Abstract
To create a rehabilitation protocol following reconstruction of the posterior cruciate ligament (PCL), through a literature review. The literature review was conducted in the Medline and Embase databases, to search for data on biomechanical concepts and analyses relating to the posterior cruciate ligament of the knee. The search strategy was set up using the following rules: problem or injury in association with anatomical location terms; or surgical intervention procedure in association with rehabilitation terms. We began the process in this manner and subsequently introduced restrictions on certain terms to improve the search specificity. To design the protocol, a table was created for better data assessment, based on the time that elapsed between surgery and the start of physiotherapy. A rehabilitation protocol was created to improve weight-bearing control in the initial weeks after surgery, with the aid of a knee brace. Our aim was to achieve gains in total range of motion of the knee, which should be attained by the third month, thereby avoiding contractures resulting from the tissue healing process. Strengthening exercises and sensory-motor training were guided accordingly, thus avoiding overload on the graft and respecting the healing phases. The protocol proposed through this review was based on the current evidence relating to this subject.
Keywords: Posterior Cruciate Ligament, Knee, Rehabilitation
INTRODUCTION
There is a lack of biomechanical, histological and clinical studies on knee rehabilitation following posterior cruciate ligament (PCL) injury, in relation both to cases treated conservatively and to cases that underwent reconstruction. The existing studies are often based on aspects of the integration and rehabilitation of the anterior cruciate ligament (ACL), transposed to the PCL. The aim of the present study was to review the points presented in the current literature and, together with tacit knowledge of the last few years at our clinic, to put forward a rehabilitation protocol.
METHODS
A search in the literature was conducted using the Medline database through the PubMed website and using the Embase database through the Patient, Intervention, Comparison and Outcome (PICO) strategy. The investigation was divided into search strategies that emphasized range of motion and therapeutic exercises, as described below.
Regarding range of motion (ROM): Surgery, Reconstruction and Posterior cruciate ligament were combined and the terms Posteromedial corner, Posterolateral corner, Arthroplasty, Prosthesis and Total knee replacement were used to clean up the search for related articles. In addition, the Mesh terms Rehabilitation and Range of motion were also combined in an attempt to only retrieve articles relating to ROM. In this manner, 33 articles were identified. Of these 11 reported the ROM and/or showed programs for ROM gain.
Regarding exercise programs: Posterior cruciate ligament [Mesh] was combined with Physical therapy modalities [Mesh], Rehabilitation [Mesh], Exercise [Mesh], Exercise therapy [Mesh] and Exercise test [Mesh] as a strategy, and 19 articles were identified. Of these, six had the objective of analyzing the rehabilitation protocol.
In addition, because few in vivo studies were available, we also used a strategy with greater sensitivity, through analyzing in vitro biomechanical studies and mathematical modeling studies on knee-related exercises.
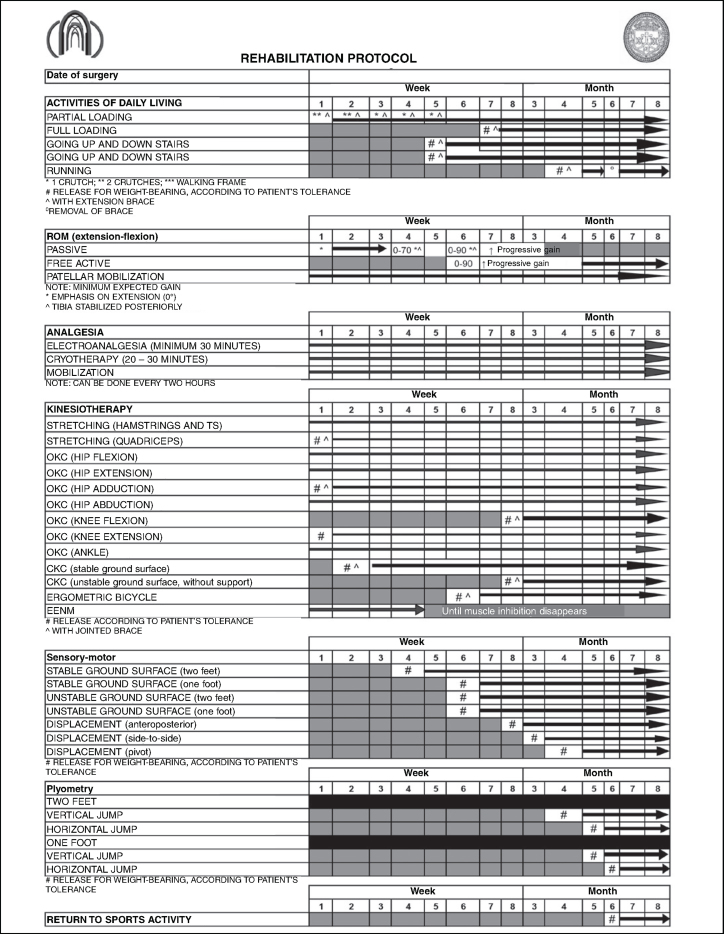
From using a filter for meta-analyses or randomized controlled clinical trials, only one study was identified, and this did not cover all aspects of rehabilitation. Thus, the present review (Table 1) was conducted mainly on basic science studies and on cadaver models, because of the few randomized controlled clinical trials found. The protocol was constructed in a spreadsheet with a format that accompanied the variable of postoperative time. Thus, the protocol was made to be easy to view and to consult (Annex 1).
Table 1.
Review with systematized search of the literature.
| Brace in extension | Weight-bearing | ROM | OKC | CKC | Hamstrings | |
|---|---|---|---|---|---|---|
| Fanelli et al (1994)(27) | 6th week | Tolerance | 0-90° without time period | 0-70° | X | X |
| Irrgang & Fitzgerald (2000)(25) | 6th to 8th week | Tolerance | 0-90° for 6 to 8 weeks | 60°, 1st to 3rd week | 45°, 4th to 6th week | Avoid, without defined date |
| Stähelin et al (2001)(15) | 6th week | Tolerance | 0-90° | X | X | X |
| Allen et al (2002)(28) | 4th week | Tolerance | X | X | 4th to 6th week | 16th week |
| Margheritini (2002)(18) | 6th week | Partial until 6th or 8th week | Progressive and slow | X | 6th to 8th week | Avoid, but without defined date |
| Bottoni & Parr (2003)(11) | Release after achieving good quadriceps control | Progressive after 8th week | 0 to 70° from 4 to 6 weeks | X | X | X |
| Noyes et al (2003)(16) | 6th week | Partial initially and full after 6th week | 3°-0°-120° | X | 0-70° | 8th week |
| Wang et al (2003)(8) | 6th week | Tolerance | Not specified | X | X | 6th week |
| Chen et al (2003)(7) | 6th week | Tolerance | 0-60° up to 6th week and 0-90° up to 8th week | X | 6th week | X |
| Faustino (2003)(17) | 6th week | Tolerance | Without stipulated limit | X | 12th week | |
| MacGillivray et al (2006)(12) | 4th week | Partial | 0 to 90° | X | X | X |
| Fanelli et al (2010)(21) | 4th to 6th week | Without loading until 6th, partial from 7th to 10th and full in 11th week | 3rd to 6th week without stipulated ROM | X | X | X |
| Fanelli (2008)(3) | 3rd to 6th week | Without loading until 6th, partial from 7th to 9th and full in 10th week | Progressive start in 4th week | 0-45° in 11th week | 0-45° in 11th week | Start in 24th week |
| McAllister & Hussain (2010)(20) | 3rd week | Without loading until 3rd-6th, partial between 3rd and 6th and full in 6th week | Start between 3rd and 6th weeks | X | X | X |
| Quelard et al (2010)(19) | 6th week | Without loading until 10th day, partial between 11th day and 6th week and full after 6th week | 0-60° until 6th week, 0-95° until 8th week and 0-120° after 8th week | 2nd week | 6th week 0-60° | 16th week |
| Fanelli et al (2010)(21) | 5th week | Without loading until 5th week, partial until 10th week and full after 10th week | 5th to 10th weeks | X | 11th week | X |
| Edson et al (2010)(22) | 5th week | Without loading until 5th week, partial until 10th week and full after 10th week | 5th to 10th weeks | After 5th week | After 10th week 0-60° | 24th week |
Annex 1.
Rehabilitaion protocol for posterior cruciate ligament
RESULTS
The protocol presented shows the period of early release for weight-bearing over the first weeks, done partially through use of two crutches and a long immobilizer locked into extension.
Passive mobilization for improving ROM should be done early on; for this, we recommend that progressive gain should be envisaged, with the parameters of 70° of flexion in the fourth week and 90° in the sixth week. Following this, full ROM needs to be achieved by the third month in order to avoid contractures resulting from the tissue healing process. Note that active flexion movement of the knee should be delayed for two months.
The post-surgical reconstruction period for the PCL may be accompanied by pain. In this case, analgesia provided through electrotherapeutic means is beneficial for the rehabilitation process, with regard to the patient's comfort. Cryotherapy should be used whenever the knee presents conditions of pain or edema.
The greatest limitation of physiotherapy in the patient rehabilitation process relates to strengthening exercises. In our protocol, we delay open kinetic chain (OKC) exercises for the knee flexors until the eighth week after the operation, while closed kinetic chain (CKC) and OKC exercises for the extensors remain in the second week.
Sensory-motor work should start together with the release to perform CKC exercises for the extensors, and the progression from stable ground surfaces to unstable surfaces should be done by around the fourth month, along with stressing for anteroposterior, side-to-side and rotational displacement, respectively. Over this period, we begin the process of plyometric training, which is reserved for the population of athletes.
The time taken for non-athletic individuals to be released for general activities is around six months, with a further two months for sports activities at competitive level.
DISCUSSION
The rehabilitation process for PCL injuries is assessed as a complementary but essential point within functional recovery of the knee(1). Rehabilitation protocols prioritize protection of the reconstructed ligament, so as to avoid excessive stress on the graft during the rehabilitation until the graft has become integrated(2). However, it is not known with any certainty what the safe tensions would be and how much provocation can be allowed during rehabilitation exercises(3).
Little is known on the structural modifications of grafts after ligament reconstruction. Bosch and Kasperczyk(4) studied the histochemical and biochemical characteristics of grafts from the central third of the patellar tendon for ACL reconstruction, in sheep, with the aim of understanding the integration process. They found a necrotic phase with diminution of the resistance to stress particularly in the eighth week after reconstruction. It is noteworthy that graft necrosis continued to be seen until the 104th week, i.e. two years after the reconstruction.
Moreover, it is a difficult task to determine the stress that the ligaments are subjected to during passive movement of the knee in weight-bearing and muscle force activities and whether these are prejudicial to the graft. Direct measurement methods such as placement of load cells (measurement devices) in the ligament are difficult to do in vivo. Thus, studies on cadavers and indirect biomechanical methods such as inverse dynamics are the methods most used.
Points relating to ROM
To avoid loss of ROM, Irrgang and Harner(5) divided the care relating to reconstructed knees into three phases: before the surgery, the focus should be on elimination of edema and pain and restoration of ROM; during the operation, ROM seems to be closely related to the positioning of the bone tunnels and to the surgical technique; after the surgery, early mobilization and gains in mobility are recommended, with extension restored after two to three weeks and flexion achieved by the third month(6).
Restrictions relating to the limits on knee flexion gains are discussed in the literature, with divergences between the rehabilitation protocols presented. Some authors have prioritized limiting the range of angles to between 0 and 60°7, 8, 9, 10, 0 and 70°(11), 0 and 90°12, 13, 14, 15 or 0 and 120°(16), or without any stimulated limit(17) or according to the patient's tolerance(18). Quelard et al(19) recommended a gradual protocol for gaining passive mobility of the knee, such that a range of 0–60° would be achieved in the first six weeks, 0–90° from the sixth to the eighth week and 0–120° from the eighth week onwards.
Some studies have used a slower protocol and have not included passive mobilization of the knee in the first weeks. McAllister and Hussain(20) started the protocol between the third and sixth weeks, Fanelli et al(21) between the fifth and tenth weeks, Fanelli(3) in the fourth week and Edson et al(22) in the fifth week.
The criteria of ROM progression are not discussed in the protocols that we found, and there is no biomechanical explanation to explain why passive gain of movement is limited. The protocols used in the literature seem to be based on personal clinical experience(22).
In situ studies on PCL tensions(23) have demonstrated that with increasing degree of passive flexion of the knee, there is also an increase in the tension in the PCL. Moreover, the varus stress and posterior shear stress in the tibia may also generate increased force on the PCL(24).
Because of this evidence, caution is needed in relation to gains in passive knee ROM. On the other hand, delayed gain in movement may have consequences such as restriction of joint ROM and functional loss.
One of the practical procedures used by many professionals during rehabilitation is to stabilize the tibia using constant anterior pressure on the posterior region of the leg, in order to avoid excessive tension on the ligament. Decreased tension on the PCL through anteriorization of the tibia has been demonstrated in studies on cadavers(24) and was advocated by Irrgang and Fitzgerald(25) in their rehabilitation protocol.
Our protocol restricts the gain in passive ROM to 70° for four weeks and progresses to 90° for another two weeks. After the sixth week, gains in passive ROM are progressive, according to the patient's tolerance, but we maintain the passive anteriorization force applied to the tibia until the tenth week.
Release for weight-bearing (walking)
Early release for weight-bearing in isolated reconstruction of the PCL is a common practice among the rehabilitation protocols cited in the literature11, 12, 16, but there is no consensus regarding how much this could be done without causing deleterious effects to the graft undergoing healing. Many protocols7, 15, 17 favor early weight-bearing according to the patient's tolerance. In other words, the introduction of weight-bearing may be completed in the first weeks of reconstruction.
Through a study with a mathematical model, Shelburne and Pandy(26) demonstrated that because of the forces exerted on the knee during weight-bearing, the tibia presents a tendency towards anterior shearing in relation to the femur, which theoretically would not overload the PCL.
Bosch and Kasperczyk(4) conducted an experiment on sheep and found that movement and early weight-bearing did not cause ruptures and did not increase the length of the graft. Corroborating this concept, Toutoungi et al(2) found that the effect of axial compression tended to diminish the femorotibial shearing and consequently the stresses generated on the central ligaments.
In the study by Noyes and Barber-Westin(6), which involved PCL reconstruction, weight-bearing was introduced progressively, with a protective orthosis locked in extension for four weeks, until full weight-bearing was reached around the fifth week. However, other studies are divergent. Some authors have recommended that weight-bearing should be introduced according to the patient's tolerance and should be started in the first week7, 8, 15, 17, 20, 27, 28, while one study restricted weight-bearing until the sixth week(16) and others until the eighth week11, 18.
In some protocols, weight-bearing is not recommended during the first days after reconstruction. Quelard et al(19) used a protocol without weight-bearing over the first 10 days, progressing to partial weight-bearing on the 11th day, which continued until the fifth week, with full weight-bearing from the sixth week onwards. McAllister and Hussain(20) did not used weight-bearing for three weeks and progressed to partial weight-bearing in the fourth and fifth weeks and full weight-bearing in the sixth week.
Edson et al(22) did not use weight-bearing for five weeks and progressed to partial weight-bearing in the sixth week and full weight-bearing in the 10th week. Other authors have used different protocols (Table 1); however, all of them used a protective orthosis locked in extension, in association with weight-bearing.
Based on the studies cited above, our group feels increasingly secure in recommending partial weight-bearing, with evolution to full weight-bearing according to the patient's tolerance, for isolated PCL injuries.
Muscle strengthening
There have been divergences of opinion regarding the use of OKC or CKC exercises as rehabilitation options for the process of muscle strengthening, in relation to efficacy of strength gains, control over knee muscles and stress on ligaments. There is a tendency towards using CKC exercises at the start of protocols, with complementation using OKC exercises at the more advanced phases29, 30, 31, 32, 33, 34. CKC exercises generate axial compression forces on the joint, which diminishes the shearing forces on the knee, as well as leading to simultaneous contraction of the quadriceps and hamstrings, which are desirable in the initial phase of rehabilitation.
In the rehabilitation protocols cited in previous studies7, 11, 17, OKC and CKC exercises were introduced in an arbitrary manner, without backing from any studies quantifying the tensions in the PCL or their consequences in relation to ligament laxity during the rehabilitation process. Quelard et al(19) recommended that OKC exercises for strengthening the quadriceps should be started from the second week. Some studies have suggested starting these exercises in the first three weeks7, 8, 9, 11, while others have introduced them only between the fourth and sixth weeks10, 13, 14, 22. Fanelli(3) only started quadriceps strengthening with OKC exercises in the 11th week, at angles of 0–45°.
Certain protective angle ranges have been recommended for OKC quadriceps strengthening exercises. Ranges of 0 to 60° have been used9, 13, 14, while other authors have recommended that this strengthening should be done from 0 to 70°(27).
Dürselen et al(23) demonstrated on cadavers, and other authors2, 33, 35 through mathematical models, that in OKC exercises, the quadriceps muscle might diminish the stress on the PCL, especially at the end of the knee extension. Thus, these were the preferred exercises at the start of the rehabilitation process.
One proviso needs to be made in relation to OKC exercises for the quadriceps and their implications in post-reconstruction rehabilitation of the PLC. By considering only the protection angles in relation to PCL grafts, excessive stress may be placed on the femoropatellar joint and may consequently cause lesions in the cartilage coating this joint(36). Therefore, the safe angle for the neoligament is between 0 and 70°(25), and protection for the femoropatellar joint involves angles from 45 to 90°(36). Thus, with the aim of protecting the graft and the femoropatellar joint, our group uses angles from 45 to 70° to stimulate the quadriceps. Since this only leaves a small range of motion, we undertake the OKC exercises in isometric form at multiple angles within this safety range. The aim of OKC exercises in the initial phase is not related to gains in strength, resistance or muscle power, but to recruitment of the maximum number of muscle fibers. Thus, we undertake OKC exercises by associating the patient's maximum voluntary contraction with neuromuscular electrostimulation, with the aim of combating the arthrogenic inhibition that is present in diseased knees(37).
The rehabilitation protocols that we found introduced CKC exercises for quadriceps strengthening at different times during the rehabilitation protocol. These times included the fourth9, 10, sixth19, 27, eighth11, 14, tenth(22), eleventh 3, 21 and twelfth weeks(17).
Regarding the protection angles, three variants were found in our investigation. Some authors started with mini-squats from 0 to 45°3, 25, others introduced CKC exercised at angles from 0 to 70°(16) and yet others19, 22 started with 0 to 60°.
In vivo studies38, 39, 40 analyzing the length of the native PCL through measurements made using magnetic resonance imaging have demonstrated that CKC exercises increased the length of the two bands of the PCL at greater flexion angles. However, this type of measurement is unable to define the amount of tension generated in the PCL during active rehabilitation exercises. Therefore, only direct measurements by means of load cells would be capable of defining these tensions, but the methodology of this procedure makes it very difficult to assess the PCL.
CKC exercises are safe in relation to anterior shearing forces on the tibia(26) and should be performed carefully in the initial phase of the rehabilitation process following PCL surgery. The factors that may have an influence on the stresses in the cruciate ligaments are the forces generated by muscle contractions, such as co-contractions and ground reaction forces(32).
Shelburne and Pandy(26) demonstrated that from 10° of flexion onwards, in CKC exercises, the PCL presents increased tension, even though the peak stress occurs at around 80° of flexion.
In our protocol, CKC exercises are started in the second week and are initially performed in situations of controlled overload. We use exercises on stable surfaces, such as leg press exercises, mini-squats and functional activities such as getting up from and sitting down on high chairs.
The ROM should respect the angles of 0 to 45°, since shearing of the tibia occurs anteriorly, which spares the PCL from excessive tension, as well as protecting the femoropatellar joint(25). Beyond 70 to 80°, the tensions increase considerably, thus causing excessive stress on the PCL(26).
When the hamstring muscles are contracted in isolation, i.e. in OKC exercises, the tension on the PCL is increased because of the traction force of these muscles on the tibia2, 26, 34, 35. Shelburne and Pandy(26) demonstrated that the hamstrings are responsible for constant posterior tension and that as the knee flexion increases, the forces favoring anteriorization of the tibia diminish.
The protocols generally postpone the introduction of exercises directed towards the hamstrings with the aim of not excessively tensioning the graft during the initial postoperative phase. There is divergence between studies regarding when to start to work on the posterior muscles of the thigh, such that the suggested start is in the sixth(8), eighth(16), ninth(13), 16th(28) or 24th week3, 19, 22. In our protocol, hamstring exercises are postponed until the eighth week with the aim of sparing the posteriorization forces during the initial phase of the rehabilitation protocol.
Andersen et al(41) found that beyond 10 to 12 weeks after PCL surgery, for patients with functional ROM, normalized gait and little or no significant clinical complaint, there was less concern regarding the type of exercise, speed at which the exercise was performed and the muscles to be emphasized for normalizing these patients’ muscle strength and restoring their remaining functional deficits.
Sensory-motor training
One of the structures that assist in proprioception for the knee is the PCL(42), because of the enormous quantity of mechanoreceptors found in this ligament. The proprioceptive effect of the PCL has mainly been studied and discussed in relation to preservation or not of this ligament in total knee prosthesis surgery. There have been divergent results regarding comparisons between knees with and without the ligament, in assessing functional outcomes for the knee(43).
Because of the PCL injury process and the role of the PCL in proprioception, sensory-motor training should always be performed. There should be progression from stable ground surfaces with static exercises to unstable surfaces with dynamic exercises that are increasingly specific to the functional objective(43).
FINAL REMARKS
The majority of the protocol proposed fits within the current evidence on this subject. The protocol has been used in our clinic with good tolerance among the patients. The present state of evidence has allowed us to analyze each phase of the rehabilitation process, but further studies of clinical nature with greater strength of evidence need to be conducted.
Footnotes
Work performed in the Knee Surgery Group, Department of Orthopedics and Traumatology, School of Medical Sciences, Santa Casa de Misericórdia de São Paulo (SCMSP), São Paulo, SP, Brazil.
REFERENCES
- 1.Wilk KE. Rehabilitation of isolated and combined posterior cruciate ligament injuries. Clin Sports Med. 1994;13(3):649–677. [PubMed] [Google Scholar]
- 2.Toutoungi DE, Lu TW, Leardini A, Catani F, O'Connor JJ. Cruciate ligament forces in the human knee during rehabilitation exercises. Clin Biomech (Bristol, Avon) 2000;15(3):176–187. doi: 10.1016/s0268-0033(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 3.Fanelli GC. Posterior cruciate ligament rehabilitation: how slow should we go? Arthroscopy. 2008;24(2):234–235. doi: 10.1016/j.arthro.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Bosch U, Kasperczyk WJ. Healing of the patellar tendon autograft after posterior cruciate ligament reconstruction—a process of ligamentization? An experimental study in a sheep model. Am J Sports Med. 1992;20(5):558–566. doi: 10.1177/036354659202000513. [DOI] [PubMed] [Google Scholar]
- 5.Irrgang JJ, Harner CD. Loss of motion following knee ligament reconstruction. Sports Med. 1995;19(2):150–159. doi: 10.2165/00007256-199519020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769–778. doi: 10.1177/036354659702500608. [DOI] [PubMed] [Google Scholar]
- 7.Chen CH, Chen WJ, Shih CH. Double-bundle posterior cruciate ligament reconstruction with quadriceps and semitendinosus tendon grafts. Arthroscopy. 2003;19(9):1023–1026. doi: 10.1016/j.arthro.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Wang CJ, Chen HS, Huang TW. Outcome of arthroscopic single bundle reconstruction for complete posterior cruciate ligament tear. Injury. 2003;34(10):747–751. doi: 10.1016/s0020-1383(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 9.Leighton MM, Bach BR. Reabilitação das lesöes dos ligamentos do joelho. In: Tria AJ, editor. Lesöes ligamentares do joelho. Revinter; Rio de Janeiro: 2002. pp. 289–319. [Google Scholar]
- 10.Weber MD, Woodall WR. Reabilitação do joelho. In: Andrews JR, Harrelson GL, Wilk KE, editors. Reabilitação física do atleta. Elsevier; Rio de Janeiro: 2005. pp. 399–456. [Google Scholar]
- 11.Bottoni CR, Parr RR. Double bundle arthroscopic posterior cruciate ligament reconstruction using a new medial femoral cortical bridge technique. Techniques in Knee Surgery. 2003;2(4):239–249. Available from: www.en.aspetar.com/mritems/pdf/pcl%20op%20techs.pdf Accessed in 2010 (Ago 11). [Google Scholar]
- 12.MacGillivray JD, Stein BE, Park M, Allen AA, Wickiewicz TL, Warren RF. Comparison of tibial inlay versus transtibial techniques for isolated posterior cruciate ligament reconstruction: minimum 2-year follow-up. Arthroscopy. 2006;22(3):320–328. doi: 10.1016/j.arthro.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 13.Kisner C, Colby LA. O Joelho. In: Kisner C, Colby LA, editors. Exercícios terapéuticos. Manole; Sâo Paulo: 1998. pp. 407–457. [Google Scholar]
- 14.Monteiro CG, Forgas CB. Assisténcia fisioterapéutica nas lesöes do ligamento cruzado posterior. In: Cohen M, Abdalla RJ, editors. Lesöes nos esportes. Revinter; Rio de Janeiro: 2003. pp. 558–559. [Google Scholar]
- 15.Stähelin AC, Südkamp NP, Weiler A. Anatomic double-bundle posterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy. 2001;17(1):88–97. doi: 10.1053/jars.2001.20661. [DOI] [PubMed] [Google Scholar]
- 16.Noyes FR, Medvecky MJ, Bhargava M. Arthroscopically assisted quadriceps double-bundle tibial inlay posterior cruciate ligament reconstruction: An analysis of techniques and a safe operative approach to the popliteal fossa. Arthroscopy. 2003;19(8):894–905. doi: 10.1016/s0749-8063(03)00651-0. [DOI] [PubMed] [Google Scholar]
- 17.Faustino CAC. Reconstrução do ligamento cruzado posterior com os enxertos dos tendöes dos músculos flexores do joelho. Acta Ortop Bras. 2003;11(2):95–101. [Google Scholar]
- 18.Margheritini F, Rihn J, Musahl V, Mariani PP, Harner C. Posterior cruciate ligament injuries in the athlete: an anatomical, biomechanical and clinical review. Sports Med. 2002;32(6):393–408. doi: 10.2165/00007256-200232060-00004. [DOI] [PubMed] [Google Scholar]
- 19.Quelard B, Sonnery-Cottet B, Zayni R, Badet R, Fournier Y, Hager JP. Isolated posterior cruciate ligament reconstruction: is non-aggressive rehabilitation the right protocol? Orthop Traumatol Surg Res. 2010;96(3):256–262. doi: 10.1016/j.otsr.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 20.McAllister DR, Hussain SM. Tibial inlay posterior cruciate ligament reconstruction: surgical technique and results. Sports Med Arthrosc. 2010;18(4):249–253. doi: 10.1097/JSA.0b013e3181faaee1. [DOI] [PubMed] [Google Scholar]
- 21.Fanelli GC, Beck JD, Edson CJ. Double bundle posterior cruciate ligament reconstruction: surgical technique and results. Sports Med Arthrosc. 2010;18(4):242–248. doi: 10.1097/JSA.0b013e3181f2faa1. [DOI] [PubMed] [Google Scholar]
- 22.Edson CJ, Fanelli GC, Beck JD. Postoperative rehabilitation of the posterior cruciate ligament. Sports Med Arthrosc. 2010;18(4):275–279. doi: 10.1097/JSA.0b013e3181f2f23d. [DOI] [PubMed] [Google Scholar]
- 23.Dürselen L, Claes L, Kiefer H. The influence of muscle forces and external loads on cruciate ligament strain. Am J Sports Med. 1995;23(1):129–136. doi: 10.1177/036354659502300122. [DOI] [PubMed] [Google Scholar]
- 24.Markolf KL, O'Neill G, Jackson SR, McAllister DR. Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32(5):1144–1149. doi: 10.1177/0363546503262198. [DOI] [PubMed] [Google Scholar]
- 25.Irrgang JJ, Fitzgerald GK. Rehabilitation of the multiple-ligament-injured knee. Clin Sports Med. 2000;19(3):545–571. doi: 10.1016/s0278-5919(05)70223-4. [DOI] [PubMed] [Google Scholar]
- 26.Shelburne KB, Pandy MG. Determinants of cruciate-ligament loading during rehabilitation exercise. Clin Biomech (Bristol, Avon) 1998;13(6):403–413. doi: 10.1016/s0268-0033(98)00094-1. [DOI] [PubMed] [Google Scholar]
- 27.Fanelli GC, Giannotti BF, Edson CJ. The posterior cruciate ligament arthroscopic evaluation and treatment. Arthroscopy. 1994;10(6):673–688. doi: 10.1016/s0749-8063(05)80067-2. [DOI] [PubMed] [Google Scholar]
- 28.Allen CR, Kaplan LD, Fluhme DJ, Harner CD. Posterior cruciate ligament injuries. Curr Opin Rheumatol. 2002;14(2):142–149. doi: 10.1097/00002281-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Draganich LF, Vahey JW. An in vitro study of anterior cruciate ligament strain induced by quadriceps and hamstrings forces. J Orthop Res. 1990;8(1):57–63. doi: 10.1002/jor.1100080107. [DOI] [PubMed] [Google Scholar]
- 30.Escamilla RF, Fleisig GS, Zheng N, Barrentine SW, Wilk KE, Andrews JR. Biomechanics of the knee during closed kinetic chain and open kinetic chain exercises. Med Sci Sports Exerc. 1998;30(4):556–569. doi: 10.1097/00005768-199804000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Escamilla RF. Knee biomechanics of the dynamic squat exercise. Med Sci Sports Exerc. 2001;33(1):127–141. doi: 10.1097/00005768-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Palmitier RA, An KN, Scott SG, Chao EY. Kinetic chain exercise in knee rehabilitation. Sports Med. 1991;11(6):402–413. doi: 10.2165/00007256-199111060-00005. [DOI] [PubMed] [Google Scholar]
- 33.Lutz GE, Palmitier RA, An KN, Chao EY. Comparison of tibiofemoral joint forces during open-kinetic-chain and closed-kinetic-chain exercises. J Bone Joint Surg Am. 1993;75(5):732–739. doi: 10.2106/00004623-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Mesfar W, Shirazi-Adl A. Biomechanics of changes in ACL and PCL material properties or prestrains in flexion under muscle force-implications in ligament reconstruction. Comput Methods Biomech Biomed Engin. 2006;9(4):201–209. doi: 10.1080/10255840600795959. [DOI] [PubMed] [Google Scholar]
- 35.Zavatsky AB, Beard DJ, O'Connor JJ. Cruciate ligament loading during isometric muscle contractions. A theoretical basis for rehabilitation. Am J Sports Med. 1994;22(3):418–423. doi: 10.1177/036354659402200320. [DOI] [PubMed] [Google Scholar]
- 36.Steinkamp LA, Dillingham MF, Markel MD, Hill JA, Kaufman KR. Biomechanical considerations in patellofemoral joint rehabilitation. Am J Sports Med. 1993;21(3):438–444. doi: 10.1177/036354659302100319. [DOI] [PubMed] [Google Scholar]
- 37.Fischer-Rasmussen T, Krogsgaard M, Jensen DB, Dyhre-Poulsen P. Inhibition of dynamic thigh muscle contraction by electrical stimulation of the posterior cruciate ligament in humans. Muscle Nerve. 2001;24(11):1482–1488. doi: 10.1002/mus.1172. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu T, Kadoya Y, Nakagawa S, Yoshida G, Takaoka K. Movement of the posterior cruciate ligament during knee flexion–MRI analysis. J Orthop Res. 2005;23(2):334–339. doi: 10.1016/j.orthres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Li G, DeFrate LE, Sun H, Gill TJ. In vivo elongation of the anterior cruciate ligament and posterior cruciate ligament during knee flexion. Am J Sports Med. 2004;32(6):1415–1420. doi: 10.1177/0363546503262175. [DOI] [PubMed] [Google Scholar]
- 40.DeFrate LE, Gill TJ, Li G. In vivo function of the posterior cruciate ligament during weightbearing knee flexion. Am J Sports Med. 2004;32(8):1923–1928. doi: 10.1177/0363546504264896. [DOI] [PubMed] [Google Scholar]
- 41.Andersen LL, Magnusson SP, Nielsen M, Haleem J, Poulsen K, Aagaard P. Neuromuscular activation in conventional therapeutic exercises and heavy resistance exercises: implications for rehabilitation. Phys Ther. 2006;86(5):683–697. [PubMed] [Google Scholar]
- 42.Pagnano MW, Cushner FD, Scott WN. Role of the posterior cruciate ligament in total knee arthroplasty. J Am Acad Orthop Surg. 1998;6(3):176–187. doi: 10.5435/00124635-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Lephart SM, Fu FH. Proprioception and neuromuscular control in joint stability. Human Kinetics; Philadelphia: 2001. [Google Scholar]