Abstract
Influenced by gravidity, bone tissue experiences stronger or lighter deformation according to the strength of the activities of daily life. Activities resulting in impact are particularly known to stimulate osteogenesis, thus reducing bone mass loss. Knowing how bone cells recognize the mechanical deformation imposed to the bone and trigger a series of biochemical chain reactions is of crucial importance for the development of therapeutic and preventive practices in orthopaedic activity. There is still a long way to run until we can understand the whole process, but current knowledge has shown a strong progression, with researches being conducted focused on therapies. For a mechanical sign to be transformed into a biological one (mechanotransduction), it must be amplified at cell level by the histological structure of bone tissue, producing tensions in cell membrane proteins (integrins) and changing their spatial structure. Such change activates bindings between these and the cytoskeleton, producing focal adhesions, where cytoplasmatic proteins are recruited to enable easier biochemical reactions. Focal adhesion kinase (FAK) is the most important one being self-activated when its structure is changed by integrins. Activated FAK triggers a cascade of reactions, resulting in the activation of ERK-1/2 and Akt, which are proteins that, together with FAK, regulate the production of bone mass. Osteocytes are believed to be the mechanosensor cells of the bone and to transmit the mechanical deformation to osteoblasts and osteoclasts. Ionic channels and gap junctions are considered as intercellular communication means for biochemical transmission of a mechanical stimulus. These events occur continuously on bone tissue and regulate bone remodeling.
Keywords: Mechanotransduction cellular, Osteogenesis, Stress mechanical, Weight-bearin, Osteocytes, Osteoblasts, Gap junctions, Ion channels
INTRODUCTION
Bone mass maintenance is regulated by various stimuli, which can be grouped into the biochemical (growth factors and hormones) and the mechanical. Regarding the latter, it is known that prolonged immobilization and situations that reduce gravity cause a reduction in bone mass, whereas the impact on bone tissue caused by physical exercise, for example, increases bone mass1, 2, 3, 4, 5, 6, 7, 8, 9, 10.
Regardless of the type of mechanical stimulation (low-power ultrasound, fluid flow, centrifugation, applied static load, vibration, or electromagnetic field), it is recognized by the bone cells after a process called mechanotransduction that is responsible for producing biochemical reactions from a mechanical (physical) phenomenon, determining a cellular response, which may be bone growth or bone resorption(8), 10, 11, 12, 13. In this manuscript, we discuss the phenomena and theories about this partially understood area of orthopedics.
1. Amplification of the mechanical stimulus
The primary function of bone tissue is to bear the load of the body. According to Wolff's law cited in Duncan and Turner(8), this tissue is able to adapt to mechanical stresses produced by the weight of the individual and the physical activities that cause deformation of the entire skeleton. Typically, the deformation suffered by bone tissue during locomotion varies from 0.04 to 0.3% and rarely exceeds 0.1%14, 15. However, in vitro studies have shown that the deformation needed for bone cells to respond to mechanical stimulation is 10 to 100 times greater than that required for bone tissue as a whole (1-10%). If the same relative deformation (strain) used to stimulate bone cells were used in bone tissue, it would fracture8, 16. This apparent contradiction between stimulation on the macroscopic level and the microscopic (cellular) level was explained and justified by the experimental mathematical model developed by You et al.(16), in which the canalicular system in which bone cells (osteocytes) are inserted serves as an amplifier of mechanical deformation generated by physical activity.
1.1. Bone histological anatomy
The structure of long bones can be understood schematically as a cylinder, containing within it a number of cylinders, the Haversian canals, which communicate with each other through the Volkmann's canals. The walls that form the Haversian canals are arranged radially and are called lamellae. These consist of bone extracellular matrix (ECM), which consists mainly of hydroxyapatite (inorganic component) and type I collagen (organic component). The bone ECM forms a structure that traps the osteocytes in lacunae within the lamellae. The osteocytes have extensions of their cytoplasm, called cytoplasmic processes (or dendrites), enveloped by canaliculi (Figure 1). Between the canalicular wall and cytoplasmic processes, there is the pericellular space, permeated by a fluid. In the pericellular space there is the pericellular organic matrix (POM) supported by transverse fibrils that anchor and center the cytoplasmic processes of osteocytes in their canaliculi16, 18 (Figure 2).
Figure 1.

Bone histological anatomy
Figure 2.
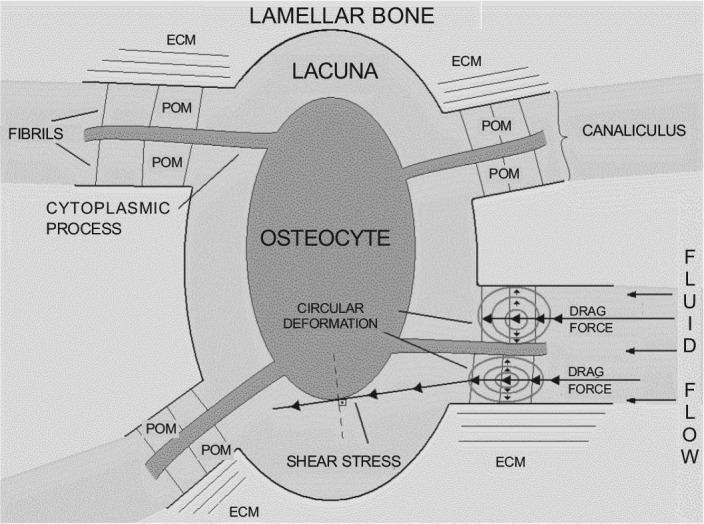
Amplification of the mechanical stimulus. The mechanical deformation of bone tissue produces fluid flow, which, at the level of bone canaliculi, exerts drag force on the cytoplasmic processes and the walls of bone canaliculi. In addition, the fluid flow exerts a tangential force on the plasma membrane of osteocytes, producing shear stress
1.2. Drag force and shear stress
Put simply, when the bone is deformed, it creates a pressure gradient in the complex system of cylinders, resulting in fluid flow within the pericellular space of cytoplasmic processes, exerting drag force on the POM. The drag force is the result of the frictional force and pressure on a body that moves in a liquid medium. In this case, what moves is the liquid (bone fluid) over the POM, but the drag force is produced in the same way.
The histological structure of bone allows the drag force to produce circular deformations (hoop strains) in the membrane-cytoskeleton system of the cell processes of osteocytes, deformations which are 20 to
100 (or more) times greater than the deformation of bone tissue as a whole. Circular deformations are the normal stresses (i.e., stress that forms a 90° angle to the tangent) to a body with circular symmetry. In other words, circular deformations produce compression and traction forces on the tissue and the greater the magnitude or frequency of the stimulus, the greater the amplification of deformation (Figure 2).
The shear stress (or tangential stress) is the deformation that a body undergoes when subjected to the action of shear (tangential) forces. While You et al.(16) did not consider shear stress to be responsible for amplifying the deformation of bone tissue at the cellular level, in the view of several authors, its importance should not be ignored8, 10, 11, 12, 16, 19, 20, 21. The two types of cell deformation likely contribute to the cellular mechanical stimulus (Figure 2).
2. The mechanosensory cell
There are three main types of cells in bone tissue: osteoblasts, osteocytes, and osteoclasts. For decades, osteoblasts and osteoclasts were considered the protagonists of bone remodeling and have therefore been studied much more than osteocytes, which account for 90-95% of all bone cells. While investigating the behavior of osteocytes, it has been observed that they, like osteoblasts, also respond to mechanical stimulation, displaying the activation of basically the same proteins as osteoblasts17, 22. Since then the question has been: what is the main mechanosensor of bone tissue, the osteoblast or the osteocyte?
Although there is no definitive scientific evidence, it has been widely reported that the osteocytes are the cells that orchestrate bone remodeling8, 22, 23, 24, releasing biochemical mediators that regulate the activity of osteoblasts and osteoclasts. In support of this theory, it has been observed that osteocytes produce prostaglandins-mediators of osteoblast and osteoclast activity-faster than the osteoblasts after mechanical stimulation(25), and that osteocytes subjected to fluid flow stimulate the osteogenic activity of osteoblasts(26). It is expected that osteoblasts are sensitive to mechanical stimulation, since osteocytes are derived from these cells. In addition, bone cells are not the only ones capable of mechanotransduction. Fibroblasts(27), chondrocytes(28), cardiomyocytes(29), endothelial cells(30), and rhabdomyocytes(31) also have this capability. However, for the scientific community, it is more logical that osteocytes are more utilized as mechanosensors in bone tissue than osteoblasts because they are part of the canalicular system of mechanical deformation amplification, while osteoblasts are located on the periphery of the bone tissue, in the periosteum.
3. Piezoelectricity
The piezoelectric effect is a biological response to mechanical stimulation that has been known for a long time, documented by Fukada and Yasuda in 1957 after observing the production of a negative electrical charge in areas of bone compression and a positive charge in the areas of traction(32). This effect was reproduced and measured by other authors, such as Butcher et al.(33) and Qin et al.(34). The hypothesis for this phenomenon was reported by Duncan and Turner(8), who believed that the fluid flow generated by mechanical stresses produced power currents (streaming potentials), modulating the cellular response. Currently, it is known that the piezoelectric effect is not mechanotransduction: it is only a marker of fluid flow. This causes the activation of mechanosensitive ion channels, especially potassium and calcium ion channels, inducing ion flux in bone cells, resulting in a change in the cell membrane potential, which may be positive (depolarization) or negative (hyperpolarization). The bone cell can quickly identify the characteristics of mechanical stimuli and respond electrophysiologically in different ways, with varying degrees of ion channel activation, resulting in the hyperpolarization or depolarization of the plasma membrane. What determines the intensity of the activation of ion channels is unclear, but it is known that the intensity and frequency of mechanical stimulation, as well as the velocity of fluid flow, regulate the activation of these channels. It is known that hyperpolarization is associated with osteogenesis, and depolarization with bone resorption. In addition to regulating the cellular response to mechanical stimulation, it is believed that activation of ion channels biochemically transmits the mechanical deformation to neighboring osteocytes, osteoblasts, and osteoclasts12, 33, 34, 35, 36, 37, 38, 39.
4. Mechanotransduction
Mechanotransduction can be interpreted as the process of producing a biochemical reaction from a mechanical stimulus. The biochemical chain reactions induced by mechanical stimuli act at the cellular level and can cause inhibition of apoptosis, increased cell proliferation, altered cell migration, among other effects.
When an external mechanical stimulus deforms bone tissue, circular deformation and shear stress occur at the cellular level, acting on the plasma membrane of osteocytes, and are transmitted throughout the cell through a complex network that connects the plasma membrane to the cell nucleus, called the integrin-cytoskeleton-nucleus extracellular (and pericellular) matrix system. Duncan and Turner(8) devised a model of mechanotransduction in which this system is critical; the mechanical stress causes strain between the constituents of this system, allowing for the transmission of deformation from the ECM to the nucleus of cells. Elaborating on this model in light of current knowledge, the authors of this review believe that its operation is like a lever system with multiple pivot points, which represent the interactions between the molecules that comprise the extracellular/pericellular matrix (collagen, fibronectin, and fibrils), the different subunits of integrins, the cytoskeleton (actin, vinculin, talin, paxillin, and α-actinin), and the nuclear membrane8, 17, 18. Therefore, the pivot points can be tailored to the type and quantity of molecules interacting with each other, which allows for various types of transmission of mechanical deformation.
4.1. Integrins
In each region of the ECM/POM-integrin-cytoskeleton-nucleus system, proteins are interacting with their constituents. The integrin region is the most understood, and is considered the most important for mechanotransduction. The name integrin refers to its function of integrating the interior of the cell (cytoskeleton) to its exterior (ECM and POM). Integrins are heterodimeric transmembrane cell adhesion glycoproteins composed of a and p subunits. In humans, there are 24 well-established types of integrins, resulting from combinations of the 18 different a subunits and eight types of p subunits. The different combinations of a and p subunits determine the specificity of integrin binding to ECM components and of the cytoskeleton and cytoplasmic proteins, as well as the degree of affinity for each ligand. Osteoblasts express the subunits α2, α3, α4, α5, αv, α6, β1, β3, and β5 in vitro40, 41, whereas in vivo, the expression of integrins is restricted primarily to α3, α5, αv, β1, and β3(42). In these cells, the subunits that are known to respond to mechanical stimuli are α2, α5, β1, and β3, and the heterodimers that have been demonstrably involved in mechanotransduction are α5β1 and αvβ3.
It is believed that after the mechanical deformation of the plasma membrane, an integrin conformational change occurs, creating high-affinity sites for chemical reactions in its chemical structure resulting in connections with other integrins and components of the cytoskeleton. In other words, mechanical stimulation functions as an enzyme that catalyzes a biochemical reaction. Links between various integrins form clusters that increase the avidity of these proteins to bind to other molecules. The clusters of integrins anchor to components of the cytoskeleton, inducing its remodeling, forming a specialized structure called focal adhesion (or focal contact). This structure is dynamic, as it forms in response to mechanical stimulation and breaks down in response to the absence of that stimulus. It is located near the plasma membrane and the ECM, and it recruits several molecules involved in mechanotransduction: tyrosine kinases, ion channels, phospholipase C, and mitogen-activated protein kinase (MAPK), among others11, 12, 43, 44, 45, 46.
4.2. Focal adhesion kinase (FAK) pathways
Of all the molecules that interact with integrins in focal adhesions, the one that is most studied and considered essential in the conversion of mechanical phenomena to biochemical phenomena is focal adhesion kinase (FAK), an adapter protein of the tyrosine kinase group, the structure of which allows it to interact with several proteins, allowing for the formation of multiple protein complexes. This feature can increase cellular response if the activity of the multiple complexes is additive, or can induce different types of cellular response if the activity of the different complexes is on different paths.
It has not been well established whether FAK is always connected to integrins, or whether it is recruited by integrins when they form the focal adhesions. The authors of this manuscript believe that the two forms exist and have their roles. After the deformation of integrins, the FAK must subsequently be deformed, also undergoing changes in its structure, resulting in autophosphorylation at tyrosine residue 397 (Tyr-397). This activates FAK, with the creation of a high-affinity site for binding to proteins containing the SH2 domain (Src-homology-2), such as Src and the p85 subunit of phosphatidylinositol 3-kinase (PI3K). In response, the activated FAK combines with Src or with PI3K, activating FAK-Src/Grb2/Sos/Ras-Raf/MEK/ERK-1/2 and FAK/PI3K/Akt/ pathways, respectively. The extracellular signal-regulated kinase-1/2 (ERK-1/2) and Akt are effector proteins of these pathways and may exercise their functions in both the cytoplasm and the nucleus of bone cells. What determines whether these proteins, including FAK, are in the nucleus or cytoplasm is not well established, but research suggests that this is determined by the amount of protein and the amount of activated protein. It has been speculated that nuclear localization involves the increased effects of these proteins (FAK, ERK-1/2 and Akt), which may be: induction of migration, cell proliferation and differentiation, and inhibition of apoptosis8, 10, 20, 30, 47, 48, 49, 50, 51, 52, 53, 54 (Figure 3).
Figure 3.

FAK-dependent mechanical stimulus signaling in bone cells. The FAK maintains a close relationship with the and subunits of integrins and cytoskeletal components (actin, paxillin, talin, and vinculin), which are also in contact with the nucleus
4.3. Biochemical transmission of the mechanical stimulus to other cells
Even the cells that are not deformed by mechanical force exhibit a biochemical response similar to that of those that were deformed. In these cases, the phosphorylation (activation) of connexin 43 was observed, which is responsible for activating the gap junctions, the intercellular communication structures located in the cytoplasmic processes of the osteocytes. Gap junctions allow for the exchange of ions and small molecules, particularly prostaglandin E2 (PGE2), between osteocytes and between osteocytes and osteoblasts.
The activation of ion channels should also contribute to the biochemical transmission of mechanical stimulation. The mechanical deformation causes activation and inactivation of ion channels, especially calcium and potassium channels, which generates a negative action potential, with membrane hyperpolarization, which is transmitted to neighboring cells, activating intracellular reactions26, 35, 37, 55, 56.
Although intriguing, this subject has gone largely unexplored, with current research focused primarily on the integrin pathway.
4.4. In vivo mechanotransduction
Most studies have been based on in vitro experimental models11, 16, 19, 21, 23, 25, 26, 35, 46. Despite the undeniable contribution of these studies in guiding subsequent research, the limitations of an in vitro study when compared with reality, that is, an in vivo study, should be considered57, 58. The beneficial effect of low-power ultrasound (lpUS) in accelerating bone healing in bones with some type of injury has been documented59, 60, 61, 62, 63, 64, 65, 66.
The authors of this review have developed a rat model to assess the “molecular” effect of 20 minutes of daily treatment with lpUS in the intact tibias of these animals. The tibias were stimulated for at least one week. After treatment, bone proteins involved in mechanotransduction (FAK, ERK-1/2, and Akt) were measured. It was observed that long-term treatment with lpUS increased synthesis of FAK and ERK-1/2 in a non-cumulative manner, that is, at a certain point, the synthesis of these proteins stopped increasing, as if something had blocked the effects of lpUS. The activation of FAK, ERK-1/2, and Akt occurred early and, at times, was sustained for 15 hours (only FAK and ERK-1/2), unlike what occurs when applying a single stimulus. The finding of a greater amount of insulin receptor substrate-1 (IRS-1), a protein activated by growth factors and hormones in one of the stimulated groups, suggests that the lpUS interferes with cellular reactions mediated by growth factors, which is a controversial issue in in vitro models that has been little investigated. In addition, increasing the amount and activation of FAK in the bones of animals stimulated with the lpUS equipment turned “off”, although lower than in animals stimulated with the equipment turned on, indicates that “muscular” stress interferes with the activity of mechanosensitive proteins in “bone” tissue67, 68.
FINAL CONSIDERATIONS
Mechanotransduction is a phenomenon that is constantly occurring in bone tissue, with important clinical implications that should be acknowledged. However, because it is a complex phenomenon that involves several types of molecules arranged in multiple systems that interact with each other, it is still poorly understood. The detailed study of all the phases involved in the chain of molecular events is essential to understanding its entirety and to act systematically in this process, which apparently remains very complex.
Footnotes
Study conducted at the Labimo-Biomaterials Laboratory of the Center for Experimental Medicine and Surgery, Faculdade de Ciencias Médicas, Universidade Estadual de Campinas (UNICAMP).
REFERENCES
- 1.Dauty M, Verbe BP, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27(2):305–309. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 2.Epstein S, Inzerillo AM, Caminis J, Zaidi M. Disorders associated with acute rapid and severe bone loss. J Bone Miner Res. 2003;18(12):2083–2094. doi: 10.1359/jbmr.2003.18.12.2083. [DOI] [PubMed] [Google Scholar]
- 3.Frey-Rindova P, de Bruin ED, Stüssi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38(1):26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- 4.LeBlanc A, Shackelford L, Schneider V. Future human bone research in space. Bone. 1998;22(5 Suppl.):113S–116S. doi: 10.1016/s8756-3282(98)00013-1. [DOI] [PubMed] [Google Scholar]
- 5.Vico L, Lafage-Proust MH, Alexandre C. Effects of gravitational changes on the bone system in vitro and in vivo. Bone. 1998;22(5 Suppl.):95S–100S. doi: 10.1016/s8756-3282(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 6.Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355(9215):1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 7.Bailey DA, Mckay HA, Mirwald RL, Crocker PRE, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14(10):1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 8.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tiss Int. 1995;57(5):344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 9.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 10.Hughes-Fulford M. Signal transduction and mechanical stress. Sci STKE. 2004;(249):RE12. doi: 10.1126/stke.2492004re12. [DOI] [PubMed] [Google Scholar]
- 11.Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and prolinerich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279(29):30588–30599. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- 12.Scott A, Khan KM, Duronio V, Hart DA. Mechanotransduction in human bone in vitro cellular physiology that underpins bone changes with exercise. Sports Med. 2008;38(2):139–160. doi: 10.2165/00007256-200838020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomura S, Takano-Yamamoto T. Molecular events caused by mechanical stress in bone. Matrix Biology. 2000;19(2):91–96. doi: 10.1016/s0945-053x(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 14.Fritton SP, Kenneth JM, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33(3):317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 15.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):271–280. [PubMed] [Google Scholar]
- 16.You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–1386. doi: 10.1016/s0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- 17.Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr Course Lect. 1996;45:371–386. [PubMed] [Google Scholar]
- 18.Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. II: Formation, form, modeling, remodeling, and regulation of cell function. Instr Course Lect. 1996;45:387–399. [PubMed] [Google Scholar]
- 19.Kapur S, Baylink D, Lau K-HW. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32(3):241–251. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- 20.Liedbert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Comm. 2006;349(1):1–5. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 21.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces β-catenin signaling in osteoblasts. Calcif Tiss Int. 2004;75(5):396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 22.Bonewald LF. Mechanosensation and transduction in osteoblasts. Bonekey Osteovision. 2006;3(10):7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherian PP, Siller-Jakson AJ, Gu S, Wang X, Bonewald LF, Sprague E. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;(16)7:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289(3)::633–:643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 25.Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9(5):441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 26.Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292(1):C545–C552. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Yang G, Khan M, Stone D, Woo SL-Y, Wang JH-C. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med. 2004;32(2):435–440. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- 28.Hsu H-C, Fong Y-C, Chang C-S, Hsu C-J, Hsu S-F, Lin J-G. Ultrasound induces cyclooxygenase-2 expression through integrin, integrin-linked kinase, Akt, NF-KB and p300 pathway in human chondrocytes. Cell Signal. 2007;19(11):2317–2328. doi: 10.1016/j.cellsig.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res. 2006;72(3):375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Chen K-D, Li Y-S, Kim M, Li S, Yuan S, Chien S. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274(26):18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 31.Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194(3):323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukada E, Yasuda I. On the piezoeletric effect of bone. J Phys Soc Japan. 1957;12(10):1158–1162. [Google Scholar]
- 33.Butcher MT, Espinoza NR, Cirilo SR, Blob RW. In vivo strains in the femur of river cooter turtles (Pseudemys concinna) during terrestrial locomotion: tests of force-platform models of loading mechanics. J Exp Biol. 2008;211(Pt 15):2397–2407. doi: 10.1242/jeb.018986. [DOI] [PubMed] [Google Scholar]
- 34.Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng. 2002;30(5):693–702. doi: 10.1114/1.1483863. [DOI] [PubMed] [Google Scholar]
- 35.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20(1):41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riddle RC, Donahue HJ. From streaming-potentials to shear stress: 25 years of bone cell mechanotransduction. J Orthop Res. 2009;27(2):143–149. doi: 10.1002/jor.20723. [DOI] [PubMed] [Google Scholar]
- 37.Salter DM, Robb JE, Wright MO. Electrophysiological responses of human bone cells to mechanical stimulation: evidence for specific integrin function in mechanotransduction. J Bone Miner Res. 1997;12(7):1133–1141. doi: 10.1359/jbmr.1997.12.7.1133. [DOI] [PubMed] [Google Scholar]
- 38.Salter DM, Wallace WH, Robb JE, Caldwell H, Wright MO. Human bone cell hyperpolarization response to cyclical mechanical strain is mediated by an interleukin-1 beta autocrine/paracrine loop. J Bone Miner Res. 2000;15(9):1746–1755. doi: 10.1359/jbmr.2000.15.9.1746. [DOI] [PubMed] [Google Scholar]
- 39.Yokota H, Tanaka SM. Osteogenic potentials with joint-loading modality. J Bone Miner Metab. 2005;23(4):302–308. doi: 10.1007/s00774-005-0603-x. [DOI] [PubMed] [Google Scholar]
- 40.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res. 1997;12(8):1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- 41.Sinha RK, Tuan RS. Regulation of human osteoblast integrin expression by orthopaedic implant materials. Bone. 1996;18(5):451–457. doi: 10.1016/8756-3282(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 42.Bennett JH, Carter DH, Alavi AL, Beresford JN, Walsh S. Patterns of integrin expression in a human mandibular explant model of osteoblast differentiation. Arch Oral Biol. 2001;46(3):229–238. doi: 10.1016/s0003-9969(00)00114-x. [DOI] [PubMed] [Google Scholar]
- 43.Van Der Flier A, Sonnenberg A. Functions and interactions of integrins. Cell Tiss Res. 2001;305(3):285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 44.Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Salter DM. Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and beta-catenin in human articular chondrocytes after mechanical stimulation. J Bone Miner Res. 2000;15(8):1501–1509. doi: 10.1359/jbmr.2000.15.8.1501. [DOI] [PubMed] [Google Scholar]
- 45.Pommerenke H, Schmidt C, Dürr F, Nebe B, Lüthen F, Muller P. The mode of mechanical integrin stressing controls intracellular signaling in osteoblasts. J Bone Miner Res. 2002;17(4):603–611. doi: 10.1359/jbmr.2002.17.4.603. [DOI] [PubMed] [Google Scholar]
- 46.Tang CH, Yang RS, Huang TH, Lu DY, Chuang WJ, Huang TF. Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, FAK, phosphatidylinositol 3-kinase and Akt pathway in osteoblasts. Mol Pharmacol. 2006;69(6):2047–2057. doi: 10.1124/mol.105.022160. [DOI] [PubMed] [Google Scholar]
- 47.Cornillon J, Campos L, Guyotat D. Focal adhesion kinase (FAK), a multifunctional protein. Med Sci (Paris) 2003;19(6-7):743–752. doi: 10.1051/medsci/20031967743. [DOI] [PubMed] [Google Scholar]
- 48.Giancotti FG, Rouslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 49.Guan J. Focal adhesion kinase in integrin signaling. Matrix Biol. 1997;16(4):195–200. doi: 10.1016/s0945-053x(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 50.Kawamura N, Kugimiya F, Oshima Y, Ohba S, Ikeda T, Saito T. Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS One. 2007;2(10):e1058. doi: 10.1371/journal.pone.0001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nature. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 52.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116(Pt 8):1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 53.Raucci A, Bellosta P, Grassi R, Basilico C, Mansukhani A. Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J Cell Physiol. 2008;215(2):442–451. doi: 10.1002/jcp.21323. [DOI] [PubMed] [Google Scholar]
- 54.Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18(5):2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donahue HJ. Gap junctions and biophysical regulation of bone cell differentiation. Bone. 2000;26(5):417–422. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 56.Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–1462. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AIUM Mechanical bioeffects from diagnostic ultrasound: AIUM consensus statements. American Institute of Ultrasound in Medicine. J Ultrasound Med. 2000;19(2):68–168. doi: 10.7863/jum.2000.19.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WFUMB World Federation for Ultrasound in Medicine and Biology Symposium on safety of ultrasound in medicine. Ultrasound Med Biol. 1998;24(Suppl 1):S1–58. [PubMed] [Google Scholar]
- 59.Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res. 2001;16(4):671–680. doi: 10.1359/jbmr.2001.16.4.671. [DOI] [PubMed] [Google Scholar]
- 60.Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg. 1983;101(3):153–159. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- 61.Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76(1):26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Jingushi S, Mizuno K, Matsushita T, Itoman M. Low-intensity pulsed ultrasound treatment for postoperative delayed union or nonunion of long bone fractures. J Orthop Sci. 2007;12(1):35–41. doi: 10.1007/s00776-006-1080-3. [DOI] [PubMed] [Google Scholar]
- 63.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 1997;79(7):961–973. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Mayr E, Frankel V, Rüter A. Ultrasound-an alternative healing method for nonunions? Arch Orthop Trauma Surg. 2000;120(1-2):1–8. doi: 10.1007/pl00021234. [DOI] [PubMed] [Google Scholar]
- 65.Shimazaki A, Inui K, Azuma Y, Nishimura N, Yamano Y. Low intensity pulsed ultrasound accelerates bone maturation in distraction osteogenesis in rabbits. J Bone Joint Surg Br. 2000;82(7):1077–1082. doi: 10.1302/0301-620x.82b7.9948. [DOI] [PubMed] [Google Scholar]
- 66.Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM, Greenleaf JF. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthopc Res. 1994;12(1):40–47. doi: 10.1002/jor.1100120106. [DOI] [PubMed] [Google Scholar]
- 67.Gusmão CVB, Pauli JR, Belangero WD. Efeito do ultra-som de baixa potência na expressão da FAK (focal adhesion kinase), ERK-2 (extracellular signal-regulated kinase-2) e IRS-1 (insulin receptor substrate-1) no osso in vivo. In: Anais do XVI Congresso Médico Acadêmico da Unicamp; 2007.
- 68.Gusmão CVB, Pauli JR, Belangero WD. O ultra-som de baixa potência altera a cinética da FAK (focal adhesion kinase), ERK-1/2 (extracellular signal-regulated kinase-1/2) e Akt em tíbias de ratos sem fratura. In: Anais do XVII Congresso Médico Acadêmico da Unicamp; 2008.


