Abstract
Purpose
To evaluate the effects of lanreotide Autogel on patient-reported outcomes and association with biochemical control, using PRIMARYS data.
Methods
PRIMARYS was a 1-year, open-label study of lanreotide Autogel (Depot in USA) 120 mg every 4 weeks in 90 treatment-naïve patients with acromegaly. Symptoms were assessed using Patient-assessed Acromegaly Symptom Questionnaire (PASQ) and health-related quality of life (HRQoL) using the AcroQoL questionnaire. Correlations between PASQ and AcroQoL scores, and between PASQ/AcroQoL and growth hormone (GH)/insulin-like growth factor-1 (IGF-1) levels were also evaluated (post hoc).
Results
Acromegaly symptoms and HRQoL significantly improved from week 12 to week 48, with modest correlations at week 48 between PASQ total score (R = –0.55, p < 0.0001) and AcroQoL global and physical scores (R = –0.67, p < 0.0001). Approximately 60 % of patients achieved a minimal important difference (MID; improvement >50 % of baseline standard deviation) in PASQ total score and >40 % achieved a MID in AcroQoL global score (post hoc). Changes in PASQ scores were similar in biochemically controlled (GH levels ≤2.5 μg/L and normal IGF-1 levels) and uncontrolled groups, while changes in global and psychological AcroQoL scores were greater in the controlled group. There was no correlation between changes in PASQ or AcroQoL scores and changes in GH or IGF-1 levels.
Conclusions
Primary treatment with lanreotide Autogel over 1 year was associated with rapid and sustained improvements in clinical signs and symptoms and HRQoL in patients with acromegaly. Improvements in HRQoL, but not symptoms, were greater in those achieving biochemical control (ClinicalTrials.gov: NCT00690898; EudraCT: 2007–000155–34).
Electronic supplementary material
The online version of this article (doi:10.1007/s11102-015-0693-y) contains supplementary material, which is available to authorized users.
Keywords: Acromegaly, Lanreotide Autogel, Quality of life, Symptoms
Introduction
Acromegaly is a multisystem disease, characterized by somatic overgrowth, multiple comorbidities (including diabetes and cardiovascular disorders), premature mortality, and physical disfigurement [1, 2]. Perhaps not surprisingly, acromegaly is associated with psychological issues such as mood swings, impaired self-esteem, and disruptions in interpersonal relationships [2]. Furthermore, studies have shown that patients with acromegaly have impaired health-related quality of life (HRQoL), measured using both generic and disease-specific (AcroQoL) questionnaires [3, 4].
Treatment options for acromegaly include surgery, drug treatment, and radiotherapy. Goals of treatment are amelioration of clinical signs and symptoms, reduction of morbidity and mortality, and control of GH and IGF-1 hypersecretion and tumor growth [1, 5, 6]. The somatostatin analogs (SSAs), which include lanreotide Autogel (Depot in the USA), are largely recognized for their role in patients whose disease persists despite surgical intervention [1, 5, 6]. In addition, a number of guidelines on the management of acromegaly advocate the first-line use of long-acting SSAs in those who cannot be cured using surgery or who are poor surgical candidates [1, 5, 6]. SSAs have been shown to improve acromegaly symptoms and limit GH/IGF-1 hypersecretion and tumor growth [7–10]. There is also evidence that SSAs improve HRQoL in acromegaly [11–13].
PRIMARYS was a robust, 1-year study designed to evaluate the effect of primary treatment with lanreotide Autogel 120 mg every 4 weeks in 90 treatment-naïve patients with acromegaly [7]. The results showed that clinically significant tumor shrinkage (≥20 %) was achieved in 63 % of patients and there were early and sustained improvements in growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels, acromegaly symptoms, and HRQoL [7]. Lanreotide Autogel was well tolerated, with no patients discontinuing treatment due to gastrointestinal intolerance. The patient population enrolled in PRIMARYS was particularly homogeneous: all patients were treatment naïve and all were treated first-line with the highest available dose of lanreotide Autogel from the outset (i.e. there was no dose titration) in order to achieve rapid efficacy. In view of this, the relatively large number of patients enrolled, and the long duration of follow-up, we conducted a more in-depth analysis to further investigate the effects of treatment on patient-reported outcomes.
Methods
The methods used for this trial (ClinicalTrials.gov: NCT00690898 and EudraCT: 2007–000155–34) have previously been described in detail [7].
Patients
The study included treatment-naïve men and women, aged 18–75 years, diagnosed with acromegaly, with mean GH levels >1 µg/L and IGF-1 levels above the normal age- and sex-matched range. All patients had a GH-secreting macroadenoma (diameter ≥10 mm) without visual field defects. Patients were excluded if they had: undergone or were likely to need pituitary surgery or radiotherapy; previously received treatment with a SSA, dopamine agonist, or GH receptor antagonist; prolactin co-secretion >100 µg/L; optic nerve disease or any visual abnormality that may have worsened during the trial; or a glomerular filtration rate <30 mL/min/1.73 m2.
Patients could be withdrawn from the study if they had an insufficient IGF-1 response (<10 % reduction in IGF–1 at Week 24 or an inadequate response in the investigator’s opinion), if there was suspicion of visual field deterioration, in the event of a post-baseline prolactin level >100 µg/L, or if there were any other safety concerns.
Trial design and interventions
This was an international, 48-week, open-label, single-arm phase 3b trial, conducted in 27 specialist endocrine centers in nine countries (Belgium, Czech Republic, Finland, France, Germany, Italy, The Netherlands, Turkey, and the United Kingdom) between May 20, 2008 and February 13, 2012. Patients received twelve injections of lanreotide Autogel 120 mg by deep subcutaneous injection every 28 days. Dose titration was not permitted.
All patients gave written informed consent to participate before the start of the trial, which was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and all local regulatory requirements. Trial documentation was approved by institutional review boards before the start of the trial.
Assessments and endpoints
Trial visits were conducted at baseline (week 1) and at weeks 12, 24, and 48. GH and IGF-1 levels were measured at each trial visit, as described previously [7]. The primary endpoint (change from baseline in pituitary tumor volume at week 48) and all secondary endpoints of the trial have been reported previously; here we present further detailed analyses on changes in acromegaly symptoms and changes in HRQoL. Symptoms were assessed at each visit using the Patient-assessed Acromegaly Symptom Questionnaire (PASQ), which allows patients to rate five key symptoms (headache, excessive perspiration, fatigue, soft tissue swelling, arthralgia) on a 9-point scale (0, no symptoms; 8, severe incapacitating symptoms) [14]. The total PASQ score is the sum of the individual symptom scores (maximum = 40). HRQoL was assessed at each visit using the AcroQoL questionnaire (except Finland and Turkey, where validated translations were not available). The AcroQoL questionnaire is a reliable, validated tool [3] that assesses global HRQoL, physical performance, and psychological wellbeing. The psychological wellbeing dimension is further divided into sub-dimensions for ‘appearance’ and ‘personal relationships’. Scores were standardized from 0 to 100, with higher scores in all cases representing better HRQoL.
Statistical analyses
Summary statistics were based on the intention-to-treat (ITT) population and calculated for PASQ and AcroQoL scores at baseline, weeks 12, 24, and 48, and last post-baseline value available (LVA), and for the change in scores from baseline at these timepoints. Post hoc analyses were conducted to determine the percentage of patients with minimally important differences (MIDs) in PASQ and AcroQoL scores, defined as a change (improvement) >50 % of the baseline standard deviation (SD) of each score [15]. Post hoc analyses were also conducted to evaluate the following correlations in the overall population: total PASQ score versus AcroQoL global, physical and psychological dimension scores (Pearson correlation); individual PASQ symptom scores versus AcroQoL global, physical and psychological dimension scores (Kendall correlation); total PASQ score versus IGF-1 and GH levels, and AcroQoL global and subscale scores versus IGF-1 and GH levels. In all cases, correlations were conducted for absolute scores at week 48 and for changes in scores from baseline to week 48. Finally, post hoc analyses were conducted to evaluate baseline characteristics, total PASQ score, and global and physical AcroQoL dimension scores in patients with and without biochemical control, defined as GH levels ≤2.5 μg/L and normal IGF-1 levels, at LVA.
Results
Patient disposition and baseline characteristics
Ninety patients received treatment and 89 were included in the ITT population; 26 patients (29 %) withdrew before week 48 and 64 patients (71 %) completed the trial. The most common reason for withdrawal was insufficient IGF-1 response as per protocol at week 12 (n = 18), followed by consent withdrawn (n = 4), adverse events (n = 3), and other reasons (n = 1). One patient in the ITT population did not have data available on biochemical control; for this reason, there were 88 patients in the overall population in the current analyses. Of these, all completed the PASQ at baseline and 83 completed the AcroQoL questionnaire.
Baseline demography and clinical characteristics are summarized in Table 1 for the overall population and for those with and without biochemical control, and in Supplementary Table S1 for the 18 patients who withdrew because of insufficient IGF-1 response. Patients with biochemical control (GH ≤2.5 µg/L and IGF-1 < ULN) at LVA were significantly older at baseline than those in the uncontrolled group at LVA, and had significantly lower baseline IGF-1 levels (Table 1). Patients with biochemical control tended to be female (70 vs. 45 % in the uncontrolled group) and tended to have lower median baseline tumor volumes (1339 vs. 1786 mm3 in the uncontrolled group). There were no statistically or clinically significant differences in baseline characteristics between the group who withdrew because of insufficient response and the ITT population excluding these 18 patients (Table 1).
Table 1.
Baseline demography and disease characteristics in the overall population and according to achievement of biochemical control
| Overall population (n = 88)a | Patients achieving biochemical control (n = 30) | Patients not achieving biochemical control (n = 58) | |
|---|---|---|---|
| Age, years | 49.5 (46.9, 52.1) | 56.1 (52.4, 59.7) | 46.1 (42.8, 49.3) |
| Female, n | 47 | 21 | 26 |
| % (95 % CI) | 53.4 (42.5, 64.1) | 70.0 (50.6, 85.3) | 44.8 (31.7, 58.5) |
| BMI, kg/m2 | 27.7 (26.7, 28.7) | 26.4 (24.9, 27.9) | 28.4 (27.1, 29.7) |
| Time since acromegaly diagnosis, days | 121.1 (84.6, 157.6) | 147.0 (44.8, 249.3) | 107.3 (87.1, 127.5) |
| Maximum tumor diameter, mm | |||
| Mean (95 % CI) | 18.9 (17.4, 20.4) | 17.3 (15.6, 19.1) | 19.8 (17.7, 21.9) |
| Median (interquartile range) | 18.1 (13.9, 21.8) | 17.2 (13.4, 20.2) | 18.2 (14.2, 23.0) |
| Maximum tumor volume, mm3 | |||
| Mean (95 % CI) | 2731 (2033, 3428) | 1822 (1211, 2433) | 3201 (2199, 4203) |
| Median (interquartile range) | 1653 (866, 3370) | 1339 (811, 2325) | 1786 (958, 4404) |
| GH level, µg/L | |||
| Mean (95 % CI) | 15.2 (11.2, 19.2) | 13.0 (6.6, 19.3) | 16.3 (11.1, 21.6) |
| Median (interquartile range) | 8.6 (3.8, 16.8) | 6.4 (2.9, 14.9) | 9.7 (4.6, 17.3) |
| IGF-1 level, µg/L | |||
| Mean (95 % CI) | 796 (735, 857) | 665 (563, 767) | 863 (792, 935) |
| Median (interquartile range) | 778 (594, 973) | 630 (472, 794) | 805 (674, 1010) |
| IGF-1 levels, % of ULN | 285 (260.6, 309.3) | 249 (211.2, 286.7) | 304 (272.6, 334.6) |
| PASQ scores, mean (95 % CI) | |||
| Total | 19.4 (17.7, 21.1) | 19.8 (16.9, 22.7) | 19.2 (17.0, 21.5) |
| Headache | 2.8 (2.2, 3.3) | 2.0 (1.2, 2.9) | 3.1 (2.5, 3.8) |
| Excessive perspiration | 4.0 (3.4, 4.5) | 4.1 (3.2, 5.1) | 3.9 (3.2, 4.6) |
| Fatigue | 4.5 (4.0, 5.0) | 5.0 (4.1, 5.9) | 4.3 (3.7, 4.9) |
| Soft tissue swelling | 4.3 (3.7, 4.8) | 4.6 (3.7, 5.5) | 4.1 (3.5, 4.7) |
| Arthralgia | 3.9 (3.4, 4.5) | 4.1 (3.2, 5.0) | 3.8 (3.1, 4.6) |
| AcroQoL scoresb, mean (95 % CI) | |||
| Global | 56.0 (52.5, 59.5) | 50.9 (45.1, 56.7) | 58.8 (54.4, 63.1) |
| Physical | 54.6 (50.4, 58.8) | 52.2 (44.6, 59.7) | 55.9 (50.7, 61.1) |
| Psychological | 56.9 (53.1, 60.6) | 50.2 (44.5, 56.0) | 60.5 (55.7, 65.2) |
| Psychological (appearance) | 41.4 (37.0, 45.7) | 34.1 (27.8, 40.4) | 45.4 (39.7, 51.0) |
| Psychological (personal relationships) | 71.7 (67.6, 75.7) | 65.1 (58.0, 72.2) | 75.0 (70.1, 79.8) |
CI confidence interval, BMI body mass index, IGF-1 insulin-like growth factor-1, GH growth hormone, SD standard deviation, ULN upper limit of normal
aExcludes one patient in the ITT population with no data available on biochemical control. Data are the mean (95 % CI) unless otherwise specified; italic cells indicate parameters with non-overlapping CIs for biochemical control and lack of biochemical control (i.e. statistically significant differences). Biochemical control defined as GH levels ≤2.5 μg/L and normal IGF-1 levels at last post-baseline value available
bn = 83 for overall population (29 for controlled, 54 for uncontrolled)
IGF-1, acromegaly symptoms, and HRQoL in the overall population
As previously reported (7), mean IGF-1 levels were greatly reduced at week 12 versus baseline, and reductions were maintained until the end of the trial.
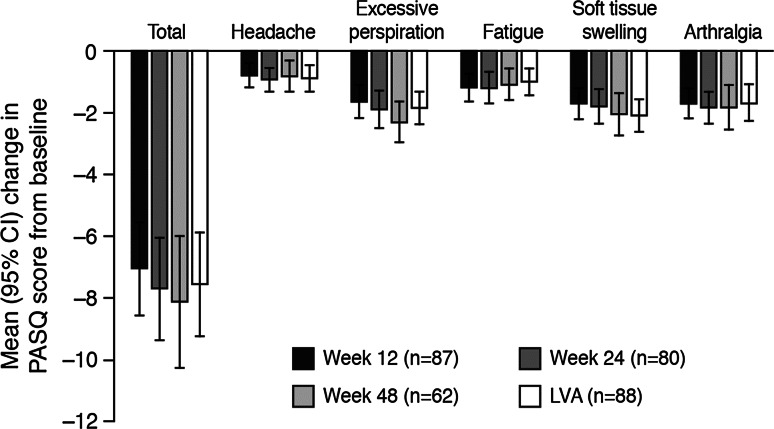
PASQ scores at baseline (n = 88) and during treatment (week 12, n = 87; week 48, n = 62) are shown in Table 1 and Fig. 1, and the percentage of patients with a MID in PASQ is shown in Supplementary Figure S1. The most troublesome symptoms at baseline were fatigue and soft tissue swelling, whereas headache was least troublesome (Table 1). The PASQ total score decreased significantly between baseline and week 12 (mean change, −7.1); this decrease was maintained at LVA (mean change, −7.6) (Fig. 1). Early and sustained reductions were also observed in each of the individual symptom scores. At LVA, greatest mean change was in excessive perspiration (−1.9) and smallest mean change was in headache (−0.9). Over half of patients experienced a MID in their PASQ total score at week 12 (59 %) and LVA (63 %). For the individual symptoms, excessive perspiration and soft tissue swelling showed greatest improvement, with MIDs in 51 %/51 % (week 12/LVA) and 49 %/62 % of patients, respectively, while the lowest MID rates were for headache with 26 %/30 % (Figure S1).
Fig. 1.
Mean (95 % CI) change in PASQ scores during treatment with Lanreotide Autogel. Each symptom is assessed on a 9-point scale, total maximum score = 40 (higher scores indicate worse symptoms); baseline scores are summarized in Table 1. Full data for this Figure are provided in Supplementary Table S2. CI confidence interval, PASQ patient-assessed acromegaly symptom questionnaire
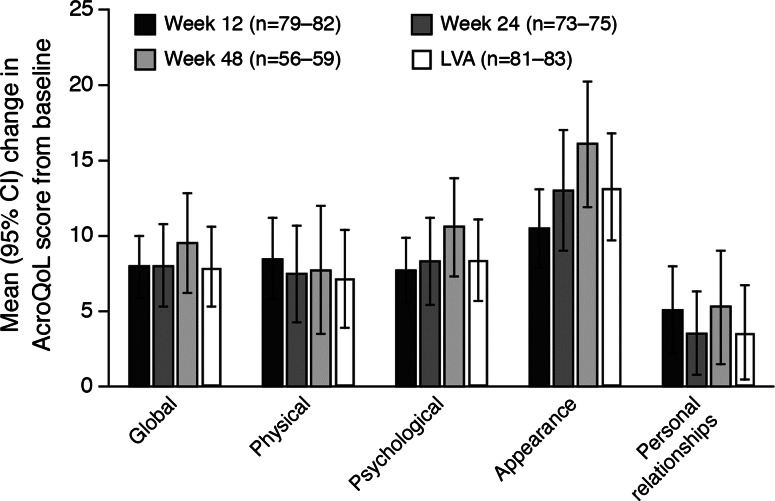
AcroQoL scores at baseline (n = 81–83) and during the study (week 12, n = 81–82; week 48, n = 57–59) are shown in Table 1 and Fig. 2, and the percentage of patients with a MID during treatment is shown in Supplementary Figure S1. At baseline, the greatest impairment was for the psychological sub-dimension, appearance and the least impairment was for the psychological sub-dimension, personal relationships (Table 1). The improvement in the global AcroQoL score from baseline was significant at week 12 (mean change, 8.0) and at LVA (mean change, 7.9) (Fig. 2). During treatment, the greatest improvements in each of the dimension or sub-dimensions were seen between baseline and week 12; these improvements continued (psychological dimension, appearance sub-dimension) or remained relatively stable (physical dimension, personal relationships sub-dimension) through to week 48. MID rates during treatment for the AcroQoL global score were 43 % at week 12 and 42 % at LVA, with appearance showing the greatest improvement (52 %/55 %), and personal relationships sub-dimension showing the least improvement (33 %/28 %) (Figure S1).
Fig. 2.
Mean (95 % CI) change in AcroQoL scores during treatment with Lanreotide Autogel. Scores for each dimension/sub-dimension are standardized from 0 to 100 (higher scores = better HRQoL); baseline scores are summarized in Table 1. Full data for this Figure are provided in Supplementary Table S2. CI confidence interval, AcroQoL acromegaly quality of life questionnaire
Correlation analyses in the overall population
For week-48 scores, there was a moderate correlation between the total PASQ and both global and physical AcroQoL scores (R = −0.55 and R = −0.67; both p < 0.0001), but not psychological AcroQoL dimension scores (R = −0.27, p = 0.0017; Supplementary Table S3). The correlations between the total PASQ score and AcroQoL domain scores were stronger than those between the individual PASQ symptom scores and the AcroQoL domain scores. For changes from baseline to week-48 scores, there were no correlations between PASQ total/individual symptom scores and AcroQoL domains. Finally, there were no correlations for week-48 scores or changes from baseline to week 48 for GH/IGF-1 levels and either PASQ or AcroQoL scores.
Acromegaly symptoms and HRQoL in patients according to biochemical response
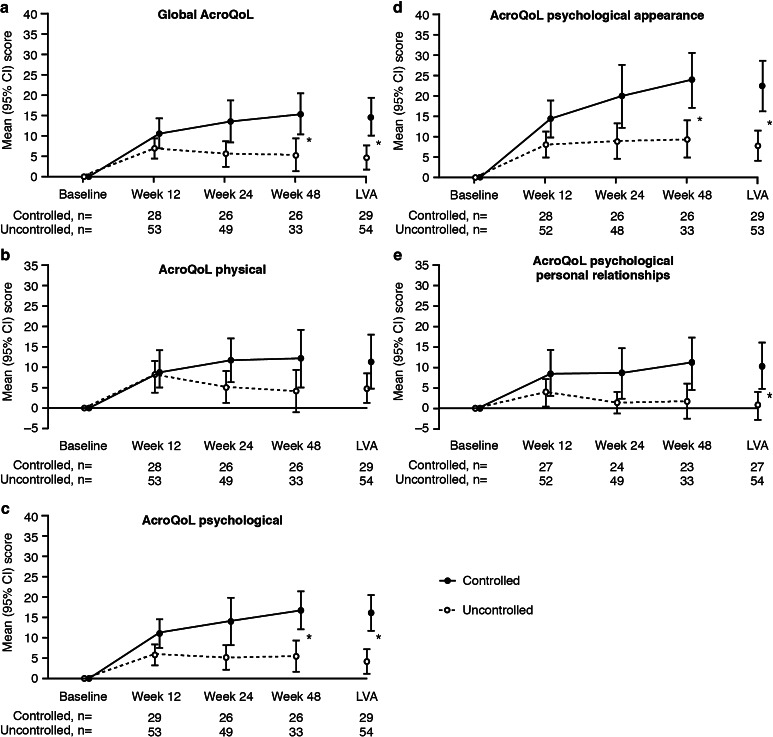
At baseline, PASQ symptom scores were similar in the two groups (Table 1). In addition, there were no significant differences in the change in symptom scores from baseline to LVA between the biochemically controlled and the uncontrolled groups (Supplementary Table S4). There was no significant difference in baseline AcroQoL scores in the groups with and without subsequent biochemical control (Table 1). However, the mean [95 % CI] change in the global AcroQoL score at LVA was significantly higher in the controlled group (14.4 [9.8–19.0]) than in the uncontrolled subjects (4.4 [1.5–7.4]), as were the changes in the psychological dimension, and the appearance and personal relationships sub-dimension scores (Fig. 3). Conversely, there was no significant difference between the controlled and uncontrolled groups for the physical AcroQoL score at LVA.
Fig. 3.
Changes in AcroQoL scores for patients with and without biochemical control during treatment with Lanreotide Autogel. AcroQoL acromegaly quality of life questionnaire, CI confidence interval. Asterisks indicate non-overlapping CIs, i.e. statistically significant between-group differences. Baseline scores are summarized in Table 1
Changes in PASQ total and AcroQoL global scores in the 18 patients who withdrew because of insufficient IGF-1 response are summarized in Supplementary Figure S2. Compared with the ITT population excluding these 18 patients, there appeared to be smaller improvements in PASQ and AcroQoL scores, but between-group differences were not statistically significant.
Discussion
The results of the current analyses show that primary medical treatment of acromegaly with lanreotide Autogel 120 mg is associated with early and sustained improvements in patient-reported symptoms and HRQoL. Improvements were noted across all symptoms evaluated (headache, excessive perspiration, fatigue, soft tissue swelling, and arthralgia) and approximately 60 % of patients had a clinically significant improvement (MID) in their total symptom score at LVA. Similarly, improvements were noted across all HRQoL dimension and sub-dimension scores (physical, psychological, appearance, and personal relationships) and >40 % of patients had a clinically significant improvement in their global HRQoL score at LVA. Amongst the AcroQoL dimensions and sub-dimensions, the greatest proportion of patients had clinically significant improvements in the appearance sub-dimension. This sub-dimension also scored worst at baseline, so the greater effect of treatment may reflect a larger scope for improvement.
As reported previously [7], mean GH and IGF-1 levels were significantly reduced at week 12 versus baseline, and improvements were maintained until the end of the trial. Improvements in acromegaly symptoms were also greatest during the first 12 weeks. Interestingly, however, the total and individual symptom scores during the study did not differ in those who achieved biochemical control (GH ≤2.5 μg/L and normal IGF-1 levels), compared with those who did not. Furthermore, there were no correlations between symptom scores and GH or IGF-1 levels. These results suggest that there may be a dissociation between hormonal control and symptom improvement, possibly reflecting patients’ long-term experience of the disease before diagnosis, the effect of comorbidities, or underlying differences in baseline characteristics between those with and without biochemical control (e.g. age and possibly gender). With regards to HRQoL, there was a modest correlation between the total PASQ and both global and physical AcroQoL scores. There were no correlations between any of these HRQoL scores and GH or IGF-1 levels, but improvements in the global and psychological AcroQoL scores were significantly greater in those who achieved biochemical control compared with those who did not. A number of previous studies have evaluated the association between HRQoL and biochemical control; the results are mixed, with some showing better HRQoL in those with biochemical control, and others showing no such association [16, 17]. Interestingly, in one study, IGF-1 control was associated with better scores on the AcroQoL appearance sub-dimension, but not for the global, physical or psychological dimensions [18]. In another study, administration of pegvisomant to patients biochemically controlled with a SSA resulted in an improvement in HRQoL despite no change in IGF-1 levels [14]. The relationship between biochemical control (as determined by circulating hormones) and HRQoL therefore remains to be determined, but it should be borne in mind that HRQoL can be affected by a number of factors associated with acromegaly and the treatment modality. These include cosmetic or orthopedic deformities or comorbidities, which may already be established in some patients, particularly in view of the time lag between symptom onset and diagnosis [16]. It has also been shown that illness perception affects HRQoL in those with acromegaly [19]. In addition, tissue sensitivity to GH and IGF-1 excess can vary [14], which may also explain the apparent disconnect between HRQoL and circulating levels of GH and IGF-1 in some studies. Finally, it should be borne in mind that the studies in which HRQoL and biochemical control have been assessed include heterogeneous populations in terms of treatments received (surgery, radiotherapy, medical treatment, or a combination of these) [14, 16–18], which could also impact on HRQoL. Indeed, one of the strengths of the current study was the homogeneity of the patient population, who were all treatment naïve.
Other studies have used the PASQ and AcroQoL to assess symptoms and HRQoL in those treated for acromegaly [9, 12, 20–23]. In the observational ACROSTUDY of the GH receptor antagonist, pegvisomant, only the PASQ score for excessive perspiration was significantly improved following 1 year’s treatment; after 2 years, numbness and soft tissue swelling were significantly reduced [20]. HRQoL was assessed using AcroQoL in a recent randomized study comparing octreotide LAR and pasireotide LAR in 358 medical treatment-naïve patients; baseline scores (55.6 and 58.4) were similar to those in the current study (56.0). After 1 year, scores increased by 4.9 in the octreotide LAR group and 7.0 in the pasireotide group; the results in the current study (increase of 9.5) compare well with these [7]. Larger increases in the AcroQoL global score (+17) were noted with octreotide LAR in an earlier observational study, but it included only 28 patients treated for 4 years, so the results are more difficult to compare [12].
One limitation of the current study was its open-label, uncontrolled design. However, this was felt to be justified because of the objective nature of the primary endpoint, tumor volume reduction [7]. Strengths of the study, particularly in the context of the patient-reported outcomes, include the relatively large size of the patient population, its homogeneity, and the long duration of follow-up. Many of the previously reported studies evaluating the effect of SSAs on HRQoL included fewer than 50 patients [12–14, 24–26]. Furthermore, unlike many of the other studies [11, 12, 14, 26], patients were treatment naïve, and as such, they represent an appropriate population for evaluating patient-reported outcomes and HRQoL in the earlier stages of treatment.
The results of the current study confirmed that medical treatment with lanreotide provided meaningful improvements not only in hormone levels, but also in clinical outcomes as evaluated by the PASQ and AcroQoL questionnaires. The relationship between biochemical and PASQ improvements was not clear, whereas the association between biochemical and AcroQoL improvements was more apparent. This divergence highlights the importance, wherever practicable, of using more than one appropriate questionnaire, in addition to biochemical measures, to fully evaluate clinical outcomes during medical treatment for acromegaly. Such an approach will offer different, but complementary, information on treatment response. In particular, it is most important to consider using a tool, such as AcroQoL, that assesses patients’ subjective perceptions of the impact of disease on their daily HRQoL, as this outcome cannot always be reliably inferred from biochemical markers or objective symptom evaluation.
In conclusion, primary treatment with lanreotide Autogel 120 mg every 4 weeks for 1 year was associated with early and sustained improvements in signs and symptoms and HRQoL in patients with acromegaly. Symptom improvement did not appear to depend exclusively on biochemical control. On the other hand, improvements in HRQoL may reflect improvements in clinical signs and symptoms in these patients as well as a decrease of GH/IGF-1 hypersecretion. These results highlight the importance of using appropriate questionnaires to fully evaluate clinical outcomes during medical treatment for acromegaly.
Electronic supplementary material
Acknowledgments
The authors thank the patients, their families, and investigators who participated in this trial. We thank Watermeadow Medical for writing assistance (funded by Ipsen).
Author contribution
P J Caron, J S Bevan, and S Petersenn were involved in: concept and design of the study; patient enrolment into the study; analysis and interpretation of the data; and drafting of the manuscript. S M Webb, A Houchard and C Sert were involved in: analysis and interpretation of the data; and drafting of the manuscript. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Sponsorship
This study was sponsored by Ipsen.
Appendix
PRIMARYS Study Group
Belgium L. Van Gaal; Czech Republic J. Marek; Finland P. Nuutila, M. Välimäki; France C. Ajzenberg, F. Borson-Chazot, T. Brue, P. Caron, O. Chabre, P. Chanson, C. Cortet Rudelli, B. Delemer, J.-M. Kuhn, A. Tabarin; Germany K. Badenhoop, C. Berg, S. Petersenn, C. Schöfl, J. Schopohl; Italy S. Cannavò, A. Colao, L. De Marinis; the Netherlands A. Stades, A.J. van der Lely; Turkey P. Kadıoğlu; UK J.S. Bevan, D. Flanagan, P. Trainer.
Compliance with ethical standards
Conflict of interest
P J Caron is a consultant and speaker for Ipsen and Novartis, and an advisory board member for Ipsen. J S Bevan is a consultant and study investigator for Ipsen, and an advisory board member for Novartis and ViroPharma. S Peterssen has received lecture fees and participated in advisory boards for Ipsen, Novartis, and Pfizer. S M Webb has received speaker fees from Ipsen and has been involved in some of their clinical trials. A Houchard and C Sert are employed by Ipsen.
Footnotes
Additional investigators are listed in the “Appendix”.
References
- 1.Katznelson L, Laws ER, Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933–3951. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 2.Pantanetti P, Sonino N, Arnaldi G, Boscaro M. Self image and quality of life in acromegaly. Pituitary. 2002;5:17–19. doi: 10.1023/A:1022145116901. [DOI] [PubMed] [Google Scholar]
- 3.Webb SM, Badia X, Surinach NL, Spanish AcroQol Study Group Validity and clinical applicability of the acromegaly quality of life questionnaire, AcroQoL: a 6-month prospective study. Eur J Endocrinol. 2006;155:269–277. doi: 10.1530/eje.1.02214. [DOI] [PubMed] [Google Scholar]
- 4.Rowles SV, Prieto L, Badia X, Shalet SM, Webb SM, Trainer PJ. Quality of life (QOL) in patients with acromegaly is severely impaired: use of a novel measure of QOL: acromegaly quality of life questionnaire. J Clin Endocrinol Metab. 2005;90:3337–3341. doi: 10.1210/jc.2004-1565. [DOI] [PubMed] [Google Scholar]
- 5.Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, van der Lely AJ, Strasburger CJ, Lamberts SW, Ho KK, Casanueva FF, Melmed S, Acromegaly Consensus Group Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10:243–248. doi: 10.1038/nrendo.2014.21. [DOI] [PubMed] [Google Scholar]
- 6.Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update. Endocr Pract. 2011;17(Suppl 4):1–44. doi: 10.4158/EP.17.S4.1. [DOI] [PubMed] [Google Scholar]
- 7.Caron PJ, Bevan JS, Petersenn S, Flanagan D, Tabarin A, Prévost G, Maisonobe P, Clermont A. Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J Clin Endocrinol Metab. 2014;99:1282–1290. doi: 10.1210/jc.2013-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colao A, Auriemma RS, Rebora A, Galdiero M, Resmini E, Minuto F, Lombardi G, Pivonello R, Ferone D. Significant tumour shrinkage after 12 months of lanreotide Autogel-120 mg treatment given first-line in acromegaly. Clin Endocrinol. 2009;71:237–245. doi: 10.1111/j.1365-2265.2008.03503.x. [DOI] [PubMed] [Google Scholar]
- 9.Colao A, Bronstein MD, Freda P, Gu F, Shen CC, Gadelha M, Fleseriu M, van der Lely AJ, Farrall AJ, Hermosillo Reséndiz K, Ruffin M, Chen Y, Sheppard M. Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab. 2014;99:791–799. doi: 10.1210/jc.2013-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercado M, Borges F, Bouterfa H, Chang TC, Chervin A, Farrall AJ, Patocs A, Petersenn S, Podoba J, Safari M, Wardlaw J. A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide L. Clin Endocrinol. 2007;66:859–868. doi: 10.1111/j.1365-2265.2007.02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lombardi G, Minuto F, Tamburrano G, Ambrosio MR, Arnaldi G, Arosio M, Chiarini V, Cozzi R, Grottoli S, Mantero F, Bogazzi F, Terzolo M, Tita P, Boscani PF, Colao A. Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in somatostatin analogue-naive patients with acromegaly. J Endocrinol Investig. 2009;32:202–209. doi: 10.1007/BF03346453. [DOI] [PubMed] [Google Scholar]
- 12.Mangupli R, Camperos P, Webb SM. Biochemical and quality of life responses to octreotide-LAR in acromegaly. Pituitary. 2014;17:495–499. doi: 10.1007/s11102-013-0533-x. [DOI] [PubMed] [Google Scholar]
- 13.Sonino N, Scarpa E, Paoletta A, Fallo F, Boscaro M. Slow-release lanreotide treatment in acromegaly: effects on quality of life. Psychother Psychosom. 1999;68:165–167. doi: 10.1159/000012326. [DOI] [PubMed] [Google Scholar]
- 14.Neggers SJ, van Aken MO, de Herder WW, Feelders RA, Janssen JA, Badia X, Webb SM, van der Lely AJ. Quality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomant. J Clin Endocrinol Metab. 2008;93:3853–3859. doi: 10.1210/jc.2008-0669. [DOI] [PubMed] [Google Scholar]
- 15.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Shlomo A, Sheppard MC, Stephens JM, Pulgar S, Melmed S. Clinical, quality of life, and economic value of acromegaly disease control. Pituitary. 2011;14:284–294. doi: 10.1007/s11102-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postma MR, Netea-Maier RT, van den Berg G, Homan J, Sluiter WJ, Wagenmakers MA, van den Bergh ACM, Wolffenbuttel BHR, Hermus ARMM, van Beek AP. Quality of life is impaired in association with the need for prolonged postoperative therapy by somatostatin analogs in patients with acromegaly. Eur J Endocrinol. 2012;166:585–592. doi: 10.1530/EJE-11-0853. [DOI] [PubMed] [Google Scholar]
- 18.Matta MP, Couture E, Cazals L, Vezzosi D, Bennet A, Caron P. Impaired quality of life of patients with acromegaly: control of GH/IGF-I excess improves psychological subscale appearance. Eur J Endocrinol. 2008;158:305–310. doi: 10.1530/EJE-07-0697. [DOI] [PubMed] [Google Scholar]
- 19.Tiemensma J, Kaptein AA, Pereira AM, Smit JW, Romijn JA, Biermasz NR. Affected illness perceptions and the association with impaired quality of life in patients with long-term remission of acromegaly. J Clin Endocrinol Metab. 2011;96:3550–3558. doi: 10.1210/jc.2011-1645. [DOI] [PubMed] [Google Scholar]
- 20.Buchfelder M, Schlaffer S, Droste M, Mann K, Saller B, Brübach K, Stalla GK, Strasburger CJ. The German ACROSTUDY: past and present. Eur J Endocrinol. 2009;161(Suppl 1):S3–S10. doi: 10.1530/EJE-09-0350. [DOI] [PubMed] [Google Scholar]
- 21.Milian M, Honegger J, Gerlach C, Psaras T. Health-related quality of life and psychiatric symptoms improve effectively within a short time in patients surgically treated for pituitary tumors–a longitudinal study of 106 patients. Acta Neurochir. 2013;155:1637–1645. doi: 10.1007/s00701-013-1809-7. [DOI] [PubMed] [Google Scholar]
- 22.Sardella C, Lombardi M, Rossi G, Cosci C, Brogioni S, Scattina I, Webb SM, Gasperi M, Martino E, Bogazz IF. Short- and long-term changes of quality of life in patients with acromegaly: results from a prospective study. J Endocrinol Investig. 2013;33:20–25. doi: 10.1007/BF03346555. [DOI] [PubMed] [Google Scholar]
- 23.Sievers C, Brubach K, Saller B, Schneider HJ, Buchfelder M, Droste M, Mann K, Strasburger CJ, Stalla GK. Change of symptoms and perceived health in acromegalic patients on pegvisomant therapy: a retrospective cohort study within the German Pegvisomant Observational Study (GPOS) Clin Endocrinol. 2010;73:89–94. doi: 10.1111/j.1365-2265.2009.03773.x. [DOI] [PubMed] [Google Scholar]
- 24.Karaca Z, Tanriverdi F, Elbuken G, Cakir I, Donmez H, Selcuklu A, Durak AC, Dokmetas HS, Colak R, Unluhizarci K, Kelestimur F. Comparison of primary octreotide-lar and surgical treatment in newly diagnosed patients with acromegaly. Clin l Endocrinol. 2011;75:678–684. doi: 10.1111/j.1365-2265.2011.04106.x. [DOI] [PubMed] [Google Scholar]
- 25.Ruchala M, Stangierska I, Gurgul E, Stangierski A, Fajfer J, Sowinski J. The effect of octreotide treatment on somatic and psychological symptoms of acromegaly. Neuro Endocrinol Lett. 2010;31:265–269. [PubMed] [Google Scholar]
- 26.Schopohl J, Strasburger CJ, Caird D, Badenhoop K, Beuschlein F, Droste M, Plöckinger U, Petersenn S. Efficacy and acceptability of lanreotide Autogel(R) 120 mg at different dose intervals in patients with acromegaly previously treated with octreotide LAR. Exp Clin Endocrinol Diab. 2011;119:156–162. doi: 10.1055/s-0030-1267244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.