Abstract
Introduction
trans-3,4′-Dimethyl-3-hydroxyflavanone (t-flavanone) is a derivative of astilbin that actively stimulates hair growth. The aim of the present study was to identify the mechanisms of action of t-flavanone on hair growth.
Methods
A double-blind usage test was performed with healthy volunteers who had androgenic alopecia (AGA). The subjects were divided into three groups with equal average baldness. The members in each group applied a vasodilator-containing hair lotion supplemented with either 0, 0.1, or 0.3% (wt) t-flavanone twice a day for 30 weeks. The efficacy of t-flavanone was evaluated based on the parietal global and microscopic images. At week 30, the anchoring strength of hair was measured by the average peak force required for plucking out a single hair in a non-bald area using a digital force gauge. Desmoglein expression in the cultured human hair follicle was analyzed by Western blotting.
Results
After 30 weeks, t-flavanone significantly improved AGA and enhanced the hair-anchoring strength in a hair diameter-independent manner. Culture of human hair follicles in vitro with t-flavanone resulted in the upregulation of desmoglein protein expression.
Conclusions
The results of our in vivo and in vitro studies demonstrated that t-flavanone enhanced the cell-cell adhesions in hair follicles; thus, reinforcement of hair rooting may be a mechanism by which t-flavanone promotes hair growth.
Funding
Kao Corp.
Keywords: Androgenic alopecia, Desmoglein, Hair anchoring strength, t-Flavanone
Introduction
Androgenic alopecia (AGA), which is characterized by recession of the frontal hairline and thinning of hair coverage on the vertical scalp, reportedly affects up to 70% of men after the age of 50 years [1–4]. Hair loss is associated with a progressive miniaturization of the hair follicles, which initially results in shorter and finer hairs and finally in a miniature follicle with no hair [5, 6]. Although the underlying causes of AGA remain poorly understood, genetic and hormonal factors clearly play significant roles [6]. At the present time, only topical minoxidil and oral finasteride are approved pharmacologic agents for the treatment of AGA in the USA.
Desmosomes are intercellular adhesive junctions that mediate cell-cell adhesion and anchor the intermediate filament network to the plasma membrane, thereby providing mechanical resilience to tissues such as the epithelial tissue. Desmosomes are expressed by epithelial cells and a few other cell types and are abundant in tissues under mechanical stress, such as the heart, skin, and hair follicles. In such desmosomal proteins, mutations to the desmoglein 3 (dsg3) and dsg4 genes have been reported in inherited hypotrichosis in both mouse and human studies [7–10]. On the other hand, loss of function of the DSG1 protein and dsg3 gene mutations were reported to be associated with alopecia because of the loss of hair anchoring by the mouse follicle [11, 12]. These reports demonstrated that desmosomal proteins, particularly DSGs, were important to protect against baldness by suppressing hair loss.
t-Flavanone is synthesized as a derivative of astilbin, a flavonoid component of hypericum extract that has been shown to actively stimulate hair growth. In our previous report, we described some biological activities of t-flavanone as a mechanism to promote hair growth [13]. In this report, we described the in vivo efficacy of t-flavanone with a blood circulation accelerator (carbon dioxide, CO2) on AGA, while focusing on the anchoring strength (tenacity) of hair. Concurrently, we evaluated DSG expression in t-flavanone-treated cultured human hair follicles in vitro as a mechanism to strengthen hair tenacity. The aim of the present study was to assess the efficacy of t-flavanone, originally developed as a component of cosmetic products for pattern baldness, in the treatment of AGA.
Methods
Materials
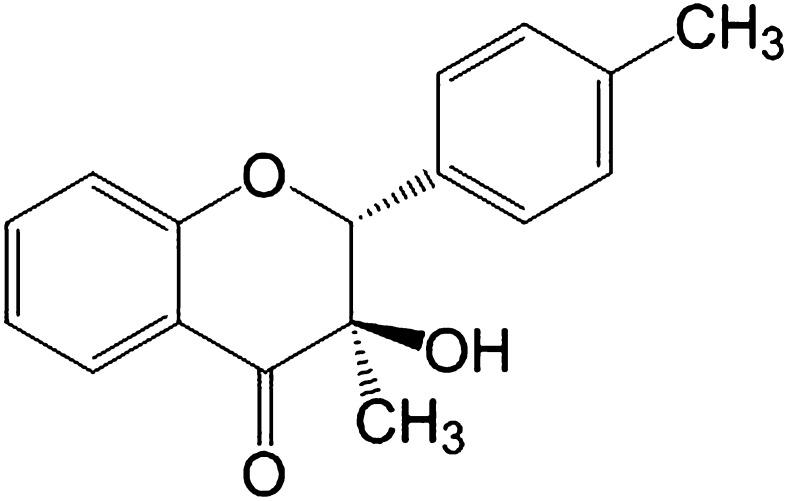
t-Flavanone (Fig. 1) was synthesized by Kao Corp. (Tokyo, Japan). Placebo lotion [57.5% (wt) ethanol–water, 0.15% (wt) l-menthol, and 2.2% (wt) CO2 with a trace amount of fragrance] and t-flavanone-containing lotion [0.1% or 0.3% (wt) t-flavanone in placebo lotion] were also formulated by Kao Corp.
Fig. 1.
Structure of t-flavanone
Human Study Design
The protocol of this randomized, placebo-controlled, double-blind human study was approved by the Institutional Review Board of Kao Corp. and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects, who were recruited in-house before enrollment. Eligible subjects who had mild to moderate hair loss were classified according to Ogata’s scale for male pattern alopecia as type II early, II middle, III early, and III middle [14] and could be categorized as type III vertex, IV, and V according to the Norwood-Hamilton scale [1]. Subjects were excluded if they had chronic dermatologic conditions of the scalp, other medical conditions that could affect hair growth, or any medical condition at the discretion of the investigators. Subjects with a history of hair transplantation or chemotherapy on the scalp within the past year as well as subjects with recent tattooing on the scalp were also excluded. Eighty-four men between the ages 20 and 60 years with mild to moderate AGA were initially enrolled at the Kao Wakayama plant. For the purpose of eliminating the effect of hair tonics that were used routinely, a control period of 8 weeks before baseline was set. The subjects were instructed to not apply any topical treatment or take any medications or supplements for hair loss, including finasteride, minoxidil, and hormonal products, during the control period and 30-week test period. After taking baseline measurements, the subjects were randomly assigned to one of three groups with equal average baldness: 0% (placebo), 0.1%, or 0.3% (wt) t-flavanone groups by the third person who did not contribute to the analyses of the results. Randomization was generated using a stratified randomization method. Approximately 2 ml of sample lotion was topically applied on the scalp twice a day over the test period, and compliance was assessed with a sample use diary. Seven subjects dropped out during the test period, and 27 refused to participate in a plucking test to assess hair tenacity. Finally, 77 subjects continued until the observational period, and 50 subjects completed the study.
Observations by the Parietal Hair and Plucking Test
At baseline and the end of the test period (week 30), a parietal global image and microscopic image of the target area (a square of approximately 0.7 × 0.7 cm) of each subject were taken using a standardized technique. At baseline, a target area was selected at the anterior edge of the bald area, and the position on the scalp was recorded to provide a stable reference point for photographic imaging throughout the test period. At baseline and week 30, the hair within the target area was clipped to approximately 1 mm in length, and a microscope image was captured 3 days later. These parietal global and microscopic images were used for assessments by a blinded investigator who rated the changes in scalp appearance from baseline to week 30 using a standardized 5-point rating scale as follows: very effective, effective, slightly effective, no change, and worse. Investigator assessments and a brief questionnaire were completed at week 30.
At week 30, the hair tenacity was electronically measured by the average peak force required for plucking out a single strand of hair in a non-bald area using the HF-1000N Digital Force Gauge Meter (Japan Instrumentation System Co., Ltd., Nara, Japan). Approximately ten strands of hair were measured, and the average hair tenacity was calculated for each subject. The diameter of each hair was also measured under a microscope at 100× magnification.
In Vitro Study
Human scalp tissue was obtained from the Skin Clinic group. This study was approved by the Institutional Review Board of Kao Corp. and was performed in accordance with the principles of the Declaration of Helsinki.
A scalp tissue sample (male; age 67 years) was sterilized by immersion in 0.1% (wt) chlorhexidine gluconate solution (Sumitomo Dainippon Pharma Co., Ltd., Tokyo, Japan) for 1 min. After washing with phosphate-buffered saline, the hair follicles were isolated under a stereoscopic microscope with tweezers and a scalpel in William E medium (Life Technologies, Carlsbad, CA, USA). The isolated hair follicles were individually cultured in 300 μl William E medium containing 2 mM l-glutamine (Life Technologies), 10 μg/ml insulin (Life Technologies), 40 ng/ml hydrocortisone (Sigma-Aldrich Corp., St. Louis, MO, USA), and 1% (vol) antibiotics-antimitotics (Life Technologies) in a 24-well plate at 37 °C in an atmosphere of 5% CO2. t-Flavanone was prepared at final concentrations of 0.01 and 0.1 μM with ethanol and then added to the medium at 0.1% (vol). An equal volume of ethanol was added to the medium as a vehicle control. After incubation for 48 h, the cultured hair follicles were examined.
The cultured hair follicles were individually homogenized and extracted in RIPA lysis buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) containing 1% (vol) protease inhibitor cocktail (Sigma-Aldrich Corp.). The lysates were centrifuged at 15,000 rpm at 4 °C for 10 min to remove insoluble contents. The individual or pooled protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 4–10% gradient gels (TEFCO Corp., Tokyo, Japan) and transferred to polyvinylidene fluoride membranes (Hybond P PDVF; GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK). After blocking with Tris-buffered saline with Tween 20 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) containing 5% non-fat milk (Wako Pure Chemical Industries, Ltd.) for 1 h at room temperature, the membranes were incubated at 4 °C overnight with the appropriate primary antibodies [rabbit anti-DSG1 (H-290), rabbit anti-DSG2 (H-145), rabbit anti-DSG3 (H-145), goat anti-DSG4 (C-17), and goat anti-actin (I-19); Santa Cruz Biotechnology, Inc.]. The proteins were visualized by enhanced chemiluminescence using horseradish peroxidase-conjugated anti-rabbit (Cell Signaling Technology, Danvers, MA, USA) or anti-goat (Santa Cruz Biotechnology, Inc.) immunoglobulin G. Band intensities were measured using the ImageQuant™ LAS 4000 biomolecular imager (GE Healthcare Life Sciences) and normalized to that of actin (n = 4–5).
Statistical Analyses
All results of the human study were statistically analyzed using the Mann-Whitney U test to identify the differences between the placebo and t-flavanone groups. The results of the in vitro study were statistically analyzed using Dunnett’s test to identify the differences between the vehicle control and t-flavanone groups. In each case, StatView for Windows version 5.0 (SAS Institute, Inc., Cary, NC, USA) was used, and a P value <0.05 denoted the presence of a statistically significant difference.
Results
t-Flavanone Improves AGA
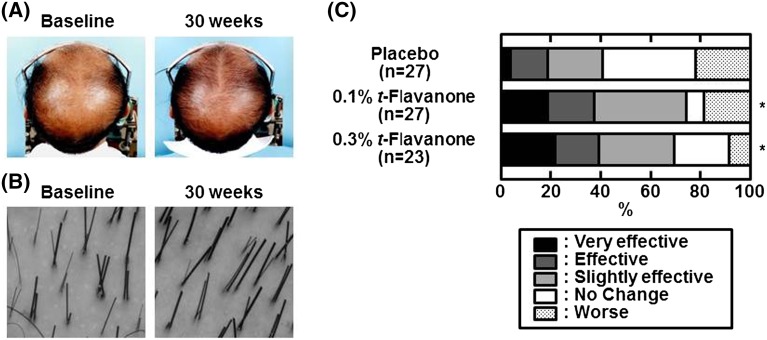
There were no significant differences in the usage of the sample lotions between the intervention and control groups (data not shown). As shown in Fig. 2, the representative parietal appearance and impression of magnified hair were improved in the 0.3% t-flavanone group, and comprehensive determination indicated significant effectiveness on AGA in the 0.1% and 0.3% t-flavanone groups (P < 0.05, respectively). However, in response to the questionnaire, many subjects in the intervention groups reported a reduction in hair loss (data not shown).
Fig. 2.
The efficacy of t-flavanone on AGA. Representative parietal global (a) and microscopic (b) images of a patient in the 0.3% (wt) t-flavanone group at baseline and week 30. Comprehensive evaluations were performed using these images, and the magnitude of improvement in each subject was classified according to five items (c). The results are expressed as the percentages of the existence ratio. Data are presented as the mean + SD values. *P < 0.05 vs. placebo group by the Mann-Whitney U test
t-Flavanone Increases Hair Tenacity
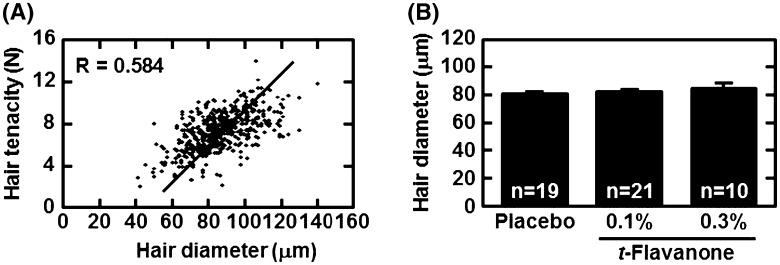
We confirmed a strong correlation between hair tenacity and diameter. Because they were strongly correlated (R = 0.584, Fig. 3a), we next analyzed the hair diameter in each group. The hair diameter was thicker in the t-flavanone treatment groups in a dose-dependent manner; however, there were no significant differences compared with the placebo group (Fig. 3b).
Fig. 3.
Correlation between hair tenacity and diameter. Hair tenacity in a non-bald area was measured using a digital force gauge at week 30. The diameter and tenacity of each hair are plotted (a), and the average of hair diameter in each group is indicated (b)
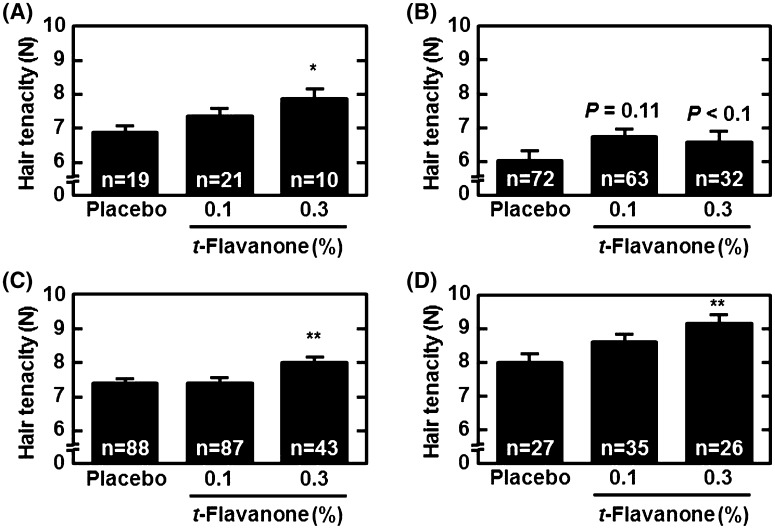
Next, we analyzed hair tenacity in each group. The average of the hair tenacity measurements in the 0.3% t-flavanone group was significantly greater than that in the placebo group (Fig. 4a, P < 0.05). Then, we divided the hair samples into three groups according to the diameters (60–80, 80–100, and 100–120 µm) and compared the average tenacity measurements among the groups (Fig. 4b–d, respectively). The results showed that even for hairs with the same diameter distribution, the hair tenacity was enhanced by t-flavanone (Fig. 4b–d), and hairs were significantly stronger in the 0.3% t-flavanone than in the placebo group (Fig. 4c, d, P < 0.01, respectively).
Fig. 4.
Effect of t-flavanone on hair tenacity. The average (a) and distribution of hair tenacity (b 60–80 μm, c 80–100 μm, and d 100–120 μm) are indicated. Data are presented as the mean + SD values. *P < 0.05 vs. placebo group using the Mann-Whitney U test
Mechanism Analysis of t-Flavanone on Enhancing the Hair Tenacity
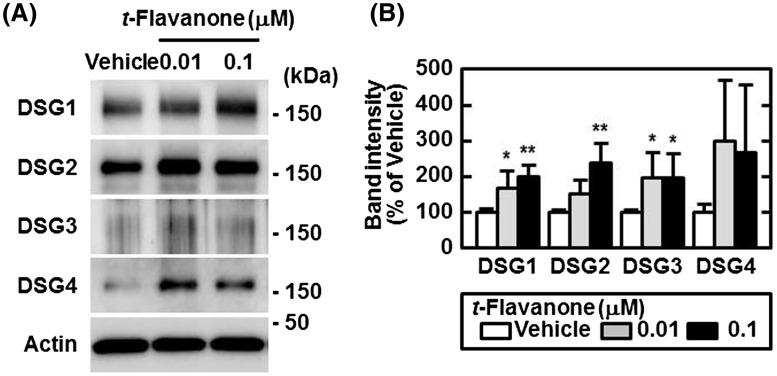
To analyze the mechanism of t-flavanone on enhancing hair tenacity, we evaluated the effect of t-flavanone on the expression of DSG proteins in cultured human hair follicles in vitro. As shown in Fig. 5, t-flavanone-treated hair follicles had the highest expression levels of DSG proteins, and expression levels of DSG1, DSG2, and DSG3 were significantly higher in the t-flavanone-treated than vehicle-treated hair follicles (P < 0.05 or P < 0.01).
Fig. 5.
t-Flavanone upregulated DSG protein expression in cultured human hair follicles. Surgically isolated human hair follicles were cultured with or without t-flavanone for 48 h. Expression levels of DSG and actin in pooled (a) and individual (b) samples were analyzed by Western blotting. Band intensities of DSGs were normalized to those of actin. The densitometry results are expressed as the percentage of the vehicle control. Data are presented as mean + SD values of four (0.01 and 0.1 μM t-flavanone groups) or five (vehicle group) experiments. *P < 0.05, **P < 0.01 vs. vehicle control using Dunnett’s test
Discussion
AGA is the most common type of hair loss among both men and women after puberty. Although AGA is not a serious health problem, the adverse effects of AGA are associated with psychological sequelae, principally a decrease in body image satisfaction [15]. The psychosocial impact of AGA is not insignificant, as suggested by the global popularity of hair growth stimulants. In view of the subjective nature of the effects of baldness, recent studies of hair growth products have focused on consumer self-perception of improvement rather than on objective measurement of hair counts [16]. Our observations showed that t-flavanone improved the parietal appearance of AGA (Fig. 2) as well as the subjective cognition of AGA (questionnaire responses, data not shown).
The results of comprehensive evaluations showed slight improvements in AGA in the placebo group (Fig. 2c). As reported previously, the subcutaneous blood flow in the early stage of male pattern baldness is significantly lower than that in normal subjects [17]. In addition, the external application of CO2 has been reported as a major accelerator of blood circulation [18, 19]. In such situations, we speculated that the weak effectiveness in the placebo group (Fig. 2c) was due to the effect of CO2 as a blood circulation accelerator. These data indicate that t-flavanone significantly improved AGA, and the combination with a blood circulation accelerator such as CO2 may lead to even better results.
Hair tenacity was previously correlated with hair diameter [20]. This report indicated the possibility that the increase in hair diameter was due to the application of t-flavanone, which could explain why hair tenacity became greater in the intervention than placebo group. Therefore, we verified the correlations in hair tenacity and diameter by comparing tenacity in the same distributions of hair diameter. As shown in Fig. 3a, the tenacity and diameter in each hair diameter group were strongly correlated. Moreover, t-flavanone increased hair tenacity, even among hairs in the same diameter distribution (Fig. 4b–d). These data suggested that the enhancement in hair tenacity by t-flavanone was not due to an increase in hair diameter. In other words, t-flavanone enhanced hair tenacity regardless of the hair diameter, possibly explaining one of the mechanisms by which t-flavanone improves AGA.
At the same time, our human study had some limitations to distinguish the patients with pure AGA from those with a combination of AGA and chronic telogen effluvium (CTE). CTE was reported as a female’s disease characterized by diffusible hair shedding [21]. Although the subjects observed in our study were males, we could not exclude the possibility that some had a combination of AGA with CTE. Recently, a method to distinguish patients with CTE was reported [22, 23]. In the near future, we believe these methods will resolve the above problem.
We previously reported that t-flavanone decreased the active form of transforming growth factor-β2 (TGF-β2) through the downregulation of urokinase-type plasminogen activator [13]. TGF-βs have been reported as strong inhibitors of hair growth [24, 25], and TGF-β2 was shown to induce the catagen phase in the hair cycle [26]. Thus, the suppression of TGF-β activity by t-flavanone effectively improves AGA. However, according to the plucking test results, we speculated that other mechanism(s) should exist that would improve AGA by t-flavanone. After measuring hair tenacity, we analyzed the mechanism of t-flavanone on the enhancement of hair tenacity using cultured human hair follicles in vitro and observed significant increases in the expression levels of DSG1, DSG2, and DSG3 following the application of t-flavanone (Fig. 5). DSG1 and DSG3 have been reported as the indicators of hair tenacity [11, 12]. These data indicated that t-flavanone enhanced cell-cell adhesions in the cultured human hair follicles in vitro, which presented a possible mechanism of hair tenacity enhancement by t-flavanone. On the other hand, DSG2 was reported to be less important for cell-cell adhesions than DSG3 [27], although the role of DSG2 in the hair follicle remains unclear. Another group predicted that the loss of DSG2 in the skin results in some structural problems in the hair follicle because DSG2 is expressed in the basal layer of the outer root sheath at the level of the bulge and below [28]. The role of DSG2 in the hair follicle is expected to be elucidated in the near future. Our analysis showed that DSG4 expression tended to be upregulated by t-flavanone, but not significantly (Fig. 5). Because DSG4 is critical to the presence of the hair shaft itself [9, 10], if t-flavanone upregulates DSG4 expression in the hair shaft, it may also influence other properties of the hair.
As described above, we aimed to identify the mechanisms of t-flavanone on hair growth [13]. The suppression of the TGF-β signal transduction pathway, including the activation of TGF-β itself, is a possible mechanism by which t-flavanone improves hair growth and prevents the progression of AGA, although t-flavanone is likely to induce other mechanisms as well. In this study, we focused on cell-cell adhesion-related factors in the hair follicle and found that t-flavanone upregulated DSG expression levels in cultured human hair follicles in vitro. Our results also suggested the enhancement of hair tenacity by t-flavanone in vivo. Hence, our data provided novel insight into the mechanisms by which t-flavanone improves AGA.
Conclusion
In this article, we aimed to identify the mechanisms of action of t-flavanone on hair growth. We observed that t-flavanone improved AGA and enhanced hair tenacity by upregulation of desmoglein protein expressions. Reinforcement of hair rooting may be a mechanism by which t-flavanone promotes hair growth.
Acknowledgments
We thank Dr. Ishii, Director of the Skin Clinic Group, for the human scalp tissue samples. Sponsorship and article processing charges for this study were funded by the Kao Corp. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Azumi Nagasawa, Etsuji Wakisaka, Hideshi Kidena, Tomoko Nomura, Mitsuyuki Hotta, Hiroyuki Taguchi, and Shigeru Moriwaki have nothing to disclose.
Compliance with Ethics Guidelines
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave their informed consent prior to inclusion in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975;68:1359–1365. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Olsen EA, Messenger AG, Shapiro J, et al. Evaluation and treatment of male and female pattern hair loss. J Am Acad Dermatol. 2005;52:301–311. doi: 10.1016/j.jaad.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann R. Male androgenetic alopecia. Clin Exp Dermatol. 2002;27:373–382. doi: 10.1046/j.1365-2230.2002.01086.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee WS, Lee HJ. Characteristics of androgenetic alopecia in asian. Ann Dermatol. 2012;24:243–252. doi: 10.5021/ad.2012.24.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergfeld WF. Alopecia: histologic changes. Adv Dermatol. 1989;4:301–320. [PubMed] [Google Scholar]
- 6.Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198:89–95. doi: 10.1016/S0303-7207(02)00372-6. [DOI] [PubMed] [Google Scholar]
- 7.Montagutelli X, Lalouette A, Boulouis HJ, Guénet JL, Sundberg JP. Vesicle formation and follicular root sheath separation in mice homozygous for deleterious alleles at the balding (bal) locus. J Invest Dermatol. 1997;109:324–328. doi: 10.1111/1523-1747.ep12335844. [DOI] [PubMed] [Google Scholar]
- 8.Pulkkinen L, Choi YW, Simpson A, et al. Loss of cell adhesion in Dsg3bal-Pas mice with homozygous deletion mutation (2079del14) in the desmoglein 3 gene. J Invest Dermatol. 2003;119:1237–1243. doi: 10.1046/j.1523-1747.2002.19645.x. [DOI] [PubMed] [Google Scholar]
- 9.Jahoda CA, Kljuic A, O’Shaughnessy R, et al. The lanceolate hair rat phenotype results from a missense mutation in a calcium coordinating site of the desmoglein 4 gene. Genomics. 2004;83:747–756. doi: 10.1016/j.ygeno.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Kljuic A, Bazzi H, Sundberg JP, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–260. doi: 10.1016/S0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 11.Koch PJ, Mahoney MG, Cotsarelis G, Rothenberger K, Lavker RM, Stanley JR. Desmoglein 3 anchors telogen hair in the follicle. J Cell Sci. 1998;111:2529–2537. doi: 10.1242/jcs.111.17.2529. [DOI] [PubMed] [Google Scholar]
- 12.Hanakawa Y, Li H, Lin C, Stanley JR, Cotsarelis G. Desmogleins 1 and 3 in the companion layer anchor mouse anagen hair to the follicle. J Invest Dermatol. 2004;123:817–822. doi: 10.1111/j.0022-202X.2004.23479.x. [DOI] [PubMed] [Google Scholar]
- 13.Sasajima M, Moriwaki S, Hotta M, Kitahara T, Takema Y. trans-3,4′-Dimethyl-3-hydroxyflavanone, a hair growth enhancing active component, decreases active transforming growth factor β2 (TGF-β2) through control of urokinase-type plasminogen activator (uPA) on the surface of keratinocytes. Biol Pharm Bull. 2008;31:449–453. doi: 10.1248/bpb.31.449. [DOI] [PubMed] [Google Scholar]
- 14.Ogata T. Origins of the baldness. Sougou rinsyou. 1953;2:101. [Google Scholar]
- 15.Cash TF. The psychological effects of androgenetic alopecia in men. J Am Acad Dermatol. 1992;26:926–931. doi: 10.1016/0190-9622(92)70134-2. [DOI] [PubMed] [Google Scholar]
- 16.Barber BL, Kaufman KD, Kozloff RC, Girman CJ, Guess HA. A hair growth questionnaire for use in the evaluation of therapeutic effects in men. J Dermatolog Treat. 1998;9:181–186. doi: 10.3109/09546639809160551. [DOI] [Google Scholar]
- 17.Klemp P, Peters K, Hansted B. Subcutaneous blood flow in early male pattern baldness. J Invest Dermatol. 1989;92:725–726. doi: 10.1111/1523-1747.ep12721603. [DOI] [PubMed] [Google Scholar]
- 18.Schnizer W, Erdl R, Schöps P, Seichert N. The effects of external CO2 application on human skin microcirculation investigated by laser Doppler flowmetry. Int J Microcirc Clin Exp. 1985;4:343–350. [PubMed] [Google Scholar]
- 19.Nishimura N, Sugenoya J, Matsumoto T, et al. Effects of repeated carbon dioxide-rich water bathing on core temperature, cutaneous blood flow and thermal sensation. Eur J Appl Physiol. 2002;87:337–342. doi: 10.1007/s00421-002-0626-0. [DOI] [PubMed] [Google Scholar]
- 20.Chase ES, Weinsier RL, Laven GT, Krumdieck CL. Trichotillometry: the quantitation of hair pluckability as a method of nutritional assessment. Am J Clin Nutr. 1981;34:2280–2286. doi: 10.1093/ajcn/34.10.2280. [DOI] [PubMed] [Google Scholar]
- 21.Rebora A. Telogen effluvium revisited. G Ital Dermatol Venereol. 2014;149:47–54. [PubMed] [Google Scholar]
- 22.Rebora A, Guarrera M, Baldari M, Vecchio F. Distinguishing androgenetic alopecia from chronic telogen effluvium when associated in the same patient: a simple noninvasive method. Arch Dermatol Am J Clin Nutr. 2005;141:1243–1245. doi: 10.1001/archderm.141.10.1243. [DOI] [PubMed] [Google Scholar]
- 23.Bittencourt C, Teixeira F, Ferraro DA, Soares TC, Moraes AM, Cintra ML. Non-invasive method distinguishes chronic telogen effluvium from mild female pattern hair loss: clinicopathological correlation. Int J Dermatol. 2015 doi: 10.1111/ijd.13200. [DOI] [PubMed] [Google Scholar]
- 24.Paus R, Foitzik K, Welker P, Bulfone-Paus S, Eichmüller S. Transforming growth factor-β receptor type I and type II expression during murine hair follicle development and cycling. J Invest Dermatol. 1997;109:518–526. doi: 10.1111/1523-1747.ep12336635. [DOI] [PubMed] [Google Scholar]
- 25.Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97:463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- 26.Soma T, Tsuji Y, Hibino T. Involvement of transforming growth factor-β2 in catagen induction during the human hair cycle. J Invest Dermatol. 2002;118:993–997. doi: 10.1046/j.1523-1747.2002.01746.x. [DOI] [PubMed] [Google Scholar]
- 27.Hartlieb E, Kempf B, Partilla M, Vigh B, Spindler V, Waschke J. Desmoglein 2 is less important than desmoglein 3 for keratinocyte cohesion. PLoS One. 2013;8:e53739. doi: 10.1371/journal.pone.0053739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Stanley JR, Cotsarelis G. Desmoglein isotype expression in the hair follicle and its cysts correlates with type of keratinization and degree of differentiation. J Invest Dermatol. 2003;120:1052–1057. doi: 10.1046/j.1523-1747.2003.12234.x. [DOI] [PubMed] [Google Scholar]