Abstract
Objective: To induce growth of a neomeniscus into the pores of a prosthesis in order to protect the knee joint cartilage. Methods: 70 knees of 35 New Zealand rabbits were operated. The rabbits were five to seven months old, weighed 2 to 3.8 kilograms, and 22 were male and 13 were female. Each animal underwent medial meniscectomy in both knees during a single operation. A bioabsorbable polymeric meniscal prosthesis composed of 70% polydioxanone and 30% L-lactic acid polymer was implanted in one side. The animals were sacrificed after different postoperative time intervals. The femoral condyles and neomeniscus were subjected to histological analysis. Histograms were used to measure the degradation and absorption of the prosthesis, the growth of meniscal tissue in the prosthesis and the degree of degradation of the femoral condyle joint cartilage. Results: The data obtained showed that tissue growth histologically resembling a normal meniscus occurred, with gradual absorption of the prosthesis, and the percentages of chondrocytes on the control side and prosthesis side. Conclusion: Tissue growth into the prosthesis pores that histologically resembled the normal rabbit meniscus was observed. The joint cartilage of the femoral condyles on the prosthesis side presented greater numbers of chondrocytes in all its layers.
Keywords: Knee, Cartilage, Prostheses and implants
INTRODUCTION
It has long been known that meniscectomy on the human knee has medium and long-term harmful effects, especially considering that the force distribution becomes significantly modified. The contact area decreases, thereby producing increased concentration of contact forces in a smaller area. This may accelerate degeneration of the joint surface, thus resulting in early osteoarthrosis1, 2, 3, 4, 5.
In the absence of better alternatives, meniscal injuries have been treated over the years purely and simply by removing the meniscus, independent of the type and duration of the injury or the patients' activities and ages. Young, physically very active patients who are sports players sometimes injure the meniscus so badly that there is no way out other that total meniscectomy.
Over the last 25 years, particularly since the advent of arthroscopy, many authors have shown concern regarding meniscus salvage6, 7, 8, 9, 10.
Different techniques have been used over recent decades with the aim of preserving the healthy parts of menisci. Sutures, transplants and meniscal prostheses have been among these. Tissue engineering suggests a very promising future. As new knowledge within the fields of medical genetics, immunology, biochemistry and bioengineering is acquired, further alternatives for treating various human diseases are emerging.
Research on the use of meniscal prostheses composed of nontoxic, biocompatible and bioabsorbable material seems to be a field of great interest, since this brings the hope of mechanical protection for knee joint cartilage following loss of the menisci. If such hopes were proven, these patients' joints would present greater longevity, with better quality of life for them.
The aim of this study, which was conducted on rabbits, was to evaluate the growth of a neomeniscus into the pores of a meniscal prosthesis and the degree of protection given to the joint cartilage of the medial femoral condyle. This prosthesis was developed from a membrane composed of bioabsorbable polymers. With degradation of the prosthesis, its components would be absorbed, thereby making room for the growth of fibrocartilaginous tissue that would histologically resemble the normal medial meniscus of rabbits.
METHODS
Rabbits
Operations were performed on 70 knees of 35 New Zealand rabbits of ages ranging from five to seven months and weights from two to 3.8 kilograms. There were 22 males and 13 females. Two groups were formed. In group A, consisting of 17 rabbits, arthrotomy and juxtacapsular medial meniscectomy were performed on the left knee, while the right knee received these procedures plus implantation of a meniscal prosthesis. In group B, with 18 rabbits, the implant was inserted in the left knee, while the right knee only received arthrotomy and medial meniscectomy.
Prosthesis
The prosthesis implanted was composed of a polymeric mixture of 70% polydioxanone and 30% L-lactic acid polymer, with 3% sodium triethyl citrate as a plasticizer. The prostheses were individually packed in small envelopes and were sterilized with ethylene oxide.
Prosthesis manufacture and characteristics
To manufacture the prosthesis, pieces of PDS®1 suture thread of 2 cm in length were cut and placed in a solution of methylene chloride at room temperature. This solution was stirred mechanically for 24 hours, in order to extract its violet pigmentation. The resulting polymer [poly(p-dioxanone)] was dissolved in 6 ml of hexafluoroisopropanolol (HFIP) solvent at room temperature and was stirred mechanically for one hour. The other polymer used in the composition of the prosthesis was poly(L-lactic acid)®2, which was obtained commercially in liquid form.
The two polymeric solutions were mixed and stirred for a further hour, until completely homogenized. The plasticizer sodium triethyl citrate was then added, with stirring for another hour. The final mixture was poured into a glass mold of 1,500 mm2 in area (50 × 30 mm) and was left to evaporate slowly in an evaporating chamber overnight, in order to form a thin porous membrane. This membrane was then dried under vacuum until the solvent had completely evaporated. It was then cut using a puncturing instrument into half-moon shapes with the following dimensions: length of 0.8 cm along the longitudinal axis, width of 0.4 cm along the transverse axis and thickness of 1 mm uniformly along the entire length (Figure 1).
Figure 1.
A) On the right, a polymeric membrane and cutting performed using the puncture tool; on the left, two prostheses after cutting them out. B) On the right, a meniscal prosthesis; on the left, a rabbit's normal medial meniscus
Surgical technique
The animals were kept under absolute fasting conditions for 12 hours before the operation. They were subjected to general anesthesia, with intramuscular administration of 5% ketamine hydrochloride at a dose of 30 mg/kg of weight, in association with 2% xylazine at a dose of 3 mg/kg of weight and atropine at a dose of 0.2 mg/kg de peso. The area of the incision was shaved immediately before starting the surgical procedure.
The rabbits were positioned on an appropriate surgical table, in horizontal dorsal decubitus. Asepsis was performed using a 50% iodated alcohol solution, applied by means of sterile gauze. The surgeon and surgical assistant both used sterile surgical gloves and surgical instruments sterilized in an autoclave at 120°C.
An anterior medial parapatellar surgical access was made longitudinally in both knees, of around 5 cm in length, followed by dissection of the subcutaneous tissue, medial parapatellar capsulotomy, lateral displacement of the extensor apparatus of the knee (quadricipital tendon, patella and patellar ligament), transverse sectioning of the medial collateral ligament at the joint interline and juxtacapsular resection of the medial meniscus, without damaging the joint cartilage of the femoral condyles. The polymeric meniscal prosthesis was implanted in one of the knees. Fixation of the implant was done by means of two simple stitches of 5-0 nylon monofilament thread: one in the anterior portion of the prosthesis and the other in the middle portion, with suturing to the juxtacapsular soft tissue that was inserted in the tibia (Figure 2).
Figure 2.
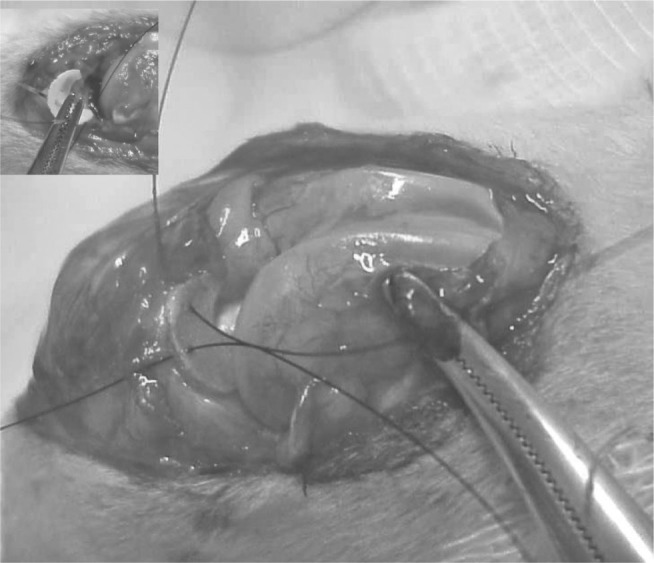
Implantation and fixation of the prosthesis to the capsule margin, using two stitches of 5-0 monofilament nylon thread
After washing with physiological serum, the medial collateral ligament was repaired and the capsule was closed using separate stitches of 5-0 nylon monofilament thread. The skin was closed using continuous stitches, with the same thread as used for the sutures. The postoperative dressing consisted of sterile gauze soaked in 50% iodated alcohol, which was covered by wrapping a Micropore adhesive bandage around the knee four times. Ampicillin was administered intramuscularly at a dose of 100 mg/kg of weight, as antimicrobial prophylaxis during the immediate postoperative period.
Postoperative follow-up
Immediately after the operation, the animals were housed in the vivarium, in suspended individual cages. They received commercial feed and water ad libitum, but were not given any medication. All the rabbits were able to support themselves on their hind legs a few hours after coming out of anesthesia.
Sacrifice technique and tissue collection
The rabbits received an injection of sodium pentobarbital at a dose of 100 mg/kg of weight intramuscularly and were then placed in a chloroform box.
After death had been confirmed (paralytic mydriasis and cessation of heartbeats respiratory movements), the animals were positioned in horizontal dorsal decubitus on a surgical table. The hind legs were shaved and asepsis was performed using 50% iodated alcohol. The joint was accessed using a lateral parapatellar route, thereby avoiding the scare from the original operation, while taking care not to damage the joint cartilage of the medial femoral condyle.
Longitudinal osteotomy was performed between the condyles of the femur, thus removing the medial condyle from both knees, along with the neomeniscus that had resulted from tissue growth in the prosthesis. The material collected was immediately placed in individually identified glass receptacles containing Bouin solution.
Histological analysis method
The specimens were processed, and the slides were stained using Masson's trichrome and picrosirius and were then examined under an ordinary optical microscope. The slides containing the neomeniscus were used to investigate whether new tissue growth had occurred in and among the pores of the polymeric prosthesis and what degree of prosthesis degradation and reabsorption had occurred. The slides containing the femoral condyles were used to perform histometry, by means of automated computer software for counting cells*. Through this method, the percentages of chondrocytes in the femoral condyles were determined, both on the side with the prosthesis implanted and on the control side. All the histological analyses were performed by an independent investigator.
Histometry
The slides stained with Masson's trichrome and picrosirius containing sections through the femoral condyles, both on the control side and on the prosthesis side, i.e. with and without the prosthesis implant, were photographed individually and analyzed using automated computer software (HLImage++97 – Histogram Tool API Western Vision Software, L.C., 1997 – version 2.0.0.0) in order to count the percentage of extracellular matrix, thereby also making it possible to establish the percentage of chondrocytes present in the joint cartilage of the condyle (Figure 3).
Figure 3.

Example of automated computed histogram
The Figure shows a computer screen image from a histogram that was obtained by photographing a slide containing a section through the joint surface of a rabbit's femoral condyle. The extracellular matrix is stained black, although in this software it is indicated as gray. The white arrow shows the number 0.906, which signifies 90.6% extracellular matrix. The percentage of chondrocytes (which are seen in white on the photograph, with some examples marked by circles) is equal to the difference between the two parts (matrix and cells). In this case, the percentage of cells is 9.4% (chondrocytes), here seen in white, and would be equal to the difference, i.e. to 9.4%. Since the software counts the extracellular matrix, there is no false counting of chondrocytes.
RESULTS
All the prostheses implanted underwent a process of gradual degradation and absorption, which could be seen starting from the third week after implantation. In the sixth week, greater degradation and invasion of the connective tissue were observed in 100% of the specimens.
Tissue growth resembling that of the normal meniscus of rabbit knees was also observed, with the presence of collagen and fibrochondrocytes, although the latter had not yet become aligned with the collagen fibers.
Neomeniscus
Three weeks after the implantation, invasion of connective tissue was observed, thereby dividing the prosthesis into smaller units that had not yet been degraded. These smaller fragments were spherulites. The presence of giant foreign-body cells was also observed (Figure 4). There were still no typical cells of normal menisci (Figure 5). The white region seen in Figure 6 represents parts of the prosthesis that had not yet become degraded. These birefringent regions were composed of spherulites from the prosthesis, surrounded by collagen fibers.
Figure 4.

Cross-section through prosthesis (P), three weeks after implantation. Start of the process of prosthesis degradation and invasion by tissue. Note the presence of a giant foreign-body cell (arrow). Masson's trichrome, 170 x
Figure 5.
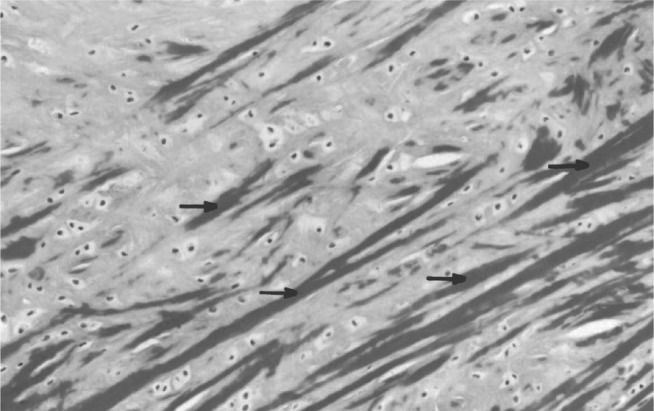
Cross-section through a rabbit's meniscus, showing fibrochondrocytes dispersed throughout an extracellular matrix and the orientation pattern of the collagen fibers (arrow). Masson's trichrome, 200 ×
Figure 6.
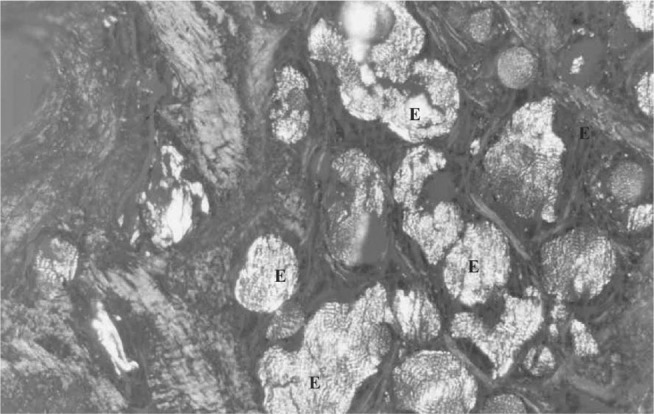
Cross-section through the prosthesis three weeks after implantation. Note the presence of spherulites (E) relating to the degraded polymer and collagen fibers. With polarization. Picrosirius, 170 x
The spherulites were only observed under polarized light, because of the difference in refractive index between the crystalline and amorphous regions. There were still no typical cells of normal menisci.
Six weeks after implantation, intense degradation of the material was seen, and consequently, greater tissue invasion than seen after three weeks (Figure 7). It was still not possible to see any fibrochondrocytes. Under polarized light, the fragments of the prosthesis were seen to be more widely dispersed, as represented by the bright regions in the photo (Figure 8). After 12 weeks, the prosthesis and giant foreign-body cells could still be seen on some slides (Figure 9). On other slides, the prosthesis could not be discerned, but fibrocartilage was seen, albeit with poorly aligned collagen fibers (Figure 10).
Figure 7.
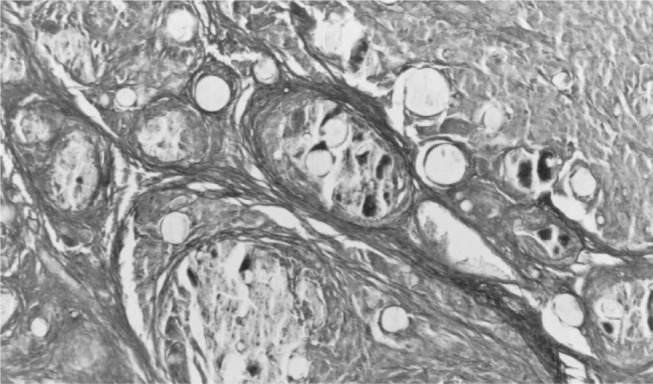
Cross-section through the prosthesis six weeks after implantation. Note the intense degradation and greater tissue invasion. Masson's trichrome, 170 x
Figure 8.

Cross-section through the prosthesis six weeks after implantation. Note the greater dispersion of polymer fragments, as represented by the bright regions in the photo. With polarization. Picrosirius, 170 x
Figure 9.

Cross-section through the prosthesis, 12 weeks after implantation, showing complete invasion by connective tissue. Note the presence of giant foreign-body cells (arrows). Masson's trichrome, 425 x
Figure 10.
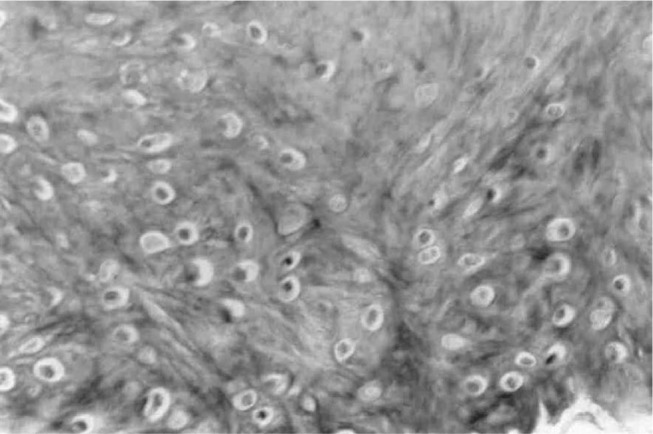
Cross-section through the prosthesis, 12 weeks after implantation, showing only the presence of fibrocartilage. Masson's trichrome, 290 x
Macroscopic appearance
Three weeks after implantation, the prosthesis presented a yellowish macroscopic appearance, with gelatinous consistency (Figure 11). From the sixth week onwards, the consistence was seen to be firmer and resistant to manipulation (Figure 12).
Figure 11.

Macroscopic appearance of a rabbit's knee three weeks after implantation of a polymeric prosthesis (arrows). The implant has a yellowish gelatinous appearance and consistency
Figure 12.

Macroscopic appearance of the neomeniscus formed from the implanted prosthesis, after six weeks (arrows). Firmer consistency, resistant to manipulation
After 12 weeks, the neomeniscus had a firm consistency and was fibrocartilaginous, with a color resembling that of normal menisci (Figure 13).
Figure 13.

Macroscopic appearance of the neomeniscus formed from the prosthesis, 12 weeks after implantation (arrows). The neomeniscus has a firm consistency, fibrocartilaginous appearance and color that resemble the characteristics of normal menisci
Femoral condyles
The histological analysis on the joint cartilage of the femoral condyle on the side with the implanted polymeric prosthesis showed that invasion of connective tissue had occurred, with many chondrocytes along the entire length of the prosthesis, three weeks after implantation. In the control condyle, three weeks after the operation, the chondrocytes were located more internally. The most superficial layer of the cartilage presented signs of cell disarrangement and greater concentration of collagen fibers (Figures 14 and 15).
Figure 14.
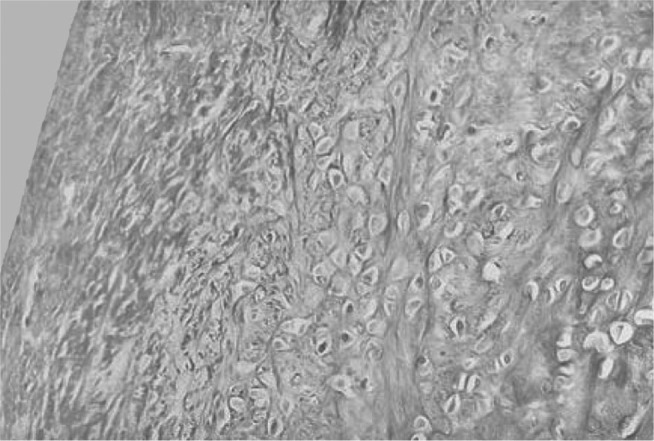
Condyle on control side, three weeks after operation, presenting few chondrocytes, which are located more internally. Note large accumulation of collagen fibers. Masson's trichrome, 50 x
Figure 15.
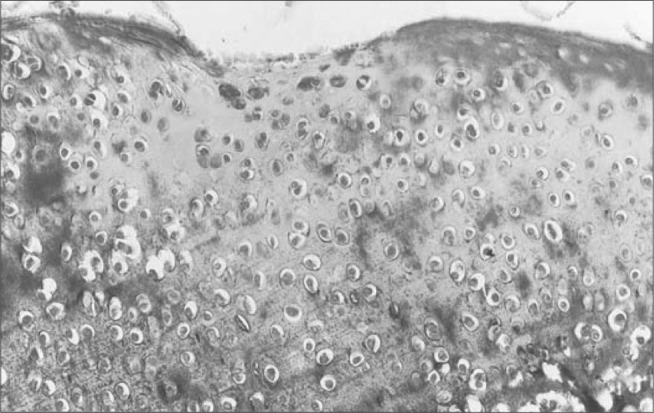
Condyle on prosthesis side, three weeks after the operation, presenting large numbers of chondrocytes in all layers. Masson's trichrome, 50 x
After six weeks, it was seen that the control condyle presented chondrocytes in the deeper layers, while the most superficial layer presented dense connective tissue that was practically acellular. On the other hand, after six weeks, the condyle with the implanted prosthesis presented chondrocytes distributed more equally across all the layers (Figures 16 and 17).
Figure 16.

Control condyle, six weeks after the operation, presenting few chondrocytes, located more internally, and dense connective tissue more superficially. Masson's trichrome, 50 x
Figure 17.

Condyle on prosthesis side, six weeks after the operation, presenting many chondrocytes in all layers of the condyle. Masson's trichrome, 50 x
Twelve weeks after implantation, the control condyle presented a larger number of chondrocytes in the more internal layers, with an appearance of cell disarrangement on the joint surface. The distribution of chondrocytes in the cartilage of the condyle with the prosthesis was more homogenous and better organized, in all layers (Figures 18 and 19).
Figure 18.

Condyle on control side, 12 weeks after the operation, presenting more chondrocytes in the interior than on the joint surface. Masson trichrome, 50 x
Figure 19.

Condyle on prosthesis side, 12 weeks after the operation, presenting similar quantities of chondrocytes on the joint surface and in the matrix. Picrosirius, 50 x
STATISTICAL ANALYSIS**
The distribution of absolute frequencies (n) and relative frequencies (%) of the nominal characteristics (qualitative) or enumerative data (attributes) was analyzed in relation to sex (male or female), side that received the prosthesis (right or left) and presence of fibrochondrocytes in the neomeniscus (absent or present).
The frequencies of occurrence of each attribute (quality) according to the groups of three weeks, six weeks and twelve weeks (time since implantation) and the TOTAL (general), were presented in contingency tables. The proportions (%) in the groups were represented by pie charts.
To analyze specific magnitudes (variables), descriptive statistics of the ordinal (quantitative) characteristics were produced: mean (M), standard deviation (SD), standard effort of the mean (SEM), maximum value (MAX), minimum value (MIN) and number of cases (N).
Scientific rounding was used, and the results were presented with up to two decimal places or until the first significant number. The confidence level of 5% was used (μ = 0.05). In the tables, proven statistical differences were shown by asterisks (*).
The descriptive statistics on magnitudes according to the groups (samples) of three weeks, six weeks and twelve weeks, and between controls and prosthesis use, were presented in statistical tables and represented in bar graphs.
In comparisons between frequencies of classes, in relation to the presence of fibrochondrocytes in the neomeniscus, the chi-square test could not be applied because the number of cells in the range from < 5 to 0 was greater than the permitted level. It was decided to investigate the difference between the groups of twelve weeks (final) and three weeks (initial) using Fisher's exact test (Table 1 and Figure 20).
Table 1.
Distribution of absolute frequencies (n) and relative frequencies (%) of the presence of fibrochondrocytes in histological sections from the neomeniscus, according to the time elapsed since implantation (three, six and 12 weeks). Comparison between three and 12 weeks according to Fisher's exact test (μ = 0.05)
| Presence of fibrochondrocytes in the neomeniscus |
||||||||
|---|---|---|---|---|---|---|---|---|
| 3 weeks | 6 weeks | 12 weeks | Total | |||||
| n | % | n | % | n | % | n | % | |
| Absent | 8 | 34.8 | 4 | 17.4 | 0 | 0.0 | 12 | 32.2 |
| Present | 1 | 4.3 | 4 | 17.4 | 6 | 26.1 | 11 | 47.8 |
| Total | 9 | 39.1 | 8 | 34.8 | 6 | 26.1 | 231 | 100.0 |
123 rabbits evaluated out of the 35 initial rabbits, due to loss of slides
Figure 20.

Distribution of relative frequencies (%) of the presence of fibrochondrocytes in the histological sections from the neomeniscus, according to the time elapsed since implantation, in weeks
In comparisons between the two groups (control and prosthesis) regarding the nonparametric variables of percentages of chondrocytes (%) at three, six and twelve weeks, the Mann-Whitney U test was used (Tables 2, 3 and 4 and Figures 21, 22 and 23).
Table 2.
Descriptive statistics on the maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles, in the various histological sections from each condyle, with or without prosthesis (neomeniscus), three weeks after implantation. Comparison using the Mann-Whitney U test (μ = 0.05)
| Percentage of chondrocytes (%) after 3 weeks |
||
|---|---|---|
| Control | Prosthesis | |
| M | 20.46 | 29.42 |
| SD | 2.38 | 11.72 |
| SEM | 1.07 | 4.78 |
| MAX | 22.9 | 48.2 |
| MIN | 17.6 | 15.8 |
| N | 5 | 6 |
Mann-Whitney UUc = 3 U = 9.0 p > 0.05
Table 3.
Descriptive statistics on the maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles, in the various histological sections from each condyle, with or without prosthesis (neomeniscus), six weeks after implantation. Comparison using the Mann-Whitney U test (μ = 0.05)
| Percentage of chondrocytes (%) after 6 weeks |
||
|---|---|---|
| Control | Prosthesis | |
| M | 10.81 | 19.23 |
| SD | 0.53 | 6.87 |
| SEM | 0.20 | 2.81 |
| MAX | 11.5 | 29.5 |
| MIN | 10.2 | 11.7 |
| N | 7 | 6 |
Mann-Whitney U Uc = 6 U = 0.0 p < 0.05
Table 4.
Descriptive statistics on the maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles, in the various histological sections from each condyle, with or without prosthesis (neomeniscus), 12 weeks after implantation. Comparison using the Mann-Whitney U test (μ = 0.05)
| Percentage of chondrocytes (%) after 12 weeks |
||
|---|---|---|
| Control | Prosthesis | |
| M | 11.30 | 16.13 |
| SD | 1.01 | 3.00 |
| SEM | 0.45 | 1.73 |
| MAX | 12.6 | 19.6 |
| MIN | 10.2 | 14.3 |
| N | 5 | 3 |
MANN-WHITNEY U UC = 0.0 U = 0.0 P < 0.05
Figure 21.

Maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles, in the various histological sections from each condyle, with or without prosthesis (neomeniscus), three weeks after implantation
Figure 22.
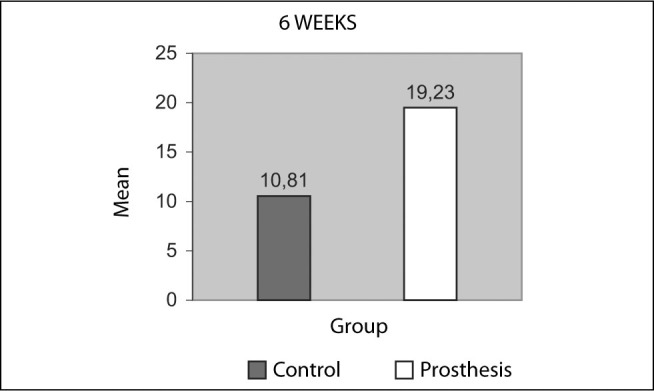
Maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles, in the various histological sections from each condyle, with or without prosthesis (neomeniscus), six weeks after implantation
Figure 23.

Maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles, in the various histological sections from each condyle, with or without prosthesis (neomeniscus), 12 weeks after implantation
In comparisons between the three groups regarding the nonparametric variables of percentage of chondrocytes (%) in condyles without a prosthesis (controls) (Table 5 and Figure 24) and percentage of chondrocytes (%) in the condyles with a prosthesis (neomeniscus) (Table 6 and Figure 25), the Kruskal-Wallis test was used and the significant differences were discriminated using the multiple comparisons test modified by Dunn (Table 5 and Figure 24).
Table 5.
Descriptive statistics on the maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles on the side without a prosthesis (control), in the various histological sections from each condyle, according to the time elapsed since implantation (three, six and 12 weeks). Comparison using the Kruskal-Wallis test and discrimination using the multiple comparisons test modified by Dunn (μ = 0.05)
| Percentage of chondrocytes (%) in the condyle without a prosthesis (control) |
|||
|---|---|---|---|
| 3 weeks | 6 weeks | 12 weeks | |
| M | 20.46 | 10.81 | 11.30 |
| SD | 2.38 | 0.53 | 1.01 |
| SEM | 1.07 | 0.20 | 0.45 |
| MAX | 22.9 | 11.5 | 12.6 |
| MIN | 17.6 | 10.2 | 10.2 |
| N | 5 | 7 | 5 |
Kruskal-Wallis H = 10.4 p = 0.006*
Dunn 3 weeks > 6 weeks
Figure 24.
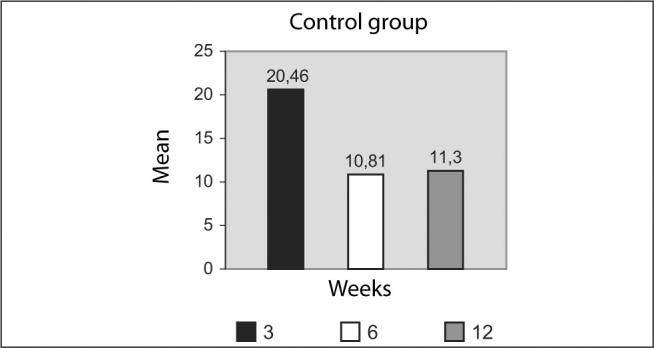
Maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles on the side without a prosthesis (control), in the various histological sections from each condyle, according to the time elapsed since implantation, in weeks
Table 6.
Descriptive statistics on the maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles on the side with a prosthesis (neomeniscus), in the various histological sections from each condyle, according to the time elapsed since implantation (three, six and 12 weeks). Comparison using the Kruskal-Wallis test (μ = 0.05)
| Percentage of chondrocytes (%) in the condyle with a prosthesis (neomeniscus) |
|||
|---|---|---|---|
| 3 weeks | 6 weeks | 12 weeks | |
| M | 29.42 | 19.23 | 16.13 |
| SD | 11.72 | 6.87 | 3.00 |
| SEM | 4.78 | 2.81 | 1.73 |
| MAX | 48.2 | 29.5 | 19.6 |
| MIN | 15.8 | 11.7 | 14.3 |
| N | 6 | 6 | 3 |
Kruskal-Wallis H = 5.27 p = 0.07
Figure 25.

Maximum percentage of chondrocytes (%) observed in the cartilage of the femoral condyles on the side with a prosthesis (neomeniscus), in the various histological sections from each condyle, according to the time elapsed since implantation, in weeks
DISCUSSION
For many years, little or no importance was given to menisci, and the treatment for meniscal injuries was limited to meniscectomy11, 12, 13.
In 1897, the Englishman Bland-Sutton apud Annandale(11) stated that menisci were structures originating from leg muscle remnants and did not have any function.
At the end of the seventeenth century, the English surgeon Thomas Annandale apud AAOS(12) published an article in the British Medical Journal, in which he demonstrated concern regarding meniscal salvage. A few years later, his compatriot Sir Robert Jones(13), who at that time was an orthopedist of worldwide renown, criticized his colleague's position and declared that suturing the meniscal cartilage should be considered to be an obsolete operation.
Menisci have a half-moon shape: their upper surface (which is in contact with the femoral condyle) is concave, while their lower surface (in contact with the tibia) is slightly convex. This annuls the geometrical incongruence of the knee bone anatomy12, 14.
They are composed of connective tissue with few cells, distributed in an abundant extracellular matrix composed of collagen (approximately 60% to 70% of their dry weight), proteoglycans, glycoproteins, elastin and water(12).
Among its important functions, it distributes the forces on the joint cartilage, absorbs shocks during dynamic loading and probably helps in joint lubrication. It adapts well to weight support, especially in areas of loading and movement such as the synovial joints(12).
The menisci of the knee are poorly vascularized structures. The vascularization decreases from the peripheral insertion at the edge of the capsule to the joint center15, 16, 17, 18.
The longest time for maintaining the implant was defined as twelve weeks because the transformation of fibrocytes into fibrochondrocytes occurs at the end of this period19, 20, 21, 22.
Rabbits were chosen for our study because they are animals that are easy to manage, very resistant to infections and of an adequate size for carrying out the experiment. The large number of studies conducted around the world using these animals also contributed towards this choice5, 7, 18, 20, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34.
In the pilot study by Pezzin(35), mechanical traction tests on membranes composed of 80% poly(para-dioxanone) and 20% poly(L-lactic acid), which were obtained by means of solvent evaporation, showed that these were the membranes with the lowest modulus of rigidity and that therefore this mixture had the greatest flexibility.
The term polymer comes from Greek: poly means many and meres means parts. Polymers are macromolecules formed by repeated united known as monomers. One of the best known examples is polyethylene, which is composed of repeated ethylene molecules.
Conceptually, biodegradable polymers are those that undergo degradation through vital activity by the organism, while bioabsorbable polymers undergo a process of hydrolysis by body fluids, which leads to their gradual absorption.
Biomaterials such as biodegradable and bioabsorbable polymers have a growing role within the therapeutic arsenal of medicine. Suture threads, skin replacements, growth guides for nerves and vessels and orthopedic devices such as plates and screws can be cited. Examples of these include Dexon® suture thread, composed of poly(glycolic acid) and PDS® (polydioxanone suture), composed of poly(para-dioxanone).
Certain criteria need to be respected with regard to implanting biomaterial in the human body. Among these, the material needs to be nontoxic, have an adequate absorption rate and present low immunological, carcinogenic and thrombogenic responses(36).
Polymeric membranes that are proposed for use as meniscal prostheses have to meet criteria relating to porosity, biocompatibility, mechanical resistance, flexibility, shape or geometrical design, and adequacy of size and composition for allowing tissue growth among the membrane pores. The porosity needs to be such that it allows invasion and fixation of biological tissue. The relationship between the sizes of the pores and the invading cells is very important34, 36, 37, 38, 39, 40, 41, 42.
Concern regarding the treatment of meniscal lesions and particularly in relation to meniscal salvage, thereby saving knees, goes back a long time5, 8, 11, 20.
Several methods have been used. The following can be highlighted: partial or selective meniscectomy2, 6, 12, 14, 43, 44, 45, 46, 47, 48, 49, use of synovial flaps7, 25, 27, 28, 32, 50, use of different replacement tissues37, 50, 51, 52, 53, meniscal sutures6, 7, 9, 10, 11, 12, 14, 43, 46, 47, 48, meniscal transplants16, 23, 51, 53, 54, 55, 56, 57, 58, 59, 60 and meniscal prostheses16, 30, 31, 34, 37, 38, 61, 62, 63.
Each of these methods presents advantages and disadvantages. Partial meniscectomy occasionally resects a significant portion of the meniscus, thus leaving a very large dead space. On the other hand, when it is done far from the peripheral insertion of the capsule of the meniscus, no regeneration of meniscal tissue is achieved, probably because of the vascularization characteristics.
The use of synovial flaps seems to fulfill a role in inducing growth of meniscal tissue, but only in small defects or as a bridge between the juxtacapsular synovium and lesions that are more central.
The use of heterologous grafts is subject to an important problem of rejection by the receptor organism. The results from using tissues with functions differing from those of the meniscus, such as tendons, even if they are homologous or autologous, do not seem to be encouraging, particularly with regard to mechanical results.
Meniscal sutures are limited to small or recent marginal lesions, i.e. those located in the zone of best vascularization that are smaller than 1.3 centimeters in length and which occurred less than two or three months ago. Different techniques and artifacts have been studied, such as suturing from inside to outside, versus from outside to inside; and fixation using polymer darts.
Transplants, along with prostheses, are perhaps the most promising form of treatment. There are still some unresolved problems regarding immunological matters and the methods for sterilization and preoperative evaluation of the correct size for the transplant(59). It is important to bear in mind that donor availability, like in relation to other organs and tissues, is still an obstacle to success for these methods.
The first meniscal transplant was performed by Milachowski et al in 1984(57). Through refinement of the technique and the indication criteria, the results have improved and it can now be said that this procedure is studied investigatively rather than experimentally. The difference in considering a study to be investigative is that some degree of success is implied, although not as predictably as would be desired.
Wickiewicz et al(60) performed the first arthroscopic meniscal transplant and presented a study on 96 cases with ten years of postoperative follow-up in which the failure rate was almost 50%. They stated that the main reason for this was that cases with moderate to severe arthrosis had been included. Careful selection of candidates for meniscal transplantation is very important.
Another important point is that the graft should be of the appropriate size. The sides (left or right), the compartment (medial or lateral) and the weight and height of the donor and receptor need to be noted. The way in the grafts are preserved and stored is also very important, given that some sterilization methods involving irradiation weaken the grafts. It is certainly recommendable to perform the surgery as early as possible, while the arthrotic changes are still not very severe. It seems unlikely that severe degenerative osteoarthrosis could be reversed through implantation of a meniscal graft(60).
Studies on prostheses have included different types of materials with a wide variety of results. There have been attempts to use polyurethane(37), Teflon(64), collagen61, 62, 63, polyester(34), carbon fiber32, 34, 65, Dacron30, 31 and combinations of these materials30, 31, 65. Carbon fiber prostheses almost always induce synovitis and cause problems that are difficult to resolve. Materials like Dacron, Teflon and polyester are abrasive, probably because of their high degree of rigidity, and do not facilitate cell growth. The use of Teflon does not impede the appearance of arthrotic abnormalities(64).
It seems that none of these prostheses restores the normal biomechanics of the knees. Nor do they impede the appearance of arthrotic abnormalities. Prostheses produced using collagen molds allow tissue growth better than do others that have been investigated. They cause fewer problems for the joint cartilage, but still have the problem of immunogenicity. Nonetheless, this seems to be a path to be investigated.
The aim of our study was evaluate the degree of degradation and reabsorption of the prosthesis and the growth of a neomeniscus.
We were able to confirm that the joint cartilage of the medial femoral condyle was in some manner protected by the prosthesis, given that greater numbers of chondrocytes could be seen in all layers of the cartilage on the side in which the device was implanted, compared with the control side.
The statistical analysis demonstrated that, three weeks after the implantation, there was no difference in the percentages of chondrocytes on the control and prosthesis sides. On the other hand, after six and twelve weeks, the percentage of chondrocytes on the prosthesis side was greater than on the control side.
In the statistical analysis on the femoral condyles, we observed that there was a greater decrease in the percentage of cartilaginous cells on the side without a prosthesis, compared with the side with an implanted prosthesis. Thus, there was greater degeneration of the cartilage and therefore greater degenerative osteoarthrosis on the control side, which coincides with previous findings in the literature1, 2, 3, 5, 66.
Our results suggest that polymeric prostheses present great potential for replacing the meniscus. We believe that porous polymeric prostheses are certainly one of the alternatives for prophylactic treatment of secondary osteoarthrosis in the human knee. This treatment is clinically applicable especially for young athletes who suffer severe and irreparable meniscal injuries.
We believe that in the future, porous polymeric molded prostheses should be one of the alternatives for prophylactic treatment of secondary osteoarthrosis, following trauma to the human knee, with important clinical application in preventing this pathological condition, particularly among young patients with severe meniscal injuries.
It is likely that polymeric membranes are going to play an essential role in structuring molded prostheses that enable the construction of tissues and organs from patients' own cells. This will make it possible to have solutions that are more biological and less aggressive, with regard to a range of pathological conditions.
Tissue engineering will probably change the history of medicine in the near future, and polymers will be part of this.
CONCLUSIONS
-
1 –
Polymeric prostheses undergo gradual degradation and absorption.
-
2 –
Tissue growth takes place into the pores of the prosthesis, with histological characteristics resemble those of rabbits' normal menisci.
-
3 –
The joint cartilage of the femoral condyles on the side with the implanted prosthesis present greater numbers of chondrocytes, in all layers.
-
4 –
The joint cartilage of the femoral condyles on the side without the implant presents fewer chondrocytes, particularly in the most superficial layer.
ACKNOWLEDGEMENTS
Prof. Dr. Ana Paula Testa Pezzin, of the Department of Environmental Engineering, UNIVILLE, Prof. Dr. Maria do Carmo Alberto Rincón, of the Department of Physiological Sciences, CCMB – PUC SP, and Prof. Dr. Cecília Amélia de Carvalho Zavaglia, of the Department of Materials Engineering, School of Mechanical Engineering, Unicamp, were important collaborators in carrying out this work.
Footnotes
Work performed at the Institute of Orthopedics and Traumatology, School of Medicine of the University of Sao Paulo, and at the Biomaterials Laboratory, Medical and Biological Sciences Center of Sorocaba, Catholic Pontificate University of Sao Paulo.
We declare that thers is no confilct of interests in this article
The statistical terminology and definitions were used in accordance with the GUIA PARA EXPRESSÃO DA INCERTEZA DE MEDICÃO, Second Brazilian Edition of the Guide to the Expression of Uncertainty in Measurement (BIPM, IEC, IFCC, ISSO, IUPAC, IUPAP, OIML, 1983). Revised Edition (August 1998) –Rio de Janeiro: ABNT, INMETRO, SBM, 1998.
REFERENCES
- 1.Aagaard H, Verdonk R. Function of the normal meniscus and consequences of meniscal resection. Scand J Med Sci Sports. 1999;9(3):134–140. doi: 10.1111/j.1600-0838.1999.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666–671. doi: 10.1302/0301-620X.66B5.6548755. [DOI] [PubMed] [Google Scholar]
- 3.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Am. 1948;30B(4):664–670. [PubMed] [Google Scholar]
- 4.Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors effecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(4):719–729. [PubMed] [Google Scholar]
- 5.Korkala O, Karaharju E, Grünblad M, Aalto K. Articular cartilage after meniscectomy. Rabbit knees studied with the scanning electron microscope. Acta Orthop Scand. 1984;55(3):273–277. doi: 10.3109/17453678408992355. [DOI] [PubMed] [Google Scholar]
- 6.DeHaven KE. Meniscus repair in the athlete. Clin Orthop Relat Res. 1985;(198):198–200. [PubMed] [Google Scholar]
- 7.Ghadially FN, Wedge JH, Lalonde JM. Experimental methods of repairing injured menisci. J Bone Joint Surg Br. 1986;68(1):106–110. doi: 10.1302/0301-620X.68B1.3753606. [DOI] [PubMed] [Google Scholar]
- 8.Hughston JC. A simple meniscectomy. J Sports Med. 1975;3(4):179–187. doi: 10.1177/036354657500300406. [DOI] [PubMed] [Google Scholar]
- 9.Wageck JM, Rockett PRP. Sutura meniscal artroscópica. Rev Bras Ortop. 1992;27(6):395–398. [Google Scholar]
- 10.Wageck JM. Suturas meniscais. In: Camanho GL, editor. Patologia do joelho. Sarvier; São Paulo: 1996. pp. 45–50. [Google Scholar]
- 11.Annandale T. An operation for displaced semilunar cartilage. Br Med J. 1885;1:779–781. doi: 10.1136/bmj.1.1268.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Orthopaedic Surgeons. Arthroscopic meniscus repair. Monograph Series, Rosemont; 1999. 63p
- 13.Jones R. Notes on derangement of the knee. Ann Surg. 1909;50:969–1001. doi: 10.1097/00000658-190912000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amatuzzi MM. Os meniscos do joelho. Acta Ortop Bras. 1993;1:97–104. [Google Scholar]
- 15.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10(2):90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 16.Arnoczky SP. Building a meniscus. Biologic considerations. Clin Orthop Relat Res. 1999;(367 Suppl):S244–S253. [PubMed] [Google Scholar]
- 17.Cohen M, Granata Junior GSM, Ejnisman B, Seixas MT, de Vicenze V. Estudo da vascularização do menisco humano. Rev Bras Ortop. 1998;33(4):264–270. [Google Scholar]
- 18.Bombelli R. Sulla vascolarizzazione dei menischi articolare del ginocchio del coniglio. Nuffield Orthopaedic Centre; Oxford: 1957. pp. 119–125. [PubMed] [Google Scholar]
- 19.Arnoczky SP, Warren RF, Kaplan N. Meniscal remodeling following partial meniscectomy–an experimental study in the dog. Arthroscopy. 1985;1(4):247–252. doi: 10.1016/s0749-8063(85)80092-x. [DOI] [PubMed] [Google Scholar]
- 20.King D. The healing of semilunar cartilages. J Bone Joint Surg Am. 1936;18:333–342. [Google Scholar]
- 21.Moon MS, Kim JM, Ok IY. The normal and regenerated meniscus in rabbits. Morphologic and histologic studies. Clin Orthop Relat Res. 1984;(182):182–189. [PubMed] [Google Scholar]
- 22.Smillie IS. Observations on the regeneration of the semilunar cartilages in man. J. Bone Joint Surg Br. 1944;31:398–401. [Google Scholar]
- 23.Cummins JF, Mansour JN, Howe Z, Allan DG. Meniscal transplantation and degenerative articular change: an experimental study in the rabbit. Arthroscopy. 1997;13(4):485–491. doi: 10.1016/s0749-8063(97)90128-6. [DOI] [PubMed] [Google Scholar]
- 24.Goertzen D, Gillquist J, Messner K. Tensile strength of the tibial meniscal attachments in the rabbit. J Biomed Mater Res. 1996;30(1):125–128. doi: 10.1002/(SICI)1097-4636(199601)30:1<125::AID-JBM16>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.He L. [An experimental study on accelerating regeneration of meniscus with synovial flap implant in the rabbit knee] Zhonghua Wai Ke Za Zhi. 1993;31(10):591–592. [PubMed] [Google Scholar]
- 26.Heatley FW. The meniscus–can it be repaired? An experimental investigation in rabbits. J Bone Joint Surg Br. 1980;62(3):397–402. doi: 10.1302/0301-620X.62B3.6893331. [DOI] [PubMed] [Google Scholar]
- 27.Huang TL, Lin GT, O'Connor S, Chen DY, Barmada R. Healing potential of experimental meniscal tears in the rabbit. Preliminary results. Clin Orthop Relat Res. 1991;(267):267–305. [PubMed] [Google Scholar]
- 28.Kim JM, Moon MS. Effect of synovectomy upon regeneration of meniscus in rabbits. Clin Orthop Relat Res. 1979;(141):141–194. [PubMed] [Google Scholar]
- 29.Messner K, Lohmander LS, Gillquist J. Cartilage mechanics and morphology, synovitis and proteoglycan fragments in rabbit joint fluid after prosthetic meniscal substitution. Biomaterials. 1993;14(3):163–168. doi: 10.1016/0142-9612(93)90018-w. [DOI] [PubMed] [Google Scholar]
- 30.Messner K, Gillquist J. Prosthetic replacement of the rabbit medial meniscus. J Biomed Mater Res. 1993;27(9):1165–1173. doi: 10.1002/jbm.820270907. [DOI] [PubMed] [Google Scholar]
- 31.Sommerlath K, Gillquist J. The effect of a meniscal prosthesis on knee biomechanics and cartilage. An experimental study in rabbits. Am J Sports Med. 1992;20(1):73–81. doi: 10.1177/036354659202000117. [DOI] [PubMed] [Google Scholar]
- 32.Veth RP, den Heeten GJ, Jansen HW, Nielsen HK. An experimental study of reconstructive procedures in lesions of the meniscus. Use of synovial flaps and carbon fiber implants for artificially made lesions in the meniscus of the rabbit. Clin Orthop Relat Res. 1983;(181):181–184. [PubMed] [Google Scholar]
- 33.Walsh CJ, Goodman D, Caplan AI, Goldberg VM. Meniscus regeneration in a rabbit partial meniscectomy model. Tissue Eng. 1999;5(4):327–337. doi: 10.1089/ten.1999.5.327. [DOI] [PubMed] [Google Scholar]
- 34.Wood DJ, Minns RJ, Strover A. Replacement of the rabbit medial meniscus with a polyester-carbon fibre bioprosthesis. Biomaterials. 1990;11(1):13–16. [PubMed] [Google Scholar]
- 35.Pezzin APT. Obtenção e caracterização de blendas de poli (paradioxanona) e poli (L-ácido láctico) (PPD/PLLA) para aplicação como prótese de menisco bioreabsorvível. Tese [doutorado]. Campinas: Faculdade de Engenharia Mecĉnica da Unicamp; 2001
- 36.Sittinger M, Reitzel D, Dauner M, Hierlemann H, Hammer C, Kastenbauer E, Planck H, Burmester GR, Bujia J. Resorbable polyesters in cartilage engineering:affinity and biocompatibility of polymer fiber structures to chondrocytes. J Biomed Mater Res. 1996;33(2):57–63. doi: 10.1002/(SICI)1097-4636(199622)33:2<57::AID-JBM1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.de Groot JH, de Vrijer R, Pennings AJ, Klompmaker J, Veth RP, Jansen HW. Use of porous polyurethanes for meniscal reconstruction and meniscal prostheses. Biomaterials. 1996;17(2):163–173. doi: 10.1016/0142-9612(96)85761-9. [DOI] [PubMed] [Google Scholar]
- 38.de Groot JH, Zijlstra FM, Kuipers HW, Pennings AJ, Klompmaker J, Veth RP, Jansen HW. Meniscal tissue regeneration in porous 50/50 copoly(L-lactide/epsilon-caprolactone) implants. Biomaterials. 1997;18(8):613–622. doi: 10.1016/s0142-9612(96)00169-x. [DOI] [PubMed] [Google Scholar]
- 39.Lam KH, Nieuwenhuis P, Molenaar I, Esselbrugge H, Feijen J, Dijkstra PJ. Biodegradation of porous versus non porous poly (L-lactic acid) films. J Mater Sci Mater Med. 1994;5(4):181–189. [Google Scholar]
- 40.Luciano RM. Síntese, caracterização e degradação de membranas de poli (ácido láctico), um polímero bioabsorvível [dissertação]. Campinas: Faculdade de Engenharia Mecĉnica da UNICAMP; 1997
- 41.Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17(14):1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 42.Vert M, Li SM, Spenlehauer G, Guerin P. Bioresorbability and biocompability of aliphatic polyesters. J Mater Sci Mater Med. 1992;3(6):432–446. [Google Scholar]
- 43.Amatuzzi MM. Estado da arte no tratamento das doenças meniscais do joelho. Rev Bras Ortop. 2000;35(1):45–52. [Google Scholar]
- 44.Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med. 1983;11(3):131–141. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- 45.Bruce J, Walmsley R. Replacement of the semilunar cartilages of the knee after operative excision. Br J Surg. 1937;25(1):17–28. [Google Scholar]
- 46.Cabaud HE, Rodkey WG, Fitzwater JE. Medical meniscus repairs. An experimental and morphologic study. Am J Sports Med. 1981;9(3):129–134. doi: 10.1177/036354658100900301. [DOI] [PubMed] [Google Scholar]
- 47.Camanho GL. [Arthroscopy in the treatment of traumatic meniscal injuries] Rev Hosp Clin Fac Med Sao Paulo. 1991;46(6):262–265. [PubMed] [Google Scholar]
- 48.Mello Júnior WA. Técnica cirúrgica. In: Camanho GL, editor. Patologia do joelho. Sarvier; São Paulo: 1996. p. 44. [Google Scholar]
- 49.Noble J, Turner PG. The function, pathology, and surgery of the meniscus. Clin Orthop Relat Res. 1986;(210):210–218. [PubMed] [Google Scholar]
- 50.Milachowski KA, Kohn D, Wirth CJ. [Meniscus replacement using Hoffa's infrapatellar fat bodies–initial clinical results] Unfallchirurgie. 1990;16(4):190–195. doi: 10.1007/BF02588774. [DOI] [PubMed] [Google Scholar]
- 51.Bruns J, Kampen J, Kahrs J, Plitz W. [Autologous meniscus replacement with rib perichondrium. Experimental results] Orthopade. 2000;29(2):145–150. doi: 10.1007/s001320050023. [DOI] [PubMed] [Google Scholar]
- 52.Cook JL, Tomlinson JL, Kreeger JM, Cook CR. Induction of meniscal regeneration in dogs using a novel biomaterial. Am J Sports Med. 1999;27(5):658–665. doi: 10.1177/03635465990270051901. [DOI] [PubMed] [Google Scholar]
- 53.Arnoczky SP, DiCarlo EF, O'Brien SJ, Warren RF. Cellular repopulation of deep-frozen meniscal autografts: an experimental study in the dog. Arthroscopy. 1992;8(4):428–436. doi: 10.1016/0749-8063(92)90003-t. [DOI] [PubMed] [Google Scholar]
- 54.Arnoczky SP, Warren RF, McDevitt CA. Meniscal replacement using a cryopreserved allograft. An experimental study in the dog. Clin Orthop Relat Res. 1990;(252):252–258. [PubMed] [Google Scholar]
- 55.Canham W, Stanish W. A study of the biological behavior of the meniscus as a transplant in the medial compartment of a dog's knee. Am J Sports Med. 1986;14(5):376–379. doi: 10.1177/036354658601400505. [DOI] [PubMed] [Google Scholar]
- 56.Cury RPL, Camargo OPA, Próspero JD, Botter FCS, Severino NR, Aihara T. Transplante homólogo de menisco: estudo experimental em coelhos. Rev Bras Ortop. 2002;37(8):341–349. [Google Scholar]
- 57.Milachowski KA, Weismeier K, Wirth CJ. Homologous meniscus transplantation. Experimental and clinical results. Int Orthop. 1989;13(1):1–11. doi: 10.1007/BF00266715. [DOI] [PubMed] [Google Scholar]
- 58.Rath E, Yassir W, Albright J. The long term outcome of meniscal allograft transplantation. Orthopaedic Today [serial online] http://www.slackinc.com/bone/ortoday/200009/rath.asp Acesso em 19 de setembro de 2001.
- 59.Shaffer B, Kennedy S, Klimkiewicz J, Yao L. Preoperative sizing of meniscal allografts in meniscus transplantation. Am J Sports Med. 2000;28(4):524–533. doi: 10.1177/03635465000280041301. [DOI] [PubMed] [Google Scholar]
- 60.Wickiewicz T, Harner CD, Noyes FR. Surgeons tout benefits of meniscal transplants following meniscectomy. Orthopaedic Today [serial online] disponível
- 61.http://www.slackinc.com/bone/ortoday/199909/surgeons.asp Acessado em 25 de novembro de 2001.
- 62.Rodkey WG, Stedman JR, Li ST. Collagen scaffolds: a new method to preserve and restore the severely injured meniscus. Sports Med Arthrosc Rev. 1999;7(1):63–73. [Google Scholar]
- 63.Stone KR, Rodkey WG, Webber RJ, McKinney L, Steadman JR. Future directions. Collagen-based prostheses for meniscal regeneration. Clin Orthop Relat Res. 1990;(252):252–255. [PubMed] [Google Scholar]
- 64.Stone KR, Rodkey WG, Webber R, McKinney L, Steadman JR. Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am J Sports Med. 1992;20(2):104–111. doi: 10.1177/036354659202000202. [DOI] [PubMed] [Google Scholar]
- 65.Toyonaga T, Uezaki N, Chikama H. Substitute meniscus of Teflon-net for the knee joint of dogs. Clin Orthop Relat Res. 1983;(179):179–180. [PubMed] [Google Scholar]
- 66.Veth RP, Jansen HW, Leenslag JW, Pennings AJ, Hartel RM, Nielsen HK. Experimental meniscal lesions reconstructed with a carbon fiber-polyurethane-poly(L-lactide) graft. Clin Orthop Relat Res. 1986;(202):202–293. [PubMed] [Google Scholar]
- 67.Zukor DJ, Cameron JC, Brooks PJ, Oakeshott RD, Farine I. The fate of human meniscal allografts. In: Ewing JW, editor. Articular cartilage and knee joint function: basic science and arthroscopy. Raven Press; New York: 1990. pp. 147–151. [Google Scholar]



