Abstract
Innate immune and differentiated T cells produce signature cytokines in response to cytokine stimulation. Optimal production requires stimulation by an NF-κB inducer, most commonly an interleukin (IL)-1 family member, and a STAT activator. Usually, there is linkage between the IL-1 family member, the activated STAT and the cytokines produced: IFNγ producers respond to the IL-1 family member, IL-18 and IL-12, a STAT4 activator; IL-13 producers respond to IL-33 (although for ILC2 cells this may be replaced by IL-25) and STAT5 activators; for cells producing IL-17A or IL-22, the combination is IL-1 and a STAT3 inducer. Cytokine-induced cytokine production may have broad significance in orchestrating innate responses to distinct infectious agents and in maintaining inflammatory responses after elimination of the inciting antigen.
Keywords: cytokine, IL-1, STAT, ILC, T helper cells, innate, effector
Cytokine-induced cytokine production: a general mechanism
Innate immunity functions as a first line host defense mechanism against infection. Cells of the innate immune system rely on recognition of a limited set of conserved pathogen-associated molecular patterns (PAMPs) by a diverse array of germ-line-encoded pattern recognition receptors (PRRs), such as the Toll-like receptors (TLRs) and the NOD-like receptors (NLRs) [1–3]. Alternatively, they respond to signals generated within the host in response to pathogens or other inducers of inflammation; these signals often are in the form of cytokines. Cells of the innate immune system include mast cells, macrophages, neutrophils, dendritic cells (DCs), basophils, eosinophils, natural killer (NK) cells, and the growing set of innate lymphoid cells (ILCs) and innate-like T lymphocytes, and are responsible for immediate, early immune responses against pathogens. They act as effectors through the production of ‘effector cytokines’ or direct cytotoxic activity.
It is now recognized that many cells operate at the crossroads of the innate and adaptive immune responses. Recent work also shows that adaptive immune cells are capable of producing effector cytokines in response to cytokine stimulation, independently of signaling through multichain immunoreceptors, such as the B cell receptor, the T cell receptor (TCR), or Fc receptors, although there are instances, such as responsiveness of natural killer T (NKT) cells or certain invariant γδ T cells, in which a T cell receptor of limited variability mediates an ‘innate’ response. Here we describe the processes through which the various cells capable of cytokine-induced cytokine production, including NK cells, ILCs, γδ T cells, NKT cells, innate CD8 T cells, mast cells, basophils, neutrophils and, perhaps most surprisingly, CD4 Th1/Th2/Th17 cells, are induced to produce such cytokines.
Innate features of conventional T cells
The adaptive immune system has the unique capacity to provide long-term, specific protective immunity to previously encountered pathogens. Memory T cells can be broadly divided into effector memory T cells (TEM cells) and central memory T cells (TCM cells) [4–6]. Resting TCM cells circulate among the secondary lymphoid tissues; upon reencounter with antigen, TCM cells quickly proliferate and differentiate into TEM cells or directly into effector T (Teff) cells. Teff cells provide a first line of defense against antigen at the entry portals.
Traditionally, induction of cytokine production by TEM cells has been thought to depend on stimulation by cognate antigen presented by antigen-presenting cells. It is now clear, however, in vitro at least, that these cells can also be stimulated to produce cytokines through an independent pathway initiated by the action of key cytokines.
T helper cell production of signature cytokines in response to IL-1 family members and STAT activators
Th1, Th2, and Th17 cells generated in tissue culture can produce several signature cytokines when challenged with other cytokines. It was reported more than a decade ago that the STAT4 activator, IL-12, and IL-18 together induce IFNγ production by Th1 cells [7–9]. TCR induced Th2 and Th17 cell induction also requires STAT activation (STAT 5 and STAT3, respectively) [10–12] and these cells express receptors for members of the IL-1 family, IL-33 in the case of Th2 and IL-1 in the case of Th17 cells [13]. These data suggest that a combination of an appropriate STAT activator and IL-1 family member might cause cytokine-induced cytokine production by each type of Th cell. Indeed, resting Th2 cells produce IL-13 if challenged with IL-33 and one of the STAT5 activators IL-2, IL-7, or TSLP [13]. IL-5 production was similarly observed but, strikingly, no IL-4 was produced while the very same cells produce both IL-4 and IL-13 in response to phorbol 12-myristate 13-acetate (PMA)/ionomycin, to anti-CD3/CD28, or to cognate antigen through antigen-presenting cells. Challenging Th17 cells with IL-1β, together with the STAT3 activators IL-23 or IL-21 leads to robust IL-17 production. Although modest IL-17 production occurred in response to added IL-23 alone, this was not the case in IL-1R1-deficient T cells, indicating a requirement for IL-1 signaling [14]. Moreover, IL-1 receptor antagonist-deficient (Il1ra−/−) CD4 T cells produce significantly more IL-17 in response to IL-23 than do their wild type counterparts [15].
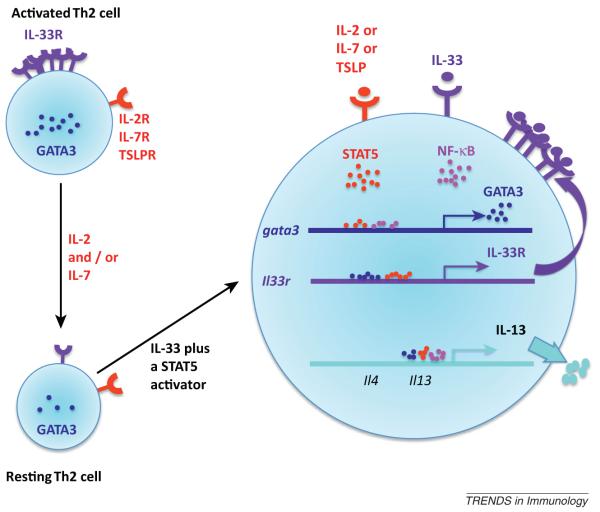
IL-1 receptor and ‘master’ transcription factor expression by Th cells might also be controlled by cytokine and STAT signaling. IL-33 receptor (IL-33R, T1-ST2) expression, as well as GATA3 mRNA, is diminished in resting Th2 cells compared with expression directly after priming (Figure 1). Both IL-33R expression and GATA3 mRNA increase in response to IL-33 and IL-2. IL-12 alone upregulated IL-18R1 and T-bet but addition of IL-18 maximized both IL-18R1 and T-bet expression. Upregulation of RORγt and of IL-1R1 by resting Th17 cells was maximal in cells cultured with IL-23 and IL-1β; IL-23 alone caused some induction whereas IL-1β alone had a very modest effect.
Figure 1.
Co-stimulating Th2 cells with IL-33 and a STAT5 activator induces IL-13 production. Recently differentiated Th2 cells express high levels of GATA3 and IL-33R. When cells are cultured in medium containing IL-2 or IL-7, which Th2 cells rely on for survival, expression of both GATA3 and IL-33R decline. Upon stimulation with IL-33 and a STAT5 activator, GATA3 transcription is substantially increased. GATA3 and possibly activated STAT5 jointly cause enhanced expression of IL-33R. Upregulated IL-33Rs allow for more efficient IL-33 signal transduction including robust activation of NF-κB, which together with GATA3 and possibly activated STAT5, leads to substantial IL-13 production.
The relevance of cytokine-induced cytokine induction in Th cells is not clear. Several fluorescent surrogates for cytokine expression are available that will in theory allow analysis of in situ cytokine production in response to either physiological (i.e., endogenous) production or pharmacological administration of the appropriate IL-1 family member and STAT activator.
Potentially, through a feedback mechanism, Th2 cells activated by antigen produce allergic inflammatory cytokines even after antigen is no longer present. In vitro, IL-33 alone (acting on recently activated Th2 cells) or with TSLP (jointly acting on resting Th2 cells) causes antigen-independent IL-13 and IL-5 production [13]. Induction of TSLP expression by keratinocytes and other epithelial cells by IL-13 or TNFα as well as by inflammatory stimuli has been reported [16]. IL-33 has been reported to largely be released upon necrotic death of cells and to be upregulated in certain instances [17]. IL-33 is a nuclear protein that can be degraded by caspase3 or caspase7 so that apoptotic death could destroy biologically active IL-33 [17]. Whether IL-33 levels can be regulated through the action of Th2 products is not clear. Nonetheless, a positive feedback loop through which activated Th2 cells lead to production of the cytokines that stimulate cytokine-induced cytokine production by resident Th2eff or Th2EM is tempting to postulate although not yet certain.
Conventional CD8 T cells
CD8+ T cells play a critical role in controlling infection by many intracellular pathogens including certain bacteria, viruses, and protozoan parasites [18]. CD8 TEM cells can produce IFNγ in response to IL-18 and IL-12 [19–22]. For example, ovalbumin-specific CD8 TEM cells secrete IFNγ 16 hours after Listeria monocytogenes infection in an IL-12- and IL-18-dependent manner [23]. TCR-independent, cytokine-dependent IFNγ production also contributes to the pathology of chronic obstructive pulmonary disease (COPD) and ectromelia virus infection [24,25].
Innate lymphoid cells (ILCs)
Effector cytokines can also be produced by a diverse array of ILCs. NK cells are the prototypic ILCs, and have recently been joined by a variety of additional cell subsets that are important in innate immunity and lymphoid tissue formation [26–29]. These newly defined ILCs lack lineage markers (Lin-) but express the lymphoid progenitor marker IL-7 receptor α chain (IL-7Rα; CD127) and the cytokine common gamma (γc) receptor chain, and they all require IL-7 for development. There are at least three distinct ILC lineages: (i) Type 1 ILCs (ILC1); (ii) LTi, Type 17 ILC (ILC17) and/or Type 22 ILC (ILC22); and (iii) Type 2 ILC (ILC2). Strikingly, these ILC subsets resemble the discrete T cell effectors, Th1, Th17, and Th2, in their cytokine profiles and transcription factors that determine their development. These various ILC populations probably represent distinct lymphocyte lineages that have unique effector pathways in various immune responses.
Type 1 ILCs
Conventional NK (cNK) cells are granular lymphocytes that eliminate infected cells by immediate cytotoxic activity and via cytokine and chemokine production [30,31]. cNK cells can be activated through crosslinkage of FcεRIII, by engagement of activating NK cell receptors or by cytokine stimulation [30]. IL-12, IL-15, or IL-18 alone induces little or no cytokine production. However, IL-12 plus IL-18 or IL-1β [32–34] stimulates robust IFNγ while stimulation with IL-15 and IL-12 induces less IFNγ but more IL-10 and TNFα [32]. TNFα and IL-2 also augment IL-12-induced IFNγ production [35]. It is notable that TNFα activates NF-κB and MAP kinases as IL1-family cytokines do, suggesting that TNFα may function on cNK cells similarly to IL-18 and IL-1β.
Thymic NK (tNK) cells are a distinct population of NK cells that represent ~0.05% of thymic cellularity in fetal and adult thymus and adult lymph node (LN) [36–38]. In contrast to cNK cells, tNK cells express CD127 and large amounts of GATA3 [39]. In response to IL-12, tNK cells express less granzyme B but more IFNγ, GM-CSF and TNFα than cNK cells [39]. Based on their ability to produce IFNγ, cNK and tNK cells could be designated as ILC1 cells that represent an innate counterpart of Th1 CD4 T cells.
LTi, Type 17 (ILC17) and/or Type 22 ILC (ILC22)
LTi cells were initially identified in mouse neonatal lymph nodes and named based on their ability to promote formation of secondary lymphoid nodes and Peyer’s patches during embryonic development [40]. LTi-like cells have now been identified in secondary and mucosal-associated lymphoid tissues in both mice and humans (reviewed in [41]). Several Th17-associated transcription factors, such as RORγt and the aryl hydrocarbon receptor (AhR), are important for LTi cell function [42–45]. LTi-like cells also share other features with Th17 cells, including expression of IL-23R and CCR6 [46–48]. Consistent with their receptor expression, LTi-like cells in mouse spleen and human fetal LN respond to IL-23 and to secrete IL-17 and IL-22 [49,50]. Depletion of LTi cells impairs IL-22 production and innate resistance to Citrobacter rodentium [48].
A unique innate lymphocyte subset sharing characteristics of both LTi cells and NK cells was recently identified in mucosal-associated lymphoid tissues in humans and mice [51–56]. These cells have been designated NKR+ LTi cells, LTi-like NK cells, NK22 cells, or ILC22 cells based on their expression of cNK cell lineage markers and ability to produce IL-22. Similar to LTi cells, ILC22 cells require RORγt and AhR [45,51–55]. ILC22 cells are potent IL-22 producers in response to IL-23 [51,54,56]. They also produce IL-26, another cytokine that can be co-secreted by Th17 cells, upon stimulation with IL-23 [51,57]. In contrast to LTi cells, which produce both IL-17 and IL-22, ILC22 cells selectively express IL-22 but not IL-17 [51,54].
ILC17 cells are detected in mouse and human intestine and express RORγt, AhR, and IL-23R [57,58]. Depletion of ILC17 cells abolishes Helicobacter hepaticus-induced acute and chronic innate colitis, demonstrating their importance in intestinal pathology. ILC17 cells respond to IL-23 by producing IL-17, IFNγ and, to a lesser degree, IL-22.
It is somewhat surprising that LTi cells, ILC22 cells, and ILC17 cells respond ex vivo to STAT-inducers without a signal mediated by an IL-1 family member or indeed any NF-κB stimulator. However, ILC22 cells have been shown to constitutively express IL-1R1 [59]. Furthermore, IL-23-stimulated ex vivo IL-22 production is severely reduced in ILC22 cells isolated from mice lacking adaptor for IL-1 signaling myeloid differentiation primary response gene 88 (MyD88) [59,60]. Diminished IL-23-induced IL-22 production was also observed in IL-1 receptor 1(IL-1R1) deficient cells and in wild type cells in the presence of anti-IL-1R1 antibody [59]. Thus, signaling through the IL-1 receptor appears essential for ILC22 cytokine production, even if exogenous IL-1 has not been provided. It is plausible that LTi, ILC22, and ILC17 cells are dependent on signals from an IL-1 family member and a STAT activator, in this case the STAT3 activator being IL-23, for signature cytokine production. This is further supported by in vitro studies showing that IL-22 production from human tonsillar ILC22 cells is enhanced by the combination of IL-23 and IL-1β, while IL-1β alone fails to induce IL-22 [61].
Type 2 ILC (ILC2)
Innate counterparts of Th2 cells were identified as Lin-CD127+ ILC subsets, present in Rag2−/− mice, that produce IL-5 and IL-13 upon stimulation with IL-25 or IL-33 [62]. These cells have been designated ‘nuocytes’, ‘natural helper (NH)’ cells, or ‘innate helper type2’ (Ih2) cells [63–70]. ILC2 cells express CD127, T1-ST2, and IL-17RB (a receptor for IL-25). Key transcription factors for ILC2 cells include RORα and GATA3 [66,71–74].
A characteristic features of ILC2 cells is their expansion and abundant production of IL-13 and IL-5 in asthma, in protease allergen-induced airway inflammation, in a chronic rhinosinusitis mouse model and during infection by the helminths Nippostrongylus brasiliensis, Trichuris muris, and Strongyloides venezuelensis [63–67,74–77]. Production of IL-13 and IL-5 by ILC2 is mediated through IL-25 and/or IL-33. IL-25R/IL-33R doubly deficient mice have a severely impaired response to helminth infection [63]. Administration of IL-25 or IL-33 to wild type mice induces IL-13 and/or IL-5 production [63,66]. Either IL-25 or IL-33 is sufficient to induce IL-13 and IL-5 production by ILC2 cells. Addition of IL-2 to IL-33 or IL-25 further enhanced IL-13 and IL-5 production from NH cells [64,67].
Do the requirements of ILC2 cells for cytokine-induced cytokine production differ from those of in vitro differentiated Th2 cells? As discussed earlier, resting in vitro differentiated Th2 cells require signals from both IL-33 and a STAT5 activator for IL-13 production. This is a stepwise process in which GATA3 must first be upregulated, then the IL-33 receptor and finally IL-13/IL-5. It is clear that both STAT5 activation and IL-33 are required for GATA3 upregulation; IL-33 appears essential for both receptor upregulation and IL-13/IL-5 transcription but the role of STAT5 in IL-33 receptor upregulation and IL-13/IL-5 transcription is uncertain. The capacity of STAT5 to bind to both the GATA3 and IL-13 genes is consistent with a direct role of STAT5 in these processes. It is possible that ILC2 require a STAT5 signal for IL-13/IL-5 production that is provided by endogenous TSLP or IL-7. The capacity of IL-25 to stimulate IL-13/IL-5 production by ILC2 is interesting because it can activate both the NF-κB and MAP kinase signaling pathways, as the IL-1 family members do. Thus IL-25 may act as a surrogate for the IL-1 family members. Also similar to Th2, ILC2 cells fail to produce IL-4 in response to IL-25 and/or IL-33 [63,66], although IL-4 production was observed after stimulation with PMA and ionomycin [64]. This is similar to the selective production of IL-13 but not IL-4 by Th2 cells in response to cytokines, despite their abundant production of both IL-4 and IL-13 upon stimulation with cognate antigen or PMA/ionomycin.
Innate-like T lymphocytes
Besides T helper cells and ILCs, cytokine-induced antigen-independent cytokine production has also been observed by innate-like T lymphocytes such as NKT cells, γδ T cells, and innate CD8 cells.
NKT cells
NKT cells recognize lipid and glycolipid antigens [78]. Invariant NKT (iNKT) cells constitute more than 80% of the NKT cell population. Three iNKT subsets have been identified based on their capacity to undergo cytokine-induced cytokine production. One subset, the conventional iNKT cells that are highly enriched in liver, respond to co-stimulation with IL-12 and IL-18 or IL-12 and IL-33 to produce IFNγ [79–85]. The second subset, recently characterized as CD4- NK1.1-, is highly enriched in peripheral LNs but represents a very small population in liver [86–88]. Ex vivo stimulation with IL-23 induces these cells to produce IL-17 [86,89]. Like IL-17- and IL-22-producing Th17 and ILC cells, these iNKT cells express RORγt, IL-1R1 and IL-23R [86,88,90]. The constitutive expression of IL-1R1 suggests the involvement of signals mediated by IL-1 in their cytokine-induced cytokine production; testing the importance of MyD88 in their cytokine response to IL-23 will be of importance. The third iNKT cell subset, the IL-17RB+ CD4+ population, is found in mouse spleen and lung but is barely detectable in liver. These cells respond to IL-25 in the presence of DCs to produce IL-13 and IL-4, but no IL-5 [91]. Whether signals from DCs are absolutely necessary for cytokine production needs to be further clarified. Adoptive transfer of such iNKT cells into NKT-deficient mice rescues the IL-25-induced airway hypersensitivity reaction [91]. Note that in contrast to Th2 cells and ILC2 cells, these iNKT cells, like basophils (see below), produce IL-4 in response to cytokine stimulation. The mechanism(s) underlying the differential pattern of Th2 cytokines produced in cytokine-induced cytokine production needs to be determined. Thus, various NKT subsets can produce distinct sets of effector cytokines in response to a STAT activator and an NF-κB activator, often an IL-1 family member.
γδ T cells
γδ T cells constitute 1–5% of circulating T cells and are enriched in epithelial and mucosal tissues [92]. Co-stimulation with IL-1β and IL-12 induces these cells to produce IFNγ [93] while stimulation with IL-1β and IL-23 induces IL-17A, IL-17F, IL-21, and IL-22 production [94,95]. Recently, the compelling idea was raised that cytokine profiles of γδT cells are pre-determined during thymic selection by a mechanism involving the expression of CD27, a TNF family member [96,97]. CD27- γδ T cells constitutively express RORγt and RUNX1 and are prepared to produce IL-17 while CD27+ γδ T cells express T-bet and are competent to produce IFNγ [97]. Haas et al., however, segregated γδ T cells based on the expression of CCR6 and NK1.1 [98]. In their report, co-stimulation with IL-12 and IL-18 induced IFNγ production from NK1.1+ γδ T cells while IL-23 induced IL-17A production by CCR6+ γδ T cells [98]. In both subsets, the pattern of cytokines produced is a property of the stimulated cell. There is generally a linkage between the IL-1 family member inducers and the cytokine products, but it appears to represent the pattern of IL-1 family cytokine receptors expressed by the cell rather than distinctive signals induced by these receptors.
Innate CD8 cells
Innate CD8 T cells have been detected in BALB/c mice [99] and human fetal thymus and fetal spleen [100]. In thymus, a subset of IL-4R+ CD8 cells captured small amounts of IL-4 secreted by promyelocytic leukemia zinc finer transcription factor+ (PLZF+) NKT cells and developed into innate CD8 cells [99]. Stimulation of these cells with IL-12 and IL-18 leads them to produce IFNγ [99].
Mast cells, basophils, and neutrophils
Mast cells are effector cells of myeloid origin enriched at epithelial or endothelial surfaces. Basophils are related cells, usually are absent in tissues but that may be recruited to inflammatory sites. After crosslinking the high affinity IgE receptor, FcεRI, mast cells release large amounts of IL-13 and relatively modest amounts of IL-4; basophils secrete large amount of both cytokines [101,102]. Both mouse bone marrow-derived mast cells (BMDM) and basophils express a large amount of T1-ST2 [103]. IL-18Rα is expressed by basophils and by mast cells in low amounts [104]. In response to IL-33, together with IL-3 that activates STAT5 and STAT3, BMDM produce significant amounts of IL-13 but no IL-4; basophils produce large amounts of both IL-13 and IL-4 [103–107]. IL-3 alone induced very small amounts of IL-13 or IL-4. Robust IL-4 production induced by IL-3, IL-18, or IL-33 was observed in recently identified TSLP-elicited basophils, a discrete subset distinct from the IL-3-elicited bone marrow-derived basophils [108]. Similar to what has been observed in Th CD4 cells and ILC cells, there is a synergistic effect for cytokine-induced cytokine production between an IL-1 family cytokine and a STAT activator. In these cells, IL-3 activates STAT5 and STAT3. Intriguingly, a large amount of both IL-4 and IL-13 are produced by basophils; as noted above, the production of IL-4 through cytokine-induced cytokine production is unusual. It has been suggested that NF-κB activation by IL-1 family cytokines would more likely stimulate IL-13 production than IL-4 production as NF-κB more efficiently binds to the murine Il13 promoter than to the murine Il4 promoter [13,109–111]. Indeed, treatment with an NF-κB inhibitor resulted in diminished anti-CD3/CD28-stimulated IL-13 production from differentiated Th2 cells but IL-4 production remained unaffected [112]. The discrepancy in patterns of cytokines produced in response to IL-33 and STAT5 activators between basophils, on the one hand, and Th2 and ILC2 cells, on the other, needs more study.
Neutrophils account for 40–70% of all white blood cells [113]. Recently, in a mouse model of kidney ischemia-reperfusion injury (IRI), CD11b+Gr1+ neutrophils were shown to be the major source of early IL-23-mediated IL-17A production [114]. Adoptive transfer of neutrophils to Il17a-deficient mice reconstituted kidney injury, which could be blocked by specific IL-17A antibody [114]. It would be interesting to examine the expression of IL-23R and RORγt on this cell population as well as the expression pattern of IL-1 receptor family members in order to determine if neutrophils follow the same set of ‘rules’ for cytokine-induced cytokine production as do the other cell types discussed here.
Concluding remarks
Despite their phenotypic and functional differences, Th cells, ILCs, and innate-like T cells share striking similarities in their cytokine dependence and transcriptional regulation.
The comparable transcriptional regulation of cytokine production among diverse subsets is the most remarkable (Table 1). RORγt was originally shown to be essential for the development of LTi cells and then for Th17 cells. The importance of RORγt for a wide variety of cell subsets all having the capacity to produce IL-17 and/or IL-22, including ILC22, ILC17 cells, IL-17-producing NKT cells, and IL-17-producing γδ T cells is now recognized. Many of these cells also share expression of the Th17-associated transcription factor, AhR. Accordingly, they all express IL-23R and IL-1R1 and, upon appropriate stimulation, produce Th17 signature cytokines.
Table 1.
Shared properties of cells capable of cytokine-induced cytokine production.
| Features of cytokine production | |||
|---|---|---|---|
| Cytokine produced | IFNγ | IL-13/IL-5/(IL-4) | IL-17/IL-22 |
| T helper cells | Th1 | Th2 | Th17 |
| Innate lymphoid cells | cNK | nuocytes | ILC22 |
| tNK | natural helpers (NH) | ILC17 | |
| innate helper type2 | LTi | ||
| Innate-like T cells | IL-18-responsive NKT subset | IL-25 responsive NKT subset | IL-23 responsive NKT subset |
| γδ T cell subset | γδ T cell subset | ||
| Innate CD8 T cells | |||
| Receptors | IL-18R | T1ST2 (IL-33R) | IL-1R1 |
| IL-12R | IL-17RB (IL-25R) | IL-23R | |
| IL-2R | |||
| IL-7R | |||
| TSLPR | |||
| Stimulating cytokines | IL-18 | IL-33/IL-25 | IL-1β |
| IL-12 | IL-2/IL-7/TSLP | IL-23 | |
| Transcription factors | T-bet (Th1, cNK, NKT subset) | GATA3 (Th2 and ILC2 cells?) | RORγt |
| Eomes (cNK and innate CD8 T) | RORα (ILC2 cells) | AhR |
Unexpectedly, another member of the RORγt family transcription factor, RORα, also associated with Th17 cells, has been shown to be essential for ILC2 cells development. The iconic IL-25-mediated-nuocyte-induced intestinal goblet-cell hyperplasia and eosinophilia is disrupted in mice with RORα deficiency [71]. ILC2 cells express high levels of GATA3. Considering that GATA3 serves as the master transcription factor driving Th2 differentiation, it will be important to address the role of GATA3 in ILC2 cells. IL-13 production by Ih2 cells was absent in N. brasiliensis-infected Il13yetCre/+Gata3fl/fl implying that IL-13 production by ILC2 cells is dependent on GATA3 [72]. However, whether this effect is cell intrinsic has not been determined since IL-13-Cre would also have deleted Gata3 gene in Th2 cells, which might regulate IL-13 production by ILC2s. Furthermore, whether GATA3 is involved in ILC2 development has not been addressed. Thus, the relative roles of RAR-related orphan receptor α (RORα) and GATA3 remain to be established.
The Th1 master transcription factor, T-bet, is also important in priming and regulating terminal maturation and peripheral homeostasis of cNK and NKT cells [115,116]. Its congener, eomesodermin, plays critical roles in innate CD8 T cells and cNK cell development [116].
Functionally, these cells share the property that they produce their signature cytokines in response to cytokine stimulation. Usually the requirement for optimal stimulation is a combination of an IL-1 family member and a STAT activator, the latter being the STAT activator usually associated with the differentiation of the Th cell that has the particular cytokine production pattern. In the case of Th2 cells, where this has been studied in detail, it appears that IL-1 family member functions through its activation of NF-κB and MAP kinases, particularly p38. As each of the IL-1 family members shares this pattern of activation, the differences in cytokine production associated with different IL-1 family members probably reflects the linkage of receptor expression with state of cellular differentiation rather than a particular targeting of signals generated by a particular IL-1 receptor family member to the transcription of a given cytokine. By contrast, the association between distinct STATs and patterns of cytokine production appears to reflect the targeting of the STAT to the promoter/enhancer of the cytokine locus in question, thus establishing a direct link between particular STAT activators and the production of particular cytokines.
Cytokine-induced cytokine production allows both innate and adaptive cells to rapidly sense perturbations during infection and inflammation, responding to distinct IL-1 family members and STAT activators with effector cytokine production (Figure 2). The importance of this mechanism is clear during certain types of infections. For example, after infection with N. brasiliensis, nuocyte numbers peaked at 5–7 days, the time point of the initiation of the Th2 response in the gut. Impaired nuocyte generation in commonγ/Rag knockout mice is associated with impaired worm expulsion, which was rescued by adoptive transfer in vitro expanded nuocytes [66]. Worm expulsion depended on the capacity of the nuocytes to produce IL-13 and to respond to IL-25 or IL-33. By contrast, during primary infection, worm clearance was not greatly impaired in mice whose CD4 T cells had a deficiency in producing IL-4 and IL-13, implying that IL-13 produced by nuocytes rather than Th2 cells plays the central role in worm expulsion [117]. Whether the dominance displayed by innate cells capable of producing IL-13 will be seen in mice that have expanded numbers of Th2 cells needs to be determined.
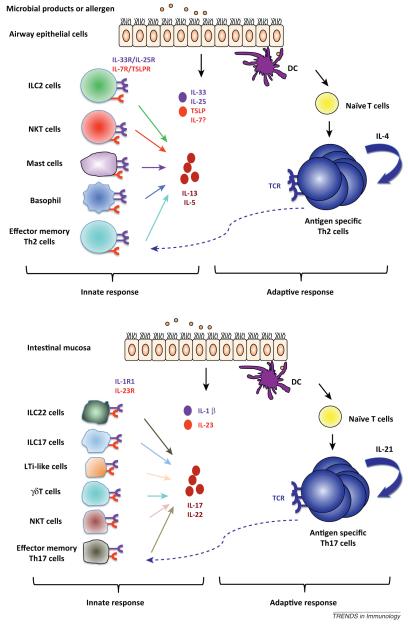
Figure 2.
IL-1 family cytokine-induced cytokine production provides a mechanism for a rapid innate response. In a type 2-dominated airway response, respiratory epithelial cells and/or myeloid cells release a variety of cytokines including IL-33, IL-25, and TSLP. These cytokines could be recognized by receptors expressed on innate lymphoid cell (ILC2), natural killer T (NKT) cells, mast cells, basophils, and effector memory Th2 cells to induce IL-13 and IL-5 production, contributing to rapid production of effector cytokines. In parallel, recognition of antigen on dendritic cells (DCs) by antigen-specific naïve and memory CD4 T cells results in cellular activation and proliferation, leading to an adaptive immune response. In intestinal mucosa, where a type 17 response plays a major role, IL-1β and IL-23 produced by mucosal epithelial cells and other cells after microbial invasion could induce innate IL-17 and/or IL-22 production from diverse cell subsets, such as ILC22, ILC17, LTi, NKT, γδ T cells, and effector memory Th17 cells. Such innate IL-17 and/or IL-22 sources are potential important sentinels for immune responses.
Cytokine-induced cytokine production may also be important in chronic inflammation, although this remains to be established. However, the capacity of both innate cells and Th cells to produce cytokines that may stimulate production of inducing cytokines raises the possibility of self-reinforcing stimulatory loops. As an example, Th2 cells produce IL-4, IL-13, and TNFα, all inducers of TSLP production by keratinocytes and other epithelial cells. TSLP and IL-33, whose production can also be turned on during various types of inflammation, jointly activate IL-13 and TNFα production by Th2 cells. Thus, antigen/TCR driven production of signature cytokines by Th2 Teff cells in the tissues (skin, lung, and gut) may activate local epithelial cells and/or macrophages/dendritic cells to produce TSLP and IL-33, respectively. In turn, this would cause antigen-independent IL-13 and TNFα production by Th2 cells with the possibility of self-reinforcing loops allowing for continued production of effector cytokines well after the inducing antigen had disappeared.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy, and Infectious Diseases, National Institutes of Health.
References
- 1.Janeway CA, Medzhitov R. Innate immune recognition. Ann. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, et al. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 3.Tschopp J, et al. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, et al. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 6.Seder RA, Ahmed R. Similarities and differences in CD4(+) and CD8(+) effector and memory T cell generation. Nat. Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 7.Robinson D, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimoto T, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 9.Yang J, et al. Induction of interferon-gamma production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur. J. Immunol. 1999;29:548–555. doi: 10.1002/(SICI)1521-4141(199902)29:02<548::AID-IMMU548>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Thieu VT, et al. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, et al. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 12.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 13.Guo L, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton C, et al. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho ML, et al. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J. Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 16.Schleimer RP, et al. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J. Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, et al. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur. J. Immunol. 2007;37:2892–2899. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter LL, Murphy KM. Lineage-specific requirement for signal transducer and activator of transcription (Stat)4 in interferon gamma production from CD4(+) versus CD8(+) T cells. J. Exp. Med. 1999;189:1355–1360. doi: 10.1084/jem.189.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kambayashi T, et al. Memory CD8+ T cells provide an early source of IFN-gamma. J. Immunol. 2003;170:2399–2408. doi: 10.4049/jimmunol.170.5.2399. [DOI] [PubMed] [Google Scholar]
- 22.Berg RE, et al. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur. J. Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Berg RE, et al. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman CM, et al. Cytotoxic potential of lung CD8(+) T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J. Immunol. 2010;184:6504–6513. doi: 10.4049/jimmunol.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, et al. IL-12p40 and IL-18 play pivotal roles in orchestrating the cell-mediated immune response to a poxvirus infection. J. Immunol. 2009;183:3324–3331. doi: 10.4049/jimmunol.0803985. [DOI] [PubMed] [Google Scholar]
- 26.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 27.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 29.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 30.Biron CA, et al. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 31.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu. Rev. Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 32.Fehniger TA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J. Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 33.Cooper MA, et al. Interleukin-1beta costimulates interferon-gamma production by human natural killer cells. Eur. J. Immunol. 2001;31:792–801. doi: 10.1002/1521-4141(200103)31:3<792::aid-immu792>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 34.Lauwerys BR, et al. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 35.Tripp CS, et al. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlyle JR, et al. Natural killer cell development and function precede alpha beta T cell differentiation in mouse fetal thymic ontogeny. J. Immunol. 1998;160:744–753. [PubMed] [Google Scholar]
- 37.Schmitt TM, et al. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J. Exp. Med. 2004;200:469–479. doi: 10.1084/jem.20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veinotte LL, et al. Unique subset of natural killer cells develops from progenitors in lymph node. Blood. 2008;111:4201–4208. doi: 10.1182/blood-2007-04-087577. [DOI] [PubMed] [Google Scholar]
- 39.Vosshenrich CAJ, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 40.Kelly KA, Scollay R. Seeding of neonatal lymph nodes by T cells and identification of a novel population of CD3-CD4+ cells. Eur. J. Immunol. 1992;22:329–334. doi: 10.1002/eji.1830220207. [DOI] [PubMed] [Google Scholar]
- 41.Cherrier M, Eberl G. The development of LTi cells. Curr. Opin. Immunol. 2012;24:178–183. doi: 10.1016/j.coi.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Eberl G, et al. An essential function for the nuclear receptor ROR gamma t in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 43.Kurebayashi S, et al. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 45.Lee JS, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2012;13:U144–U158. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 47.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonnenberg GF, et al. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cupedo T, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC(+) CD127(+) natural killer-like cells. Nat. Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 51.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crellin NK, et al. Human NKp44(+)IL-22(+) cells and LTi-like cells constitute a stable RORC(+) lineage distinct from conventional natural killer cells. J. Exp. Med. 2010;207:281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luci C, et al. Influence of the transcription factor ROR gamma t on the development of NKp46(+) cell populations in gut and skin. Nat. Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 54.Sanos SL, et al. ROR gamma t and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46(+) cells. Nat. Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satoh-Takayama N, et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3(−)NKp46(+) cell subsets from Id2-dependent precursors. J. Exp. Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46(+) cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Geremia A, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reynders A, et al. Identity, regulation and in vivo function of gut NKp46+RORgammat+ and NKp46+RORgammat- lymphoid cells. EMBO J. 2011;30:2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 61.Cella M, et al. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 63.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 65.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mjosberg JM, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 68.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikutani M, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 70.Bartemes KR, et al. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong SH, et al. Transcription factor RORalpha is critical for nuocyte development. Nat. Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang HE, et al. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2012;13:U58–U83. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Q, et al. Cutting edge: natural helper cells derive from lymphoid progenitors. J. Immunol. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halim TY, et al. Lung natural helper cells are a critical source of th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 75.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barlow JL, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 2012;129:191–198. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 77.Yasuda K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc. Natl. Acad. Sci. U.S.A. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bendelac A, et al. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 79.Leite-De-Moraes MC, et al. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J. Immunol. 1999;163:5871–5876. [PubMed] [Google Scholar]
- 80.MacDonald HR, Eberl G. Rapid death and regeneration of NKT cells in anti-CD3 epsilon- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 81.Bendelac A, et al. The contribution of NKT cells, NK cells, and other gamma-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J. Immunol. 2003;170:1197–1201. doi: 10.4049/jimmunol.170.3.1197. [DOI] [PubMed] [Google Scholar]
- 82.Benedict CA, et al. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brenner MB, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dustin ML, et al. Activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J. Immunol. 2008;180:2024–2028. doi: 10.4049/jimmunol.180.4.2024. [DOI] [PubMed] [Google Scholar]
- 85.Herbelin A, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur. J. Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 86.Rachitskaya AV, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coquet JM, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Michel ML, et al. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doisne JM, et al. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J. Immunol. 2011;186:662–666. doi: 10.4049/jimmunol.1002725. [DOI] [PubMed] [Google Scholar]
- 90.Doisne JM, et al. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J. Immunol. 2009;183:2142–2149. doi: 10.4049/jimmunol.0901059. [DOI] [PubMed] [Google Scholar]
- 91.Terashima A, et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 93.Skeen MJ, Ziegler HK. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J. Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 94.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Shibata K, et al. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 96.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haas JD, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur. J. Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 99.Weinreich MA, et al. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat. Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Min HS, et al. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J. Immunol. 2011;186:5749–5757. doi: 10.4049/jimmunol.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galli SJ, et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 102.Karasuyama H, et al. Nonredundant roles of basophils in immunity. Annu. Rev. Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 103.Kondo Y, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 104.Yoshimoto T, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kroeger KM, et al. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J. Leukoc. Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Min B, Paul WE. Basophils and type 2 immunity. Curr. Opin. Hematol. 2008;15:59–63. doi: 10.1097/MOH.0b013e3282f13ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ho LH, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J. Leukoc. Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 108.Siracusa MC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Silbermann K, et al. Stimulation of interleukin-13 expression by human T-cell leukemia virus type 1 oncoprotein Tax via a dually active promoter element responsive to NF-kappaB and NFAT. J. Gen. Virol. 2008;89:2788–2798. doi: 10.1099/vir.0.2008/003699-0. [DOI] [PubMed] [Google Scholar]
- 110.Casolaro V, et al. Inhibition of NF-AT-dependent transcription by NF-kappa B: implications for differential gene expression in T helper cell subsets. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11623–11627. doi: 10.1073/pnas.92.25.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Neumann M, et al. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J. 1995;14:1991–2004. doi: 10.1002/j.1460-2075.1995.tb07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Das J, et al. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat. Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 113.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 114.Li L, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 116.Gordon SM, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sullivan BM, et al. Genetic analysis of basophil function in vivo. Nat. Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]