pKa value correlated positively with iron affinity.
Table 1.
Iron binding by bipy and biheteroaryl compounds.
| Compound | Heteroaryl pKaa (ref.) | pKab | Fe(II) Complex λmaxc |
Stoichiometry (Ligand:Fe)d |
Fe20-EC50e |
|---|---|---|---|---|---|
| bipy | 5.2 (38) | 4.3 | 523 | 3:1 | 43 ± 2 |
| pypyrid | 2.3 (39) | <3 | 522 | 3:1 | 124 ± 1 |
| pypyraz | 0.8 (40) | <3 | 523 | 3:1 | 400 ± 7 |
| pyim | 7.1 (41) | 5.4 | 484 | 3:1 | 900 ± 30 |
| pypyr | 2.5 (42) | 4.1 | 441 | NDf | 2250 ± 100 |
| pythi | 2.5 (43) | <3 | 524 | 3:1 | 5100 ± 100 |
| pyox | 0.8 (44) | <3 | NOg | NOg | >18,000 |
pKa value of the conjugate acid of pyridine (bipy) or the non-pyridyl ring.
pKa value of the conjugate acid as estimated by titration of the entire compound; bipy, ref. 45.
Determined using spectrophotometry (see: Supporting Information).
Determined by titration of 20 μM FeSO4; bipy, ref. 25. Values are the mean (±SE) of 3 replicates.
Not determined due to prohibitive competing absorbance from iron.
Not observed.
Biheteroaryl Dicarboxylates in Vitro
Inspired by the low affinity of the biheteroaryl compounds for free iron, we prepared a small library of biheteroaryl dicarboxylates (Figure 2) and interrogated those compounds as both iron chelators and inhibitors of human CP4H. With the exceptions of pyimDC (which was synthesized via a classical route to substituted imidazoles28) and pypyrDC (which was synthesized by a 1,3-dipolar cycloaddition of a 2 ethynylpyridine with ethyl diazoacetate29), all of the biheteroaryl dicarboxylates were synthesized by either palladium-catalyzed direct arylation of an appropriate heterocycle (pypyrazDC, pypyridDC, pyoxDC, pyoxDC*, pythiDC or pythiDC*) with a functionalized 2 bromopyridine30–34 or palladium-catalyzed oxidative cross-coupling (pypyrroleDC) between a functionalized pyridine N-oxide and an N protected pyrrole.35 Typically, direct arylation using methyl- or ethyl-protected carboxylate esters allowed synthesis of the target compounds in 2–4 steps with an acceptable yield. For pyoxDC and pythiDC, cross-coupling yields using the typical inner-sphere base pivalic acid (PivOH) were prohibitively low (<5%, data not shown). We found that the addition of 1 adamantanecarboxylic acid rather than PivOH improved yields markedly (see: Supporting Information) and encourage the continued investigation of 1 adamantanecarboxylic acid as an inner-sphere base in palladium-catalyzed direct arylation reactions.
We investigated iron chelation by the biheteroaryl dicarboxylates in a manner similar to that for the parent scaffolds. To our surprise, we were not able to detect complex formation by spectrophotometry for any of the biheteroaryl dicarboxylates at concentrations up to 1 mM, suggesting that the affinity of these compounds for free iron would be negligible in a biological context. Previously, we reported that various bipyDCs have Fe20-EC50 values that are similar to that of bipy itself,25 so our discovery that biheteroaryl dicarboxylates investigated herein have an Fe20-EC50 value >1 mM represents an improvement of at least an order of magnitude.
Next, we assessed the biheteroaryl dicarboxylates as inhibitors of human CP4H1. To separate any inhibitory effect that derives from iron sequestration rather than enzymic binding, we employed previously described assay conditions (10 µM compound and 50 µM FeSO4) in which potent chelators like bipy do not cause inhibition.25 In this initial screen (Figure S2), we found that some biheteroaryl dicarboxylates showed little or no inhibition of human CP4H1, consistent with the inability of their heteroatoms to participate in an enzymic interaction. (Both pypyridDC and pypyrDC even showed modest activation under these conditions by a mechanism that is unclear.) Notably, we found that pyimDC, pyoxDC, and pythiDC were inhibitors of human CP4H1, with pyimDC and pythiDC demonstrating potency only a bit weaker than that of the bipyDCs. Importantly, the regioisomers pythiDC* and pyoxDC* did not show significant inhibition, suggesting that proper regiochemistry is essential for inhibition.
Unlike oxazole or thiazole, imidazole exists as two tautomers, one with a proton on N1 (as in the depiction of pyimDC in Figure 2) and another with a proton on N3. Although we did not observe the formation of a complex between pyimDC and free iron by spectrophotometry, we examined this issue more thoroughly. We found that pyimDC was able to deter the formation of the Fe(bipy)32+ complex in a dose-dependent manner (Figure S3). Moreover, competition required a free carboxylate on the imidazole ring. These data are consistent with the formation of a Fe(pyimDC)2 complex with N1 bound to iron. To eliminate this mode of binding, we synthesized NMe-pyimDC (Figure 2), which is an analogue of pyimDC that is methylated on N1. We found that NMe-pyimDC was able to deter the formation of the Fe(bipy)32+ complex, but only at high concentrations (Figure S3). We also found that NMe pyimDC is an inhibitor of human CP4H in vitro, but that its potency is less than that of pyimDC (Figure S2).
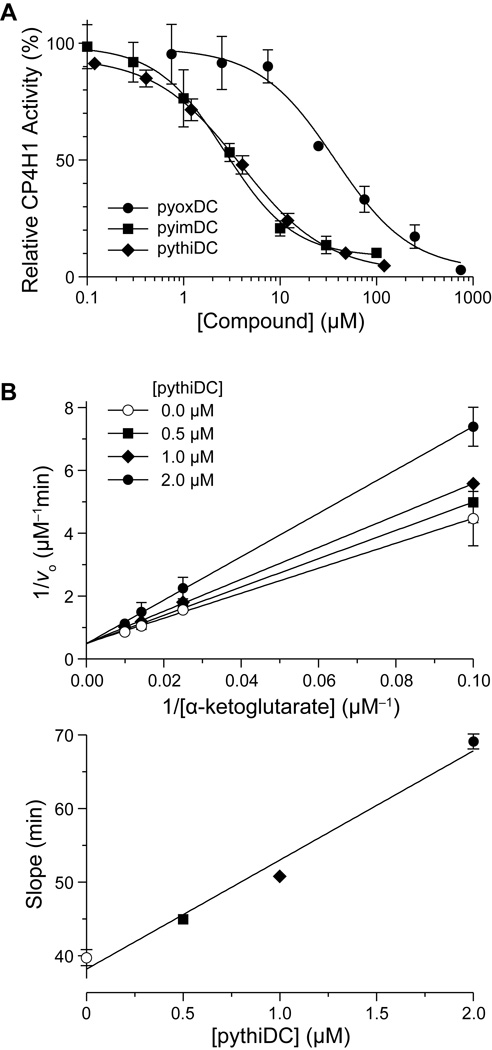
In subsequent dose–response experiments, we found the inhibition curves for pyimDC, pythiDC, and pyoxDC to be sigmoidal, yielding IC50 values in the low-micromolar range (Figure 3A). The potencies of pyimDC and pythiDC were approximately 10-fold greater than that of pyoxDC. A Lineweaver–Burke analysis of inhibition of CP4H by pythiDC demonstrated competitive inhibition with respect to the AKG cosubstrate (Figure 3B).
Figure 3.
Biheteroaryl dicarboxylates as inhibitors of human CP4H1. (A) Individual points represent the mean (±SD) of three independent experiments. Data were fitted to a dose–response equation to determine IC50 values: pyimDC, (2.6 ± 0.1) µM; pyoxDC, (33 ± 8) µM; pythiDC, (4.0 ± 0.2) µM. (B) Lineweaver–Burke analysis of inhibition by pythiDC. The rate of the reaction catalyzed by CP4H1 with increasing α-ketoglutarate concentration (10–100 µM) was determined in the presence of a fixed concentration of pythiDC (0.0, 0.5, 1.0, or 2.0 µM). Individual points represent the mean (±SE) of two independent experiments. Data were fitted by linear regression to determine a Ki value of (0.39 ± 0.04) µM for competitive inhibition.
Like bipy45′DC and bipy55′DC,26,25 pyimDC, pyoxDC, and pythiDC could bind in the AKG binding pocket and use their second carboxyl group to form additional interactions with residues in the active site of CP4H. If so, then the biheteroaryl dicarboxylates should exhibit structure–activity relationships similar to those of bipy45′DC and bipy55′DC for the inhibition of PHD2, another P4H enzyme that has been characterized extensively. We find that pyimDC, pythiDC, and pyoxDC inhibit human PHD2 only weakly (Figure S4), with pythiDC displaying especially low potency.
Biheteroaryl Dicarboxylates in Cellulo
Encouraged by the results in vitro, we next sought to determine if pyimDC and pythiDC could be suitable for use in cellulo. First, we determined the effect of these compounds on iron metabolism in human cells. Because human CP4Hs are validated therapeutic targets for breast cancer, we chose the MDA-MB-231 human breast cancer cell line as a primary model system.6 We performed additional analyses in human embryonic kidney (HEK) cells. We assessed iron metabolism with immunoblots for ferritin, the transferrin receptor (TfR), and the transcription factor HIF-1α, all of which are known to give distinct phenotypes depending on the status of iron in a human cell.24 More specifically, levels of ferritin and TfR are regulated by iron regulatory proteins 1 and 2 (IRPs), which have iron-dependent RNA-binding activity that modulates the expression of target genes at the level of translation.36,37 The stability of HIF-1α is dependent on the prolyl 4-hydroxylase activity of PHD2, which is an activity that is inherently sensitive to the iron status of the cell. Thus, iron-deficient cells exhibit ferritin levels that are lower and TFR and HIF-1α levels that are higher than those of untreated cells.
Ethyl dihydroxybenzoate (EDHB) an AKG mimic that is commonly used as a “P4H” inhibitor in cell culture models, served as our benchmark.23,24 Calculations suggested that the diethyl esters of pyimDC and pythiDC have logP values conducive to cellular uptake (Table S1), and we synthesized those two esters. Moreover, the iron affinity of these diethyl esters remained sufficiently low (see: Section XXII in the Supporting Information and Figure S1), encouraging their use in cellular assays. Cultured MDA-MB-231 cells are known to secrete large amounts of collagen.6 Due to the importance of CP4H-dependent hydroxylation for collagen stability, the level of collagen secreted by MDA-MB-231 cells is dependent on the activity of this enzyme. Thus, MDA-MB-231 cells are ideal for investigations of both iron deficiency and CP4H activity.
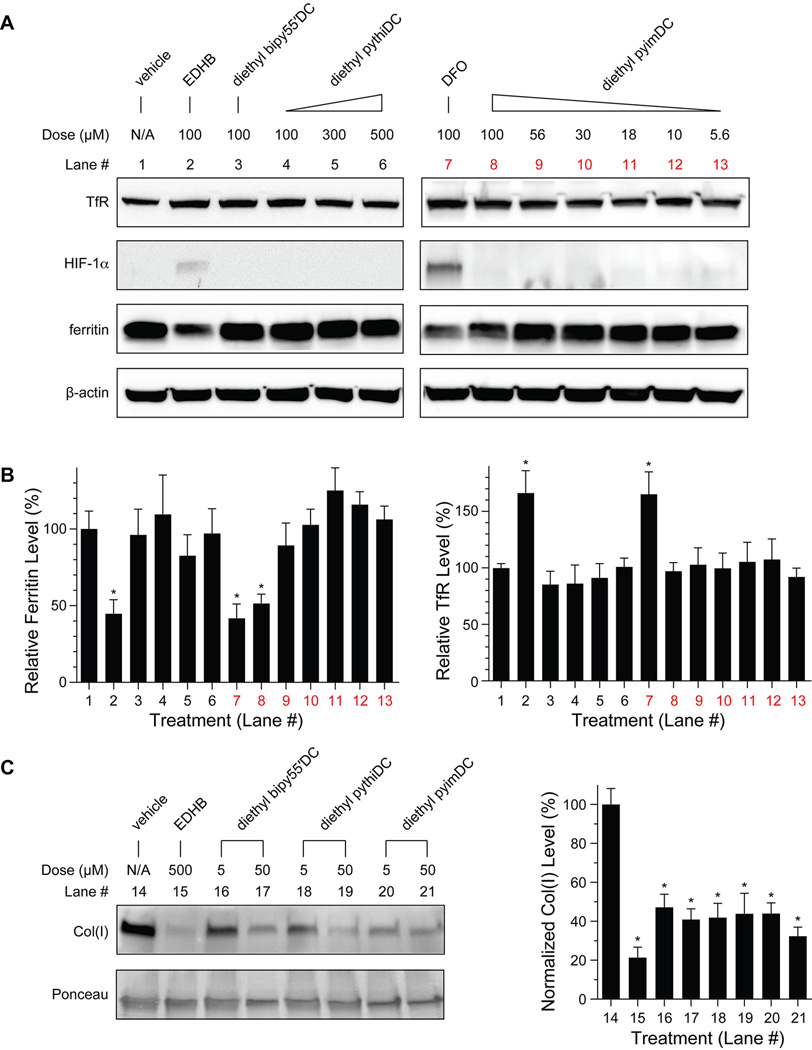
Toward this end, we treated MDA-MB-231 cells with biheteroaryl dicarboxylates, and assayed for cytotoxicity and indicators of iron deficiency. None of the esterified biheteroaryl dicarboxylates exhibited cytotoxic activity at high micromolar concentrations (see: Section XI in the Supporting Information). Cells treated with EDHB demonstrated a strong iron-deficient phenotype, as expected (Figures 4A and 4B). In contrast, cells treated with diethyl pythiDC appeared to be normal at concentrations as high as 500 µM. Interestingly, diethyl pyimDC showed an intermediate phenotype characterized by a significant decrease in ferritin levels but without an associated accumulation of TfR or HIF-1α. This phenotype was, however, not observable at diethyl pyimDC concentrations ≤56 µM. The results in MDA-MB-231 cells were replicated in HEK cells. Again, treatment with diethyl pythiDC and low levels of diethyl pyimDC (as well as simply pyim and pythi; Figure S5) did not affect the level of TfR, HIF-1α, or ferritin (Figure S6).
Figure 4.
Effect of esterified compounds on iron metabolism and collagen secretion. MDA-MB-231 breast cancer cells were treated with deferoxamine (DFO), ethyl dihydroxybenzoate (EDHB), biheteroaryl compounds, or vehicle (DMSO) and then analyzed with an immunoblot (*, p < 0.05). (A) Effect of esterified biheteroaryl compounds on iron metabolism. Blots are representative of at least 3 replicates. (B) Densitometric quantitations (n ≥ 3) corresponding to the blots in panel A and normalized to β-actin. The dose of EDHB (500 µM) is known to diminish collagen secretion significantly.6 (C) Effect of esterified biheteroaryl compounds on collagen secretion into conditioned media. Blots are representative of at least 5 replicates and quantitations (right) are normalized to total protein using the Ponceau S stained blot.
Next, we assessed the function of IRPs in cells treated with the esterified biheteroaryl compounds. Using a radiolabeled RNA ligand, we performed electrophoretic mobility shift assays on IRPs from treated MDA-MB-231 cells. Whereas DFO, EDHB, and bipy all caused significant increases in RNA-binding by IRPs, diethyl pyimDC and diethyl pythiDC did not (Figure S7). This result is again consistent with these compounds having little effect on cellular iron levels.
Lastly, we examined the effect of treating MDA-MB-231 cells with the esterified biheteroaryl compounds on the cellular secretion of type I collagen (Figure 4C), which relies on CP4H activity.6 We found that treatment with either diethyl pyimDC or diethyl pythiDC reduced the level of secreted collagen significantly (Figure 4C). Moreover, the efficacy of diethyl pyimDC or diethyl pythiDC was indistinguishable from that of diethyl bipy55′DC. No inhibition of catalysis by CP4H1 or PHD2 was observed in vitro with these diethyl esters at a concentration of 100 µM (data not shown), confirming that the inhibitory potential of these compounds is masked by esterification. Lastly, treatment with diethyl pyimDC and diethyl pythiDC did not appear to affect the levels of human CP4H1 itself (Figure S8), consistent with the observed reduction in collagen secretion arising from inhibition of CP4H.
Conclusions
We have identified compounds that can replace EDHB in experiments with cultured cells. Unlike EDHB, we found that diethyl pythiDC, diethyl pyimDC, and even diethyl bipy55′DC inhibit CP4H activity in cultured cells at concentrations that do not cause iron deficiency. Given the subtle ferritin phenotype caused by diethyl pyimDC and the measurable iron affinity of bipy55′DC and related bipyDCs in vitro,25 we put forth diethyl pythiDC as a preferred replacement for EDHB. Moreover, an esterified pythiDC could serve as a lead for clinical development.
Supplementary Material
Acknowledgments
This work was supported by Grant R01 AR044276 to R. T. R. (NIH) and Grant R01 DK066600 to R. S. E. (NIH). J. D. V. was supported by Molecular Biosciences Training Grant T32 GM007215 (NIH). K. A. A was supported by a PhRMA predoctoral fellowship and by Molecular and Cellular Pharmacology Training Grant T32 GM008688 (NIH). The Micromass LCT® mass spectrometer was purchased with funds from Grant CHE-9974839 (NSF). This study made use of the National Magnetic Resonance Facility at Madison, which is supported by Grant P41 GM103399 (NIH).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental procedures, compound characterization, Table S1, and Figures S1–S9. This material is available free of charge via the Internet at http://pubs.acs.org
The authors declare no competing financial interest.
REFERENCES
- 1.Shoulders MD, Raines RT. Collagen structure and stability. Annu. Rev. Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbloom J, Castro SV, Jimenez SA. Narrative review: Fibrotic diseases: Cellular and molecular mechanisms and novel therapies. Ann. Intern. Med. 2010;152:159–166. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- 3.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedi A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisinger-Mathason TS, Zhang M, Qiu Q, Skuli N, Nakazawa MS, Karakasheva T, Mucaj V, Shay JES, Stangenberg L, Sadri N, Puré E, Yoon SS, Kirsch DG, Simon MC. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3:1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, Hubbi ME, Wirtz D, Semenza GL. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73:3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox TR, Bird D, Baker A-M, Barker HE, Ho MW-Y, Lang G, Erler JT. Lox-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2014;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Gorres KL, Raines RT. Prolyl-4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg RA, Prockop DJ. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple helix of collagen. Biochem. Biophys. Res. Comm. 1973;52:115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- 11.Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann. Med. 2008;40:402–417. doi: 10.1080/07853890801986594. [DOI] [PubMed] [Google Scholar]
- 12.Hanauske-Abel HM, Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: Applicability to classification and design of inhibitors. J. Theor. Biol. 1982;94:421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- 13.Hausinger RP. Fe(II)/α-ketoglutarate-dependent hydroxylases and related enzymes. Crit. Rev. Biochem. Mol. 2004;39:21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- 14.Costas M, Mehn MP, Jensen MP, Que LJ. Dioxygen activation at mononuclear nonheme iron active sites: Enzymes, models, and intermediates. Chem. Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 15.Abraham RJ, McLauchlan KA. The proton resonance spectra and conformations of the prolines. Part I. Mol. Phys. 1962;5:195–203. [Google Scholar]
- 16.Hieta R, Kukkola L, Permi P, Pirilä P, Kivirikko KI, Kilpeläinen I, Myllyharju J. The peptide-substrate binding domain of human collagen prolyl 4-hydroxylases. Backbone assignments, secondary structure, and binding of proline-rich peptides. J. Biol. Chem. 2003;278:34966–34974. doi: 10.1074/jbc.M303624200. [DOI] [PubMed] [Google Scholar]
- 17.Pekkala M, Hieta R, Bergmann U, Kivirikko KI, Wierenga RK, Myllyharju J. The peptide-substrate-binding domain of collagen prolyl 4-hydroxylase is a tetratricopeptide repeat domain with functional aromatic residues. J. Biol. Chem. 2004;279:52255–52261. doi: 10.1074/jbc.M410007200. [DOI] [PubMed] [Google Scholar]
- 18.Anantharajan J, Koski MK, Kursula P, Hieta R, Bergmann U, Myllyharju J, Wierenga RK. The structural motifs for substrate binding and dimerization of the α subunit of collagen prolyl 4-hydroxylase. Structure. 2013;21:2107–2118. doi: 10.1016/j.str.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Myllylä R, Tuderman L, Kivirikko KI. Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur. J. Biochem. 1977;80:349–357. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- 20.Rose NR, Mcdonough MA, King ONF, Kawamura A, Schofield CJ. Inhibition of 2-oxoglutarate dependent oxygenases. Chem. Soc. Rev. 2011;40:4364–4397. doi: 10.1039/c0cs00203h. [DOI] [PubMed] [Google Scholar]
- 21.Dowell RI, Hadley EM. Novel inhibitors of prolyl 4-hydroxylase. J. Med. Chem. 1992;35:800–804. doi: 10.1021/jm00083a001. [DOI] [PubMed] [Google Scholar]
- 22.Franklin TJ, Morris WP, Edwards PN, Large MS, Stephenson R. Inhibition of prolyl 4-hydroxylase in vitro and in vivo by members of a novel series of phenanthrolinones. Biochem. J. 2001;353:333–338. doi: 10.1042/0264-6021:3530333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki T, Majamaa K, Uitto J. Reduction of collagen production in keloid fibroblast cultures by ethyl-3,4-dihydroxybenzoate. J. Biol. Chem. 1987;262:9397–9403. [PubMed] [Google Scholar]
- 24.Wang J, Buss JL, Chen G, Ponka P, Pantopoulos K. The prolyl 4-hydroxylase inhibitor ethyl-3,4-dihydroxybenzoate generates effective iron deficiency in cultured cells. FEBS Lett. 2002;529:309–312. doi: 10.1016/s0014-5793(02)03389-6. [DOI] [PubMed] [Google Scholar]
- 25.Vasta JD, Raines RT. Selective inhibition of prolyl 4-hydroxylases by bipyridinedicarboxylates. Bioorg. Med. Chem. 2015;23:3081–3090. doi: 10.1016/j.bmc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hales NJ, Beattie JF. Novel inhibitors of prolyl 4-hydroxylase. 5. The intriguing structure–activity relationships seen with 2,2′-bipyridine and its 5,5′-dicarboxylic acid derivatives. J. Med. Chem. 1993;36:3853–3858. doi: 10.1021/jm00076a014. [DOI] [PubMed] [Google Scholar]
- 27.Frey PA, Reed GH. The ubiquity of iron. ACS Chem. Biol. 2012;7:1477–1481. doi: 10.1021/cb300323q. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin JJ, Kasinger PA, Novello FC, Sprague JM, Duggan DE. 4-Trifluoromethylimidazoles and 5-(4-pyridyl)-1,2,4-triazoles, new classes of xanthine oxidase inhibitors. J. Med. Chem. 1975;18:895–900. doi: 10.1021/jm00243a007. [DOI] [PubMed] [Google Scholar]
- 29.Vuluga D, Legros J, Crousse B, Donnet-Delpon D. Synthesis of pyrazoles through catalyst-free cycloaddition of diazo compounds to alkynes. Green Chem. 2009;11:156–159. [Google Scholar]
- 30.Campeau L-C, Rosseaux S, Fagnou K. A solution to the 2-pyridyl organometallic cross-coupling problem: Regioselective catalytic direct arylation of pyridine N-oxides. J. Am. Chem. Soc. 2005;127:18020–18021. doi: 10.1021/ja056800x. [DOI] [PubMed] [Google Scholar]
- 31.Martin T, Verrier C, Hoarau C, Marsais F. Direct C-2 arylation of alkyl 4-thiazolecarboxylates: New insights in synthesis of heterocyclic core of thiopeptide antibiotics. Org. Lett. 2008;10:2909–2912. doi: 10.1021/ol801035c. [DOI] [PubMed] [Google Scholar]
- 32.Campeau L-C, Stuart DR, Leclerc J-P, Bertrand-Laperle M, Villemure E, Sun H-Y, Lasserre S, Guimond N, Lecavallier M, Fagnou K. Palladium-catalyzed direct arylation of azine and azole N-oxides: Reaction development, scope, and applications in synthesis. J. Am. Chem. Soc. 2009;131:3291–3306. doi: 10.1021/ja808332k. [DOI] [PubMed] [Google Scholar]
- 33.Strotman NA, Chobanian HR, Guo Y, He J, Wilson JE. Highly regioselective palladium-catalyzed direct arylation of oxazole at C-2 or C-5 with aryl bromides, chlorides, and triflates. Org. Lett. 2010;12:3578–3581. doi: 10.1021/ol1011778. [DOI] [PubMed] [Google Scholar]
- 34.Duric S, Tzschucke CC. Synthesis of unsymmetrically substituted bipyridines by palladium-catalyzed direct C–H arylation of pyridine-N-oxides. Org. Lett. 2011;13:2310–2313. doi: 10.1021/ol200565u. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Yu X, Li Y, Kuang C. Palladium-catalyzed oxidative CH/CH cross-coupling of pyridine N-oxides with five-membered heterocycles. Chem. Commun. 2014;50:9291–9294. doi: 10.1039/c4cc04129a. [DOI] [PubMed] [Google Scholar]
- 36.Muckenthaler MU, Bruno G, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- 37.Anderson CP, Shen L, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim. Biophys. Acta. 2012;1823:1468–1483. doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson RG, Williams FV. Rates of ionization of pseudo acids. V. Steric effects in the base-catalyzed ionization of nitroethane. J. Am. Chem. Soc. 1953;75:3073–3075. [Google Scholar]
- 39.Albert A, Goldacre R, Phillips J. The strength of heterocyclic bases. J. Chem. Soc. 1948:2240–2249. [Google Scholar]
- 40.Ford PC, DeForest PR, Gaunder R, Taube H. Synthesis and properties of pentaamminepyridineruthenium(II) and related pentaammineruthenium complexes of aromatic nitrogen heterocycles. J. Am. Chem. Soc. 1968;90:1187–1194. [Google Scholar]
- 41.Walba H, Isensee RW. Acidity constants of some arylimidazoles and their cations. J. Org. Chem. 1961;26:2789–2791. [Google Scholar]
- 42.Catalan J, Elguero J. Basicity and acidity of azoles. Adv. Heterocycl. Chem. 1987;41:187–274. [Google Scholar]
- 43.Zoltewicz JA, Deady LW. Quaternization of heteroaromatic compounds: Quantitative aspects. Adv. Heterocycl. Chem. 1978;22:71–121. [Google Scholar]
- 44.Brown DJ, Ghosh PB. The spectra, ionization, and deuteriation of oxazoles and related compounds. J. Chem. Soc. B. 1969:270–276. [Google Scholar]
- 45.Krumholz P. Ferrous mono-α-α′-dipyridyl. J. Am. Chem. Soc. 1949;71:3654–3656. [Google Scholar]
- 46.Job P. Formation and stability of inorganic complexes in solution. Annali di Chimica Applicata. 1928;9:113–203. [Google Scholar]
- 47.Huang CY. Determination of binding stoichiometry by the continuous variation method: The Job plot. Methods Enzymol. 1982;87:509–525. doi: 10.1016/s0076-6879(82)87029-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.