Abstract
The most common result of BRCA1/2 mutation testing when performed in a family without a previously identified mutation is an uninformative negative test result. Women in these families may have an increased risk for breast cancer because of mutations in non-BRCA breast cancer predisposition genes, including moderate- or low-risk genes, or shared environmental factors. Genetic counselors often encourage counselees to share information with family members, however it is unclear how much information counselees share and the impact that shared information may have on accuracy of risk perception in family members. We evaluated 85 sisters and daughters of women who received uninformative negative BRCA1/2 results. We measured accuracy of risk perception using a latent variable model where accuracy was represented as the correlation between perceived risk (indicators = verbal and quantitative measures) and calculated risk (indicators = Claus and BRCAPRO). Participants who reported more information was shared with them by their sister or mother about her genetic counseling session had greater accuracy of risk perception (0.707, p = 0.000) than those who reported little information was shared (0.326, p = 0.003). However, counselees shared very little information; nearly 20 % of family members reported their sister or mother shared nothing with them about her genetic counseling. Family members were generally not aware of the existence of a genetic counseling summary letter. Our findings underscore the need for effective strategies that facilitate counselees to share information about their genetic counseling sessions. Such communication may help their relatives better understand their cancer risks and enhance risk appropriate cancer prevention.
Electronic supplementary material
The online version of this article (doi:10.1007/s10897-015-9866-0) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Genetic counseling, Uninformative negative BRCA1/2, Accuracy of risk perception, Risk assessment, Summary letter, Family communication, Claus, BRCAPRO, Gail, Numeracy, Cancer related distress
Introduction
Breast cancer risk assessment has implications for both patients and their family members. A cancer risk assessment includes evaluating the family pedigree and other risk factors, as well as providing individualized interpretation of genetic test results. Genetic counselors can help their patients understand what test results mean to them as well as their family members in terms of risk for future cancer and appropriate medical management. Counselors typically encourage their patients to share information and test results with family members and to encourage family members, in turn, to share information with their primary care providers (Riley et al. 2012). The purpose of this study was to determine whether the accuracy of sisters’ and daughters’ perceptions of their own risk for future breast cancer are improved when more information is shared by their family members who received genetic counseling and received uninformative negative BRCA1/2 test results. We hypothesized that women who receive more information from their family members will have risk perceptions more concordant with their calculated risks for breast cancer than those receiving less information.
Background
Genetic counselors are responsible for identifying at-risk family members and helping their patients communicate information to family members. However, patients often do not share information discussed during genetic counseling sessions with their family members, and what they do share is often inaccurate (Vos et al. 2011b). The National Society of Genetic Counselors (NSGC) recommends that an essential element of disclosure is to “identify at-risk family members and provide [the] patient with tools to inform and educate family members” (Riley et al. 2012, p. 158). Because of concerns about patient privacy the most common method counselors use to disseminate risk information within the family is to suggest that patients share the information with relatives (Forrest et al. 2010). Although patients are often willing to share information, research has shown that information is often not disseminated to all family members who may benefit from receiving it and the information that is shared is often inaccurate (Forrest et al. 2003; Hayat Roshanai et al. 2010; MacDonald et al. 2007; Vos et al. 2011b). Thus, many at-risk relatives lack critical information that could help them better understand their cancer risks and be aware of appropriate preventive and screening measures (Ersig et al. 2009; Vos et al. 2011b).
Much of the research on communication and risk perception among families at risk for breast cancer has focused on families with a known BRCA1/2 mutation. Yet, the most common outcome of BRCA1/2 testing is an uninformative negative result, meaning a negative result in the absence of a known family mutation. Members of these families may still be at elevated cancer risk. It is recommended that in the absence of an identified mutation, risk be estimated based on a pedigree evaluation including the types and ages of onset of cancer in a family (National Comprehensive Cancer Network 2013c). Pedigrees may reveal a familial pattern of cancer with an increased risk for breast cancer related to shared environment or low penetrance gene mutations (Berliner et al. 2013). While these families may not carry the same level of risk as BRCA1/2 positive families, risk may still be high enough to recommend earlier onset of screening, screening breast MRI and/ or chemoprevention (Freedman et al. 2011; National Comprehensive Cancer Network 2013b). Thus, risk assessment, and individualized communication about genetic test results by the counselor are important to family members beyond the counselor’s immediate patient, even in the presence of an uninformative negative BRCA1/2 test result.
A primary goal of genetic counseling is to help people accurately understand risk and make informed decisions based on personal risk (Hilgart et al. 2012; Riley et al. 2012; Smerecnik et al. 2009). It is believed that if people more accurately understand their risk, they are better prepared to take appropriate actions to reduce and manage this risk (Haas et al. 2005). Research assessing accuracy of risk perception related to breast cancer in families at elevated risk has primarily focused on women who received genetic counseling rather than their family members (Hilgart et al. 2012; Tilburt et al. 2011; Vos et al. 2011a). In the presence of an uninformative negative test result within the family, family members do not often come in for individual counseling. If they receive information about their risk, family members must generally rely on second-hand information shared by the counselee. Women with uninformative negative test results are less likely to share information obtained during genetic counseling sessions than those who tested positive for mutations (Patenaude et al. 2006).
Risk perceptions can be measured using verbal or numeric indicators. Health care providers tend to view successful risk communication as the transmission of precise information, expecting that patients should understand their risk as the health care provider does (Collins and Street 2009, p. 1507). Patients, on the other hand, may focus more on experiential reasoning to understand risk communications, drawing upon personal life experience and emotion (Collins and Street 2009). Indeed, many women have difficulty interpreting risk information especially when it is presented in a numeric format (Leventhal et al. 1999; Schwartz et al. 1997). Often women’s verbal and numerical risk estimates are not congruent (Smerecnik et al. 2009). Women tend to underestimate risk when using verbal comparative scales but overestimate their numeric risk (Lipkus et al. 2000; Woloshin et al. 1999). Thus, multiple measures of risk perception may more accurately assess the concept than a single indicator.
Methods
Study Population
Participants included sisters and daughters of women who had a personal history of breast cancer and received uninformative negative BRCA1/2 test results from a board certified genetic counselor. Participants were between the ages of 40–74. Women were excluded if they ever received breast cancer-related genetic testing, had received a prophylactic bilateral mastectomy or oopherectomy, had a personal history of any type of cancer other than non-melanoma skin cancer, and/or if they were of Ashkenazi Jewish descent as the associated high-risk status with this ancestry necessitates special consideration in evaluating risk.
Participants were referred to the study by their sisters or mothers with breast cancer who had received genetic counseling either as part of the Risk Education & Assessment for Cancer Heredity (REACH) study, a population-based randomized equivalency/non-inferiority cluster randomized trial of remote in-person vs. telephone BRCA1/2 counseling and testing or through the clinical genetic counseling service at Huntsman Cancer Institute (Kinney et al. 2014). The counselees who referred participants to our study had received both pre- and post-test genetic counseling either by telephone or in person (based on random assignment) by one of five genetic counselors from a single clinic. Additionally, counselees received standardized summary letters indicating that close relatives may be at increased risk for breast cancer and may need more intensive breast cancer surveillance, possibly including breast MRI. Counselees were encouraged to share this information with their close relatives (our potential participants) and encourage their relatives to share that information with their primary care providers.
Procedures
All procedures were approved by and in accordance with the ethical standards of the University of Utah Institutional Review Board and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study. Potential participants were mailed introductory letters followed by telephone calls to assess for eligibility and invite them to participate. Eligible women who agreed to participate were sent a packet in the mail with a survey and a family history collection tool. They also completed a telephone interview to review their family pedigree and ask additional questions. Prior to the telephone interview a pedigree was drawn from the self-reported family history. Women were asked if they wanted to receive their 5-year and lifetime risk estimates by the three models used. Women who completed the study were thanked with a mailed $25.00 prepaid gift card and, for those who desired, their breast cancer risk estimates.
Conceptual Framework
Our study was based broadly on the Common Sense Model of Self-regulation (CSM) by Leventhal et al. (2003). The CSM proposes that people respond to health threats with both cognitive and emotional reactions. Cognitive and emotional responses simultaneously influence one another and are the primary drivers of actions that people take to control the threat and control fear. It has been proposed that the CSM provides a strong framework for studying risk perception based on family history information (Marteau and Weinman 2006; Sivell et al. 2008). We view risk perception as a cognitive response to the health threat of breast cancer (having a close family member with breast cancer who has received genetic counseling and testing). This cognitive response can be simultaneously influenced by other cognitive factors (numeracy, knowledge, health literacy) as well as emotional factors (cancer related distress). Our primary aim was to evaluate the accuracy of perceived risk (as compared to calculated risk levels) while controlling for cognitive and emotional factors known to influence risk perception (Tilburt et al. 2011). Additionally we aimed to assess the moderating effect of the amount of information shared by the counselee on the accuracy of risk perception in the family member. Supplemental Figure 1 illustrates the original model with proposed covariates.
Measures
Risk Perception
The mailed survey included two questions about perceived risk for breast cancer. The initial question asked women to rate their risk perception verbally asking, “In your opinion, compared to other women your age, what are your chances of getting breast cancer?” Women could respond on a 5-point scale ranging from “much lower” to “much higher.” The second question presented women with a graphic showing 12 of 100 women shaded dark and stated, “On average 12 women out of 100 will get breast cancer in their lifetime.” Then the question instructed, “Picture yourself in a room with 100 women exactly like you (same risk-factors). How many of you will get breast cancer in your lifetime?” This question was accompanied by a picture of 100 women with none shaded and the statement, “you can pick any number between 0 and 100.” A frequency format with graphic has been shown to have a lower risk estimation error when compared to the percentage scales when estimating lifetime risk for breast cancer (Cameron et al. 2011; Schapira et al. 2004). Asking the qualitative question first and providing an anchor population has been shown to increase accuracy of risk perception (Apicella et al. 2009; Dillard et al. 2006; Taylor et al. 2002).
Calculated Risk
Five-year and lifetime risks were calculated using the Claus, BRCAPRO and Gail models. The ACS guideline specifically recommends using models such as BRCAPRO, Claus or Tyrer-Cuzick models (American Cancer Society 2014; Saslow 2007), while the NCCN guidelines list Claus, BRCAPRO, BOADICEA, and Tyrer-Cuzick as potential models to be used when calculating lifetime risk for breast cancer for the purpose of recommending breast MRI screening (National Comprehensive Cancer Network 2013b). We selected the BRCAPRO and Claus models because they were the models used by our site’s genetic counselors during data collection. Claus and BRCAPRO lifetime risk estimates were used as indicators for the latent variable calculated risk. We chose not to include the Gail calculations in the latent variable model because it does not take extensive family history into account and is therefore not an appropriate model to use for lifetime risk calculation when determining medical management (American Cancer Society 2014). The Gail model considers risk factors beyond the family history and is the most frequently used model in primary care.
Accuracy of Risk Perception
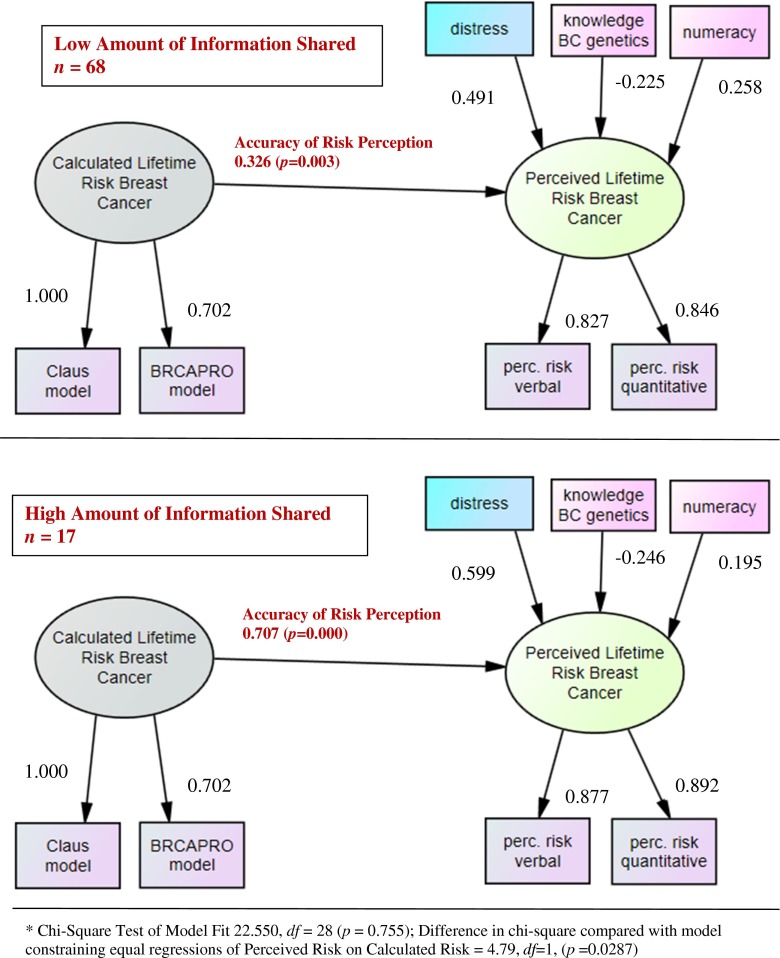
Some refer to the agreement between perceived risk and calculated risk as accuracy. Inherent in this terminology is the assumption that the calculations are correct and women are “accurate” if their perceptions are close to calculated estimates. However, every model calculates risk based on different factors and estimates may vary widely between models. Therefore we use the term “accuracy” recognizing that it is a common term in the literature, but the term “agreement” may hold less bias. We selected a novel approach to measuring accuracy of risk perception that does not require the calculation of a difference score or an arbitrary break in risk categories. We operationalized the concept of accuracy of risk perception as the level of agreement, or in statistical terms, the path coefficient, between the latent variables “calculated lifetime risk” for breast cancer and “perceived lifetime risk” (see Fig. 1 and section on statistical analysis).
Fig. 1.
Path diagrams of final model* illustrating the level of agreement between calculated risk and risk perception (accuracy of risk perception) based on the amount of information shared by sisters and mothers about genetic counseling sessions (standardized estimates)
Information Shared
Women were asked to rate how much information their sister or mother with breast cancer shared about her genetic counseling session on a scale of 0–5 with 0 indicating that the family member shared no information and 5 indicating a great deal of information shared. Similarly, women were asked to rate how well they understood the information shared on a 0–5 scale with 0 indicating that she understood none of the information and 5 indicating that she understood a great deal. We also asked whether women were aware of the summary letter, or the informational pamphlet that was provided to their sisters/mothers following their relative’s post-test genetic counseling.
Numeracy, Knowledge and Health Literacy
We assessed three cognitive variables known to influence risk perception including numeracy, knowledge and health literacy (Tilburt et al. 2011). Numeracy was measured using the 8-item Rausch-based numeracy scale that assesses the users’ ability to understand, manipulate and use numerical information including probabilities. Possible scores range from 0 to 8 with higher scores indicating higher levels of numeracy (Weller et al. 2012). The 27-item Breast Cancer Genetic Counseling Knowledge Questionnaire (BGKQ) was used to assess knowledge about breast cancer genetics. Scores could range from 0 to 27 with higher scores indicating higher levels of knowledge (Erblich et al. 2005). We assessed health literacy using the Set of Brief Questions developed by Chew et al. (2004). Each of the three questions has five response options that were scored from 0 to 4. Questions included, “How often do you have someone help you read hospital materials?” (“never” to” always”), “How confident are you filling out medical forms by yourself (“not at all” to “extremely”) and “How often do you have problems learning about your medical condition because of difficulty understanding written information?” (“never” to “always”). Total scores could range between 0 and 12 with higher levels indicating higher levels of health literacy.
Psychological Distress
Distress was measured to assess the emotional response to being at risk for breast cancer. We used the 15 item Impact of Event Scale (Horowitz et al. 1979) which asks about how frequently certain statements were true for the participant during the past 7 days ranging from “not at all” to “often.” Instructions to women were, “thinking about your family history of cancer, how often would you say…” followed by a list of comments made by people after stressful life events that are thought to be indicators of distress.
Statistical Analyses
Descriptive statistics (percent and frequencies) were calculated using IBM SPSS software version 21. Latent variable modeling was completed using Mplus software version 7 to test the hypothesis that the amount of information shared by women’s sisters or mothers who received genetic counseling would increase the accuracy of perceptions about their personal breast cancer risk.
Accuracy of risk perception was defined as the level of agreement (continuous variable) between risk perception and calculated risk. Figure 1 illustrates the accuracy of risk perception as the path arrow between “perceived lifetime risk for breast cancer” and “calculated lifetime risk for breast cancer.”
We treated “perceived lifetime risk for breast cancer” and “calculated lifetime risk for breast cancer as latent variables. Latent variables are constructs that effect outcome but are measured by other indicators (Borsboom et al. 2003; Byrne 2012; Kline 2010). For example, we expect that women have a certain perception of risk and this perception will influence the words they use to describe their risk (e.g., a verbal indicator such as “higher” or “lower” than average) and the number they select to describe their risk (e.g., a numeric indicator such as “15 %”). These measures capture overlapping aspects of risk perception and using both gives a more comprehensive view of risk perception.
Similarly, the latent variable “calculated lifetime risk” lacks a consensus or “gold standard” for measurement. Treating “calculated lifetime risk for breast cancer” as a latent variable with two observed indicators (Claus and BRCAPRO) allows stronger relationships to be revealed than using either measure alone. With latent variable modeling, relationships between the two latent variables can be more robust than multiple comparisons involving four observed indicators measured with error.
Although we calculated Gail scores we did not include those as indicators of calculated lifetime risk in our latent variable model because medical management for familial cancer risk is primarily based on selected features of a patient’s family history (National Comprehensive Cancer Network 2013a, b).
Given that risk perception is a complex concept our goal was to control for significant covariates that could complicate interpretation of our results. Selection of initial covariates was informed in part by (Tilburt et al. 2011). Only significant covariates were included in the final model. Covariates that were evaluated but ultimately not included in the final model included age, education and health literacy (see initial model in Supplemental Figure 1). Retained, significant covariates included numeracy, knowledge about breast cancer genetics and distress (see Fig. 1).
Moderating Effect of Information Shared on Accuracy of Risk Perception
To evaluate the key hypothesis, we relied on a classical understanding of moderation: is the relationship between two variables different at different levels of a third variable? In this study, we define “accuracy” as a relationship, the regression of perceived risk on calculated risk. A high regression relationship denotes high accuracy in this sense. The moderation question is whether the regression of perceived risk on calculated risk is different at high and low levels of information shared (the third variable).
To evaluate the moderating effect of information shared on the relationship between perceived and calculated lifetime risk for breast cancer we compared two models: one with moderation (allowing different slopes and intercepts for the regression of perceived risk on calculated risk) and one with no moderation (with slopes and intercepts equal across levels of information shared). We hypothesized that the amount of information shared by sisters and mothers about genetic counseling would moderate the relationship between a woman’s perception of her risk and objectively calculated risk (accuracy of risk perception).
The variable “amount of information shared” was stratified into high and low groups based on the amount of information women reported their family member had shared with them about the genetic counseling session. The low shared information group (n = 68) included women who responded between 0 and 3 and the high group (n = 17) included women who responded 4–5 on the Information Shared scale. Stratifying on the basis of all six options (0–5) was not feasible due to the small sample size.
Results
Demographics
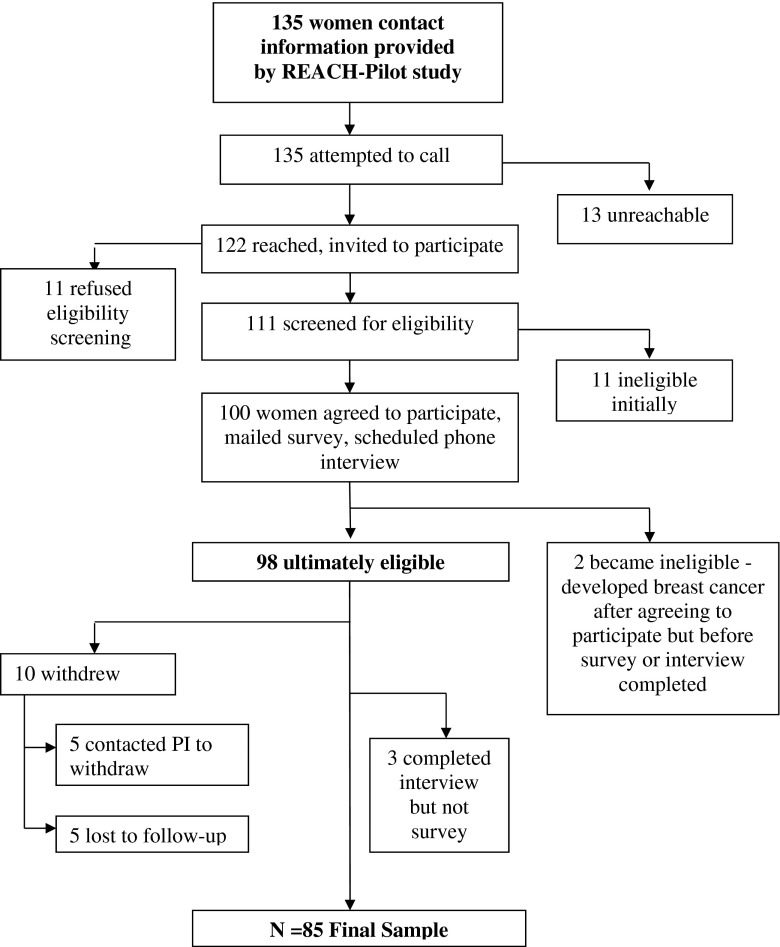
Of the 135 women who were mailed introductory letters, 98 were ultimately eligible and 85 completed both the survey and the telephone interview (see Fig. 2). Participant ages ranged from 40 to 71 with a mean of 52.2 (SD = 8.9). Nearly all women (98.8 %) reported their race and ethnicity as non-Hispanic White with one woman reporting her race as Asian. Age, race/ethnicity and educational level were similar between nonparticipants who shared demographic information (n = 11) and study participants (see Table 1).
Fig. 2.
Flow of Participants
Table 1.
Demographics of participants and nonparticipants
| Category | participants | nonparticipantsa | ||||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | M | (SD) | n | (%) | M | (SD) | |
| Age | 52.2 | (8.9) | 55.4 | 9.8 | ||||
| Race/ ethnicity | ||||||||
| Non-Hispanic White | 84 | (98.8) | 11 | (100.0) | ||||
| Asian | 1 | (1.2) | 0 | (0.0) | ||||
| Education | ||||||||
| High school/ GED | 13 | (15.3) | 0 | (0.0) | ||||
| Some college/ vocational | 32 | (37.6) | 6 | (54.5) | ||||
| 4 year degree | 28 | (32.9) | 2 | (1.8) | ||||
| Graduate degree | 12 | (14.1) | 3 | (2.7) | ||||
| Total | 85 | (100.0) | 11 | (100.0) | ||||
a Nonparticipant data were provided by 11 of 23 women who did not participate because they, refused screening, withdrew, were lost to follow-up, or became ineligible. Other nonparticipants refused to provide demographic data. Percentages are based on nonparticipants who provided data
Risk Perceptions
More women perceived their lifetime risk for breast cancer as being “higher” or “much higher” (53 %) than other women their age as opposed to “the same” (32.5 %), or “lower” or “much lower” (13.2 %) using verbal measures. On average women estimated their quantitative lifetime risk to be 25.62 % (SD = 19.94) with a range of 1-95 %.
Calculated Risk
Calculated 5-year and lifetime risk estimates by risk prediction model are presented in Table 2. Additionally, the percentage of women with lifetime risks greater than 15 and 20 % are delineated.
Table 2.
Calculated risk
| Model | [Range %] | M | (SD) | Lifetime | Lifetime | ||
|---|---|---|---|---|---|---|---|
| ≥15 % risk | ≥20 % risk | ||||||
| n | (%) | n | (%) | ||||
| Gail | |||||||
| 5-year | [0.6–12.0] | 3.14 | (2.3) | ||||
| Lifetime | [8.3–38.8] | 20.07 | (6.6) | 72 | (84.5) | 29 | (34.1) |
| Claus | |||||||
| 5-year | [0.2–5.7] | 2.15 | (1.2) | ||||
| Lifetime | [2.0–38.3] | 11.84 | (6.7) | 14 | (16.5) | 9 | (10.6) |
| BRCAPRO | |||||||
| 5-year | [0.8–2.2] | 1.24 | (0.5) | ||||
| Lifetime | [4.0–14.7] | 9.53 | (2.0) | 0 | (0.0) | 0 | (0.0) |
Summary Letter & Informational Pamphlet
Women were asked whether they were aware that their sister or mother received a summary letter about their genetic counseling session. Only 12 women (14.3 %) reported that they were aware of such a letter. Of those who were aware of a letter, seven women saw the letter and two were given a copy to keep. Three women reported that they shared information about the letter with their primary care provider. Two women reported that they provided their primary care provider a copy of the letter. The 12 women who indicated they were aware of the letter were asked to rate how strongly they felt that some of the information in the letter applied to them with “0” meaning that none of the information applies to them and “5” indicating that some of the information applies strongly to them. Of the 12, seven women rated the applicability of their sister or mother’s summary letter at “4” or greater.
Four women reported that they were aware of an informational pamphlet that their sister or mother received as a part of the genetic counseling session. Two women reported that they saw the pamphlet and one reported that she read it.
Covariates of Perceived Risk
Distress, knowledge, health literacy, numeracy, age and education were all considered as variables that could influence perceived risk. As shown in Table 3, overall, women had low levels of cancer-related distress, high levels of health literacy and average levels of numeracy and knowledge about breast cancer genetics. Health literacy, age and education were not significantly associated with risk perception and therefore were not included in the final latent variable model. The relationships between retained covariates and perceived risk are illustrated in Fig. 1 as the standardized path coefficients. Numeracy and distress were associated with higher risk perception, but knowledge was inversely associated with risk perception. Standardized path coefficients less than 0.10 indicate weak relationships, values around 0.30 represent a moderate association and values of 0.50 or more represent a strong relationship between constructs (Kline 2010).
Table 3.
Instrument performance: range, mean (SD) and estimates of internal consistency reliability
| Variable | [range] | mean | (SD) | Cronbach’s Alpha |
|---|---|---|---|---|
| Distress (Impact of Events Scale) | [1–46] | 8.20 | (11.1) | 0.890 |
| Health Literacy (Set of Brief Questions) | [6–12] | 10.91 | (1.3) | 0.511 |
| Knowledge about Breast Cancer Genetics (BGKQ) | [1–24] | 10.26 | (5.5) | 0.854 |
| Numeracy (Rausch Based Numeracy Scale) | [2–8] | 4.48 | (1.5) | 0.530 |
Note: n = 85 for all instruments except numeracy. One participant refused to answer all numeracy questions and was excluded from analysis of that instrument. The Impact of Events Scale categories are high distress > 19, medium 8.5–19 and low <8.5 (Horowitz et al. 1979)
Amount of Information Shared and Understanding
Generally, women rated the amount of information shared by their sisters and mothers about their genetic counseling sessions was low (see Table 4). Overall 18.8 % of women reported that their sisters or mothers did not share any information; these women were not asked how well they understood information shared. Women reported high levels of understanding the small amounts of information shared. As one woman stated during the interview, “all she told me was ‘I’m negative, but you should still get your mammograms’ – so that’s not hard to understand.” The families from which we recruited were not identified as having any BRCA1/2 mutations, therefore none of the sisters or mothers were likely to have a true negative test result. All of their relatives’ test results were uninformative negatives.
Table 4.
Sharing/ understanding of information from family member’s genetic counseling session
| Question | Response | n | (%) | M | SD | |
|---|---|---|---|---|---|---|
| Please rate on a scale of 0–5 how much information your sister/ mother shared with you about what she learned in her genetic counseling session, with zero being she shared nothing about the session to five being she shared a great deal. | Shared nothing | 0 | 16 | (18.8) | 2.04 | 1.53 |
| 1 | 20 | (23.5) | ||||
| 2 | 17 | (20.0) | ||||
| 3 | 15 | (17.6) | ||||
| 4 | 11 | (12.9) | ||||
| Shared a great deal | 5 | 6 | (7.1) | |||
| Total | 85 | (99.9) | ||||
| Please rate how well you understand the information she shared on a scale of 0–5 with zero being that you don’t understand it at all to five being that you understand a great deal. | Don’t understand at all | 0 | 0 | (0.0) | 3.57 | 1.49 |
| 1 | 4 | (4.7) | ||||
| 2 | 5 | (5.9) | ||||
| 3 | 15 | (17.6) | ||||
| 4 | 20 | (23.5) | ||||
| Understand a great deal | 5 | 25 | (29.4) | |||
| Total Valid | 69 | (81.2) | ||||
| Nothing was shared | 16 | (18.8) | ||||
| Total | 85 | (100.0) | ||||
Moderating Effect of Information Shared on Accuracy of Risk Perception
The amount of information shared by counselees about their genetic counseling sessions had a significant moderating effect on the accuracy of risk perception in their sisters and daughters who did not attend genetic counseling (see Fig. 1). In our sample, relatives who rated the amount of information shared by counselees as high had more than twice the accuracy (standardized path =0.707, p = 0.000) as those who rated the amount of information shared as low (standardized path = 0.326, p = 0.003) while controlling for distress, numeracy and knowledge about breast cancer genetics. (Perfect correlation between perceived and calculated risk (perfect accuracy) would yield a path coefficient of 1.0.) Thus in families where counselees were perceived to share high amounts of information, sisters and daughters had more accurate perceptions of their own lifetime risk for breast cancer.
A chi square test was conducted to assess significance of the difference of deviance between the models. A difference in chi-square between the model with moderation and the model where the groups were constrained to be equal was Chi square (df =1) =4.79, p = 0.0287. This indicates significant improvement in model fit when the high and low amount of information shared groups are not constrained to be equal. Fit indices for the alternative model assessing moderation effect of amount of information shared were: Chi-Square Test of Model Fit (df =28) = 22.550, p = .7552, RMSEA = 0.0000, 90 % CI [0.000–0.086]. A non-significant chi-square indicates that the model-implied covariance matrix is consistent with the population covariance matrix and supports the model, in other words, the model and the data are not significantly different (Kline 2010, pp. 193, 200). RMSEA is below 0.05 indicating good model fit. Thus the model that allowed for moderation was the better fitting model, indicating that increased amount of information shared by counselees about their genetic counseling sessions influences the accuracy of risk perception in their sisters and daughters who did not attend genetic counseling (see Fig. 1).
Discussion
A familial cancer risk assessment by its very nature produces information that is valuable to the entire family. Our study is among the first to demonstrate that the accuracy of risk perceptions is better among counselees’ relatives when they share more information about their genetic counseling session with them. In fact, with high amounts of information sharing, accuracy of risk perception in family members more than doubled. The salience of this finding is underscored given that genetic counselors often encourage their patients to share information with family members. Our study provides evidence that sharing makes a difference. It is noteworthy that the majority of our study’s participants reported that limited information was shared with them. Nearly 20 % reported that nothing at all was shared about their family member’s genetic counseling session and over 80 % were considered in the low amount of information shared group. Thus, our findings suggest that communication could be improved in families where an uninformative negative BRCA1/2 test has been found. These findings are consistent with other literature indicating that women with uninformative negative BRCA1/2 results are less likely to share their test results with family (Cheung et al. 2010; Patenaude et al. 2013). Our study is unique in that it elicited information about perceptions of the amount of information shared and did not focus specifically on test results.
The low amount of shared information does not necessarily mean that families do not communicate. Indeed, our participants were referred to the study by their sisters and mothers indicating that they had some contact with or knowledge about their family members. It is possible that the low amount of information shared about genetic counseling could indicate that the information provided during genetic counseling was not something counselees deemed worth sharing with their close biological relatives because the genetic test result might have been perceived as “negative”. Some women reported that their sisters or mothers told them the cancer was, “not hereditary.” The observed low level of information sharing in our study may be because the information was perceived as too complex to share in depth, or much of the information was shared but our participants had limited recall.
The sisters and mothers of our participants received genetic counseling according to a standardized protocol. A summary letter and an informational pamphlet were provided as part of test disclosure. Previous clinic patients had expressed the desire for an informational pamphlet specifically to help them explain cancer genetics to their family members. We initially thought that family members who were aware of or read the summary letter and the informational pamphlet might have more accurate risk perceptions. However, few women were aware of the letter (14.1 %) or pamphlet (3.5 %), and even fewer reported reading them; therefore we did not include these variables in our latent variable model. Although very few women were aware of a summary letter per se, we cannot rule out that the summary letter may have helped counselees convey information to their family members. Genetic counseling summary letters are not necessarily intended to be shared with family members; they are typically written for the woman herself, perhaps with a section that applies to the extended family.
It has been suggested that misperception of risk can increase or decrease use of screening and preventive services (Tilburt et al. 2011). Higher risk perceptions are generally associated with higher levels of screening (Katapodi et al. 2004; Walker et al. 2013). However, the ultimate goal is not to undiscerningly increase screening and risk perception, but to give women accurate risk information and achieve screening congruent with risk-based guidelines. The American Cancer Society and the National Comprehensive Cancer Network recommend that women with lifetime risk for breast cancer of greater than 20 % be screened annually with breast magnetic resonance imaging (MRI) in addition to mammography when risk is calculated with models that depend largely on family history (National Comprehensive Cancer Network 2013b; Saslow 2007). The American Cancer Society further suggests that there is currently not enough evidence to recommend for or against screening breast MRI when a woman’s estimated lifetime risk is moderately increased (between 15 and 20 %) (American Cancer Society 2014). Over 10 % of our participants were considered to be ≥20 % lifetime risk according to the Claus model, yet none of them had been offered or received screening breast MRI (Himes, et al., in preparation). Thus, whether they had high or low levels of accuracy or high or low amounts of information shared, the highest risk women in our study had not been offered risk-appropriate breast cancer screening.
Practice Implications
It is important that genetic counselors consider disclosure methods that balance confidentiality with obligation to identify and inform family members that may be at higher risk (Godard et al. 2006). The duty to warn would suggest that, at least for close biological relatives in whom high levels of risk are suspected, the genetic counselor should facilitate the sharing of information among family members (Offit et al. 2004; Stol et al. 2010; Suthers et al. 2006). Our findings suggest that encouraging patients to share information with family members and providing a summary letter is not enough. Women with an elevated lifetime risk for breast cancer may benefit from annual breast MRI (Berg et al. 2012) (Kriege et al. 2004). Yet most women at high risk are not receiving annual MRI (Cohen 2010). Approximately 10 % of sisters and daughters in our study population were considered at high risk but had never received an MRI nor had one recommended by their primary care provider (Himes, et al., in preparation). Women with uninformative negative BRCA1/2 tests may require more active psycho-educational interventions about what information to share with family members and strategies for sharing it. Genetic counselors might consider a letter addressed to family members that can be copied and hand delivered or mailed by the counselee or request consent from the counselee for a direct mailing of this information to their at-risk relatives. Online sharing portals can also be used for sharing information among family members. If family members are not aware of their risk they may not be aware of all screening and prevention options available to them.
Research Recommendations
Evidence-based genetic counseling interventions are needed to promote effective family communication, and readily provide consultation to family members and their primary care providers. It has been suggested that because many women have a difficult time understanding familial cancer risk information, interpreting it, and communicating this information to relatives, counselors should routinely either guide their patients in the communication process or inform relatives directly about risk and test results with counselee consent (Chan-Smutko et al. 2008; Godard et al. 2006; Seymour et al. 2010; Stol et al. 2010; Suthers et al. 2006; Vos et al. 2011a). There is some evidence that family members prefer receiving risk information from a healthcare provider rather than from their family member (Tunin et al. 2009). Future research is needed to determine the feasibility, acceptability and effectiveness of these strategies in the presence of uninformative negative BRCA1/2 results when pedigree analysis indicates family members may be at elevated risk. Specifically, researchers should seek to establish the link between accurate risk perception and adherence to risk-based surveillance among those at increased familial risk. Additionally, if this study is replicated in the future it may be helpful to include the Tyrer-Cuzick model for estimating breast cancer risk as part of the latent variable model.
Study Limitations
Our study population was virtually all non-Hispanic white, well-educated and limited to a single genetic counseling center limiting the generalizability of our findings to more underserved populations. Whenever family history is used to assess risk there is the potential for inaccurate or incomplete cancer information. However, the women in our study had sufficient time to collect the required family history data. Further, many participants conferred with other family members to obtain data as accurately as possible, enhancing validity of family history information. Studies comparing self-reported family history with verified cases have yielded a high sensitivity for breast cancer when compared to validation by chart review (83–97 %) (Kerber and Slattery 1997) (Parent et al. 1997). Because our study participants had participated in a previous study about breast cancer prevention and that we asked them to collect family history information for pedigree analysis, it is possible that they may have heightened awareness of breast cancer yielding higher distress scores and inflated risk perceptions. However, this does not appear to be the case given that overall risk perceptions and distress scores were relatively low.
Conclusion
The most common result of BRCA1/2 mutation testing when performed in a family without a previously identified mutation is the uninformative negative test result. Women in these families may have an increased risk for breast cancer based on family history. We evaluated sisters and daughters of women who received an uninformative negative BRCA1/2 results and found that most of them reported receiving very little information about the counseling session from their sister or mother. When more information was shared about the genetic counseling session, sisters and daughters had more accurate perceptions of their own risks for breast cancer. However, our study participants reported that very little information was shared. At-risk female relatives of counselees are generally not aware of the existence of a genetic counseling summary letter. Genetic counselors need to explore new ways to help their patients share information with family members to help family members perceive their risk for breast cancer more accurately which could potentially allow family members to pursue risk appropriate screening and prevention measures.
Electronic supplementary material
(DOCX 67 kb)
Acknowledgement
Thanks to Amanda Gammon, MS CGC, Genetic Counselor, Huntsman Cancer Institute.
This study was funded by the Dr. Elaine D. Dyer Research Endowment Award as well as a grant from the Brigham Young University Research and Scholarship Council. Previous research with first-degree relatives of current participants was supported by grants from the National Cancer Institute at the National Institutes of Health (1R01CA129142 to AYK and U01 CA152958, K05 CA096940, and U01 CA183081) and the Huntsman Cancer Foundation. The project was also supported by the Shared Resources (P30 CA042014) at Huntsman Cancer Institute (Biostatistics and Research Design, Genetic Counseling, Research Informatics, and the Utah Population Database [UPDB]); the Utah Cancer Registry, which is funded by Contract No. HHSN261201000026C from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program with additional support from the Utah State Department of Health and the University of Utah; the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764).
Conflict of Interest
Authors Deborah O. Himes, Margaret F. Clayton, Gary Donaldson, Lee Ellington, Saundra Buys and Anita Y. Kinney declare that they have no conflict of interest.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
References
- American Cancer Society. (2014). Breast Cancer: Early detection. Retrieved 1/20/2014, 2014, from http://www.cancer.org/acs/groups/cid/documents/webcontent/003165-pdf.pdf.
- Apicella C, Peacock SJ, Andrews L, Tucker K, Daly MB, Hopper JL. Measuring, and identifying predictors of women’s perceptions of three types of breast cancer risk: population risk, absolute risk and comparative risk. British Journal of Cancer. 2009;100(4):583–589. doi: 10.1038/sj.bjc.6604910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. The Journal of the American Medical Association. 2012;307(13):1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner JL, Fay AM, Cummings SA, Burnett B, Tillmanns T. NSGC practice guideline: risk assessment and genetic counseling for hereditary breast and ovarian cancer. Journal of Genetic Counseling. 2013;22(2):155–163. doi: 10.1007/s10897-012-9547-1. [DOI] [PubMed] [Google Scholar]
- Borsboom D, Mellenbergh GJ, van Heerden J. The theoretical status of latent variables. Psychological Review. 2003;110(2):203–219. doi: 10.1037/0033-295X.110.2.203. [DOI] [PubMed] [Google Scholar]
- Byrne BM. Structural equation modeling with Mplus. New York: Routledge; 2012. [Google Scholar]
- Cameron LD, Marteau TM, Brown PM, Klein WM, Sherman KA. Communication strategies for enhancing understanding of the behavioral implications of genetic and biomarker tests for disease risk: the role of coherence. Journal of Behavioral Medicine. 2011 doi: 10.1007/s10865-011-9361-5. [DOI] [PubMed] [Google Scholar]
- Chan-Smutko G, Patel D, Shannon KM, Ryan PD. Professional challenges in cancer genetic testing: who is the patient? The Oncologist. 2008;13(3):232–238. doi: 10.1634/theoncologist.2007-0203. [DOI] [PubMed] [Google Scholar]
- Cheung EL, Olson AD, Yu TM, Han PZ, Beattie MS. Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiology, Biomarkers & Prevention. 2010;19(9):2211–2219. doi: 10.1158/1055-9965.EPI-10-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Family Medicine. 2004;36(8):588–594. [PubMed] [Google Scholar]
- Cohen, N. N. (2010). Breast cancer screening and risk reduction measures in high-risk women: Are women receiving recommendations in accordance with guidelines and are they performing these strategies? (M.S. 1485005), New York: Sarah Lawrence College. Retrieved from http://search.proquest.com/docview/518769099?accountid=4488 ProQuest Dissertations & Theses (PQDT) database.
- Collins DL, Street RL., Jr A dialogic model of conversations about risk: coordinating perceptions and achieving quality decisions in cancer care. Social Science & Medicine. 2009;68(8):1506–1512. doi: 10.1016/j.socscimed.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Dillard AJ, McCaul KD, Kelso PD, Klein WM. Resisting good news: reactions to breast cancer risk communication. Health Communication. 2006;19(2):115–123. doi: 10.1207/s15327027hc1902_3. [DOI] [PubMed] [Google Scholar]
- Erblich J, Brown K, Kim Y, Valdimarsdottir HB, Livingston BE, Bovbjerg DH. Development and validation of a breast cancer genetic counseling knowledge questionnaire. Patient Education And Counseling. 2005;56(2):182–191. doi: 10.1016/j.pec.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Ersig AL, Williams JK, Hadley DW, Koehly LM. Communication, encouragement, and cancer screening in families with and without mutations for hereditary nonpolyposis colorectal cancer: a pilot study. Genetics in Medicine. 2009;11(10):728–734. doi: 10.1097/GIM.0b013e3181b3f42d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest K, Simpson SA, Wilson BJ, van Teijlingen ER, McKee L, Haites N, Matthews E. To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clinical Genetics. 2003;64(4):317–326. doi: 10.1034/j.1399-0004.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- Forrest LE, Delatycki MB, Curnow L, Skene L, Aitken M. Genetic health professionals and the communication of genetic information in families: practice during and after a genetic consultation. American Journal of Medical Genetics. 2010;152A(6):1458–1466. doi: 10.1002/ajmg.a.33385. [DOI] [PubMed] [Google Scholar]
- Freedman AN, Yu B, Gail MH, Costantino JP, Graubard BI, Vogel VG, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. Journal of Clinical Oncology. 2011;29(17):2327–2333. doi: 10.1200/JCO.2010.33.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard B, Hurlimann T, Letendre M, Egalite N. Guidelines for disclosing genetic information to family members: from development to use. Familial Cancer. 2006;5(1):103–116. doi: 10.1007/s10689-005-2581-5. [DOI] [PubMed] [Google Scholar]
- Haas JS, Kaplan CP, Des Jarlais G, Gildengoin V, Perez-Stable EJ, Kerlikowske K. Perceived risk of breast cancer among women at average and increased risk. Journal of Women’s Health. 2005;14(9):845–851. doi: 10.1089/jwh.2005.14.845. [DOI] [PubMed] [Google Scholar]
- Hayat Roshanai A, Lampic C, Rosenquist R, Nordin K. Disclosing cancer genetic information within families: perspectives of counselees and their at-risk relatives. Familial Cancer. 2010;9(4):669–679. doi: 10.1007/s10689-010-9364-3. [DOI] [PubMed] [Google Scholar]
- Hilgart, J. S., Coles, B., & Iredale, R. (2012). Cancer genetic risk assessment for individuals at risk of familial breast cancer. Cochrane Database Systematic Reviews, (2). doi: 10.1002/14651858.CD003721.pub3. [DOI] [PubMed]
- Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosomatic Medicine. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Preventive Medicine. 2004;38(4):388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer. Data in a case–control study. American Journal of Epidemiology. 1997;146(3):244–248. doi: 10.1093/oxfordjournals.aje.a009259. [DOI] [PubMed] [Google Scholar]
- Kinney, A. Y., Butler, K. M., Schwartz, M. D., Mandelblatt, J. S., Boucher, K. M., Pappas, L. M., et al. (2014). Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. Journal of the National Cancer Institute, 106(12). doi: 10.1093/jnci/dju328. [DOI] [PMC free article] [PubMed]
- Kline RB. Principles and practice of structural equation modeling. 3. New York: Guilford Press; 2010. [Google Scholar]
- Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. The New England Journal of Medicine. 2004;351(5):427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Brissette I, Leventhal EA. The Common-Sense Model of self-regulation of Health and Illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. New York: Routledge; 2003. pp. 42–65. [Google Scholar]
- Leventhal H, Kelly K, Leventhal EA. Population risk, actual risk, perceived risk, and cancer control: a discussion. Journal of the National Cancer Institute. 1999;25:81–85. doi: 10.1093/oxfordjournals.jncimonographs.a024214. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Kuchibhatla M, McBride CM, Bosworth HB, Pollak KI, Siegler IC, Rimer BK. Relationships among breast cancer perceived absolute risk, comparative risk, and worries. Cancer Epidemiolgy, Biomarkers & Prevention. 2000;9(9):973–975. [PubMed] [Google Scholar]
- MacDonald DJ, Sarna L, van Servellen G, Bastani R, Giger JN, Weitzel JN. Selection of family members for communication of cancer risk and barriers to this communication before and after genetic cancer risk assessment. Genetics in Medicine. 2007;9(5):275–282. doi: 10.1097/GIM.0b013e31804ec075. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Social Science & Medicine. 2006;62(6):1360–1368. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2013a). NCCN clinical practice guidelines in oncology (NCCN Guidelines): Breast cancer risk reduction. Version 1.2013. 2014, from NCCN.org.
- National Comprehensive Cancer Network. (2013b). NCCN clinical practice guidelines in oncology (NCCN Guidelines): Breast cancer screening and diagnosis. Version 2.2013. 2014, from NCCN.org.
- National Comprehensive Cancer Network. (2013c). NCCN clinical practice guidelines in oncology (NCCN Guidelines): Genetic/familial high-risk assessment: Breast and ovarian. Version 4.2013. 2014, from NCCN.org.
- Offit K, Groeger E, Turner S, Wadsworth EA, Weiser MA. The “duty to warn” a patient’s family members about hereditary disease risks. The Journal of the American Medical Association. 2004;292(12):1469–1473. doi: 10.1001/jama.292.12.1469. [DOI] [PubMed] [Google Scholar]
- Parent ME, Ghadirian P, Lacroix A, Perret C. The reliability of recollections of family history: implications for the medical provider. Journal of Cancer Education. 1997;12(2):114–120. doi: 10.1080/08858199709528465. [DOI] [PubMed] [Google Scholar]
- Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, Garber JE. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. Journal Of Clinical Oncology: Official Journal Of The American Society Of Clinical Oncology. 2006;24(4):700–706. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- Patenaude AF, Tung N, Ryan PD, Ellisen LW, Hewitt L, Schneider KA. Young adult daughters of BRCA1/2 positive mothers: what do they know about hereditary cancer and how much do they worry? Psycho-Oncology. 2013 doi: 10.1002/pon.3257. [DOI] [PubMed] [Google Scholar]
- Riley BD, Culver JO, Skrzynia C, Senter LA, Peters JA, Costalas JW, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the national society of genetic counselors. Journal of Genetic Counseling. 2012;21(2):151–161. doi: 10.1007/s10897-011-9462-x. [DOI] [PubMed] [Google Scholar]
- Saslow D. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA - A Cancer Journal for Clinicians. 2007;57(3):185. doi: 10.3322/canjclin.57.3.185. [DOI] [PubMed] [Google Scholar]
- Schapira MM, Davids SL, McAuliffe TL, Nattinger AB. Agreement between scales in the measurement of breast cancer risk perceptions. Risk Analysis. 2004;24(3):665–673. doi: 10.1111/j.0272-4332.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Annals of Internal Medicine. 1997;127(11):966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- Seymour KC, Addington-Hall J, Lucassen AM, Foster CL. What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. Journal of Genetic Counseling. 2010;19(4):330–342. doi: 10.1007/s10897-010-9296-y. [DOI] [PubMed] [Google Scholar]
- Sivell S, Elwyn G, Gaff CL, Clarke AJ, Iredale R, Shaw C, et al. How risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: systematic review. Journal of Genetic Counseling. 2008;17(1):30–63. doi: 10.1007/s10897-007-9132-1. [DOI] [PubMed] [Google Scholar]
- Smerecnik CM, Mesters I, Verweij E, de Vries NK, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. Journal of Genetic Counseling. 2009;18(3):217–228. doi: 10.1007/s10897-008-9210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stol YH, Menko FH, Westerman MJ, Janssens RM. Informing family members about a hereditary predisposition to cancer: attitudes and practices among clinical geneticists. Journal of Medical Ethics. 2010;36(7):391–395. doi: 10.1136/jme.2009.033324. [DOI] [PubMed] [Google Scholar]
- Suthers GK, Armstrong J, McCormack J, Trott D. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. Journal of Medical Genetics. 2006;43(8):665–670. doi: 10.1136/jmg.2005.039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KL, Shelby RA, Schwartz MD, Ackerman J, LaSalle VH, Gelmann EP, McGuire C. The impact of item order on ratings of cancer risk perception. Cancer Epidemiology, Biomarkers & Prevention. 2002;11(7):654–659. [PubMed] [Google Scholar]
- Tilburt JC, James KM, Sinicrope PS, Eton DT, Costello BA, Carey J, et al. Factors influencing cancer risk perception in high risk populations: a systematic review. Hereditary Cancer in Clinical Practice. 2011;9:2. doi: 10.1186/1897-4287-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunin R, Uziely B, Wolosik-Wruble AC. First degree relatives of women with breast cancer: who’s providing information and support and who’d they prefer. Psycho-Oncology. 2009;19:423–430. doi: 10.1002/pon.1596. [DOI] [PubMed] [Google Scholar]
- Vos J, Jansen AM, Menko F, van Asperen CJ, Stiggelbout AM, Tibben A. Family communication matters: the impact of telling relatives about unclassified variants and uninformative DNA-test results. Genetics in Medicine. 2011;13(4):333–341. doi: 10.1097/GIM.0b013e318204cfed. [DOI] [PubMed] [Google Scholar]
- Vos J, Menko F, Jansen AM, van Asperen CJ, Stiggelbout AM, Tibben A. A whisper-game perspective on the family communication of DNA-test results: a retrospective study on the communication process of BRCA1/2-test results between proband and relatives. Familial Cancer. 2011;10(1):87–96. doi: 10.1007/s10689-010-9385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MJ, Chiarelli AM, Knight JA, Mirea L, Glendon G, Ritvo P. Perceived risk and adherence to breast cancer screening guidelines among women with a familial history of breast cancer: a review of the literature. Breast. 2013;22(4):395–404. doi: 10.1016/j.breast.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Weller JA, Dieckmann NF, Tusler M, Mertz CK, Burns WJ, Peters E. Development and testing of an abbreviated numeracy scale: a Rasch analysis approach. Journal of Behavioral Decision Making. 2012 doi: 10.1002/bdm.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshin S, Schwartz LM, Black WC, Welch HG. Women’s perceptions of breast cancer risk: how you ask matters. Medical Decision Making. 1999;19(3):221–229. doi: 10.1177/0272989X9901900301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 67 kb)