Abstract
Spontaneous coronary artery dissection (SCAD) is a rare entity. It has been described in various settings like pregnancy, collagen vascular diseases, cocaine abuse, heavy exercise, variant angina, eosinophilic arteritis, or fibro muscular dysplasia. It is also easy to miss a dissection during angiography, as the typical radiolucent lumen seen in coronary angiography may be absent in many cases.
In this report, we describe the case of a 35-year-old female who presented with acute ST elevation myocardial infarction due to spontaneous coronary dissection. She had been having episodic chest pain for one year and had been seen by two different cardiologists but was thought to have non-cardiac symptoms. Even during the index hospitalization, she underwent coronary angiography three times before coronary dissection could be identified as the cause of her symptoms.
She underwent coronary artery bypass graft surgery uneventfully. However, even after myocardial revascularization, she has had multiple episodes of chest pain requiring hospitalization. However, we have not been able to find a specific cause for it and the cause of her recurrent chest pain remains an enigma. This case highlights the problems, which arise while managing a case of SCAD. More research is needed to find the exact etiology and long-term prognosis of this condition.
Keywords: Spontaneous coronary artery dissection, Coronary artery disease, Coronary spasm
1. Case
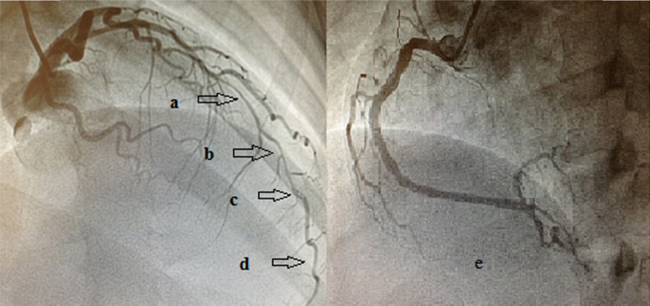
A 35-year-old female presented to emergency room with chest pain of 1-h duration. ECG showed ST elevation in V2–V6. She was immediately taken up for primary PCI. Coronary angiography showed diffuse narrowing in distal left anterior descending (LAD) artery with a discrete filling defect suggestive of thrombus. The left circumflex (LCX) and the right coronary arteries (RCA) had no significant abnormalities (Fig. 1).
Fig. 1.
Coronary angiogram showing diffuse narrowing of Left anterior descending artery (a, b, d) and a discrete filling defect (c) suggestive of thrombus. The right coronary artery showing mild luminal irregularities in proximal and mid segments (e).
Detailed history revealed that she was having episodic chest pain at rest for the past one year, which was not related to exertion. She had no history of smoking, hypertension, diabetes mellitus, dyslipidemia, family history of premature coronary artery disease, or any substance abuse. She had been evaluated by two different cardiologists and had undergone stress myocardial perfusion imaging and CT coronary angiography in the past one year and both were normal. She was thought to have non-cardiac chest pain before she finally presented to us with acute ST elevation myocardial infarction. There was no history of oral contraceptive use and her last pregnancy was more than two years ago. There was no history of any significant illness in the past.
Immediately after the angiogram, her pain improved and ECG changes resolved and she was discharged on request with medications for acute coronary syndrome. However, 12 h later, she was readmitted with chest pain. She was taken up for coronary angiography for a second time which showed resolution of filling defect from distal LAD with persisting diffuse narrowing, suggestive of coronary spasm. She was continued on medications with a presumptive diagnosis of coronary spasm.
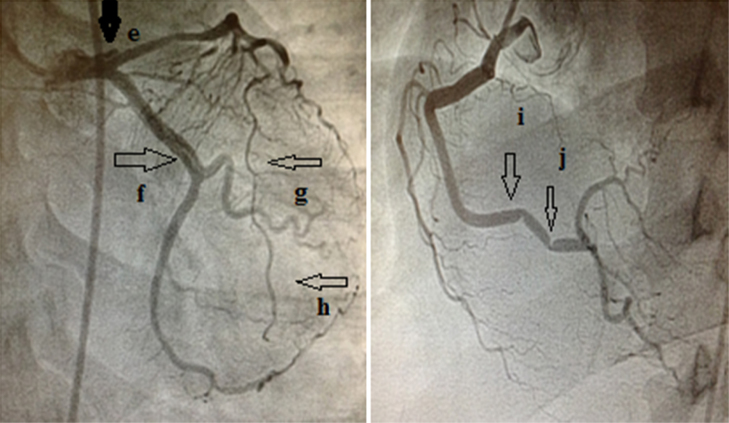
After remaining well for the next three days, she again developed chest pain with ST-T changes in ECG. Coronary angiography was done for the third time, and this time it showed dissection involving the left-main extending into the proximal LAD and LCX artery, and the RCA showed focal spasm (Fig. 2). Retrospectively, on careful study, we found that her second angiogram also showed a small dissection of LCX, which we had missed earlier.
Fig. 2.
Coronary angiogram showing dissection flap in left main extending into the left anterior descending artery, LAD (e), dissection is also seen in the left circumflex (f). The diffuse narrowing of the (LAD) persists (g, h). RCA shows focal spasms in its distal segments (i, j), which were not present in earlier angiograms.
She underwent CABG with venous grafts to LAD, diagonal, and obtuse marginal vessels. Intra-operatively, the distal LAD and diagonal vessels were found to be discolored and intramural hematoma was seen in the LAD. There was difficulty in finding the true lumen of the vessels. Immediately after putting the graft to LAD and diagonal, the color of these vessels improved dramatically. Postoperative period was uneventful and the patient was discharged on seventh postoperative day.
After discharge, she has had multiple visits to the emergency room with chest pain for which we have not found a specific cause.
2. Discussion
Spontaneous coronary artery dissection (SCAD) is a rare entity. The incidence of SCAD has been variously described from 0.07 to 1.1%.1 SCAD affects young, predominantly female population, most of the time presenting as acute coronary syndrome.1
Pathophysiology of spontaneous coronary dissection is heterogeneous. It has been described in various settings like pregnancy, collagen vascular diseases, cocaine abuse, heavy exercise, variant angina, eosinophilic arteritis, or fibro muscular dysplasia.1, 2, 3
The cause of dissection in our patient is not clear. In follow-up, we did CT angiogram of arch vessels, vertebral, and extracranial carotid arteries, but these vessels were normal. CT angiogram of iliac and renal arteries has not been done yet but there is no abdominal bruit or history of flank pain. On evaluation, she did not have evidence of connective tissue disorder.
Episodic chest pain, spontaneous resolution of ST elevation, and focal spasm of the RCA in the third angiogram all suggest that coronary spasm could be the cause of her chest pain.
High index of suspicion is required to diagnose SCAD or coronary spasms. Our patient was evaluated by two cardiologists for her pain, which was thought to be of non-cardiac nature. It is possible that she might be having episodic coronary spasms before presenting with acute coronary syndrome due to dissection.
Even during angiography, it is easy to miss a dissection (as happened in this case). During angiography, generally we seek the appearance of stereotypical multiple radiolucent lumen to diagnose SCAD, which unfortunately could be absent.4 In our patient, the initial angiogram had a filling defect in distal LAD with diffuse spasm. We thought that it was a thrombotic lesion. Retrospectively, we realize that the apparent coronary spasm as well as the thrombotic lesion seen in the angiogram could have been due to dissection. The false lumen caused by dissection can compromise the vascular lumen and that can be mistaken for coronary spasm. Similarly, intramural thrombus can mimic an intravascular thrombus. In the second angiogram, there was a small dissection of LCX, which was overlooked. This case highlights the importance of carefully evaluating the angiogram for the presence of dissection.
This case also highlights the value of Optical Coherence Tomography (OCT) and Intravascular Ultrasound (IVUS) in coronary interventions. OCT is the gold standard for diagnosing coronary dissection. It is also helpful in performing percutaneous interventions in cases of coronary dissection. Unfortunately, we did not have OCT or IVUS in our center at that time. The diagnosis of coronary dissection would have been made much earlier if we had used these modalities.
Optimal treatment of SCAD is undetermined and it needs to be individualized.4 We opted for CABG because of left main involvement and ongoing ischemia. CABG is associated with unique problems, as it might be difficult to find the true lumen of the coronary artery. Moreover, the distal vessel might be unhealthy at the anastomotic site. OCT-guided revascularization (especially using Bioresorbable Vascular Scaffolds) is an option worth considering in these patients, especially given the nature of the disease and difficulty in identifying the true lumen during surgery.
However, even after myocardial revascularization, the prognosis is unclear and the risk of recurrence of dissection remains. In the past one year, she has had multiple visits to the emergency room with complaints of chest pain, the cause of which has remained an enigma. She is on aspirin, nitrates, diltiazem, and statins. She has been repeatedly evaluated for her pain but no obvious cause has been found. ECGs and cardiac enzymes during the episodes of pain are always normal and pain is not relieved with nitrates.
CT coronary angiogram has been done twice after the CABG and it is normal. We also gave her a trial of oral steroids keeping the possibility of eosinophilic coronary arteritis in mind but there was no response. Prior to her current illness, she never had any symptoms requiring assessment for the presence of anxiety or depression. However, recently, she was evaluated by a psychiatrist and was found to have depression and has been started on antidepressant medications.
This case highlights the problems which arise while managing a case of SCAD. A recent series has suggested a not so benign outlook with recurrent SCAD in 17%.2
Since the exact etiology and long-term prognosis of SCAD is not clear, patients might develop anxiety regarding their future. Our patient has a constant fear that there might be a recurrence of dissection. Repeated inexplicable pain has led to a feeling of helplessness. A recent study has shown that a significant number of these patients require counseling or antidepressants.5 Since chest pain unrelated to exertion is a significant factor promoting anxiety in these patients, our patient is now under regular follow-up of cardiologist as well as a psychiatrist. It seems prudent that evaluation by a psychologist should be done early after SCAD as repeated visits to the hospital might drain the patient's finances as well as cause psychosocial stress.
We, as her treating physicians, are in a dilemma as to which of her many emergency room visits is actually a coronary event and what is the cause of her recurrent chest pain. More research is needed to find the exact etiology and long-term prognosis of this condition.
Conflicts of interest
The authors have none to declare.
References
- 1.Vanzetto G., Berger-Coz E., Barone-Rochette G. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250. doi: 10.1016/j.ejcts.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Tweet M.S., Hayes S.N., Pitta S.R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–588. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 3.Kajihara H., Tachiyama Y., Hirose T. Eosinophilic coronary periarteritis (vasospastic angina and sudden death), a new type of coronary arteritis: report of seven autopsy cases and a review of the literature. Virchows Arch. 2013:462–470. doi: 10.1007/s00428-012-1351-7. [DOI] [PubMed] [Google Scholar]
- 4.Adlam D., Cuculi F., Lim C. Management of spontaneous coronary artery dissection in the primary percutaneous coronary intervention era. J Invasive Cardiol. 2010;22:549–553. [PubMed] [Google Scholar]
- 5.Liang J.J., Tweet M.S., Hayes S.E. Prevalence and predictors of depression and anxiety among survivors of myocardial infarction due to spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2014;34:138–142. doi: 10.1097/HCR.0000000000000030. [DOI] [PubMed] [Google Scholar]