Abstract
Maternal diets low in choline, an essential nutrient, increase the risk of neural tube defects and lead to low performance on cognitive tests in children. However, the consequences of maternal dietary choline deficiency for the development and structural organization of the cerebral cortex remain unknown. In this study, we fed mouse dams either control (CT) or low-choline (LC) diets and investigated the effects of choline on cortical development in the offspring. As a result of a low choline supply between embryonic day (E)11 and E17 of gestation, the number of 2 types of cortical neural progenitor cells (NPCs)—radial glial cells and intermediate progenitor cells—was reduced in fetal brains (P < 0.01). Furthermore, the number of upper layer cortical neurons was decreased in the offspring of dams fed an LC diet at both E17 (P < 0.001) and 4 mo of age (P < 0.001). These effects of LC maternal diet were mediated by a decrease in epidermal growth factor receptor (EGFR) signaling in NPCs related to the disruption of EGFR posttranscriptional regulation. Our findings describe a novel mechanism whereby low maternal dietary intake of choline alters brain development.—Wang, Y., Surzenko, N., Friday, W. B., Zeisel, S. H. Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring.
Keywords: neurogenesis, EGFR, cortical development
Neural progenitor cells (NPCs) of the developing brain are influenced by many genetic and environmental factors, including nutrients. However, the precise roles of specific nutrients during brain development remain poorly understood. Choline is an essential nutrient needed for the structural integrity and signaling functions of cell membranes, for acetylcholine synthesis, and for methyl group metabolism (1). A woman who consumes a diet low in choline has a 4-fold increased risk of having an infant with a neural tube defect and a 1.7-fold increased risk of having one with a cleft palate (2, 3). Children born to mothers who consume more choline during pregnancy have enhanced cognitive function at 7 y of age when compared to children of mothers who consume less choline (4). These observations in humans are supported by studies in rodents. Rat and mouse dams fed low choline (LC) diets during pregnancy carry embryos that have diminished NPC proliferation and increased apoptosis in the hippocampus at embryonic day (E)17 (5, 6). These changes in NPC proliferation persist for more than 100 d after birth (7). Pups of mothers that consume more choline during the perinatal period have increased sensitivity to long-term potentiation and enhanced visuospatial and auditory memory in adulthood, when compared to pups of mothers that consume less choline (8–11). In addition, several investigators have reported that, in rats, maternal diets high in choline protect the fetus against certain environmental insults, such as alcohol exposure (12), effects of seizures (13), and treatment with the NMDA receptor antagonist dizocilpine (14). The effect of maternal choline on fetal brain development occurs during a sensitive window in development (E11–17). This period in mouse brain development is equivalent to the period from 8 wk of gestation through early postnatal years in human brain development. However, whether an LC diet results in altered neuronal and glial composition in distinct brain areas, and the mechanisms underlying these effects of choline on NPCs are not known.
Choline is present in high-fat foods, but dietary intake in the United States is marginal in many people (15), and the intake in developing countries is even lower (16, 17). It is important to note that maternal choline stores become markedly diminished by pregnancy and lactation, and dietary supplementation with choline restores them (18). Thus, choline supplementation during pregnancy and lactation does not constitute a pharmacological manipulation, but rather can be viewed as rehabilitation of a naturally occurring deficiency state that develops because of the extra demands for choline presented by the fetus and the suckling neonate.
Estrogen induces the expression of PEMT, a gene that encodes the enzyme that can form a choline moiety de novo, thereby sparing the dietary requirement for this nutrient (19). Concentrations of estrogen achieved during pregnancy maximally induce the enzyme, but almost half of women in the United States have a single-nucleotide polymorphism in PEMT that abrogates the ability of estrogen to induce the gene (20). Thus, the availability of choline to the fetus varies greatly.
In the developing mammalian brain, a large number of neurons are generated from a relatively small number of NPCs. Many environmental signals are indispensable for progenitor/stem cell maintenance and neuronal and glial differentiation. For instance, late embryonic and adult NPCs are responsive to epidermal growth factor (EGF) family ligands (21). Activation of the EGF receptor (EGFR; a member of the tyrosine kinase receptor family) in late progenitor cells stimulates cell proliferation, migration, and astrocyte differentiation (22). Differences in the level of EGFR expression determine how progenitor cells interpret an extrinsic signal at specific stages of development. The acquisition of responsiveness to EGF is associated with the appearance of a subpopulation of progenitor cells that express relatively high levels of EGFR. EGFR is upregulated at midgestation in the mouse telencephalon.
We have shown that EGF stimulation restores cell proliferation and protects cells from choline-deficiency–induced apoptosis in hepatocytes (23). These findings suggest that EGFR expression in NPCs is modulated by choline and that EGFR signaling is one of the mechanisms whereby choline regulates brain development. We examined EGFR expression in whole brains and in NPCs isolated from the embryos whose mothers received LC or control (CT) diets during pregnancy. We report that an LC diet reduced EGFR expression in NPCs (in vitro and in vivo) and led to a diminished number of NPCs, resulting in reduced brain size and major defects in cortical layering. We identified a novel molecular mechanism whereby low maternal dietary intake of choline alters brain development.
MATERIALS AND METHODS
Animals
The mice were bred and maintained at the David H. Murdock Research Institute (DHMRI), Center for Laboratory Animal Science facility (Kannapolis, NC, USA). All experiments were performed in accordance with the standards of the U.S. National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals and were approved by the DHMRI Institutional Animal Care and Use Committee. Nestin-CFPnuc transgenic mice were generously provided by Dr. Grigori Enikolopov (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA) (24), and were maintained on a C57B6 background. Nestin-CreERT2 and Ai9 mouse lines were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained on a C57B6/J background (25, 26).
Diets
Two dietary groups were used: CT (1.1 g choline/kg) and LC (0 g choline/kg) (AIN-76A; Dyets, Inc., Bethlehem, PA, USA).
Tissue dissociation, cell culture, and treatments
NPCs were isolated from E14 mouse brain according to a published protocol (22). The cells were cultured as described (27) in custom choline-free Neurobasal medium (Thermo Scientific–Gibco, Grand Island, NY, USA) containing 5 μM choline chloride (low choline), 70 μM choline chloride (CT choline), or 315 μM choline chloride (high choline).
Immunohistochemistry, microscopy, and data analysis
Brains were fixed in 4% paraformaldehyde and cryoprotected in a gradient of 10–30% sucrose/1× PBS solution. Coronal sections (20 µm) were prepared by cryosectioning. Brain sections were permeabilized with 0.1% Triton X-100 in blocking solution (5% normal goat serum in 1× PBS) for 2 h at room temperature. Incubation with primary antibodies was performed at 4°C overnight. Antigen retrieval [10 mM sodium citrate (pH 6.0)/steam heat for 25 min] was performed for detection of Ki67. Secondary antibodies were applied for 1 h at room temperature. The antibodies are listed in Supplemental Table S1.
Immunostaining in culture
Cultured cells were fixed in 4% paraformaldehyde for 15 min at room temperature. The cells were permeabilized with 0.1% Triton X-100 in blocking solution (5% normal goat serum in PBS) for 2 h at room temperature. The Click-iT EdU kit (Thermo Scientific–Life Technologies, Carlsbad, CA, USA) was used to assess DNA synthesis. To induce differentiation, NPCs were cultured in medium containing 2% fetal bovine serum without the growth factors for 9 d. Immunocytochemistry was performed to identify differentiating cell types.
Flow cytometry
The cerebral hemispheres (hippocampus and cortex) of E17 embryos were dissociated to single cells in Accutase (Innovative Cell Technologies, San Diego, CA, USA). A population of cells with cyan fluorescent protein (CFP) expression was obtained by flow sorting (FACSAria III cell sorter; BD Biosciences, Franklin Lakes, NJ, USA). Flow data were acquired and analyzed with FlowJo software (Tree Star, Inc., Ashland, OR, USA). These sorted populations were immediately collected for protein and RNA analysis. For cell cycle analysis, single cells were fixed in 70% ethanol at 4°C overnight, centrifuged at 300 g for 5 min, washed twice in PBS, incubated with RNase A (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 90 min, and stained with propidium iodide (PI) staining solution (Sigma-Aldrich) for at least 30 min at room temperature before flow cytometric analysis. Approximately 30,000 events were collected per sample, and cell cycle data were modeled with FlowJo software (Tree Star, Inc.).
Western blot analysis
Protein extracts were prepared from cell pellets or tissues by using RIPA lysis buffer and protease and phosphatase inhibitor cocktail (Thermo Scientific-Pierce, Rockford, IL, USA) and quantified with the bicinchoninic acid (BCA) assay (Bio-Rad, Hercules, CA, USA). Proteins were loaded into SDS-PAGE gels and blotted with antibodies listed in Supplemental Table S1. ECL (Thermo Scientific, Waltham, MA, USA) was used to detect the proteins.
EGFR degradation assays
Growth factor–starved NPCs were incubated in the presence of LC or CT choline medium (12 h), and 50 ng/ml EGF was added for various periods. When required, cycloheximide (CHX; 100 μg/ml; Sigma-Aldrich) was added to the cells 1 h before addition of EGF. The cells were chilled on ice, and the cell pellets were processed for Western blot analysis.
Immunoprecipitation and nascent EGFR synthesis assay
A nascent EGFR protein assay was performed by a modified protocol of Melemedjian et al. (28). Methionine stores were depleted in progenitors by incubating them in methionine-free medium for 1 h, followed by stimulation of the cells in the presence of azidohomoalanine (AHA), an analog of methionine, for 2 h. EGFR proteins were immunoprecipitated and labeled with biotin, by using click chemistry to label only proteins that had incorporated AHA. The nascent EGFR and total EGFR were probed by streptavidin and EGFR antibody, respectively. To control for the labeling procedures, AHA was added to some samples, and, to control for the immunoprecipitation, AHA immunoprecipitated by the appropriate CT IgG was added to other samples.
Real-time quantitative PCR
mRNA was isolated from both CT and LC-sorted progenitors with the RNeasy mini kit (Qiagen, Hilden, Germany) and cDNA was synthesized from 1 µg of total RNA using the RT2 first-strand kit (Qiagen). Real-time quantitative (q)PCR was performed using RT2 SYBR Green qPCR mastermix (Qiagen) with a Realplex4 Mastercycler (Eppendorf, Hauppauge, NY, USA). EGFR primers (PPM03714F) were from Qiagen. Quantification was performed with REST 2009 software (http://www.gene-quantification.de/rest-2009.html) with data normalized to the level of TATA box binding protein (Tbp) mRNA.
Virus infection
EGFR-eGFP and CLE-eGFP plasmids were a kind gift from Dr. Steven Rosenfeld (Cleveland Clinic, Cleveland, OH, USA). The 293T (Clontech, Palo Alto, CA, USA) cells were used to produce the virus. Viral titers were determined in 293T cells. Virus was added to monolayer cultures at 24 h after plating, and the resulting clones were analyzed 3 d after infection. Anti-EGFP antibody was used to quantify infected cells.
Transfections of NPCs
NPCs were plated as monolayers for 24 h before transfection. Transient transfections (100 nM, DharmaFECT 1 Transfection Reagent; GE Life Sciences–Dharmacon, Lafayette, CO, USA) were conducted for 72 h. siRNA against mouse EGFR (Smartpool, E-040411-00-0005; GE Life Sciences–Dharmacon) was delivered in Accell medium.
Statistical analyses
All results are expressed as means ± sem. Statistical analyses were performed with Prism 5 (GraphPad Software, San Diego, CA, USA). The test used for each experiment is described in the corresponding figure legend. Student’s t test was used for 2-population comparisons, 1-way ANOVA with Tukey’s post hoc test for multiple comparisons, and 2-way ANOVA with Tukey’s post hoc test for comparing EGFR half-life and degradation.
RESULTS
Choline is essential for the maintenance of the NPC pool
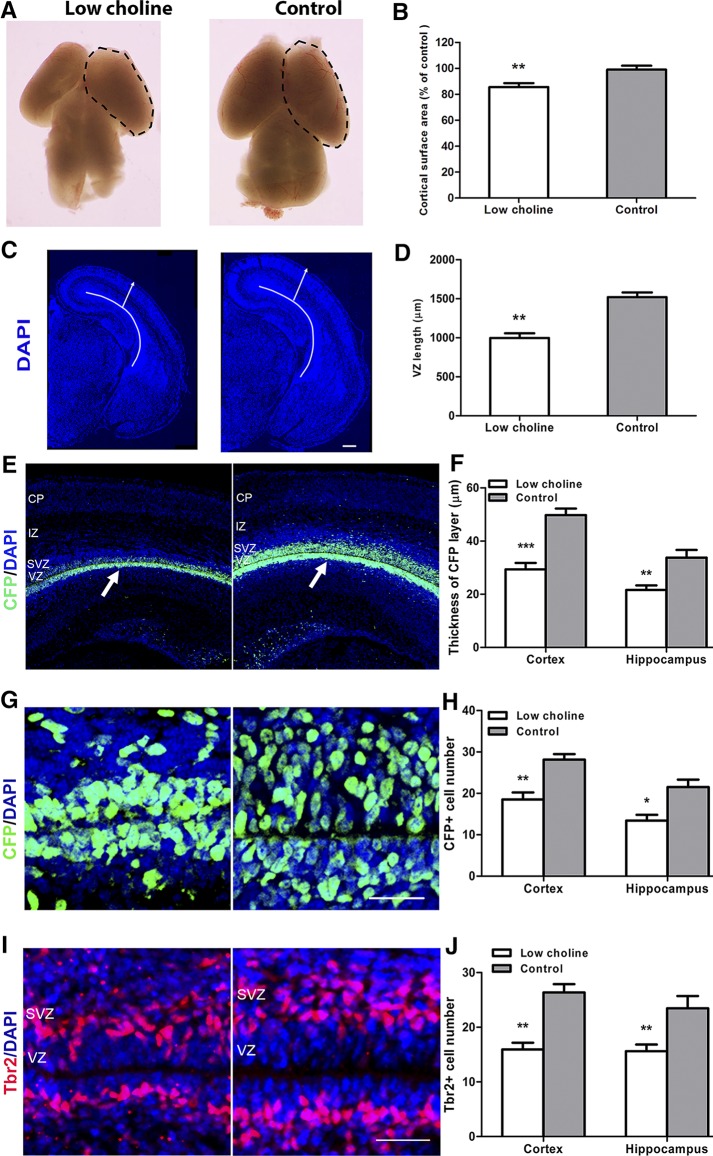
To evaluate in detail the consequences of low maternal intake of choline for fetal brain development, we first examined the effects of an LC maternal diet on the size and morphology of E17 mouse brain. An LC diet was fed to pregnant females between E11 and E17—a critical period for the effects of maternal choline intake on the offsprings’ cognitive ability (29). Although the gross morphology of the LC embryonic brains appeared normal at E17, the cortical hemisphere surface area was reduced compared to CT diet brains (∼14% decrease; P < 0.01; Fig. 1A, B), as was brain weight (∼38% decrease; P < 0.05; Supplemental Fig. S1A). A remarkable reduction in the mean radial thickness of the cortex (377 µm in the LC group vs. 495 µm in the CT group; P < 0.05; arrows in Fig. 1C; Supplemental Fig. S1B) and in the length of the ventricular surface was observed (997 µm in the LC group vs. 1520 µm in the CT group; P < 0.01; Fig. 1C, D, curved lines).
Figure 1.
Maternal dietary choline is essential to maintain the NPC pool in the E17 cortex and hippocampus. On E11, timed-pregnant mice were randomly assigned to 2 feeding groups—LC (0 g/kg diet choline chloride) or CT (1.1 g/kg choline chloride)— and fed these diets until E17. A) Dorsal view of LC and CT E17 mouse brains; the cortical surface is marked by dotted lines. B) Quantitative comparison of cortical surface areas in LC and CT E17 brains. C) DAPI staining revealed the preservation of the gross morphology of the brain structure in the E17 LC mice, albeit at a reduced size. Solid lines: length of the VZ; arrows: cortical thickness. D) Quantification of dorsolateral VZ length. E) Confocal images of E17 coronal sections from LC and CT brains stained for CFP (green). F) Quantification of the thickness of the CFP cell layer. G, I) RGCs and IPCs were identified by staining for CFP (green, G) and Tbr2 (red, I), respectively, in the LC and CT E17 brains. H, J) Quantification of CFP+ RGCs (H) and Tbr2+ IPCs (J). Sections were counterstained with DAPI (blue). At least 3 brains were analyzed for each group. CP, cortical plate; IZ, intermediate zone. See also Supplemental Fig. S1. Scale bars, 200 µm (C); 50 µm remaining images. Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, by Student’s t test.
Using a reporter transgenic mouse line encoding a CFP fused to a nuclear localization signal (CFPnuc) driven by the Nestin regulatory element (Nestin-CFPnuc) (24), we evaluated the thickness of the CFP+ NPC layer in LC vs. CT E17 brains. CFP+ layer thickness was significantly reduced in both the cortical and hippocampal regions (P < 0.001 and P < 0.01, respectively; Fig. 1E, F) of LC brains. Next, we quantified the number of CFP-expressing NPCs lining the ventricular surface (ventricular zone, VZ) of the developing cortex and hippocampus. Coexpression of Nestin-CFP with Pax6, but not with T-box transcription factor (Tbr)-2 (Supplemental Fig. S1C), showed that CFP-expressing NPCs in E17 brains were primarily radial glial cells (RGCs) and not intermediate progenitor cells (IPCs) that occupy the subventricular zone (SVZ). We found that the number of CFP+ RGCs was reduced in LC E17 cortical and hippocampal regions (P < 0.01 and P < 0.05, respectively; Fig. 1G, H) compared to those in CT brains. Consistent with this, we found that the numbers of Tbr2-expressing IPCs, derived from the RGCs, were also significantly reduced in LC brains (P < 0.01; Fig. 1I, J). Furthermore, we confirmed that a decrease in the number of NPCs in LC E17 brains was associated with the reduced number of mitotic cells, marked by phospho-histone 3, in the cortical and hippocampal ventricular zones (P < 0.001 and P < 0.01, respectively; Supplemental Fig. S1D, E), consistent with our previous findings (6).
To determine whether the reduction in NPCs in LC brains was related to apoptosis, we evaluated the expression of an apoptotic cell marker, activated caspase 3, in E17 LC and CT brains. We found that the number of apoptotic cells was increased in LC vs. CT cortical and hippocampal regions (P < 0.001 and P < 0.01, respectively; Supplemental Fig. S1F, G). However, expression of activated caspase 3 was not localized to the ventricular or subventricular NPC layers (arrowheads in Supplemental Fig. S1F), but to the developing cortical and hippocampal neuronal layers, suggesting that apoptosis is not the main cause of NPC reduction by the LC maternal diet. Together, these data demonstrate that an LC maternal diet leads to defects in NPC proliferation and results in a decreased number of RGCs and IPCs and in overall reduction in brain size in E17 embryos.
LC alters cell cycle progression in NPCs
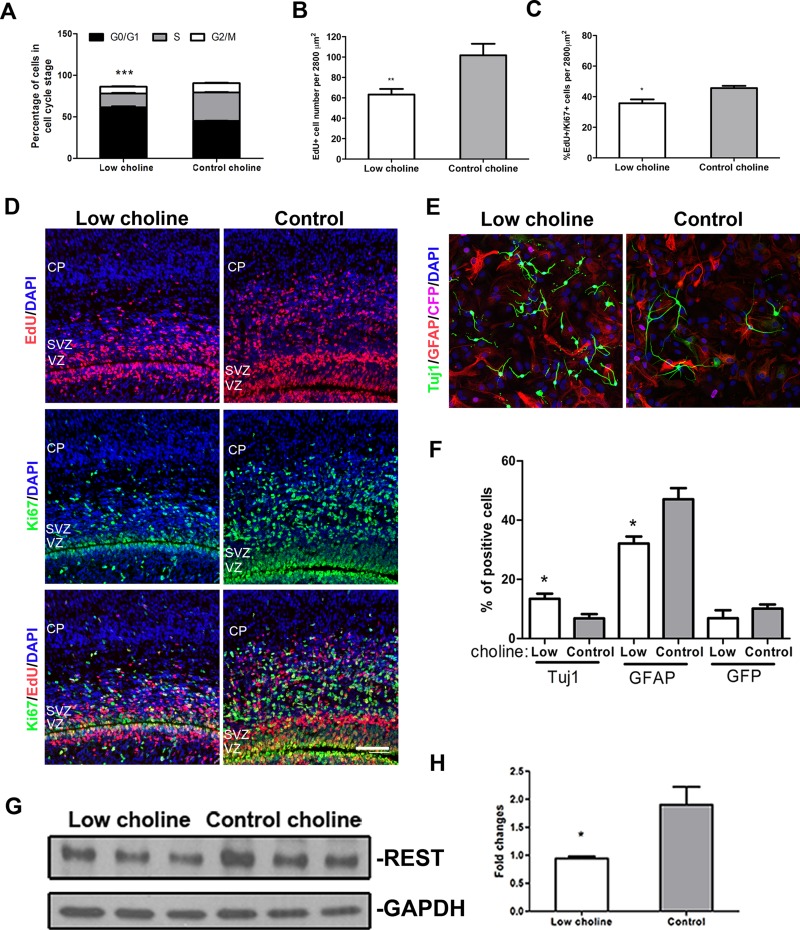
We next examined whether reduction in RGCs and IPCs in LC brains occurs as a result of altered progression of NPCs through the cell cycle. To this end, we first isolated NPCs from the telencephalons of Nestin-CFPnuc+/− embryos and expanded them in neurosphere cultures. When cultured in both neurosphere and adherent conditions, all NPCs maintained expression of Nestin-CFP, suggesting that they retained the properties of RGCs we studied in vivo. We determined the proportions of cultured cells in each phase of the cell cycle using PI staining and fluorescence-activated cell sorting (FACS). As expected, the proportion of NPCs in the G1/G0 phase of the cell cycle was significantly increased in cells cultured in LC conditions (5 μM choline chloride) for 48 h, compared to cells maintained in CT conditions (70 μM choline chloride; P < 0.001; Fig. 2A). On the contrary, the proportion of cells in the G2/M phase of the cell cycle was decreased in LC vs. CT conditions (P < 0.001).
Figure 2.
Maternal dietary choline is essential for NPC self-renewal. A) NPCs were cultured as neurospheres in LC (5 µM) or CT (70 µM) conditions for 48 h (n = 3), stained with PI, and analyzed for DNA content (cell cycle) by flow cytometry. Graph shows the proportions of cells in G0/G1, S, and G2/M phases of the cell cycle. B, C) EdU was administered to pregnant females at E16, and embryos were analyzed 30 h later, at E17. Graphs illustrate quantitative comparison of the proportions of NPCs that were EdU+ (B) and EdU/Ki67 double positive (C) in the LC vs. CT developing cortex. D) Fewer EdU+ (red) and Ki67+ (green), and EdU/Ki67 double positive cells (yellow) are present in the VZ, SVZ, and cortical plate (CP) of LC vs. CT brains. E) Immunostaining of differentiated NPCs with antibodies against the indicated markers: Tuj1 (green), a pan-neuronal marker; glial fibrillary protein (red), an astrocyte marker; Nestin-CFP (magenta), an NPC marker; and DAPI (blue). F) Quantification of the percentages of cells labeled with Tuj1, GFAP, and CFP in LC vs. CT conditions. G) Western blot of CFP+ NPCs isolated from E17 brains by FACS, probed for REST. H) Quantification of REST protein present in LC vs. CT NPCs. At least 3 samples were analyzed for each group. See also Supplemental Fig. S2. Scale bar, 100 µm. Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test.
To determine whether an LC diet induces NPCs to exit the cell cycle prematurely in vivo, we labeled cells in the S phase of the cell cycle by injecting pregnant females with 5-ethynyl-2′-deoxyuridine (EdU) at E16. We analyzed the proportions of EdU-labeled NPCs that were still cycling 30 h later, at E17, by quantifying coexpression of EdU with the proliferating cell marker Ki67 (Fig. 2B–D). We found that the total number of EdU-labeled cells was significantly reduced in the VZ and SVZ of LC brains (P < 0.01; Fig. 2B, D). As expected, the ratio of EdU-labeled cells that expressed Ki67 30 h after EdU administration was also reduced in LC brains compared with that in CT brains (P < 0.05; Fig. 2C, D), suggesting that a fraction of remaining LC NPCs exit the cell cycle prematurely.
Cell cycle exit of embryonic NPCs is tightly associated with their neuronal differentiation. Therefore, we compared the proportions of NPCs that acquired neuronal marker expression in LC vs. CT differentiating culture conditions. We found that a larger proportion of NPCs that were induced to differentiate in monolayer cultures expressed the neuronal marker β-tubulin III (Tuj1) in LC vs. CT conditions (P < 0.05; Fig. 2E, F).
The transcriptional repressor RE1–silencing transcription factor (REST) is a key repressor of numerous neuronal genes during embryonic development (30), and its down-regulation can serve as a marker of precocious neuronal differentiation. Aberrant REST binding to its target neuronal genes has been reported to be caused by an LC maternal diet (27). In our study, we examined whether altered REST expression in LC NPCs in vivo is associated with their precocious cell cycle exit and differentiation. We found that REST protein levels were significantly reduced in Nestin-CFP+ NPCs isolated from LC vs. CT E17 embryos by using FACS (P < 0.05; Fig. 2G, H). Together, these data suggest that choline is essential for preventing premature cell cycle exit of NPCs in the developing brain.
Choline is essential for proper neuronal layer organization in the cerebral cortex
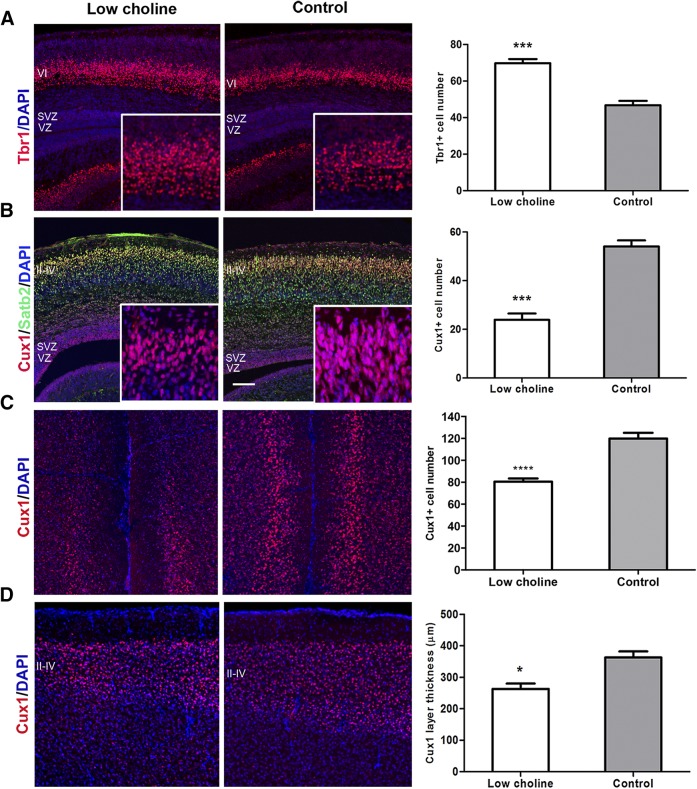
Precocious cell cycle exit and depletion of NPCs can ultimately alter the temporal progression of neurogenesis, resulting in production of early-born neurons at the expense of late-born cell types. The cerebral cortex is composed of 6 layers derived from inside–out patterning; the deep cortical layers are composed of early-born neurons, and the superficial layers are composed of later-born neurons (31). To determine whether depletion of NPCs in LC brains alters the temporal pattern of cortical neurogenesis, we immunostained E17 brains with layer-specific markers: Tbr1 for layer VI and cut-like homeobox 1 (Cux1) and special AT-rich sequence binding protein 2 (Satb2) for layers II–IV. We found that the number of early-born T-box, brain-1 (Tbr1)-expressing neurons in the developing deep cortical layer (VI) in E17 brains were increased in the LC group (P < 0.001; Fig. 3A, insets), whereas the late-born Cux1+ and Satb2+ neurons in the upper cortical layers (layers II–IV) were dramatically reduced in density (P < 0.001; Fig. 3B, insets). These data suggest that the LC diet–induced cell cycle exit of NPCs is associated with the production of the early-born cortical neurons marked by Tbr1, along with the reduction in the number of later-born upper layer cortical neurons.
Figure 3.
Low maternal choline availability leads to aberrant cortical layer development. A) Confocal images of coronal sections through LC and CT E17 brains stained with cortical layer VI marker Tbr1 (red). Graph shows an increased number of Tbr+ cells in LC compared to CT brains. B) E17 coronal brain sections were stained for cortical layer II–IV marker Cux1 (red) and layer II–V marker Satb2 (green). Graph shows a reduced number of Cux1+ cells in LC vs. CT brains. C) Coronal sections through adult, 4-mo-old mouse brains were stained with Cux1. The density of Cux1+ cells was measured in the medial prefrontal cortex. Graph shows reduced density of Cux1 cells in LC brains. D) Coronal sections through adult, 4-mo-old mouse brains were stained with Cux1. Graph shows reduced thickness of the Cux1 layer in the LC brains in the sensory cortex at the level of the corpus callosum. Nuclei were counterstained with DAPI (blue). At least 3 brains were analyzed for each group. See also Supplemental Fig. S3. Scale bars, 100 µm. Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by Student’s t test.
We next used an independent method to verify that the reduced expression of Cux1 in the developing cortical layers II–IV is caused by diminished production of pyramidal neurons. We first crossed transgenic NestinCreERT2 mice with Ai9 Cre reporter mice to produce NestinCreERT2; Ai9 double-transgenic embryos, in which NestinCreERT2 expressing NPCs and all their progeny can be permanently labeled in a Cre-dependent temporally controlled manner with the fluorescent protein tdTomato (25, 26). We next induced activation of Cre by administering tamoxifen to pregnant females at E14, and analyzed brains of postnatal day 0 (P0) pups for expression of tdTomato. In CT brains, most of the tdTomato-expressing differentiating upper layer neurons born after E14 displayed typical morphology: elongated cell body shape and dendritic and axonal projections extending basally and apically, respectively (Supplemental Fig. S2A; arrows in Supplemental Fig. S2B; higher magnification images from Supplemental Fig. S2A). On the contrary, tdTomato-expressing cells in layers II–IV in LC brains were reduced in number, and many displayed rounded body morphology (Supplemental Fig. S2A; arrows in Supplemental Fig. S2B). We found that the reduction in the Cux1 layer’s thickness, assessed in the medial prefrontal cortex (P < 0.0001; Fig. 3C) and sensory cortex, persisted into adulthood (4 mo) (P < 0.05; Fig. 3D). Together, these results indicate that an LC maternal diet affects the temporal pattern of cortical neurogenesis and permanently disrupts proper cortical layer formation.
EGFR signaling is reduced in LC NPCs because of decreased EGFR protein levels
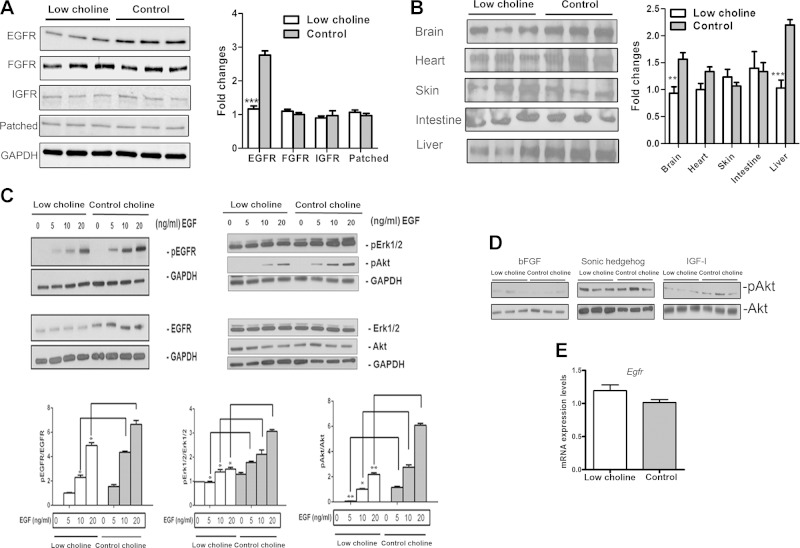
Multiple signaling pathways are implicated in the regulation of NPC proliferative state. To determine which signaling pathways are sensitive to maternal dietary choline supply, we analyzed the expression of EGFR, fibroblast growth factor receptor (FGFR), insulin-like growth factor receptor (IGFR), and the receptor for Sonic hedgehog (Shh), and Patched in NPCs isolated from LC and CT embryos by FACS, and in NPCs cultured in LC and CT conditions. Western blot analyses revealed a significant reduction in the EGFR protein expression in LC vs. CT NPCs isolated from E17 brains (P < 0.001; Fig. 4A). Meanwhile, there were no changes detected in other receptors. The difference in EGFR protein levels in NPCs was detected as early as E14 (Supplemental Fig. S3A). Moreover, we observed a large increase in EGFR protein levels in NPCs between E14 and E17 (Supplemental Fig. S3B), consistent with previous reports of its role during late embryonic and early postnatal development (22, 32, 33).
Figure 4.
Low maternal dietary choline results in reduced EGFR signaling in NPCs isolated from E17 fetal brain. A) The cortex and hippocampus of E17 embryos were dissociated into single cells, and CFP+ NPCs were isolated by FACS. Cell lysates were analyzed by Western blot with antibodies to EGFR, FGFR, IGFR, Patched, and GAPDH. The bar graph is a summary of densitometric measurements of Western blots of cell extracts from 3 different embryos from different litters. B) Western blot for EGFR from various E17 tissues. The bar graph is a summary of densitometric measurements of Western blots performed on lysates of 3 different embryos. C) E14 neurospheres were prepared, cultured in LC (5 µM) or CT (70 µM) conditions for 48 h, and stimulated with different doses of EGF. The activation (phosphorylation) of EGFR and its downstream signals Erk1/2 and Akt were assessed by Western blot. The bars are the densitometric analysis of 3 independent Western blots. D) Western blots show no changes in activation of downstream signals of Akt induced by other ligands in NPCs grown in LC vs. CT conditions. Cells were stimulated with bFGF (20 ng/ml), Shh (1 µg/ml), or IGF-I (3 nM). A representative blot from 3 independent experiments is shown. E) Expression of Egfr mRNA was tested in sorted CFP+ cells from E17 mouse brains by real-time RT-PCR. mRNA expression of Egfr relative to Tbp gene. At least 3 samples were analyzed for each group, results are averages of 3 independent experiments. See also Supplemental Fig. S4. Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test.
To test whether low maternal dietary choline reduces EGFR protein expression in tissues other than brain, we assessed EGFR protein in E17 tissues, including brain, liver, skin, heart, and intestine. Western blot analyses showed that LC diet-induced changes in EGFR expression levels differed between tissues. Embryonic brain and liver were the only tissues in which EGFR protein levels were significantly reduced (P < 0.01 and P < 0.001, respectively; Fig. 4B). To further confirm EGFR expression changes due to an LC maternal diet, we performed immunohistochemical analysis in LC and CT E17 brains. In agreement with the Western blot data, we found a significant decrease in EGFR fluorescence intensity in LC vs. CT cortical and hippocampal regions (P < 0.05; Supplemental Fig. S3C). High choline treatment (315 μM) did not increase EGFR protein levels in cultured NPCs compared to CT conditions (Supplemental Fig. S3D), indicating that CT levels of choline were sufficient to maintain optimal EGFR protein levels.
We then asked whether downstream members of the EGFR signaling pathway are affected by LC. In cultured NPCs, challenge with EGF produced marked increases in phosphorylated (p)EGFR, which was accompanied by increases in the phosphorylation of its downstream signaling targets Erk1/2 and Akt (PKB, protein kinase B). Both the pEGFR and total EGFR levels were significantly decreased in the LC-treated NPCs when compared with levels observed in CT-treated cells (P < 0.05; Fig. 4C). Also, Erk1/2 and Akt phosphorylation in response to EGF was decreased in LC NPCs (P < 0.05 and P < 0.01, respectively). No changes were seen in total Akt or Erk1/2 levels. Because pErk1/2 and pAkt are indicative of the MAPK and PI3K signaling pathways that mediate cell proliferation and survival, these results indicate that LC decreased signaling along the signaling pathway downstream of EGFR. To verify that this decrease in downstream signaling was specifically related to LC-mediated changes in EGFR, we stimulated NPCs with bFGF, Shh, and IGF-1, all of which can elicit Akt activation in progenitor cells. No significant differences in pAkt levels were detected when comparing NPCs incubated in LC and CT conditions (Fig. 4D).
RT-qPCR results demonstrated that Egfr mRNA expression levels remained unchanged in LC compared to CT CFP+ NPCs isolated from whole E17 brains by FACS sorting (Fig. 4E). These results suggest that an LC diet leads to a reduction in EGFR protein levels and signaling in NPCs, whereas Egfr mRNA expression is not affected.
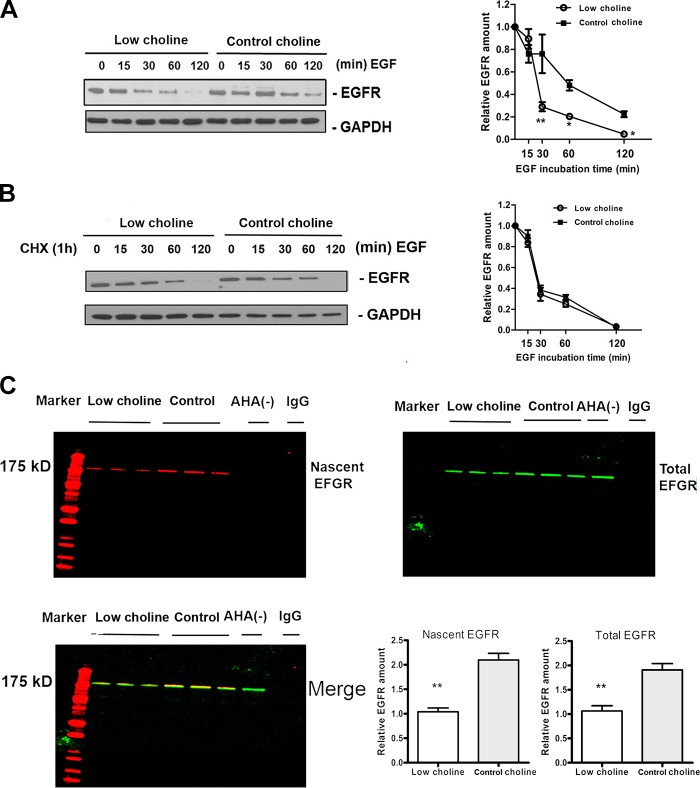
LC alters EGFR protein synthesis in NPCs
EGFR is activated upon ligand binding and the activation leads to internalization of the receptor and trafficking to the early endosomal compartment of the cell, from where the receptor can either be degraded via lysosomes or recycled to the cell surface (34). Thus, EGFR concentrations are the result of both protein synthesis and protein removal. To determine whether LC decreases the rates of EGFR synthesis rather than increasing the rates of EGFR removal, we analyzed how choline modulates the kinetics of EGFR in NPCs after stimulation with EGF. We found that at 30–120 min after EGF stimulation, EGFR concentrations were significantly lower in LC- vs. CT-cultured NPC (P < 0.01; Fig. 5A). When we inhibited EGFR synthesis using cycloheximide, the difference was abolished (Fig. 5B). Thus, the removal of EGFR proceeded at similar rates under LC and CT conditions. This result indicates that LC reduces the synthesis of EGFR protein rather than increases the degradation of EGFR. To further test that possibility, we used AHA, an analog of methionine, and immunoprecipitated EGFR total protein to assess the rates of nascent EGFR synthesis. LC conditions led to a robust decrease in newly synthesized EGFR in NPCs (P < 0.01; Fig. 5C). These results strongly suggest that altered EGFR synthesis induced by LC is the cause of reduced EGFR signaling in NPCs.
Figure 5.
EGFR protein synthesis is inhibited in LC conditions. A, B) Representative Western blots of the time course of EGFR degradation in NPCs in the absence (A) or presence (B) of CHX incubated in LC or CT conditions for the indicated times. Graphs show densitometric analysis of EGFR total protein at different times after various exposures. Each point is the mean ± sem of results in 3 different experiments. *P < 0.05, **P < 0.005 by 2-way ANOVA with Tukey’s post hoc test. C) Representative double-labeled blots of nascent EGFR (red) and total EGFR (green) are shown. NPCs were cultured in LC (5 µM) and CT (70 µM) conditions in the presence of AHA to tag nascently synthesized proteins. EGFR was immunoprecipitated and AHA was labeled with biotin using click chemistry. The bar graph is a summary of densitometric measurements. At least 3 samples were analyzed for each group. Results are averages of 3 independent experiments. Data are means ± sem. **P < 0.01 by Student’s t test.
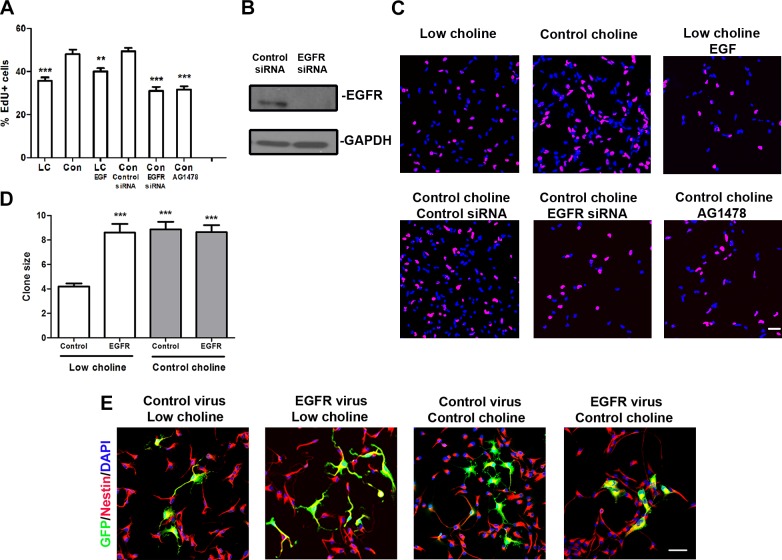
EGFR mediates the effect of LC on NPC self-renewal
Increase in EGFR levels in NPCs stimulates their proliferation (22). We therefore hypothesized that the self-renewal defect observed in LC NPCs is a result of reduced EGFR signaling. To test this hypothesis, we first inhibited EGFR protein expression by adding EGFR-siRNA to cultured NPCs and observed significantly impaired proliferation rates in NPCs cultured in CT conditions (P < 0.001; Fig. 6A–C), as assessed by incorporation of the thymidine analog EdU. In addition, we found that the EGFR kinase inhibitor AG1478 significantly impaired proliferation of NPCs in CT conditions (P < 0.001; Fig. 6A, C), indicating that the function of EGFR was dependent on its tyrosine kinase activity, which is consistent with other reports (35, 36).
Figure 6.
Ectopic Egfr expression restores cell proliferation in NPCs grown in LC conditions. A) Quantification of percentages of proliferating NPCs, as measured by incorporation of EdU. B) EGFR immunoblot in monolayer NPCs transfected with CT siRNA or siRNA against Egfr. C) Representative images of NPCs analyzed for incorporation of EdU (red) during the last hour of the 48 h culture period. DAPI, blue. D) Analysis of the sizes of EGFP+ clones (number of cells/clone) in monolayer cultures infected with CT or EGFR-expressing viruses in the indicated culture conditions. E) Representative images of NPCs infected with either CT or EGFR viruses in LC (5 µM) and CT (70 µM) conditions. After 3 d of culture, NPCs were stained for EGFP (green) and Nestin (red) and counterstained with DAPI (blue). Results are averages of 3 independent experiments. For each condition, ≥3 wells; for each well, ≥10 clones. See also Supplemental Fig. S5. Scale bars, 50 µm. Data are means ± sem. **P < 0.01, ***P < 0.001 by 1-way ANOVA with Tukey’s post hoc test.
In addition to receptor numbers or density, the level and duration of EGFR activation also depends on ligand availability (22). Previous work has demonstrated 2 major EGF family ligands (TGF-α and HB-TGF) that are expressed in the embryonic cortex (37, 36). We asked whether an LC diet changes the concentrations of these ligands in E17 brains. Western blot analysis indicated that the levels of both of these ligands were comparable between LC and CT brains (Supplemental Fig. S4). Furthermore, we supplemented LC NPCs in culture with one of these ligands, EGF, but did not observe restoration of their proliferation rates (Fig. 6A). Thus, changes in ligand availability were not responsible for the decreased EGFR signaling observed in LC NPCs.
Finally, we hypothesized that the proliferation defects observed in LC NPCs can be rescued by ectopic misexpression of EGFR protein. Therefore, we misexpressed EGFR in cultured NPCs via retroviral infection, which can deliver a single copy of a gene strictly to mitotic NPCs. NPCs were grown as monolayers: one group was infected with a CT CLE-eGFP virus and the other with CLE-EGFR-eGFP virus, encoding EGFR-eGFP fusion protein (39, 40). NPCs were grown in LC or CT conditions for 72 h, and the number of cells in individual clones resulting from the low viral titer infections of individual NPCs (∼1 × 105 pfu/ml) was quantified according to the expression of EGFP. We found that misexpression of EGFR restored the clone size in LC NPCs compared to uninfected LC NPCs (P < 0.001; Fig. 6D, E). The clone size in LC NPC cultures infected with CLE-EGFR-eGFP was restored to the levels observed in CT NPCs. There was no effect of EGFR misexpression in NPCs cultured in CT conditions. These results demonstrate that ectopic expression of an extra EGFR copy can restore the NPC cell cycle progression defect induced by LC conditions, supporting the hypothesis that diminished EGFR protein is a major cause of decreased EGFR signaling and altered self-renewal in LC NPCs.
Together, our data show, for the first time, that reduced EGFR signaling in NPCs as a result of an LC maternal diet between E11 and E17 leads to defects in NPC self-renewal and thereby their premature depletion. The reduction in NPCs leads to aberrant cortical neurogenesis, such that the production of the upper layer cortical neurons is compromised. The findings prompt further investigation into the cognitive consequences of maternal choline deficiency.
DISCUSSION
Since the discovery that maternal choline intake during pregnancy is important for offsprings’ brain development and memory performance throughout life, the central questions of how choline alters brain structure and how it regulates brain development are unresolved. We report, for the first time, that decreased availability of choline between days 11 and 17 of mouse gestation resulted in fewer neurons and led to defective layering of the cortex in the fetus. We show that RGCs, IPCs, and upper layer late-born neurons, are specifically affected. Furthermore, LC led to smaller cortical size and shorter length of the ventricular surface along the dorsolateral axis, as well as to reduced brain weight compared to that of CT mice. Our data indicate that altered EGFR-mediated signaling resulting in premature differentiation of cortical progenitors underlies these changes that persist into adulthood.
The observed defect in the genesis of layer II–IV cortical neurons in LC brains may be due to the depletion of both Nestin-CFP–expressing RGCs and Tbr2+ IPCs that we observed in LC brains. Our data suggest that depletion of NPC populations in LC brains occurs through the premature cell cycle exit of NPCs, coupled with aberrant neuronal differentiation and apoptosis.
First, we found that a larger fraction of NPCs cultured in the presence of mitogens remained in the G1 phase of the cell cycle in LC conditions compared to CT cells, and fewer of them progressed through the G2/M cell cycle phases. Second, we showed that while reducing the overall number of NPCs marked by EdU incorporation at E16, an LC diet induced their cell cycle exit, as evidenced by a smaller fraction of cells coexpressing EdU and Ki67 at E17 in LC compared to CT brains.
In previous reports, we suggested that LC results in premature differentiation of NPCs based on increased expression of genes and proteins (calretinin, TOAD-64, and MAP-1) that mark neurons in the developing hippocampus (5, 41, 42). Moreover, increased expression of calretinin, a calcium-binding protein enriched in neurons, was still detectable in the hippocampus in aged (24-mo-old) mice of mothers fed LC diets (42). In this study, we showed that a larger proportion of differentiating NPCs cultured in LC conditions acquired expression of a neuronal marker, TuJ1, compared to CT conditions, suggesting that LC promotes differentiation of NPCs along a neuronal lineage. REST is a key repressor of numerous neuronal genes during embryonic development (30) and can serve as a marker of precocious neuronal differentiation. Thus, the decreased levels of REST protein that we observed in LC E17 NPCs may mediate the premature expression of neuronal genes that results in NPC depletion in the LC brain. An LC diet between E11 and E17 resulted in increased production of early-born cortical layer VI neurons marked by Tbr1 at the expense of later-born layer II–IV neurons, supporting the idea that choline is necessary to prevent premature cell cycle exit and differentiation of NPCs.
Precocious cell cycle exit and neuronal differentiation are reportedly associated with apoptosis of the newborn cortical neurons (43). Our previous experiments demonstrated that maternal LC diet during pregnancy results in increased apoptosis in the fetal hippocampus (6). We now report that and increased number of apoptotic cells was observed in the developing cortex of LC embryos and that these cells were excluded from the VZ and SVZ layers populated by NPCs and instead were localized to the developing cortical layers.
Thus, we propose that increased apoptosis is a secondary consequence of premature neuronal differentiation at a time in development before appropriate neuronal survival factors are present. Together with previous data, we interpret our findings to suggest that dietary choline affects cortical and hippocampal neurogenesis via the mechanisms related to the maintenance of the self-renewing undifferentiated state of NPCs.
Many of the changes that we observed in the LC brain were mediated by diminished EGF signaling. EGF is a proliferative, self-renewal cue for late NPCs during cortical development, and a mediator of cortical neuronal and glial cell survival (21, 22, 44). Consistent with this, we find that EGFR levels in NPCs increase from E14 to E17. Increased EGFR expression promotes neural precursor migration in the telencephalon and controls cell fate decisions of NPCs in culture (45, 46). However, in our study we observed deficits in the genesis of the late-born upper layer cortical neurons resulting from an LC diet, suggesting decreased EGFR signaling. EGFR knockout mice exhibit dramatic neurodegeneration of the frontal cortex and the olfactory bulbs that occurs during early postnatal development, accompanied by a reduction in the size of the hippocampus and in the number of astrocytes (47, 48). We are the first to report the effects of reduced EGFR signaling in the developing brain. EGFR protein was markedly reduced in whole embryonic brain and specifically in NPCs by an LC diet. Remarkably, the effect of the LC diet on signaling pathways regulating growth and cell proliferation was limited to EGFR, as signaling through several other growth factor receptors remained unchanged. Thus, diminished EGFR signaling could be the major reason for the aberrant neurogenesis in LC brain.
EGFR plays an essential role in regulating the G1/S cell cycle phase transition (49). We found that an extra copy of Egfr introduced into cultured NPCs by retroviral infection, was sufficient to reverse the cell cycle progression deficits caused by LC conditions. In our earlier studies we reported that choline availability could affect the capacity of NPCs to progress through the cell cycle via epigenetic mechanisms; LC alters the methylation of regulatory sequences of genes and of histones in NPCs (50–52). An LC diet induces an increase in the expression of genes that inhibit cell cycle progression (CDKN3,p15Ink4B, and p27Kip1) in NPCs of the fetal hippocampus (51, 53). Further detailed studies are necessary to tease out the specific contributions of EGFR and other components of the cell cycle machinery to the regulation of self-renewal properties of NPCs in vivo.
We observed that, despite normal EGFR gene expression and normal rates of its degradation, EGFR protein levels in NPCs remained decreased. We propose that the posttranscriptional processing of EGFR mRNA is altered in the LC fetal brain. Consistent with this idea, choline supplementation does not induce an increase in EGFR levels in cultured NPCs. Micro-RNAs (miRNAs) are noncoding RNAs capable of inhibiting protein synthesis from the mRNAs in a sequence-specific manner (54). As noted earlier, low dietary choline leads to hypomethylation of many genes resulting in their overexpression (27, 50–52, 55). Thus, aberrant expression of miRNAs targeting Egfr mRNA is one of the possible mechanisms by which EGFR synthesis is altered in LC NPCs. Moreover, many miRNAs that are enriched in neurons are targets of transcriptional repressor REST (56). In our study, we showed that REST protein levels are reduced in LC NPCs, suggesting that mRNAs normally repressed by REST is aberrantly expressed in NPCs and alter their self-renewal capacity. Our results warrant further investigation into the regulation of miRNAs by choline.
Our new observations confirmed our previous findings that hippocampal development is dependent on choline availability (57). In this article, we report, for the first time, that the inadequate intake of choline early in life leads to persistent changes in the cortical structure, affecting primarily layers II–IV. Much behavioral evidence supports the view that rodents given prenatal choline supplementation have increased memory capacity and precision and are able to form longer lasting memories in adulthood (8, 9, 58). Moreover, offspring born to mothers fed an LC diet during pregnancy exhibit deficits in tests of proactive interference—behavior that involves both hippocampal and cortical functions (29, 59). Cerebral cortex participates with other brain regions in many aspects of learning and memory, attention, and motivation, in part through its central role in working memory (60). Injuries to the frontal cortex, or deficits in its function, are associated with impairments in executive functions, altered initiative, “personality” change, and reduced creativity (61). Based on our new findings, choline deficiency during gestation may affect numerous complex behaviors that rely on proper cortical structure and function. Thus, further studies of choline-mediated changes in cortical layer II–IV–dependent functions should be undertaken.
Together, our findings provide a mechanistic basis for understanding how dietary choline modulates fetal brain development and implicate abnormal EGFR signaling as an important mechanism. This work highlights the importance of assuring good maternal nutrition during pregnancy.
Supplementary Material
Acknowledgments
The authors thank Dr. G. Enikolopov for the Nestin-CFP mouse line; Dr. S. Rosenfeld for the EGFR-eGFP and CLE-eGFP; S. Orena for help with flow cytometry; M. G. Mehedint for help with the NPC culture; K. D. Corbin for help with Western blot and immunoprecipitation analysis; and the other members of the Zeisel laboratory for technical assistance. This work was supported by North Carolina Biotechnology Center Grant 2012-CFG-8005 and U.S. National Institutes of Health, Diabetes and Digestive and Kidney Diseases Grant DK056350. S.H.Z. owns equity in Nutrigene Sciences, and is a consultant for Dupont, Metabolon and Nestle. None of these activities are in conflict with this paper. The authors declare no conflicts of interest.
Glossary
- AHA
azidohomoalanine
- Akt (PKB)
protein kinase B
- bFGF
basic fibroblast growth factor
- CFP
cyan fluorescent protein
- CHX
cycloheximide
- CT
control
- Cux1
cut-like homeobox 1
- E
embryonic day
- EdU
5-ethynyl-2′-deoxyuridine
- EGF
epidermal growth factor
- EGFP
enhanced green fluorescent protein
- EGFR
epidermal growth factor receptor
- FACS
fluorescence-activated cell sorting
- FGFR
fibroblast growth factor receptor
- IGFR
insulin-like growth factor receptor
- IPC
intermediate progenitor cell
- LC
low choline
- miRNA
micro-RNA
- NPC
neural progenitor cell
- P
postnatal day
- PEMT
phosphatidylethanolamine N-methyltransferase
- qPCR
quantitative PCR
- REST
repressor element-1–silencing transcription factor
- RGC
radial glial cell
- Satb2
special AT-rich sequence binding protein 2
- Shh
sonic hedgehog
- SVZ
subventricular zone
- Tbr1
T-box, brain, 1
- Tbr2
T-box transcription factor (Eomes)
- VZ
ventricular zone
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Zeisel S. H. (2006) Choline: critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 26, 229–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw G. M., Carmichael S. L., Laurent C., Rasmussen S. A. (2006) Maternal nutrient intakes and risk of orofacial clefts. Epidemiology 17, 285–291 [DOI] [PubMed] [Google Scholar]
- 3.Shaw G. M., Carmichael S. L., Yang W., Selvin S., Schaffer D. M. (2004) Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 160, 102–109 [DOI] [PubMed] [Google Scholar]
- 4.Boeke C. E., Gillman M. W., Hughes M. D., Rifas-Shiman S. L., Villamor E., Oken E. (2013) Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 177, 1338–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albright C. D., Tsai A. Y., Friedrich C. B., Mar M. H., Zeisel S. H. (1999) Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. Dev. Brain Res. 113, 13–20 [DOI] [PubMed] [Google Scholar]
- 6.Craciunescu C. N., Albright C. D., Mar M. H., Song J., Zeisel S. H. (2003) Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 133, 3614–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glenn M. J., Gibson E. M., Kirby E. D., Mellott T. J., Blusztajn J. K., Williams C. L. (2007) Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur. J. Neurosci. 25, 2473–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meck W. H., Smith R. A., Williams C. L. (1988) Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev. Psychobiol. 21, 339–353 [DOI] [PubMed] [Google Scholar]
- 9.Meck W. H., Williams C. L. (1999) Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res. Dev. Brain Res. 118, 51–59 [DOI] [PubMed] [Google Scholar]
- 10.Mellott T. J., Williams C. L., Meck W. H., Blusztajn J. K. (2004) Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 18, 545–547 [DOI] [PubMed] [Google Scholar]
- 11.Pyapali G. K., Turner D. A., Williams C. L., Meck W. H., Swartzwelder H. S. (1998) Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J. Neurophysiol. 79, 1790–1796 [DOI] [PubMed] [Google Scholar]
- 12.Thomas J. D., Biane J. S., O’Bryan K. A., O’Neill T. M., Dominguez H. D. (2007) Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav. Neurosci. 121, 120–130 [DOI] [PubMed] [Google Scholar]
- 13.Holmes G. L., Yang Y., Liu Z., Cermak J. M., Sarkisian M. R., Stafstrom C. E., Neill J. C., Blusztajn J. K. (2002) Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 48, 3–13 [DOI] [PubMed] [Google Scholar]
- 14.Guo-Ross S. X., Clark S., Montoya D. A., Jones K. H., Obernier J., Shetty A. K., White A. M., Blusztajn J. K., Wilson W. A., Swartzwelder H. S. (2002) Prenatal choline supplementation protects against postnatal neurotoxicity. J. Neurosci. 22, RC195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace T. C., McBurney M., Fulgoni V. L. III (2014) Multivitamin/mineral supplement contribution to micronutrient intakes in the United States, 2007-2010. J. Am. Coll. Nutr. 33, 94–102 [DOI] [PubMed] [Google Scholar]
- 16.Dominguez-Salas P., Moore S. E., Cole D., da Costa K. A., Cox S. E., Dyer R. A., Fulford A. J., Innis S. M., Waterland R. A., Zeisel S. H., Prentice A. M., Hennig B. J. (2013) DNA methylation potential: dietary intake and blood concentrations of one-carbon metabolites and cofactors in rural African women. Am. J. Clin. Nutr. 97, 1217–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gossell-Williams M., Fletcher H., McFarlane-Anderson N., Jacob A., Patel J., Zeisel S. (2005) Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med. J. 54, 355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeisel S. H., Mar M. H., Zhou Z., Da Costa K. A. (1995) Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J. Nutr. 125, 3049–3054 [DOI] [PubMed] [Google Scholar]
- 19.Resseguie M., Song J., Niculescu M. D., da Costa K. A., Randall T. A., Zeisel S. H. (2007) Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 21, 2622–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resseguie M. E., da Costa K. A., Galanko J. A., Patel M., Davis I. J., Zeisel S. H. (2011) Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J. Biol. Chem. 286, 1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds B. A., Tetzlaff W., Weiss S. (1992) A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 12, 4565–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burrows R. C., Wancio D., Levitt P., Lillien L. (1997) Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron 19, 251–267 [DOI] [PubMed] [Google Scholar]
- 23.Albright C. D., da Costa K. A., Craciunescu C. N., Klem E., Mar M. H., Zeisel S. H. (2005) Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell. Physiol. Biochem. 15, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Encinas J. M., Vaahtokari A., Enikolopov G. (2006) Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA 103, 8233–8238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagace D. C., Whitman M. C., Noonan M. A., Ables J. L., DeCarolis N. A., Arguello A. A., Donovan M. H., Fischer S. J., Farnbauch L. A., Beech R. D., DiLeone R. J., Greer C. A., Mandyam C. D., Eisch A. J. (2007) Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 27, 12623–12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R., Lein E. S., Zeng H. (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehedint M. G., Niculescu M. D., Craciunescu C. N., Zeisel S. H. (2010) Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 24, 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melemedjian O. K., Tillu D. V., Asiedu M. N., Mandell E. K., Moy J. K., Blute V. M., Taylor C. J., Ghosh S., Price T. J. (2013) BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol. Pain 9, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meck W. H., Williams C. L. (2003) Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 27, 385–399 [DOI] [PubMed] [Google Scholar]
- 30.Chong J. A., Tapia-Ramírez J., Kim S., Toledo-Aral J. J., Zheng Y., Boutros M. C., Altshuller Y. M., Frohman M. A., Kraner S. D., Mandel G. (1995) REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 80, 949–957 [DOI] [PubMed] [Google Scholar]
- 31.Rakic P. (2009) Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seroogy K. B., Gall C. M., Lee D. C., Kornblum H. I. (1995) Proliferative zones of postnatal rat brain express epidermal growth factor receptor mRNA. Brain Res. 670, 157–164 [DOI] [PubMed] [Google Scholar]
- 33.Eagleson K. L., Ferri R. T., Levitt P. (1996) Complementary distribution of collagen type IV and the epidermal growth factor receptor in the rat embryonic telencephalon. Cereb. Cortex 6, 540–549 [DOI] [PubMed] [Google Scholar]
- 34.Segatto O., Anastasi S., Alemà S. (2011) Regulation of epidermal growth factor receptor signalling by inducible feedback inhibitors. J. Cell Sci. 124, 1785–1793 [DOI] [PubMed] [Google Scholar]
- 35.Yaish P., Gazit A., Gilon C., Levitzki A. (1988) Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science 242, 933–935 [DOI] [PubMed] [Google Scholar]
- 36.Lemcke H., Kuznetsov S. A. (2013) Involvement of connexin43 in the EGF/EGFR signalling during self-renewal and differentiation of neural progenitor cells. Cell. Signal. 25, 2676–2684 [DOI] [PubMed] [Google Scholar]
- 37.Kornblum H. I., Zurcher S. D., Werb Z., Derynck R., Seroogy K. B. (1999) Multiple trophic actions of heparin-binding epidermal growth factor (HB-EGF) in the central nervous system. Eur. J. Neurosci. 11, 3236–3246 [DOI] [PubMed] [Google Scholar]
- 38.Lazar L. M., Blum M. (1992) Regional distribution and developmental expression of epidermal growth factor and transforming growth factor-alpha mRNA in mouse brain by a quantitative nuclease protection assay. J. Neurosci. 12, 1688–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivkovic S., Canoll P., Goldman J. E. (2008) Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J. Neurosci. 28, 914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y., Goderie S. K., Temple S. (2005) Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron 45, 873–886 [DOI] [PubMed] [Google Scholar]
- 41.Albright C. D., Mar M. H., Craciunescu C. N., Song J., Zeisel S. H. (2005) Maternal dietary choline availability alters the balance of netrin-1 and DCC neuronal migration proteins in fetal mouse brain hippocampus. Brain Res. Dev. Brain Res. 159, 149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albright C. D., Siwek D. F., Craciunescu C. N., Mar M. H., Kowall N. W., Williams C. L., Zeisel S. H. (2003) Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr. Neurosci. 6, 129–134 [DOI] [PubMed] [Google Scholar]
- 43.Silver D. L., Watkins-Chow D. E., Schreck K. C., Pierfelice T. J., Larson D. M., Burnetti A. J., Liaw H. J., Myung K., Walsh C. A., Gaiano N., Pavan W. J. (2010) The exon junction complex component Magoh controls brain size by regulating neural stem cell division. Nat. Neurosci. 13, 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong R. W., Guillaud L. (2004) The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 15, 147–156 [DOI] [PubMed] [Google Scholar]
- 45.Burrows R. C., Lillien L., Levitt P. (2000) Mechanisms of progenitor maturation are conserved in the striatum and cortex. Dev. Neurosci. 22, 7–15 [DOI] [PubMed] [Google Scholar]
- 46.Caric D., Raphael H., Viti J., Feathers A., Wancio D., Lillien L. (2001) EGFRs mediate chemotactic migration in the developing telencephalon. Development 128, 4203–4216 [DOI] [PubMed] [Google Scholar]
- 47.Sibilia M., Steinbach J. P., Stingl L., Aguzzi A., Wagner E. F. (1998) A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J. 17, 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Threadgill D. W., Dlugosz A. A., Hansen L. A., Tennenbaum T., Lichti U., Yee D., LaMantia C., Mourton T., Herrup K., Harris R. C., Barnard J. A., Yuspa S. H., Coffey R. J., Magnuson T. et al. (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269, 230–234 [DOI] [PubMed] [Google Scholar]
- 49.Lo H. W., Hung M. C. (2006) Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br. J. Cancer 94, 184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niculescu M. D., Craciunescu C. N., Zeisel S. H. (2005) Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Res. Mol. Brain Res. 134, 309–322 [DOI] [PubMed] [Google Scholar]
- 51.Niculescu M. D., Craciunescu C. N., Zeisel S. H. (2006) Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 20, 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehedint M. G., Craciunescu C. N., Zeisel S. H. (2010) Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc. Natl. Acad. Sci. USA 107, 12834–12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albright C. D., Mar M. H., Friedrich C. B., Brown E. C., Zeisel S. H. (2001) Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev. Neurosci. 23, 100–106 [DOI] [PubMed] [Google Scholar]
- 54.Fabian M. R., Sonenberg N., Filipowicz W. (2010) Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379 [DOI] [PubMed] [Google Scholar]
- 55.Waterland R. A., Dolinoy D. C., Lin J. R., Smith C. A., Shi X., Tahiliani K. G. (2006) Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis 44, 401–406 [DOI] [PubMed] [Google Scholar]
- 56.Gebhardt M. L., Reuter S., Mrowka R., Andrade-Navarro M. A. (2014) Similarity in targets with REST points to neural and glioblastoma related miRNAs. Nucleic Acids Res. 42, 5436–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeisel S. H. (2006) The fetal origins of memory: the role of dietary choline in optimal brain development. J. Pediatr. 149(5, Suppl)S131–S136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meck W. H., Smith R. A., Williams C. L. (1989) Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav. Neurosci. 103, 1234–1241 [DOI] [PubMed] [Google Scholar]
- 59.Henson R. N., Shallice T., Josephs O., Dolan R. J. (2002) Functional magnetic resonance imaging of proactive interference during spoken cued recall. Neuroimage 17, 543–558 [PubMed] [Google Scholar]
- 60.Jones M. W. (2002) A comparative review of rodent prefrontal cortex and working memory. Curr. Mol. Med. 2, 639–647 [DOI] [PubMed] [Google Scholar]
- 61.Miller E. K., Cohen J. D. (2001) An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.