Abstract
Introduction
A relatively novel application for dermoscopy and reflectance confocal microscopy (RCM) is their use in the monitoring of topical treatment response for non-melanoma skin cancer. Actinic keratosis (AK) is the early phase of a multistep biologic continuum leading to invasive squamous cell carcinoma. A number of topical therapies are now available for the treatment of AK but their disadvantages include long treatment duration and prolonged local reactions. Ingenol mebutate is a newer therapy for AK which is only applied for 2 or 3 days.
Case Report
Dermoscopy and RCM findings in two patients with AK treated with ingenol mebutate confirm that it induces rapid lesion necrosis and specific neutrophil-mediated, antibody-dependent cellular cytotoxicity. Necrosis occurs via mitochondrial membrane disruption, with subsequent eradication of residual tumor cells via transient inflammation. Local skin reactions to ingenol mebutate should be considered part of the drug’s mechanism of action rather than an adverse effect.
Conclusion
Ingenol mebutate is a valuable therapy for the treatment of AK. This case report adds further evidence to the usefulness of dermoscopy and RCM in the assessment and monitoring of treatment outcome.
Electronic supplementary material
The online version of this article (doi:10.1007/s13555-016-0094-9) contains supplementary material, which is available to authorized users.
Keywords: Actinic keratosis, Dermoscopy, Field cancerization, Ingenol mebutate, Reflectance confocal microscopy
Introduction
Treatments for the so-called field cancerization in actinic keratosis (AK) include imiquimod, fluorouracil, diclofenac and photodynamic therapy [1, 2]. Drawbacks to the self-applied topical therapies currently available include long treatment duration and consequently prolonged local reactions, which could lead to less-than-ideal adherence to therapy. Recently, ingenol mebutate gel has been developed as a new topical agent for the treatment of AK and approved by the US Food and Drug Administration and European Medicines Agency. It is a macrocyclic diterpene ester, extracted from the plant Euphorbia peplus, which has been previously used as a traditional remedy for common skin lesions, including skin cancer [3, 4]. Its mechanism of action is to induce rapid and direct cell death and an immune response mediated by specific activation of protein kinase C delta, including neutrophil-mediated oxidative burst and clearance of tumors [5–7]. Thus, local skin reactions (LSR) can range from mild inflammation to severe vesiculation, pustules and erosions [8]. Dermoscopy and reflectance confocal microscopy (RCM) are currently the most used noninvasive imaging techniques that help clinicians not only to achieve high diagnostic accuracy for skin cancer therapies, but also to monitor treatment over time [9, 10]. Maier et al. recently reported successful noninvasive monitoring of morphological changes in AKs treated with ingenol mebutate using a combination of optical coherence tomography and RCM [11]. This type of monitoring was superior to clinical evaluation to detect nonresponding lesions.
Herein, we report two patients treated with ingenol mebutate gel and monitored over time by means of dermoscopy and RCM to assess skin changes and treatment response.
Case 1
A 74-year-old man with skin phototype II had several AKs on his left cheek (Fig. 1). No previous treatments for AKs were performed. Dermoscopically, the AKs were typified by a reddish pseudo network and whitish scales. Topical treatment with ingenol mebutate was initiated and, after 7 days, a moderate-to-severe reaction was noticed with confluent vesicopustules observed clinically and dermoscopically. After 15 days, complete healing of the treated area was noted, as well as a complete treatment response with no residual AKs. At baseline, RCM revealed typical findings seen in AKs, such as the presence of parakeratosis and irregular keratinocytes (Fig. 2). Notably after 7 days, vesicle formation with several inflammatory cells was seen followed, after 30 days, by a complete recovery of the epidermis that showed regularly spaced keratinocytes with a well-defined contour.
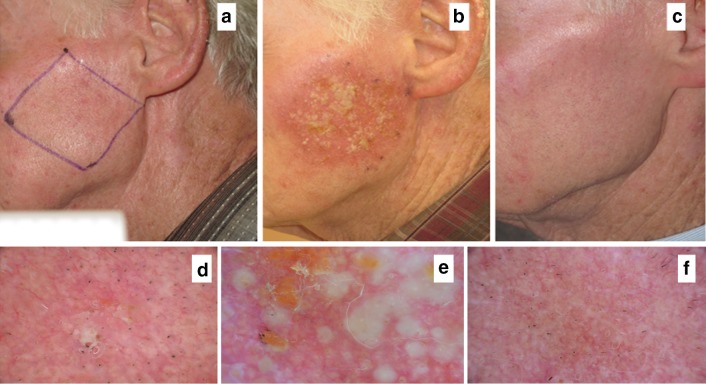
Fig. 1.
a A 74-year-old man with several AKs on his left cheek. b, e After 7 days’ treatment with ingenol mebutate, a moderate-to-severe reaction is visible clinically and dermoscopically, with the occurrence of confluent vesicles and pustules. c, f After 15 days, complete healing of the treated area is seen, with absence of residual AKs. d Dermoscopically, AKs are typified by a reddish pseudo network and whitish scales. AK Actinic keratosis
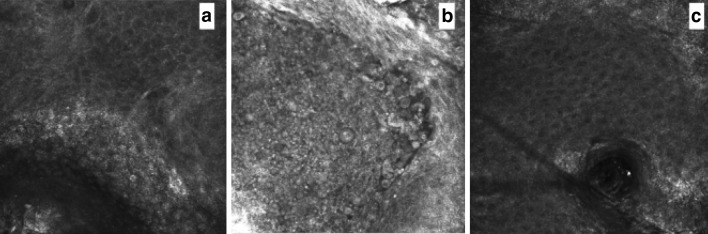
Fig. 2.
a At baseline, RCM (0.5 × 0.5 mm) reveals the typical findings seen in AKs, such as the presence of parakeratosis and irregular keratinocytes. b After 7 days, vesicles formation with several inflammatory cells are seen, followed, after 30 days, by a complete recovery of the epidermis that shows regularly spaced keratinocytes with a well-defined contour (c). RCM Reflectance confocal microscopy, AK Actinic keratosis
Case 2
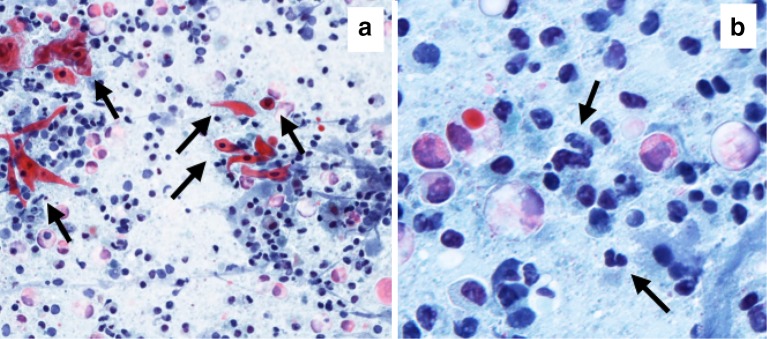
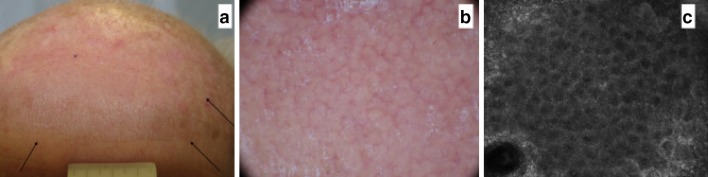
A 67-year-old man with skin phototype II had several AKs on his forehead (Fig. 3). Dermoscopically all AKs were typified by a red pseudo network and focal whitish scales. RCM depicted the presence of parakeratosis, scales and severely atypical keratinocytes. Topical treatment with ingenol mebutate was initiated and, after 7 days, erythema and vesicles located within the treated area were present. Interestingly, RCM revealed the presence of vesicles and, at higher magnification, large cells with lobed nuclei corresponding to neutrophils were detected together with necrotic keratinocytes (Fig. 4). Cytology smear of the pustules and vesicles has been performed (Papanicolaou test) to correlate the RCM findings: necrotic keratinocytes (in pink) are seen together with inflammatory cells and neutrophils with lobed nuclei (Fig. 5).
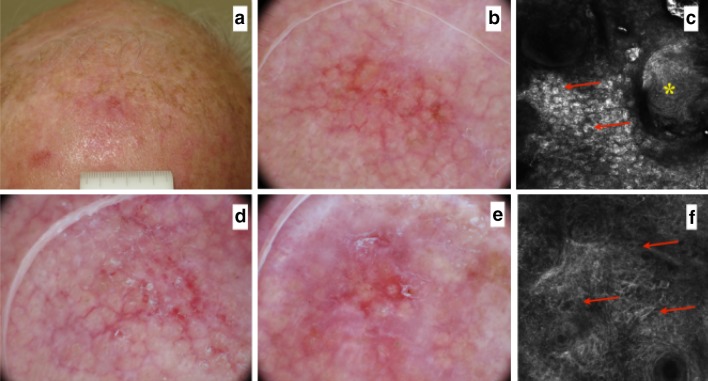
Fig. 3.
a A 67-year-old man with several AKs on his forehead. b, d, e Dermoscopically all AKs are typified by a red pseudo network and focal whitish scales. c RCM (0.5 × 0.5 mm) depicts the presence of parakeratosis (arrows), scales (asterisk) and f severely atypical keratinocytes (arrows). AK Actinic keratosis, RCM Reflectance confocal microscopy
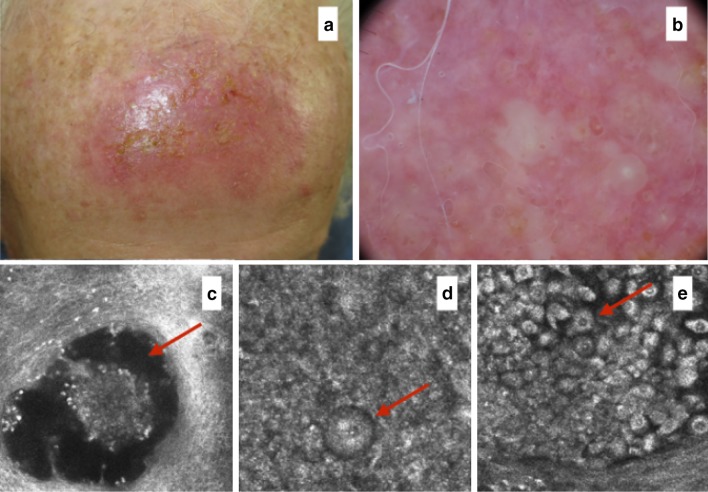
Fig. 4.
a After 7 days treatment. b Erythema and vesicles located within the treated area are present. c RCM (0.5 × 0.5 mm) reveals the presence of vesicles and, at higher magnification, d large cells with lobed nuclei corresponding to neutrophils (arrow) are seen together with necrotic keratinocytes (arrow) (e). RCM Reflectance confocal microscopy
Fig. 5.
Smear test (papanicolaou test) after 7 days treatment. a Overview image (magnification 20×) displaying necrotic keratinocytes (arrows). b High magnification (40×) image showing neutrophils (arrows)
After 15 days, a complete response was noted with healing of the treated area that appeared significantly better than the surrounding non-treated area (Fig. 6). Both dermoscopy and RCM confirmed the clinical response by showing normal skin and regular keratinocytes, respectively.
Fig. 6.
a After 15 days, the same patient of Figs. 3 and 4 achieves complete response, with healing of the treated area that appears significantly different from the surrounding non-treated area (arrows). b, c Both dermoscopy and RCM (0.5 × 0.5 mm) confirm the clinical response by showing normal skin and regular keratinocytes, respectively. RCM Reflectance confocal microscopy
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Discussion
AK is the early phase of a multistep biologic continuum leading to invasive squamous cell carcinoma [1, 12]. Currently little is known about factors predicting which lesions have the potential to become invasive squamous cell carcinoma and which are likely to remain confined to the epidermal layer. Based on the concept that AK is a very early malignant lesion, there is consensus that patients with AK should be treated and periodically followed-up [2]. During the past two decades, new and effective treatment options have been developed and are currently available on the market. Different therapeutic approaches are employed depending on whether the patient has single, isolated lesions or so-called field cancerization where multiple AKs are present along with coexisting subclinical lesions [2].
Ingenol mebutate has recently been approved for use as a topical treatment for AK in the US and Europe. The drug is a field-directed therapy and it is self-administered over two consecutive days for lesions of the trunk and extremities and over three consecutive days for lesions of the face and scalp. The rate of complete clearance was higher with ingenol mebutate than with placebo (42.2% vs. 3.7%, P < 0.001) in four randomized controlled trials [13]. LSR include erythema, flaking or scaling, crusting, swelling, vesiculation, pustulation, and erosion or ulceration [8]. Analysis of four randomized controlled trials has shown that ingenol mebutate has promising characteristics given its shorter treatment course, and overall intensity and duration of LSR measured with a global LSR score [13].
In our two cases we demonstrated the in vivo skin changes occurring during ingenol mebutate treatment by using dermoscopy and RCM. Both cases were typified by the occurrence of vesicles and pustules formed by serum and inflammatory cells, respectively. Notably, using RCM we were able to visualize the presence of neutrophils within the observed lesions. It is well known that neutrophils are among the first appearing inflammatory cells migrating towards the site of inflammation. They migrate through the blood vessels, then through interstitial tissue in a process called chemotaxis. They are the predominant cells in pus, accounting for its whitish/yellowish appearance seen clinically and dermoscopically. Another relevant RCM finding in our case is that there is a necrosis occurring at epidermal level that is composed of necrotic polygonal and detached keratinocytes as result of the direct and fast destruction of cells.
Conclusion
Taken together, our dermoscopy and RCM findings confirm that ingenol mebutate has a dual mechanism of action: (1) rapid lesion necrosis and (2) specific neutrophil-mediated, antibody-dependent cellular cytotoxicity. Because of the rapid destruction of AKs after application of ingenol mebutate gel, treatment is necessary for only 2 or 3 days. The necrosis of cytologically atypical cells occurs by disrupting the mitochondrial membrane and thus, with subsequent eradication of residual tumor cells via transient inflammation on the site of application. Dermoscopy and confocal microscopy have shown their capability to assess microscopically and non-invasively the possibility to monitor treatment reaction and shed new light into the in vivo mechanism of action of this new drug.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. Editorial assistance in the preparation of this manuscript was provided by Dr. Carmen Innes on behalf of Springer Healthcare Communications, this assistance was funded by the authors. We thank Dr Simonetta Piana for her assistance in the preparation of smear test and microscopic related pictures. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
C. Longo, S. Borsari, E. Benati, E. Moscarella, R. Alfano and G. Argenziano have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Werner RN, Stockfleth E, Connolly SM, et al. Evidence- and consensus-based (S3) guidelines for the treatment of actinic keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—Short version. J Eur Acad Dermatol Venereol. 2015;29:2069–2079. doi: 10.1111/jdv.13180. [DOI] [PubMed] [Google Scholar]
- 2.Peserico A, Neri L, Calzavara Pinton P, et al. Key Opinion Leader (KOL) Consensus for actinic keratosis management in Italy: the AKTUAL Workshop. G Ital Dermatol Venereol. 2013;148(5):515–524. [PubMed] [Google Scholar]
- 3.Green AC, Beardmore GL. Home treatment of skin cancer and solar keratoses. Australas J Dermatol. 1988;29:127–130. doi: 10.1111/j.1440-0960.1988.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 4.Ramsay JR, Suhrbier A, Aylward JH, et al. The sap from Euphorbia peplus is effective against human nonmelanoma skin cancers. Br J Dermatol. 2011;164:633–636. doi: 10.1111/j.1365-2133.2010.10184.x. [DOI] [PubMed] [Google Scholar]
- 5.Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123–8132. doi: 10.4049/jimmunol.177.11.8123. [DOI] [PubMed] [Google Scholar]
- 6.Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833–2839. doi: 10.1158/0008-5472.CAN-03-2837. [DOI] [PubMed] [Google Scholar]
- 7.Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion specific immune response. J Am Acad Dermatol. 2012;66(3):486–493. doi: 10.1016/j.jaad.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 8.Longo C, Neri L, Argenziano G, et al. Management of local skin reactions after the application of ingenol mebutate gel for the treatment of actinic keratosis: four illustrative cases. J Eur Acad Dermatol Venereol. 2014 doi: 10.1111/jdv.12714. [DOI] [PubMed] [Google Scholar]
- 9.Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of basal cell carcinoma to photodynamic therapy. Dermatology. 2012;225(3):264–270. doi: 10.1159/000345106. [DOI] [PubMed] [Google Scholar]
- 10.Fargnoli MC, Kostaki D, Piccioni A, et al. Dermoscopy in the diagnosis and management of non-melanoma skin cancers. Eur J Dermatol. 2012;22(4):456–463. doi: 10.1684/ejd.2012.1727. [DOI] [PubMed] [Google Scholar]
- 11.Maier T, et al. Treatment monitoring of topical ingenol mebutate in actinic keratoses with the combination of optical coherence tomography and reflectance confocal microscopy: a case series. Br J Dermatol. 2015;172(3):816–818. doi: 10.1111/bjd.13304. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt AR, Bordeaux JS. Actinic neoplasia syndrome and an update on the epidemiology of basal cell carcinoma, squamous cell carcinoma, and actinic keratosis. Curr Dermatol Rep. 2013;2(1):42–47. doi: 10.1007/s13671-012-0031-9. [DOI] [Google Scholar]
- 13.Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. NEJM. 2012;366(11):1010–1019. doi: 10.1056/NEJMoa1111170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.