Abstract
This is a case report on a giant cell tumor of the juxta-articular proximal tibia with a pathological fracture. A female patient presented pain and increased local volume after falling from her own height. She underwent clinical examination, radiographic examination and puncture biopsy. A diagnosis of giant cell tumor was made. The patient was then treated with tumor resection and use of an unconventional partial endoprosthesis of the tibia with preservation of the joint surface of the tibial plateau. The patient evolved with improvement of symptoms and maintenance of joint function of the operated limb, absence of recurrence and complications, without any need for reoperation over 18 years of follow-up.
Keywords: Giant Cell Tumors, Tibia, Knee, Prostheses and Implants
INTRODUCTION
Giant cell tumors are aggressive benign tumors with vascularized tissue and the presence of fusiform or ovoid cells, and characterized by the presence of giant osteoclastic cells(1). They were initially described by Sir Astley Cooper(2) and were named brown tumors by Paget(3). Bloodgood(4) was the author who defined them as benign giant cell tumors. According to Geschikter and Copeland(5) and Willis(6), giant cell tumors are neoplasms of osteoclasts surrounded by mesenchymal stroma, because of the similarity between the giant cell and nonneoplastic osteoclasts. Jaffe et al(7) described their origin as derived from stroma cells, and this hypothesis was corroborated by Schajowics(8), through histochemical analysis and cell cultures, thereby confirming that there were no significant differences between tumor osteoclasts and normal osteoclasts.
The anatomopathological grade is given by the stroma and not by the giant cells themselves, which may be present in other tumoral or pseudotumoral lesions, such as brown tumors, aneurysmatic bone cysts, epiphyseal chondroblastoma, osteoblastoma and non-osteogenic fibroma(9).
These tumors usually affect patients aged between 20 and 40 years, i.e. generally with the growth plate closed. The incidence of giant cell tumors within the age group up to 15 years is 1.7% among the cases of this pathological condition. Other studies have demonstrated an incidence of around 0.8% for cases of metaphyseal giant cell tumors in patients with an open growth plate(10). These tumors affect both sexes with similar frequency11, 12. They mainly develop in the epiphyseal-metaphyseal region, and are most frequent in the distal epiphysis of the femur (28.2%) and the proximal epiphysis of the tibia13, 14. Only 5% occur in flat bones; the sacrum is the commonest site of occurrence in the axial skeleton and may give rise to neurological manifestations1, 15.
These are tumors of puffy appearance, generally rounded or oval-shaped, and are distributed eccentrically. They may destroy the epiphyseal and joint regions, although the joint cartilage usually tends to be spared. Occurrences of fractures in the cortical bone of the segment affected is common, and are most frequent in the distal femur(16). Because these are epiphyseal tumors, joint impairment with functional abnormalities and joint effusion may be present, thereby simulating meniscal-ligamentous or arthritic processes14, 17. The main symptoms are local pain and increased volume, and the start of the clinical history generally correlates with a traumatic event18, 19.
Metastases occur in around 1% of the cases, especially to the lungs(20). They are non-aggressive and have been correlated with multiple manipulations and local recurrence.
The aim of this study was to report on a case of giant cell tumor in the proximal epiphysis of the tibia, with a pathological fracture, in which an unconventional endoprosthesis was used, thereby preserving the joint surface of the tibial plateau, with gratifying long-term functional, esthetic and clinical results.
CASE REPORT
This was a 17-year-old female patient who presented pain and progressively increasing volume of the anterior region of the right proximal tibia that had been evolving for five months. After falling from her own height, she presented comminution of the anterior cortical bone on radiological examination, which was treated in another clinic with plaster-cast immobilization from the groin to the foot. She was then referred to the Orthopedic Surgery Service of Hospital Erasto Gaertner for evaluation. Upon physical examination, visible and palpable tumor development was noted in the anterior region of the right proximal tibia, with pain and functional impotence of the right knee.
Radiographic examination (Figures 1A) demonstrated an epiphyseal-metaphyseal lesion one centimeter from the joint surface of the knee, extending distally for 15 centimeters in the direction of the metaphysis. It had a puffy, rounded appearance, tapering onto the cortical bone in an eccentric manner, and with comminution of the anterior cortex in the middle third of the lesion. The patient was restricted to bed at that time.
Figure 1.
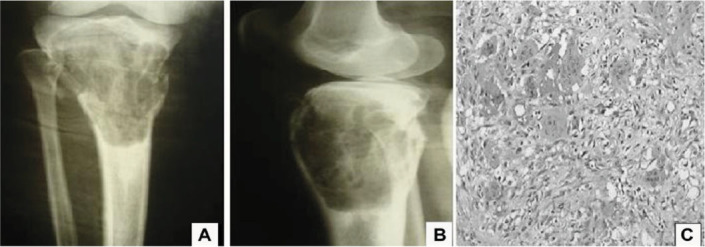
A) Preoperative radiograph: frontal view showing puffy lesion with comminution of the anterior cortical bone in the proximal third of the right tibia. B) Preoperative radiograph in lateral view. C) Image of the lamina showing giant cell surrounded by mesenchymal stroma.
It was proposed to perform a closed biopsy with the use of a trephine, in order to obtain diagnostic confirmation of the lesion. From the anatomopathological examination, a giant cell tumor was diagnosed (Figure 1C). In the light of these findings, the patient's youth and the aggressive nature of the tumor, it was decided to resect the bone segment that contained the tumor, while preserving the joint surface of the knee, with placement of an unconventional endoprosthesis for the proximal tibia (Figure 2A). The patient underwent intravenous antibiotic therapy during the induction phase of the anesthesia. Then, after performing antisepsis on the skin, the tumor was approached by means of a longitudinal anterior incision in the knee. The bone segment affected was resected, while preserving the extensor mechanism. Following this, the diaphyseal medullary canal was prepared with the use of progressively larger milling tools, in order to adapt the nail to the bone. After this first phase, the prosthesis was adapted proximally below the surface of the tibial plateau and was fixed using spongy screws. While closing the anatomic planes, a vacuum drain was inserted. During the postoperative period, the patient was kept without the use of orthoses, and she made an early start to assisted isometric exercises. The leg was released for load-bearing after the tenth week, with the aid of crutches.
Figure 2.
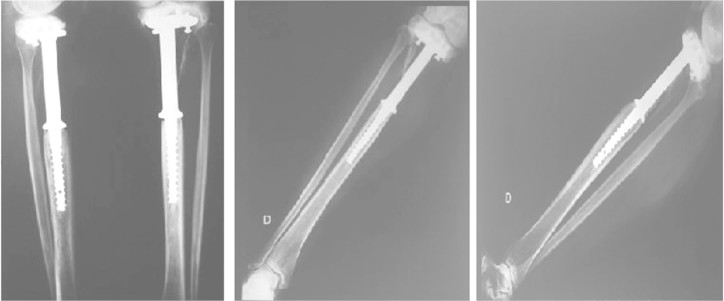
A) Immediate postoperative period: radiographs in frontal and lateral views (May 1991). B) Control radiograph in frontal view after 18 years of follow-up (May 2009). C) Control radiograph in lateral view after 18 years of follow-up.
The endoprosthesis used was developed and manufactured from titanium and polyethylene in the bioengineering laboratory of Hospital Erasto Gaertner within the same year (Figure 2). Its dimensions were, for the titanium GR.S Ø 19.10 mm and, for the polyethylene, GR.S Ø 10 mm.
Today, the patient presents full performance in her work activities (in the home), while reporting occasional pain when carrying out walking activities for prolonged periods or doing endurance exercises (Figures 3A, 3B and 3C). She currently presents a discrepancy, measured by means of scanometry (Table 1).
Figure 3.

Current assessment, 18 years after the operation: A) Standing on two feet in frontal view. B) Standing on two feet in lateral view. C) Standing on one foot with flexion of the operated limb. D) Patient in squatting position.
Table 1.
Scanometry on lower limbs.
| Scanometry measurements | |
|---|---|
| Right femur | 47.00 cm |
| Left femur | 47.00 cm |
| Right tibia | 38.50 cm |
| Left tibia | 38.75 cm |
| Right lower limb | 85.50 cm |
| Left lower limb | 85.75 cm |
Radiographic evaluation on the operated knee showed preservation of the joint relations, absence of arthrotic abnormalities and absence of remarkable features in the soft tissues. The same characteristics were observed in the non-operated limb (Figure 4)(Figure 2B) and 2C).
Figure 4.
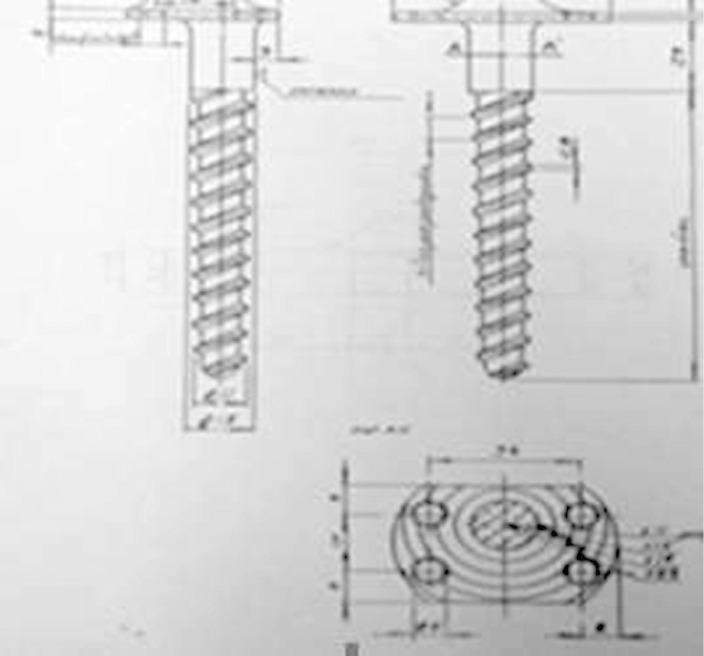
Original design for the prosthesis used, which was developed and manufactured in the Bioengineering Laboratory of Hospital Erasto Gaertner, 1990.
Control computed tomography on the lower limbs was also performed, and this showed bone demineralization in the operated femur, but with preserved joint relations and without evidence of tumor recurrence. The left knee presented bone elements with normal shape and structure, and lateralization of the patella (Figures 5A and 5B).
Figure 5.
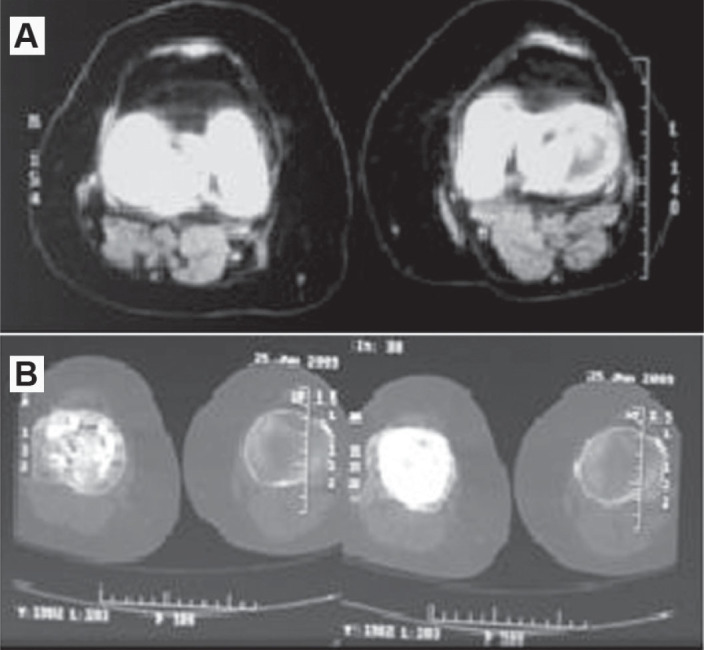
A) Tomographic images of the right and left distal femur. B) Tomographic image of the right and left proximal tibia: postoperative, with 18 years of evolution.
The physiotherapeutic evaluation consisted of assessing the pain, analyzing the gait and making an anthropometric evaluation. A visual analogue scale was applied with the aim of quantifying the intensity of this pain. The patient graded her pain as six, thus classifying it as moderate, which hindered her activities but did not impede them (Table 2).
Table 2.
Visual analogue scale.
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| No pain | Slight pain | Moderate pain | Strong or incapacitating pain | Intolerable pain | ||||||
| Does not hinder activities | Hinders activities but does not impede them | Impedes activities | Impedes activities and causes lack of control | |||||||
The visual analogue scale(21) consists of an aid for measuring the intensity of patients' pain. It is an important instrument for monitoring patients' evolution over the course of their treatment, and even at each consultation, in a more faithful manner. It is also useful for enabling analysis on whether the treatment is having any effect, which procedures are having the best results and whether there are any deficiencies in the treatment, according to the degree of improvement or worsening of the pain.
The visual analogue scale can be used at the start and end of each attendance session, to register the resultant evolution. To use the visual analogue scale, the attending professional should ask the patient about the degree of pain felt, such that 0 signifies total absence of pain and 10 is the maximum level of pain that the patient can tolerate.
With regard to gait, a visual analysis was made, in which the patient presented steps of longer duration and slower speed, although all the phases of gait were present. It cannot be said precisely whether these changes were due to the prosthesis or whether they were due to the gait pattern of obese individuals, given that this patient presented a body mass index greater than 32.
The patient underwent anthropometric analyses by means of a pachymeter and measuring tape (Table 3), which showed minimal trophic alterations registered in the lower limbs.
Table 3.
Body measurements for evaluation of trophic abnormalities.
| Height | 165.7 | |
| Body mass | 90.5 | |
| Width between the iliac spines | 24 | |
| Right | Left | |
| Mid-thigh circumference | 64.3 | 66 |
| Lower leg length (epicondyle to lateral malleolus) | 41.5 | 42 |
| Lower leg circumference (region with greatest volume) | 39.5 | 39 |
| Knee diameter | 12.7 | 11.3 |
| Height of malleolus (from lateral malleolus to the ground) | 6 | 6 |
| Diameter of the malleolus (lateral and medial malleolus) | 6.7 | 6.5 |
| Width of foot (widest part of foot) | 10.4 | 10.1 |
The range of motion on the operated side consisted of painless flexion-extension 150-0, which was measured with the aid of a goniometer.
DISCUSSION
The bases for treating giant cell tumors are the site of occurrence, extent of the lesion and degree of tumor aggressiveness.
Complete bone resection may be performed in some cases without severe functional harm to the patient, such as in cases involving the ulna, fibula and small bones of the hand and foot. The incidence of tumor recurrence is related to the type of treatment undertaken, and it is greater in cases treated with curettage, with or without associated use of bone grafting22, 23. Curettage should be accompanied by local adjuvant treatment, in an attempt to avoid survival of remaining cells(10). Current treatment methods for some lesions that are non-aggressive and in favorable locations include the use of methyl methacrylate, cryotherapy, electrocauterization, phenol and alcohol. In cases of aggressive lesions, with more accentuated bone destruction, the alternatives may include amputation proximal to the lesion, which is indicated for lesions of an advanced nature, cases presenting infection or cases that are undergoing malignant transformation. However, more usually, en-bloc resection is performed, with reconstruction by means of unconventional endoprostheses or biological reconstruction using grafts (autologous and/or homologous) or bone transportation. Radiotherapy has been proscribed because of the risk of malignant transformation during the treatment of these lesions, and embolization may be used for tumors that are shown to have abundant or non-resectable vascularization(1).
Reconstruction of the segment after lesion resection has been based on the radiographic staging proposed by Campanacci et al24, 25.
-
1–
Quiescent or intraosseous lesion;
-
2–
Active lesion with intact periosteum;
-
3–
Aggressive lesion, with destruction of the periosteum and invasion of the soft tissues.
In type I lesions, curettage can be performed in isolation from the lesion, although there may be recurrences. In type 2 and 3 lesions, complete resection of the lesion is indicated. Among the various ways of reconstructing the segment from which the tumoral lesion was resected, the main methods used are autografts, homografts, arthrodesis and unconventional endoprostheses.
The use of autografts is reserved for reconstruction of small defects within the treatment for quiescent and intraosseous tumors (Campanacci grade 1). Homografting techniques, with the use of grafts from a bone bank, have their place in cases of en-bloc resection, for cases with severely compromised joint surface. Arthrodesis, with the use of synthesis materials and some types of graft, can be performed in the presence of current infection or when the use of endoprostheses is impossible, particularly in cases of younger patients with greater physical demands and favorable life expectancy. The use of non-conventional endoprostheses has been described for reconstruction of large resected bone segments. However, because this is a benign neoplasm, some authors have preferred to use reconstruction methods that are more biological in nature for treating these lesions, and have emphasized the disadvantage of successive changes of prosthesis, which are expected particularly among affected patients in younger age groups, and because of the long survival presented by these patients(1).
According to Cassone et al(26), the reconstruction methods used after performing en-bloc resection of the proximal tibia encompass three basic problems: the major bone loss, the instability of the knee and the loss of the extensor mechanism. These authors stated that the main complications found were of mechanical and infectious nature. From a mechanical point of view, wear on the polyethylene and breakage due to weakness of the nail of the tibial component occur at low rates because the nail in the tibia coincides with the mechanical and anatomical axes of the limb, thereby reducing the stress.
MacDonald et al(27) reported that pathological fractures did not increase the chances of tumor recurrence. O'Donnell et al(28) and Renard et al(29) concluded that there was a correlation between occurrences of pathological fractures and tumor recurrence. Garcia Filho et al(1) postulated that perhaps the fracture would make the surgical approach more difficult and make the curettage less effective. They did not observe any relationship between tumor occurrences and pathological fractures with joint invasion, in cases of giant cell tumor.
The patient with a giant cell tumor of the present report had an active giant cell tumor and an intact periosteum. She had suffered a pathological fracture due to a fall from her own height, which resulted in worsened pain and functional impotence with regard to walking. It was decided to preserve the patient's joint surface by means of segmental resection and the use of an unconventional endoprosthesis for the proximal tibia because of her youth, the longevity observed among patients with this type of tumor and the possibility of successive surgical interventions to which the patient would be subjected if an endoprosthesis replacing the knee joint surface were to be implanted. The use of simple curettage and autografting were dismissed because of the extent and aggressiveness presented by the tumor, and the use of homografting was not available at our clinic at the time of the treatment that was implemented.
The patient now has few painful symptoms, satisfactory knee joint functional levels, range of motion within the limits of normality, without manual work limits (housework) without limits on activities of daily living. She has not presented any clinical or radiological evidence (radiographs and axial computed tomography scans) of tumor recurrence, and she remains without any need for surgical revision at present. The use of a partial cementless endoprosthesis for the tibia that preserved the knee joint was shown to have gratifying long-term clinical, radiological, functional and esthetic results.
ACKNOWLEDGEMENTS
Our thanks to: Juliana Carvalho Schleder – Specialist in Oncological Physiotherapy at Hospital Erasto Gaertner (HEG), Coordinator of the Postgraduate Course on Hospital Physiotherapy with Emphasis on Oncology, at HEG, person responsible for teaching and research in the Physiotherapy Sector at HEG and MSc student of Health Technology at PUCPR; Juliana Elizabet Jung – Pathologist at Hospital Erasto Gaertner; and Tânia Maria Carvalho Frigo – Secretary of CEPEP, Hospital Erasto Gaertner.
Footnotes
Work performed in the Orthopedic Surgery Service, Hospital Erasto Gaertner, Curitiba, PR.
REFERENCES
- 1.Garcia Filho RJ. Diagnóstico e tratamento de tumores ósseos. Elsevier; Rio de Janeiro: 2005. pp. 285–309. [Google Scholar]
- 2.Cooper A, Travers B. Surgical essays. Cox and Son; London: 1818. [Google Scholar]
- 3.Paget J. Lectures on surgical pathology. Longmans; London: 1853. [Google Scholar]
- 4.Bloodgood JC. Benign giant cell tumor of bone: its diagnosis and conservative treatment. Am J Surg. 1923;37:105–106. [Google Scholar]
- 5.Geschikter DF, Copeland NM. Tumors of bone. J.B. Lippincott; Philadelphia: 1949. [Google Scholar]
- 6.Willis GE. The pathology of osteoclastoma or giant cell tumors of bone. J Bone Joint Surg Br. 1949;31:236–238. [PubMed] [Google Scholar]
- 7.Jaffe HL, Lichtenstein L, Portis R. Giant cell tumor of bone. Pathologic appearance, grading, supposed variants and treatment. Arch Pathol. 1940;30:993–995. [Google Scholar]
- 8.Schajowics F. Giant cell tumors of bone (osteoclastoma): a pathological and histochemical study. J Bone Joint Surg Am. 1961;43(1):1–3. [Google Scholar]
- 9.Camargo OP. O estado da arte no diagnóstico e tratamento do tumor de células gigantes. Rev Bras Ortop. 2002;37(10):424–429. [Google Scholar]
- 10.Croci AT, Camargo OP, Oliveira NRB, Campos Filho R, Okane SY. Tumor de células gigantes em pacientes com imaturidade esquelética. Rev Bras Ortop. 1994;29(9):677–680. [Google Scholar]
- 11.Aegerter E, Kirkpatrick JA. Orthopaedic diseases. W.B. Saunders; Philadelphia: 1975. [Google Scholar]
- 12.Larsson SE, Lorentzon R, Boquist L. Giant-cell tumor of bone. A demographic, clinical, and histopathological study of all cases recorded in the Swedish Cancer Registry for the years 1958 through 1968. J Bone Joint Surg Am. 1975;57(2):167–173. [PubMed] [Google Scholar]
- 13.Lichtenstein L. Bone tumors. St. C.V. Mosby; Louis: 1972. [Google Scholar]
- 14.Jaffe HL. Tumors and tumorous conditions of the bones and joints. Lea & Febiger; Philadelphia: 1958. [Google Scholar]
- 15.Ottolenghi CE. Massive osteo and osteoarticular bone graft. Technic and results of 62 cases. Clin Orthop Relat Res. 1972;(87):87–94. [PubMed] [Google Scholar]
- 16.Garcia Filho RJ, Wajchenberg M, Justino MAF, Korukian M, Yshihara HI, Ponte FM. Tumor de células gigantes Análise da invasão articular, fratura patológica recidiva local e metástase para o pulmão. Rev Bras Ortop. 1997;32(11):854–856. [Google Scholar]
- 17.Cameron GW. Giant-cell tumor of the patella. J Bone Joint Surg Am. 1955;37(1):184–187. [PubMed] [Google Scholar]
- 18.Hutter RV, Worcester Junior JN, Francis KC. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653–690. doi: 10.1002/1097-0142(196207/08)15:4<653::aid-cncr2820150402>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Levine HA, Eurile F. Giant-cell tumor of patellar tendon coincident with Paget's disease. J Bone Joint Surg Am. 1971;53(2):335–341. [PubMed] [Google Scholar]
- 20.Seradge H. Distal ulnar translocation in the treatment of giant-cell tumors of the distal end of the radius. J Bone Joint Surg Am. 1982;64(1):67–68. [PubMed] [Google Scholar]
- 21.Carvalho DS, Kowacz PA. Avaliação da intensidade de dor. Migrâneas Cefaléias. 2006;9(4):164–168. [Google Scholar]
- 22.Campbell CJ, Akbarnia BA. Giant-cell tumor of the radiusrested by massive resection and tibial bone graft. J Bone Joint Surg Am. 1975;57(7):982–986. [PubMed] [Google Scholar]
- 23.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52(4):619–663. [PubMed] [Google Scholar]
- 24.Campanacci M. Giant cell tumor bone and soft tissue tumors. Wien: Springer-Verlg. p. 117-51.
- 25.Campanacci M, Baldini N, Boriani S. Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106–114. [PubMed] [Google Scholar]
- 26.Cassone AE, Capannacci R, Campanacci M. Reconstrução da tíbia proximal com prótese modular não cimentada após ressecção de tumores ósseos. Rev Bras Ortop. 1996;31(5):415–418. [Google Scholar]
- 27.McDonald D.J., Sim FH, Mcleod RA, Sim FH. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235–242. [PubMed] [Google Scholar]
- 28.O'Donnell RJ, Springfield DS, Motwani HK Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76(12):1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Renard AJ, Veth RP, Pruszczynski M, Wobbes T, Lemmens JA, van Horn JR. Giant cell tumor of bone: oncologic and functional results. J Surg Oncol. 1994;57(4):243–251. doi: 10.1002/jso.2930570408. [DOI] [PubMed] [Google Scholar]