Abstract
Objective: To evaluate the resistance to femoral fractures among rats treated with the immunosuppressant tacrolimus FK-506 and compare these to untreated rats and rats treated with placebo. Methods: Ninety male Wistar rats were used. The animals were nine weeks old and weighed between 220 g and 280 g. The immunosuppressive agent tacrolimus was used in this study at a dose of 2 mg/kg/day, administered orally. The suspension was administered using an insulin syringe, and the maintenance therapeutic dose was sufficient to maintain the immunosuppressive activity. The animals were randomly divided into three groups (n = 30): group 1, no substance administered; group 2, administration of the immunosuppressant tacrolimus FK-506; and group 3, administration of the vehicle alone. Treatment with FK-506 was administered for 28 days. Total leukocyte counts and differential counts (lymphocytes, monocytes, eosinophils and neutrophils) were evaluated in order to monitor the immunosuppressive effect. Bone densitometry analysis by means of dual-energy x-ray absorptiometry (DXA) was also performed before and after administration of the drug. To evaluate the resistance to flexion, a support device was developed so that mechanical tests using an EMIC universal testing machine could be carried out. Results: The results from the flexion resistance tests showed statistical differences between groups 1 and 2 (p = 0.001) and between groups 2 and 3 (p = 0.001). No statistical difference was found between groups 1 and 3 (p = 0.995). Conclusions: The femurs of rats treated with the immunosuppressive agent had lower mechanical strength than did those of normal rats and those that received placebo.
Keywords: Femoral fractures, Densitometry, Tacrolimus
INTRODUCTION
Osteoporosis is a systemic disease characterized by diminution of the bone mass per unit volume, without any significant reduction in the ratio between the mineral and organic phases, or any qualitative changes in the matrix(1). It is associated with changes to bone microarchitecture and results in increased fragility of the skeleton, with a heightened risk of occurrences of fractures caused by minimal trauma1, 2, 3. Bone in situations of osteoporosis has normal biological behavior regarding osteoid mineralization. What occurs is disequilibrium between reabsorption (osteoclastic activity) and formation (osteoblastic activity)(1).
Although the commonest idea is that osteoporosis primarily affects postmenopausal women, other populations are also at high risk. One of these groups is formed by organ transplant recipients. The longer these patients survive, the longer their exposure will be to be risks of fractures(4). Over recent decades, transplantation has become an effective therapy for end-stage kidney, liver, heart and lung diseases. Brazil has accompanied this progression in the number of transplantations(5).
The survival following transplantation ranges from 50% of lung recipients after five years to 74% of kidney recipients from identical HLA live donors after 10 years(5).
Calcineurin-phosphatase inhibitors (cyclosporin and tacrolimus) are partly responsible for the greater survival of transplanted patients and for reductions in glucocorticoid levels. However, like glucocorticoids, calcineurin-phosphatase inhibitors also cause diminution of bone mass, and the greatest bone loss occurs during the first six months following transplantation, at the time when the immunosuppressive therapy is most aggressive(6). Despite the current trend towards using lower total doses of immunosuppressants, many patients with transplants still develop fractures as a complication5, 6.
The incidence of fractures is lower following kidney transplantation (7% to 11% among non-diabetic kidney recipients) and higher among the recipients of other organs: 17.2% to 42% after liver transplantation, 18% to 50% after heart transplantation and 25% to 29% after lung transplantation(4).
However, the estimates regarding the prevalence or incidence of fractures following transplantation vary greatly. This perhaps reflects factors such as differences in patient selection, immunosuppressant regimens and criteria used for diagnosing vertebral fractures(5).
The aim of the present study was to evaluate the resistance to fracturing of femurs in rats that were treated with the immunosuppressant tacrolimus FK-506, in comparison with untreated rats and rats treated with placebo.
METHODS
Animals used
Ninety nine-week-old male Wistar rats (Rattus norvegicus) weighing 220 to 280 g were used. The rats were provided by Cecal (Fiocruz, RJ) and were kept in cages (three animals per cage), in an air-conditioned environment at a temperature of 19 to 22°C and humidity of 40% to 50%, with light/dark cycles of 12 hours each. The animals were fed with granulated feed (NuvilabCR1, Nuvital, Curitiba, Paraná, Brazil) and non-gassy water, in accordance with the guidance for animal research of the Osvaldo Cruz Foundation (Fiocruz), RJ.
MEDICATION
The medication used in this study was the immunosuppressant tacrolimus (PROGRAF®, Astellas, Ireland), administered orally (intragastrically) at a dose of 2.0 mg/kg/day (maintenance dose) in a volume of 2 ml/kg/day, as a suspension in water and 5% dextrose capsules (PROGRAF® 5 mg capsules, Astellas, Ireland) over a 28-day experimentation period. The suspension was administered with the aid of an insulin syringe (Plastipak® 1 ml, Becton Indústria Cirúrgica Ltda., Brazil). The animals' weight was measured at the time of each application and the dose was adjusted as required. The animals were divided randomly into three groups:
Group 1: no substance administered;
Group 2: administration of the immunosuppressant tacrolimus FK-506 (PROGRAF® 5 mg, Astellas, Ireland); and
Group 3: administered vehicle only.
BLOOD TESTS
The total leukocyte counts and differential leukocyte counts (lymphocytes, monocytes, eosinophils and neutrophils) were evaluated on day 0 and at the time when the animals were sacrificed (day 28), with the aim of monitoring the immunosuppressant effect of the medication. Blood samples were collected from a cut made at the tip of the rats' tails. The leukocyte counts were made using an optical microscope (Olympus BX40, Tokyo, Japan).
MECHANICAL TESTS
The mechanical tests were performed on a universal machine (Emic DL 10.00, São José dos Pinhais, Brazil). For this, two devices were constructed: one in the shape of a claw, which was attached to the top of the machine and served for pulling the femur; and another device at the bottom of the machine that served as the fixation base for the bone (Figure 1). The speed used was 0.5 mm/s. The load value and the displacement were recorded and the maximum force required for fracturing the bone (Fmax) was noted.
Figure 1.

A) Device used for performing the mechanical tests; B) Close-up of a femur undergoing traction during the mechanical test
DENSITOMETRIC ANALYSIS
The bone mineral content (BMC) was measured and divided by the area in order to obtain the bone mineral density (BMD) of each animal's femurs. A dual-energy X-ray absorptiometry (DXA) machine was used (Prodigy, GE/Lunar, USA). The femur was placed in a receptacle containing rice, to simulate the soft tissue. The bone scanning and data analysis were performed using the Prodigy software, version 1.3 (Prodigy, GE/Lunar, USA) for small animals. The resolution (size in pixels) was 0.3 × 0.6 mm, with a collimator of 0.84 mm in diameter. The width of the scanned area was 9.9 mm and the length was 11.8 mm. The scan duration per femur was 0.5 min. The femur was scanned and its total area was selected (Figure 2) for the bone density evaluation.
Figure 2.
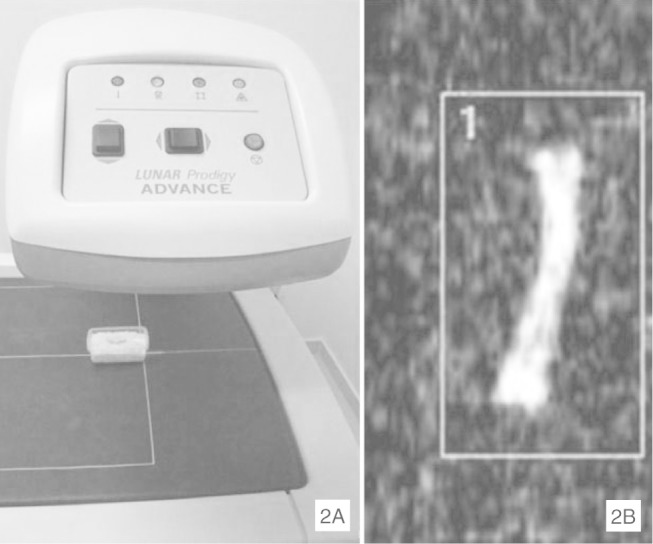
A) Machine used for densitometry analysis (Prodigy, GE/Lunar, USA); B) Scanned image of femur from X-ray densitometry (DXA), showing area selected for bone density evaluation
STATISTICAL METHODS
The data obtained from the fracture resistance tests, densitometry, lymphocyte counts and total leukocyte counts were analyzed statistically with the aid of the SPSS 13.0 software (SPSS Inc., Chicago, Illinois, USA). The values were subjected to analysis of variance (Anova), in order to determine whether there were any statistical differences between the groups, followed by the Tukey test. The results were considered to be statistically significant when P < 0.05.
RESULTS
Lymphocyte count and total leukocyte count
The lymphocyte count at time 0 was 7.71 ± 0.7 cells/mm3. After 28 days, these values had decreased to 4.54 ± 0.3 cells/mm3), and thus there was a statistical difference between times 0 and 28. There was also a reduction in the leukocyte count, going from 10.24 ± 0.8 to 6.75 ± 0.6 cells/mm3 (p < 0.05).
Resistance to flexion
The results relating to resistance to flexion and fracturing showed statistical differences between the group treated with immunosuppressant and the untreated and placebo groups (Table 1).
Table 1.
Means, standard deviations and statistical analysis on the groups evaluated
| Groups | Mean (SD) | Statistics* |
|---|---|---|
| 1 | 171.18 (± 26.07) | A |
| 2 | 117.46 (± 23.19) | B |
| 3 | 169.98 (± 27.11) | A |
SD = standard deviation
= statistical analysis, in which the same letters represent lack of statistical difference (p > 0.05)
Bone densitometry
Bone densitometry showed values that differed between the group that received the medication (140.9 ± 12.0 μg/cm2) and the two groups that did not receive it (152.2 ± 3.4 and 153.5 ± 1.2 μg/cm2 respectively for groups 1 and 3). This difference was e valuated statistically as p < 0.05.
DISCUSSION
Tacrolimus is a macrolide antibiotic that was discovered in a Japanese laboratory in 1984, and it has been shown to have an efficient immunosuppressant effect. This drug is produced through fermentation of the bacterium Streptomyces tsukubaensis and is capable of suppressing the humoral and cell-mediated immune responses, thus representing an alternative to cyclosporin-A(7).
The clinical use of tacrolimus is increasing, and it is now considered to be the baseline drug in more than 80% of liver transplantations and 30% of kidney transplantations(8). It acts through T cell activation and calcineurin blockade(8). One of the adverse effects from its use is that it acts negatively on the skeleton, thereby increasing bone reabsorption and leading to significant bone loss.
Reductions in mineral mass lead to the notion that the number of fractures in such individuals may increase. Based on this premise, the present study had the aim of evaluating the resistance to flexion and fracturing of femurs in rates that had been treated with this medication.
For this, a dose of 2.0 mg/kg/day was used. This was the same as used by Sabry et al(9), who evaluated three different doses of tacrolimus in rats that had received transplants (3.2 mg/kg/day, 2.0 mg/kg/day and 1.0 mg/kg/day, in the form of oral suspension). They observed that the dose of 2.0 mg/kg/day was sufficient to maintain the therapeutic serum levels without causing weight loss and with minimal side effects, thereby supporting the findings of Hayakawa et al(10). In the present study, we did not observe any weight loss among the rats treated with tacrolimus, or any clinically significant side effects such as pruritus(11), risk of infections(12) or diarrhea(12), which occur frequently in humans.
To monitor the immunosuppressant effect of the medication, total and differential leukocyte counts were made during the experiment. After 28 days, there had been a decrease in the total leukocytes, which presented a statistically difference between the times 0 and 28 days.
According to Cvetkovic et al(13), from the 14th day of tacrolimus use onwards, an imbalance in osteoblastic/osteoclastic activity occurs. They stated that this could be associated with a significant increase in PTH levels (oral suspension of FK-506 at a dose of 3.2 mg/kg/day). This was confirmed by Kirino et al(14), who reported that there was a significant increase in the second week, with a maximum peak in the third week (intraperitoneal injection of FK-506 at a dose of 1.0 mg/kg/days), and that through continued administration of FK-506, the increase in PTH led to the start of bone tissue loss. To attempt to evaluate the loss of mineral mass, densitometric analysis was performed. The results showed that there was a statistical difference between the group that received tacrolimus and the other groups. This result corroborated the findings of Cvetkovic et al(13) and Kirino et al(14).
Reports in the literature with contradictory results, and intense discussions regarding tacrolimus, have demonstrated that tacrolimus may induce bone loss in humans6, 15 and in experimental animal models13, 16, 17.
The results relating to the decrease in mineral density presented between the animals in groups 1 and 3 and the animals in group 2 (tacrolimus group) were confirmed through the mechanical tests on the fracture resistance of these animals' femurs. There was a significant decrease in the resistance to fracturing of the femurs from the treated rats (p < 0.05).
CONCLUSIONS
It can be concluded from this study that the femurs of the rats treated with the immunosuppressant tacrolimus FK-506 presented lower bone mineral density and lower resistance to flexion and fracturing than did the femurs of the rats treated with placebo and the femurs of the untreated rats.
Footnotes
Work performed at the Federal University of Rio de Janeiro and Osvaldo Cruz Foundation (Fiocruz), RJ.
We declare that thers is no confilct of interests in this article
REFERENCES
- 1.Wang Y, Tao Y, Hyman ME, Li J, Chen Y. Osteoporosis in china. Osteoporos Int. 2009;20(10):1651–1662. doi: 10.1007/s00198-009-0925-y. [DOI] [PubMed] [Google Scholar]
- 2.Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA. Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J Bone Miner Res. 2009;24(11):1835–1840. doi: 10.1359/jbmr.090515. [DOI] [PubMed] [Google Scholar]
- 3.Solomon DH, Rekedal L, Cadarette SM. Osteoporosis treatments and adverse events. Curr Opin Rheumatol. 2009;21(4):363–368. doi: 10.1097/BOR.0b013e32832ca433. [DOI] [PubMed] [Google Scholar]
- 4.Negri AL, Plantalech LC, Russo Picasso MF, Otero A, Sarli M. [Post-transplantation osteoporosis] Medicina (B Aires) 1999;59(6):777–786. [PubMed] [Google Scholar]
- 5.Cipriani R, Farias ML. [Osteoporosis after solid organs transplantation] Arq Bras Endocrinol Metabol. 2005;49(3):369–377. doi: 10.1590/s0004-27302005000300007. [DOI] [PubMed] [Google Scholar]
- 6.Cohen A, Shane E. Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int. 2003;14(8):617–630. doi: 10.1007/s00198-003-1426-z. [DOI] [PubMed] [Google Scholar]
- 7.Goto S, Stepkowski SM, Kahan BD. Effect of FK 506 and cyclosporine on heart allograft survival in rats. Transplant Proc. 1991;23(1 Pt 1):529–530. [PubMed] [Google Scholar]
- 8.Garcia SC. Cyclosporine A and tacrolimus: a review. J Bras Patol Med Lab. 2004;40(6):393–401. [Google Scholar]
- 9.Sabry A, El-Agroudy A, Sheashaa H, Hawas S, El-Shahat FB, Barakat N. Coadministration of ketoconazole and tacrolimus therapy: a transplanted rat model. Int Urol Nephrol. 2006;38(3-4):713–718. doi: 10.1007/s11255-006-0062-x. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa K, Hata M, Nishiyama T, Ohashi M, Ishikawa M. Maintenance of unresponsiveness by short-term pulse therapy with FK 506 in rat transplantation. Transplant Proc. 1996;28(3):1830–1831. [PubMed] [Google Scholar]
- 11.Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003;63(12):1247–1297. doi: 10.2165/00003495-200363120-00006. [DOI] [PubMed] [Google Scholar]
- 12.Plosker GL, Foster RH. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs. 2000;59(2):323–389. doi: 10.2165/00003495-200059020-00021. [DOI] [PubMed] [Google Scholar]
- 13.Cvetkovic M, Mann GN, Romero DF, Liang XG, Ma Y, Jee WS. The deleterious effects of long-term cyclosporine A, cyclosporine G, and FK506 on bone mineral metabolism in vivo. Transplantation. 1994;57(8):1231–1237. doi: 10.1097/00007890-199404270-00016. [DOI] [PubMed] [Google Scholar]
- 14.Kirino S, Fukunaga J, Ikegami S, Tsuboi H, Kimata M, Nakata N. Regulation of bone metabolism in immunosuppressant (FK506)-treated rats. J Bone Miner Metab. 2004;22(6):554–560. doi: 10.1007/s00774-004-0523-1. [DOI] [PubMed] [Google Scholar]
- 15.Rodino MA, Shane E. Osteoporosis after organ transplantation. Am J Med. 1998;104(5):459–469. doi: 10.1016/s0002-9343(98)00081-3. [DOI] [PubMed] [Google Scholar]
- 16.Katz IA, Takizawa M, Jaffe II, Stein B, Fallon MD, Epstein S. Comparison of the effects of FK506 and cyclosporine on bone mineral metabolism in the rat. A pilot study. Transplantation. 1991;52(3):571–574. [PubMed] [Google Scholar]
- 17.Abdelhadi M, Ericzon BG, Hultenby K, Sjüden G, Reinholt FP, Nordenstrüm J. Structural skeletal impairment induced by immunosuppressive therapy in rats:cyclosporine A vs tacrolimus. Transpl Int. 2002;15(4):180–187. doi: 10.1007/s00147-002-0413-1. [DOI] [PubMed] [Google Scholar]


