Abstract
The acetyltransferase, E1a-binding protein (p300), is proposed to regulate various aspects of skeletal muscle development, metabolism, and mitochondrial function, via its interaction with numerous transcriptional regulators and other proteins. Remarkably, however, the contribution of p300 to skeletal muscle function and metabolism, in vivo, is poorly understood. To address this, we used Cre-LoxP methodology to generate mice with skeletal muscle-specific knockout of E1a-binding protein (mKO). mKO mice were indistinguishable from their wild-type/floxed littermates, with no differences in lean mass, skeletal muscle structure, fiber type, respirometry flux, or metabolites of fatty acid and amino acid metabolism. Ex vivo muscle function in extensor digitorum longus and soleus muscles, including peak stress and time to fatigue, as well as in vivo running capacity were also comparable. Moreover, expected adaptations to a 20 d voluntary wheel running regime were not compromised in mKO mice. Taken together, these findings demonstrate that p300 is not required for the normal development or functioning of adult skeletal muscle, nor is it required for endurance exercise-mediated mitochondrial adaptations.—LaBarge, S. A., Migdal, C. W., Buckner, E. H., Okuno, H., Gertsman, I., Stocks, B., Barshop, B. A., Nalbandian, S. R., Philp, A., McCurdy, C. E., Schenk, S. p300 is not required for metabolic adaptation to endurance exercise training.
Keywords: acetyltransferase, physiology, metabolomics, mitochondria, knockout
Reversible lysine acetylation is a widely conserved posttranslational modification that modulates numerous cellular pathways, including DNA damage repair, metabolism, RNA splicing, and the cell cycle (1–3). The acetylation state of a protein is balanced between the activity of deacetylases (DACs) and lysine acetyltransferases (KATs), which remove and add an acetyl group to lysine residues, respectively (1–3). In recent years, research has focused almost exclusively on the action of DACs in regulating protein acetylation, muscle metabolism, and mitochondrial biogenesis, with the majority of studies focusing on the sirtuin (SIRT) proteins: SIRT1 and SIRT3 (4–7). Remarkably, little is known about how KATs modulate these processes, particularly in vivo.
There are 22 known KATs in the human genome, which are separated into 3 main families: GCN5 N-acetyltransferase, p300 (E1a-binding protein)/CBP (cAMP response element-binding protein-binding protein), and Moz, Ypf2/sas3, Sas2, Tip160 protein 1 (1, 8). The p300/CBP family of KATs is an orphan class of KATs, due to the fact that it does not contain a true consensus histone acetyltransferase domain like the Moz, Ypf2/sas3, Sas2, Tip160 protein 1 and GCN5 N-acetyltransferase families, but it does have intrinsic KAT activity (8). p300 was first described through its interaction with the adenovirus p300 and functions broadly in transcriptional regulation, including pathways central to metabolism and skeletal muscle development (9). For example, p300 contributes to the transcription of metabolic genes in COS (CV-1 in origin with SV40 genes) cells via modulation of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a master regulator of mitochondrial biogenesis (10). Importantly, in cell culture models of skeletal muscle differentiation, p300 is required for myotube formation (11–14), and whole-body heterozygous or homozygous knockout (KO) of p300 results in incorrect muscle development and embryonic lethality (13, 15); whether improper muscle development is due to loss of p300 in skeletal muscle, or occurs secondary to its loss in other tissues, is unknown.
To our knowledge, no mouse models have been generated to study the contribution of p300 to skeletal muscle biology, in vivo. To address this, we used Cre-LoxP methodology to generate mice with muscle-specific knockout of E1a-binding protein (mKO). Given that p300 has been reported as a transcriptional regulator of numerous muscle-required target genes and to be required for myotube differentiation (11–20), we hypothesized that mKO mice would exhibit impairments in muscle development, structure, and function, as well as a reduced ability to exercise and adapt to endurance exercise training (ExT).
MATERIALS AND METHODS
Animals
All studies were conducted in male mice on a C57BL/6 background. To generate mKO mice, mice harboring LoxP sites flanking exons 9 of the p300 gene (21) (kindly provided by Dr. Paul Brindle, St. Jude Children’s Research Hospital, Memphis, TN, USA) were crossed with mice expressing Cre recombinase under the control of the muscle creatine kinase promoter (Cre-MCK); after Cre-mediated recombination, exon 9 is removed (21). Floxed mice that lack Cre-MCK are referred to herein as wild-type (WT) and were used as controls for all studies. Mice were housed on a 12:12 h light-dark cycle, and all studies were conducted in 13-wk-old littermates. All experiments were approved by, and were conducted in accordance with, the Animal Care Program at the University of California, San Diego.
Tissue collection
Tissue was excised from unfed (4 h) and anesthetized mice. Skeletal muscles, liver, and epididymal adipose tissue (AT) were rinsed in sterile saline, blotted dry, weighed, and frozen in liquid nitrogen. Skeletal muscle [tibialis anterior (TA)] for sectioning was pinned on cork and frozen in liquid nitrogen-cooled isopentane. All tissues were stored at −80°C for subsequent analysis.
Voluntary wheel running
ExT was conducted by voluntary wheel running (VWR), as previously described (22). Briefly, mice had free access to a running wheel for 20 d. Time spent running, distance run, and average speed were digitally recorded throughout VWR, with data collected daily at 10:00 AM. Running wheels were removed after 20 d of running, which was 24 h before tissue collection.
Run to exhaustion
Mice were acclimated to the treadmill by running for 15 min at 10 m/min on 2 consecutive days. On the third day, a run-to-exhaustion (RTE) test was performed, during which running speed and incline were progressively increased. The protocol was as follows: 0–10 min, 25 m/min at 10° incline; 10–70 min, 25 m/min at 15° incline; and 70–90 min, 25 m/min at 20° incline, which at 90 min, speed was increased to 30 m/min and increased by 2 m/min every 5 min thereafter. A shock grid was not used. Instead, a stiff bristled brush at the back of the treadmill provided a stimulus for mice to run. Exhaustion was defined as the inability to continue running in response to this stimulus.
Histologic analysis
Muscle fiber type was determined in plantaris, TA, and gastrocnemius (GA) by assessment of myosin heavy-chain composition, as previously described (23, 24). Fiber cross-sectional area (CSA) was determined in TA muscle by laminin staining (25). Briefly, muscle cross sections (10 μm) were taken from the muscle midbelly and were blocked with 1% bovine serum albumin and normal goat and rat serum. Sections were incubated overnight with a polyclonal anti-laminin antibody (L9393; Sigma-Aldrich, St. Louis, MO, USA) and then with a secondary antibody (Alexa Fluor 594, goat anti-rabbit IgG; A-11037; Life Technologies, Carlsbad, CA, USA). Tile scan images were taken using a Leica DM6000 microscope equipped with a Leica DFC365 FX camera (both from Leica Microsystems, Buffalo Grove, IL, USA) using a ×10 objective and a TX2 filter set. Fiber CSAs were counted automatically using a custom-written macro in ImageJ (National Institutes of Health, Bethesda, MD, USA) with 12 randomly selected fields per section. Filtering criteria were applied to ensure measurement of tiles containing actual muscle fibers. These criteria rejected regions with areas ˂50 or >5600 μm2 to eliminate neurovascular structures and optically fused fibers, respectively. Fibers touching the edge of the field were excluded because they were assumed to be incomplete. Regions with circularity <0.30 or >1.0 were excluded to prevent inclusion of fibers that were obliquely sectioned.
Muscle mechanics
Muscle function was assessed in the soleus (SOL) and fifth toe muscle of the extensor digitorum longus (EDL), as previously described (22). Briefly, muscles were continuously perfused with Ringer solution [137 mM NaCl, 5 mM KCl, 2 mM CaCl2, 24 mM NaHCO3, 1 mM NaH2PO4, 11 mM glucose, 1 mM MgSO4, and 0.01% tubocurarine chloride (pH 7.5)] at 25°C that was bubbled with 100% O2. Muscles were electrically stimulated (model S88; Astro-Med, West Warwick, RI, USA) via parallel platinum electrodes (∼35 V, 300 ms train duration, and 0.3 ms pulse duration) with single twitches to set the length for maximal twitch tension. After a 10-min rest, muscles were stimulated at different frequencies (1–120 Hz for EDL and 1–100 Hz for SOL) with 120 s intervals between contractions to determine the force-frequency (F-F) relationship. The F-F curves were fitted by a nonlinear regression equation [(P = Pmin + (P0XnH/F50nH + XnH)], where Pmin is the minimum tension developed, F50 is the midpoint of the curve (Hz), and nH is the Hill coefficient. After the F-F protocol, muscles rested for 10 min, and the fatigue resistance was determined by a time-to-fatigue (TTF) protocol, which involved repeated isometric contractions at the calculated F50 frequency. During the TTF protocol, rest intervals occurred every 1 s. TTF was defined as the time it took each muscle to reach 40% of the force generated in the first contraction of the fatigue protocol. Force development was normalized to the muscle physiologic CSA (26).
Immunoprecipitation
Nuclear extract (NE-PER Nuclear and Cytoplasmic Extraction Kit, 78835; Thermo Scientific, Waltham, MA, USA) or whole-cell lysate from the triceps (TRI), GA, or quadriceps was used for immunoprecipitation (IP) experiments, as previously described (22). Protein (150 μg) was rotated end over end with anti-p300 (sc-585; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or anti-acetyl-lysine (AAC01; Cytoskeleton, Denver, CO, USA), for 1 h (4°C) and then rotated overnight (4°C) with protein A and protein G magnetic beads (50/50 μl; Bio-Rad Laboratories, Hercules, CA, USA). The following morning, beads were washed 3 times with PBS 0.1% Tween 20, and antigens were eluted with 1× Laemmli sample buffer (40 μl; Bio-Rad Laboratories, Hercules, CA, USA). Samples were boiled for 10 min, and lysate was removed from protein A/G magnetic beads via a magnetic rack (Bio-Rad Laboratories) prior to SDS-PAGE.
Immunoblotting
SDS-PAGE was performed using standard methods, as previously described (22). Primary antibodies were from Abcam Inc., Cambridge, MA, USA [complex I–V (ab110413), acyl-coenzyme A dehydrogenase long chain (ACADL; ab82853), and acyl-coenzyme A dehydrogenase very long chain (ACADVL; ab155138)]; Santa Cruz Biotechnology [p300 (sc-585), mitochondrial-specific transcription factor A (mtTFA; sc-23588), glucose transporter type 4 (sc-53566), myogenic factor 5 (Myf5; sc-302), myogenic factor 6 (Myf6; sc-301), and myocyte enhance factor 2 (pan-Mef2; sc-313)]; EMD Millipore, Billerica, MA, USA [PGC-1α (AB3242)]; Cell Signaling Technology, Danvers, MA, USA [CBP (7389), hexokinase II (HKII; 2867), myogenic factor 3 (MyoD; 13812), and pyruvate dehydrogenase (PDH; 2784)]; Developmental Studies Hybridoma Bank [myogenic factor 4 (myogenin; F5D was deposited to the Developmental Studies Hybridoma Bank by Woodring E. Wright, University of Southwestern Medical Center, Dallas, TX, USA)]; and Fitzgerald Industries International, Acton, MA, USA [glyceraldehyde-3-phosphate dehydrogenase (10R-G109a)]. Gels used for silver staining were trimmed and assayed according to the Thermo Scientific Pierce Silver Stain Kit protocol (24612; Thermo Scientific), and bands were quantitated using Image Lab Software (Bio-Rad Laboratories).
PCR analysis
PCR analysis of tissue-specific deletion of exon 9 was performed with primer sets (5′–3′): A, TGG ACT GGT TAT CGG TTC ACC; B, CTC TAC ATC CTA AGT GCT AGG; and C, CAG TAG ATG CTA GAG AAA GCC (Fig. 1A). RNA extraction, cDNA synthesis, and quantitative PCR analysis were conducted, as previously described (27).
Figure 1.
Generation of a mouse with mKO. A) Schematic of floxed exon (Ex) 9 of the p300 gene with arrows to indicate the primer pairs (A–B and A–C) used to determine KO of p300. B) Semiquantitative PCR analysis using the aforementioned primers directed across exon 9 of the p300 gene in skeletal muscle [GA, SOL, and quadriceps (Q)], AT, and liver (L) of WT and mKO mice; top arrow represents product from primer set A–C and the bottom arrow from primer set A–B. Primer set A–C only produces a product when exon 9 is excised. C) To determine p300 abundance, we immunoprecipitated from nuclear extracts with an antibody to p300 and immunoblotted (IB) for p300. Representative image of IP of p300 (left) and quantitation of p300 abundance (right) are shown. rp300, recombinant human p300. D) Representative image (left) and quantitation (right) of IB for CBP from whole-muscle lysates. For both (C) and (D), arrowheads represent molecular mass marker at 250 kDa. Data were corrected for loading based on Ponceau staining of the band at ∼200 kDa and then normalized to WT (n = 3–4 per group for C, and n = 7–8 per group for D). *P < 0.05 vs. WT. E) Silver stain of acetyl-lysine immunoprecipitates from WT and mKO muscle. F) Quantitation of these bands (excluding the IgG). For each lane, quantitation was normalized to the IgG band at ∼25 kDa. All data are reported as means ± sem.
Enzymatic activity
Powdered GA (30–50 mg) was homogenized on ice with glass-on-glass homogenizing tubes in 0.5 ml ice-cold Zheng buffer [210 mM mannitol, 70 mM sucrose, 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 1 mM EGTA (pH to 7.2 using potassium hydroxide)]. Sample homogenates were divided into aliquots and then underwent 3 freeze-thaw cycles using a methanol/dry ice bath for enzyme analysis of complex II. Homogenates were further disrupted by sonication for analysis of complex I and citrate synthase (CS). Enzyme activities were measured by spectrophotometric assays as previously described (28, 29), with minor modifications. All assays were performed in a 96-well plate using a Synergy HT spectrophotometer (BioTek, Winooski, VT, USA) at 30°C, in a final volume of 0.25 ml, in 50 mM potassium phosphate buffer (pH 7.4), unless otherwise indicated. For all assays, muscle homogenate was diluted to varying degrees with potassium phosphate buffer, and reactions were evaluated for up to 40 min to find optimal dilution needed to maximize the linearity of the reaction.
High-resolution respirometry
High-resolution respirometry was performed using an Oroboros O2K (Oroboros Instruments, Innsbruck, Austria) as previously described with minor modifications (30). Briefly, excised TRI muscle preserved in biopsy preservation solution (BIOPS; 2.77 mM CaK2EGTA, 7.23 mM K2EGTA, 5.7 mM Na2ATP, 6.56 mM MgCl2, 20 mM taurine, 15 mM Na2Phosphocreatine, 20 mM imidazole, 0.5 mM DTT, and 50 mM MES) was mechanically separated under a dissecting microscope and permeabilized with 50 μg/ml saponin for 20 min followed by two 15 min washes in MiR05 buffer [0.5 mM EGTA, 3 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 110 mM sucrose, and 1 g/L fatty acid-free bovine serum albumin]. All reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. All data were collected at 37°C in hyperoxygenated (200–400 μM) conditions in MiR06 (MiR05 plus catalase) to avoid limitations with oxygen diffusion. There were 2 substrate-uncoupler-inhibitor titrations (SUITs) performed. SUIT 1 respiration protocol was the following: 0.5 mM malate, 5 mM pyruvate, 5 mM ADP, 10 μM cytochrome c, 10 mM glutamate, 10 mM succinate, and 0.5 μM carbonyl cyanide m-chloro phenyl hydrazone, followed by 0.5 μM titrations carbonyl cyanide m-chloro phenyl hydrazone, 0.5 μM rotenone, and 2.5 μM antimycin A (Ama). SUIT 2 respiration protocol was the following: 0.5 mM malate, 0.2 mM octanoylcarnitine, 5 mM ADP, 10 μM cytochrome c, 10 mM glutamate, 10 mM succinate, and 0.5 μM carbonyl cyanide m-chloro phenyl hydrazone, followed by 0.5 μM titrations carbonyl cyanide m-chloro phenyl hydrazone and 2.5 μM Ama.
Metabolites
Metabolite analysis was performed using a targeted liquid chromatography mass spectroscopy method with an AB Sciex 5600 TripleTOF (Framingham, MA, USA), as previously described (31). For this, 20 mg powdered GA was homogenized (Dounce) in ice-cold methanol (2 ml; final concentration, 80%), which contained stable isotope standards, for 1 min. The sample was vortexed for 1 min and placed at −20°C for 1 h and then centrifuged (13,000 g) for 15 min. The supernatant was removed and evaporated to dryness using a SpeedVac (Thermo Scientific). Samples were resuspended in 100 μl 10% acetonitrile/0.1% formic acid, and 5 μl of each resuspension was loaded for each liquid chromatography mass spectroscopy run. MultiQuant software (AB Sciex) was used for peak integration and metabolite quantification. MetaboAnalyst, a server-based metabolomics processing and statistical analysis tool, was used to evaluate pathway-based differences (32).
Energy expenditure and body composition
Oxygen consumption (Vo2), CO2 production, respiratory quotient, and physical activity (total movement, ambulatory activity, and rearing activity) were assessed using the Comprehensive Lab Animals Monitoring System (Columbus Instruments, Columbus, OH, USA). Measurements were made for 3 consecutive days, and values were averaged from the light and dark phases recorded on d 2 and 3. Body composition was measured by MRI (EchoMRI, Houston, TX, USA).
Statistics
Statistical analyses were performed using Prism 6 (GraphPad Software Incorporated, La Jolla, CA, USA). Data were analyzed by an unpaired Student’s t test or 2-way ANOVA (with repeated measures, where appropriate) for main effects of exercise and genotype, followed by a Tukey post hoc analysis, with significant differences at P < 0.05. All data are expressed as means ± sem.
RESULTS
mKO model reveals distinct loss of p300 in skeletal muscle
Mice harboring LoxP sites flanking exon 9 (which contains the KIX domain that is responsible for transactivation of nuclear factors) were crossed with Cre-MCK mice. To test for efficient deletion of exon 9 of p300 in the DNA, primers were designed in the intron between exons 8 and 9 (primers A–B) or were designed to span exon 9 (primers A–C) (Fig. 1A). For primers A–B, a product is expected in all tissues, whereas for primers A–C, a product only occurs when exon 9 is excised, due to insufficient time for a product to be produced when present. As expected, primers A–B produced a band in all WT and mKO tissues, including skeletal muscle, whereas a product for primers A–C was only produced in skeletal muscles of mKO mice (Fig. 1B). To assess loss of p300, we performed an IP/immunoblotting in the nuclear fraction isolated from whole muscle. This revealed an essentially complete (∼94%) knockdown of p300 in skeletal muscle from mKO vs. WT (Fig. 1C). Despite KO of p300, there was no up-regulation of CBP abundance (Fig. 1D). These results demonstrate that p300 is efficiently knocked out in skeletal muscle in mKO mice, with no compensatory increase in CBP. Functional loss of p300 was confirmed by an ∼30% reduction in total protein acetylation in skeletal muscle of mKO vs. WT, as measured in immunoprecipitates using a pan acetyl-lysine antibody (Fig. 1F; WT, 1.0 ± 0.12; mKO, 0.72 ± 0.03; P = 0.055).
Body mass, composition, and energy expenditure are comparable between WT and mKO mice
Body mass, lean mass, fat mass, and percent body fat (WT, 11.8 ± 0.5%; mKO, 11.3 ± 0.5%; P > 0.05) were not different between WT and mKO mice (Fig. 2A). Similarly, skeletal muscle, heart, liver, and AT weights were not different between genotypes (Table 1). For energy expenditure, whereas expected diurnal rhythms in Vo2, respiratory exchange ratio (RER), and total activity (i.e., all x axis beam breaks) were observed, there were no genotype differences (Fig. 2B–D).
Figure 2.
Loss of p300 in skeletal muscle does not alter in vivo energy expenditure or activity. A) Body mass (BM), lean mass (LM), and fat mass (FM) determined by MRI for WT (open bars) and mKO (closed bars) mice (n = 29–34). B–D) Measurements were made using the Comprehensive Lab Animals Monitoring System over 3 consecutive days. Data presented are averages for the light and dark cycles of d 2 and 3 for WT (open bar) and mKO (closed bar) mice (n = 6–9 per group). Vo2 (B) and RER (C) were measured by indirect calorimetry, and total activity (D) was measured as all x axis beam breaks. RQ, respiratory quotient. Data are reported as means ± sem. *P < 0.05 vs. light.
TABLE 1.
Tissue weights
| Sed |
VWR |
|||
|---|---|---|---|---|
| Tissue | WT | mKO | WT | mKO |
| GA | 109 ± 2 | 113 ± 2 | 112 ± 4 | 114 ± 3 |
| TA | 40 ± 1 | 42 ± 1 | 42 ± 1 | 42 ± 1 |
| Quadriceps | 151 ± 5 | 153 ± 3 | 155 ± 6 | 155 ± 4 |
| Heart | 113 ± 7 | 99 ± 3 | 124 ± 5* | 121 ± 4* |
| Liver | 1056 ± 80 | 1160 ± 51 | 1273 ± 47* | 1300 ± 37* |
| AT | 390 ± 39 | 426 ± 47 | 200 ± 17* | 188 ± 15* |
Tissue weights were measured in milligrams. *P < 0.05, main effect of VWR.
Loss of p300 does not affect skeletal muscle fiber-type expression or muscle structure
Type I, IIa, IIx, and IIb protein expression in the GA, TA, and plantaris (data not shown) was similar between WT and mKO (Fig. 3A–D). Fiber size determination by laminin staining revealed a normal histologic structure in mKO vs. WT mice (Fig. 3E). Interestingly, average fiber CSA between 2400 and 2800 μm2 was significantly lower in mKO vs. WT and trended lower between 2000 and 2400 μm2 (P = 0.09; Fig. 3F).
Figure 3.
Skeletal muscle fiber type and CSA in mKO and WT mice. A–D) Representative silver stain image for myosin heavy-chain (MHC; type I, IIa, IIx, and IIb) expression (A, B) and quantification (C and D) in GA (A, C) and TA (B and D) from WT (open bars) and mKO (closed bars) mice. Std, standard. E) Antilaminin-stained cross sections. F) Mean fiber CSA from TA of WT (open bars) and mKO (closed bars) mice. Data are reported as means ± sem (n = 8–15 per genotype in A–C, and n = 4 per genotype in E, F). *P < 0.05; ∧P = 0.09.
Markers of skeletal muscle development are unaffected by loss of p300
Considering the proposed role of p300 in muscle development (11–16, 19, 21, 33), particularly in regulating MyoD and Mef2, we assessed the abundance of key skeletal muscle developmental proteins. Myf5, Myf6, Mef2, myogenin, and MyoD protein abundance was not different between mKO and WT mice (Fig. 4).
Figure 4.
Skeletal muscle development-related proteins are similarly expressed in WT and mKO mice. A) Myf5, Myf6, Mef2, myogenin (MyoG), MyoD, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein abundance was assessed by SDS-PAGE in whole-muscle lysates. B) Quantitation of protein abundance. Values were normalized to GAPDH and are presented relative to WT. Data are reported as means ± sem (n = 3 per genotype).
Targeted metabolomics reveals that loss of p300 does not affect skeletal muscle citric acid, fatty acid, or amino acid metabolism
Utilizing a targeted metabolomics approach, we compared key metabolites of the citric acid cycle and fatty acid (i.e., acyl carnitines) and amino acid metabolism, in skeletal muscle. The majority of metabolites were not statistically different between mKO and WT mice, except for 2-methylbutyroylcarnitine (2MBC) and glutamine, which were lower in mKO mice (Table 2). Isovalerylcarnitine (IVC) also trended lower in mKO vs. WT mice (P = 0.07; Table 2); both IVC and 2MBC are markers of branched-chain amino acid (BCAA) metabolism. Despite these differences, subsequent pathway analysis revealed no significant differences between WT and mKO in the citric acid cycle or fatty acid and amino acid metabolism.
TABLE 2.
Targeted metabolomics in WT and mKO
| KEGG pathway | WT | mKO |
|---|---|---|
| Lactate | 1024 ± 252 | 736 ± 202 |
| Citric acid cycle | ||
| Citrate (a.r.) | 0.6 ± 0.15 | 0.7 ± 0.16 |
| α-Ketoglutarate | 3.6 ± 1.2 | 2.8 ± 1.4 |
| Succinate | 29.6 ± 10.1 | 17.6 ± 8.6 |
| Fumarate | 2.4 ± 0.3 | 1.7 ± 0.3 |
| Malate | 50.1 ± 6.3 | 38.9 ± 7.3 |
| Acylcarnitines | ||
| Acetylcarnitine | 8.8 ± 5.0 | 7.5 ± 2.5 |
| Octanoylcarnitine (nM) | 35.7 ± 39.0 | 20.1 ± 19.0 |
| Decanoylcarnitine (a.r.) | 0.2 ± 0.2 | 0.1 ± 0.1 |
| Dodecanoylcarnitine (h.r.) | 0.03 ± 0.03 | 0.01 ± 0.01 |
| IVC | 42.5 ± 19.9 | 22.3 ± 10.2^ |
| 2MBC (a.r.) | 0.5 ± 0.25 | 0.2 ± 0.06* |
| Amino acid metabolism | ||
| Proline | 23.8 ± 2.6 | 20.8 ± 1.8 |
| 5-Oxoproline | 5.7 ± 1.0 | 7.1 ± 0.5 |
| Valine | 25.9 ± 2.7 | 24.1 ± 2.4 |
| Ketoisovaleric | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Arginine | 44.8 ± 10.7 | 34.8 ± 7.7 |
| Glutamine | 363.8 ± 10.3 | 325.8 ± 7.1* |
| Glutamate | 159.2 ± 61.2 | 138.0 ± 40.0 |
| Aspartate | 55.7 ± 6.5 | 47.1 ± 5.0 |
| Threonine | 29.9 ± 2.9 | 26.1 ± 2.3 |
| Methionine | 11.4 ± 1.1 | 9.4 ± 0.5 |
| Cystine | 25.1 ± 2.2 | 22.5 ± 1.1 |
| Asparagine | 20.0 ± 1.0 | 18.1 ± 1.6 |
| Serine | 43.3 ± 4.3 | 34.3 ± 2.1 |
| Lysine | 342.5 ± 14.3 | 312.0 ± 15.2 |
| Tyrosine | 14.4 ± 1.1 | 11.6 ± 0.8 |
| Phenylalanine | 8.7 ± 1.2 | 7.5 ± 1.2 |
| Tryptophan | 2.5 ± 0.3 | 2.0 ± 0.2 |
| Isoleucine | 10.2 ± 1.1 | 10.4 ± 1.6 |
| Leucine | 8.4 ± 1.8 | 9.1 ± 3.0 |
Data are quantified as micromolar (or nanomolar) or are estimated as noted [by ratios of area (a.r.) or heath (h.r.)]. KEGG, Kyoto Encyclopedia of Genes and Genomes. *P < 0.05 vs. WT. ^P = 0.07 vs. WT.
Mitochondrial respiration is comparable in WT and mKO muscle
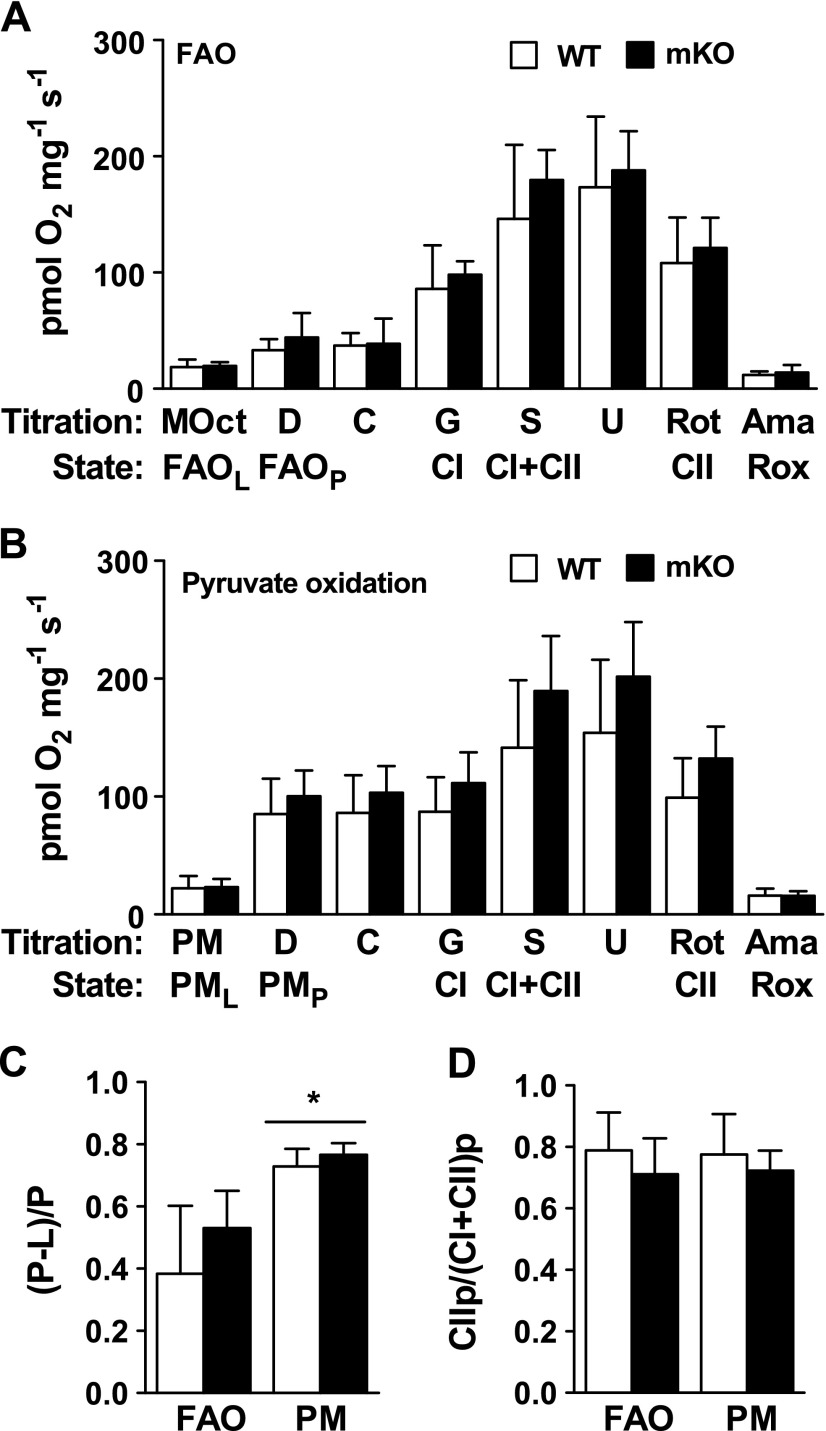
Mitochondrial respiration was determined in permeabilized muscle bundles from the TRI of WT and mKO mice using fatty acid substrate [malate plus octanoylcarnitine (MOct)] or in the presence of pyruvate [pyruvate plus malate (PM)]. We found no differences in oxidative phosphorylation capacity, electron transport system capacity, leak respiration (in the absence of adenylates), or residual oxygen consumption between WT and mKO in the presence of either MOct or PM (Fig. 5A, B). Furthermore, no genotype differences were observed in the oxidative phosphorylation-coupling efficiency; however, statistically greater coupling efficiency occurred in the presence of PM compared to MOct (Fig. 5C). Additionally, no differences in the contribution of complex II capacity compared to total complex I plus complex II capacity were observed between genotypes or substrate (Fig. 5D).
Figure 5.
High-resolution respirometry in WT and mKO TRI muscle. A) Respiratory flux normalized to muscle weight in the presence of MOct (leak respiration in the absence of adenylates), ADP (D), cytochrome c (C; mitochondrial integrity), glutamate [G; complex I (CI) capacity], succinate [S; complex I + complex II (CII) capacity], carbonyl cyanide m-chloro phenyl hydrazone (U; maximal respiration), rotenone (Rot; complex II capacity), and Ama [residual oxygen consumption (Rox)]. B) Respiratory flux normalized to muscle weight in the presence of PM followed by the same titrations in (A). C) Fatty acid oxidation (FAO) and pyruvate oxidation (PM)-coupling control factor, calculated as (P − L)/P, respectively. D) Complex II-linked flux control ratio, calculated as complex II/(complex I + complex II). Data are reported as means ± sem (n = 6 per genotype). *P < 0.05 PM vs. FAO.
Loss of p300 does not affect skeletal muscle function or adaptations to endurance ExT
To determine if loss of p300 affected muscle function, we assessed the active mechanical properties in the SOL and EDL. Twitch properties (net peak tension, time-to-peak tension, and half-relaxation time) (Fig. 6A–C) and F-F characteristics (peak stress, F-F, half-fusion frequency, and TTF) (Fig. 6D–H) were not different between genotypes. We next wanted to see if the ability of skeletal muscle of mKO mice to ExT would be impaired. First, during a 20 d VWR physiologic intervention, no genotype differences were observed among average daily running speed (Fig. 7A), running distance (data not shown), and average time spent running per 24 h (WT, 348 ± 12 min/d; mKO, 353 ± 17 min/d; P > 0.05) throughout the VWR. The effectiveness of the VWR was confirmed by an ∼92% higher RTE time in VWR vs. sedentary (Sed) mice, although no genotype differences were found (Fig. 7B). Although skeletal muscle weights were similar between Sed and ExT groups, epididymal fat weight was lower and liver and heart weights were higher in VWR vs. Sed (Table 1). At the level of skeletal muscle, VWR increased the abundance of mitochondrial markers (∼2- to 6-fold), including complex I–V, PGC-1α, ACADL, ACADVL, HKII, PDH, and mtTFA, and the maximal activity of CS, complex I, and complex II (Fig. 7C–H); there was no effect of genotype in Sed or VWR muscle.
Figure 6.
Ex vivo muscle function is not different between WT and mKO mice. Ex vivo twitch (A–C) and F-F (D–H) characteristics were measured in isolated EDL and SOL muscles. A) Net peak force. B) Time-to-peak tension (TTPT). C) Half-relaxation time (HRT). D) Maximal tetanic tension. E–H) Representative F-F curves for EDL (E) and SOL (F), half (1/2)-fusion frequency (freq.) (G) and TTF (i.e., time to reach 40% of initial force) (H). Data are reported as means ± sem (n = 6–14 per group).
Figure 7.
Endurance ExT-induced adaptations in skeletal muscle do not require p300. A) Average speed (Avg. Sped) over 24 h in WT (open squares) and mKO (closed squares) mice (n = 17–19 per group). B) RTE in WT and mKO Sed and 20 d exercised trained (ExT) mice (n = 4–6 per group). C, D) Representative images (C) and quantitation (D) of PGC-1α, mtTFA, ACADL, ACADVL, HKII, PDH, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in TRI muscle from Sed and ExT WT and mKO mice. All values were normalized to GAPDH and are presented relative to WT-Sed (n = 6–10 per group). E, F) Representative images (E) and quantitation (F) of electron transport chain complex [complex I (CI), complex II (CII), complex III (CIII), complex IV (CIV), and complex V (CV)] proteins. All values were normalized to GAPDH and are presented relative to WT-Sed (n = 6–10 per group). G, H) Specific activity of CS (G) and complex I and complex II (H) (n = 6–10 per group). Data are reported as means ± sem. *P < 0.05 vs. Sed, within each genotype.
DISCUSSION
In recent years, the contribution of reversible protein acetylation to various aspects of skeletal muscle biology and physiology, particularly mitochondrial metabolism and exercise-induced adaptations, has garnered much attention (4, 7). The vast majority of this research has focused on the role of DACs, especially SIRT1 and SIRT3 (4–7), with, to our knowledge, no skeletal muscle-specific mouse models having been generated to study KAT function and muscle biology, in vivo. To address this gap, we developed a mouse with mKO. Our results demonstrate that loss of p300 does not affect normal skeletal muscle development, nor does it impair skeletal muscle function or ExT-induced metabolic adaptation.
Many substrates of p300, such as PPARα (34) and PGC-1α (35, 36), are important regulators of cellular metabolism. The PGC-1α–p300 transcriptional cocomplex augments the transcriptional activity of nuclear respiratory factor-1, which in turn regulates the expression of mtTFA (10, 37). Additionally, Mef2a and MyoD are targets of p300 (16, 18, 33), and ExT increases acetylation of Mef2a and its binding to the carnitine palmitoyltransferase 1b promoter in skeletal muscle (38). Interestingly, in cardiac muscle and cultured ventricular myocytes, overexpression of a dominant-negative mutant p300 attenuated Mef2C-dependent PGC-1α promoter activity and transcriptional activity, which resulted in reduced mitochondrial gene expression and dysfunctional mitochondria (39). Additionally, it is clear that histone modifications contribute to exercise-induced gene transcription and, ultimately, changes in protein abundance and that these changes are associated with increased acetylation on specific histones, such as H3Lys36 (40, 41). Although these changes occur in parallel with nuclear histone deacetylase 5 export (thereby reducing the potential for histone deacetylation) (40, 41), it is likely that increased KAT activity also plays a role. Thus, we sought to determine if loss of p300 in skeletal muscle would affect markers of mitochondrial function, or the adaptive response to endurance ExT. Our results demonstrate that baseline electron transport chain enzyme activity, as well as the protein abundance of various metabolic enzymes of fatty acid and glucose metabolism and the transcriptional regulators, PGC-1α and mtTFA, is not affected by KO of p300. Moreover, VWR-induced mitochondrial biogenesis was identical between genotypes. We also observed no effects on functional measures of metabolism (respirometry flux) or mitochondrial function, such as whole-body energy expenditure, ex vivo muscle endurance (as measured by TTF), or RTE time.
Mass spectrometry-based measurement of the acetylome has revealed that many enzymes central to glucose, fatty acid, and amino acid metabolism are acetylated (2, 42–45). This is important because reversible acetylation of these enzymes can regulate their activity and, in turn, can regulate metabolic flux (42, 45, 46). For instance, the glycolytic enzyme, phosphoglycerate mutase, is acetylated by p300/CBP-associated factor, and deacetylation increases its activity (46). Similarly, lysine-to-glutamine mutation (which mimics constitutive acetylation) of the glycolytic enzymes, aldolase B and glycerol-3-phosphate dehydrogenase, inhibits their enzymatic activity, although the KAT that acetylates these proteins is unknown (42). More broadly, the in vivo role for acetylation in regulating metabolism, and the specific KATs that contribute to this, is poorly understood. Accordingly, we used targeted metabolomics to investigate whether loss of p300 affects metabolic flux, with our main focus on metabolites of the citric acid cycle and fatty acid and amino acid metabolism. On the whole, pathway analysis revealed no major differences between genotypes, although 2 markers of BCAA metabolism, 2MBC and IVC, were lower in mKO vs. WT mice. A possible explanation could be due to the fact that PGC-1α can regulate BCAA metabolism in skeletal muscle (47, 48), and as described above, a PGC-1α–p300 transcriptional cocomplex can regulate aspects of metabolism (35, 36).
Normal skeletal muscle development has long been thought to require p300 (11–13, 15–18, 20, 21). At the start of C2C12 myoblast differentiation, p300 expression and KAT activity are rapidly up-regulated, and this underlies activation of muscle-specific gene expression and promotes muscle differentiation (11, 20). Furthermore, treating C2C12 myoblasts with anti-p300 antibodies or Lys coenzyme A (a chemical inhibitor of CBP/p300) impairs exit from the cell cycle and induction of the differentiation program (11, 18). This had been attributed to p300-mediated acetylation of MyoD and potentiation of MyoD and Mef2a promoter activity (11, 16, 18, 20, 33). Complimenting these in vitro studies, mice with whole-body heterozygous or homozygous KO of p300 display defects in various aspects of neural, cardiac, and muscle development and die during embryogenesis. Indeed, embryonic lethality coincides with a lack of induction of MyoD and myogenin at d 14.5 of embryogenesis, suggesting that p300 is required for expression of these myogenic regulatory factors and the development of myogenic precursor cells (13, 15). Importantly, in our mKO mouse, it would be expected that KO of p300 would occur before this time point because muscle creatine kinase expression (which drives Cre recombinase in our model) is initially observed by d 13 of embryogenesis and is certainly up-regulated by d 15 (49, 50). Therefore, if p300 is required for normal skeletal muscle development, particularly by d 14.5 of embryogenesis, we would expect to see this manifest in the mKO mouse. Our results, however, clearly demonstrate that mKO mice are viable, develop functional skeletal muscle, and can exercise comparably to WT littermates. They also have normal expression of various myogenic regulatory factors, including myogenin, MyoD, Myf5, and Myf6. A potential explanation for this lack of effect on development in mKO mice is that whereas p300 interacts with MyoD and enhances MyoD-dependent gene transcription, it is not required (18, 33). Instead, p300/CBP-associated factor or CBP could compensate for the loss of p300 (12), which will be important to ascertain in future studies; although, we found no up-regulation of skeletal muscle CBP abundance. In addition, CBP and p300 contribute to motor neuron growth (51). As such, defective muscle development in the whole-body models (13, 15) could, in part, result from poor innervation of the developing muscle, an issue that would be circumvented in our mKO model.
The ribosomal proteins, p70 S6 kinase 1 and 2 (S6K1/2), are important regulators of skeletal muscle mass and myofiber CSA (52, 53). Cultured myotubes and TA muscle cross sections from mice with whole-body KO of S6K1/2 have lower muscle mass and reduced myofiber CSA compared to WT littermates (54). Interestingly, p300 interacts with and acetylates S6K1 in vitro and in vivo, which increases its activity (55, 56). Thus, we expected that muscle weight or fiber CSA might be reduced in mKO mice. Although we found no differences between mKO and WT mice in lean mass or muscle weights, mKO mice had fewer myofibers with a CSA between 2400 and 2800 μm2 (and trended to be lower between 2000 and 2400 μm2). This suggests that p300 plays a role in regulating muscle CSA, and it will be interesting in future studies to examine the role of p300 in modulating adaptive skeletal muscle growth and hypertrophy.
In summary, whereas it is clear that reversible protein acetylation plays an important role in regulating various aspects of muscle biology, physiology, and metabolism, the contribution of KATs to this regulation is poorly understood. Our results demonstrate that loss of p300 in skeletal muscle does not impair adult skeletal muscle development, skeletal muscle function, or metabolic adaptation to exercise, although potential effects on muscle growth and fiber CSA require more detailed investigation. Given the high-sequence homology of p300 and its paralog, CBP (57–62), in future studies, it will also be interesting to determine the separate and combined contributions of CBP and p300 to skeletal muscle biology and function.
Acknowledgments
The authors sincerely thank Shannon Bremner [Department of Orthopaedic Surgery, University of California San Diego (UCSD)] for expertise and technical assistance with muscle mechanics testing. The authors also thank the UCSD Animal Care Program Phenotyping Core for Comprehensive Lab Animals Monitoring System measurements and Dr. Jianhua Shao (Department of Pediatrics, UCSD) for use of the magnetic resonance imaging machine. This work was supported in part by U.S. National Institutes of Health Grants R01 AG043120 (National Institute on Aging), R24 HD050837 (Eunice Kennedy Shriver National Institute of Child Health and Human Development), P30 DK063491 (National Institute of Diabetes and Digestive and Kidney Diseases via a Pilot and Feasibility Award from the UCSD/University of California, Los Angeles, Los Angeles, CA, USA/Cedars-Sinai, Los Angeles, CA, USA/Salk Diabetes Research Center), P30 AR058878 to (National Institute of Arthritis and Musculoskeletal and Skin Diseases; to S.S.), a postdoctoral fellowship from the UCSD Frontiers of Innovation Scholars Program (to S.A.L.), and a Biotechnology and Biological Sciences Research Council Award (BB/L023547/1; to A.P.). S.A.L. and S.S. conceived and designed the experiments; S.A.L., C.W.M., E.H.B., H.O., B.S., S.R.N., C.E.M., and S.S. conducted experiments and contributed to data analysis presented in Figs. 1–7; I.G. and B.A.B. performed metabolomics analysis, and B.S. and A.P. analyzed data in Fig. 4; S.A.L., C.W.M., and S.S. prepared the figures; S.A.L. wrote the manuscript, which was edited by S.S.; and all authors reviewed the results and approved the final version of the manuscript. S.S. is associated with the Biomedical Sciences Graduate Program (School of Medicine, UCSD). The authors declare no conflicts of interest.
Glossary
- 2MBC
2-methylbutyroylcarnitine
- ACADL
acyl-coenzyme A dehydrogenase long chain
- ACADVL
acyl-coenzyme A dehydrogenase very long chain
- Ama
antimycin
- AT
adipose tissue
- BCAA
branched-chain amino acid
- CBP
cAMP response element-binding protein-binding protein
- Cre-MCK
Cre recombinase under the control of the muscle creatine kinase promoter
- CS
citrate synthase
- CSA
cross-sectional area
- DAC
deacetylase
- EDL
extensor digitorum longus
- ExT
exercise training
- F-F
force-frequency
- GA
gastrocnemius
- HKII
hexokinase II
- IP
immunoprecipitation
- IVC
isovalerylcarnitine
- KAT
lysine acetyltransferase
- KO
knockout
- Mef2
myocyte enhance factor 2
- mKO
muscle-specific knockout of E1a-binding protein
- MOct
malate plus octanoylcarnitine
- mtTFA
mitochondrial-specific transcription factor A
- Myf5
myogenic factor 5
- Myf6
myogenic factor 6
- MyoD
myogenic factor 3
- myogenin
myogenic factor 4
- p300
E1a-binding protein
- PDH
pyruvate dehydrogenase
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator-1α
- PM
pyruvate plus malate
- RTE
run to exhaustion
- Sed
sedentary
- SIRT
sirtuin
- SOL
soleus
- SUIT
substrate-uncoupler-inhibitor titration
- TA
tibialis anterior
- TRI
triceps
- TTF
time to fatigue
- Vo2
oxygen consumption
- VWR
voluntary wheel running
- WT
wild-type
REFERENCES
- 1.Kim G. W., Yang X. J. (2011) Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem. Sci. 36, 211–220 [DOI] [PubMed] [Google Scholar]
- 2.Choudhary C., Weinert B. T., Nishida Y., Verdin E., Mann M. (2014) The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 [DOI] [PubMed] [Google Scholar]
- 3.Verdin E., Ott M. (2015) 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 16, 258–264 [DOI] [PubMed] [Google Scholar]
- 4.Menzies K., Auwerx J. (2013) An acetylation rheostat for the control of muscle energy homeostasis. J. Mol. Endocrinol. 51, T101–T113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philp A., Rowland T., Perez-Schindler J., Schenk S. (2014) Understanding the acetylome: translating targeted proteomics into meaningful physiology. Am. J. Physiol. Cell Physiol. 307, C763–C773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White A. T., Schenk S. (2012) NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am. J. Physiol. Endocrinol. Metab. 303, E308–E321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantó C., Auwerx J. (2012) Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol. Rev. 64, 166–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K. K., Workman J. L. (2007) Histone acetyltransferase complexes: one size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 8, 284–295 [DOI] [PubMed] [Google Scholar]
- 9.Dancy B. M., Cole P. A. (2015) Protein lysine acetylation by p300/CBP. Chem. Rev. 115, 2419–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puigserver P., Spiegelman B. M. (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 24, 78–90 [DOI] [PubMed] [Google Scholar]
- 11.Puri P. L., Avantaggiati M. L., Balsano C., Sang N., Graessmann A., Giordano A., Levrero M. (1997) p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J. 16, 369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puri P. L., Sartorelli V., Yang X. J., Hamamori Y., Ogryzko V. V., Howard B. H., Kedes L., Wang J. Y., Graessmann A., Nakatani Y., Levrero M. (1997) Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol. Cell 1, 35–45 [DOI] [PubMed] [Google Scholar]
- 13.Roth J. F., Shikama N., Henzen C., Desbaillets I., Lutz W., Marino S., Wittwer J., Schorle H., Gassmann M., Eckner R. (2003) Differential role of p300 and CBP acetyltransferase during myogenesis: p300 acts upstream of MyoD and Myf5. EMBO J. 22, 5186–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sartorelli V., Puri P. L., Hamamori Y., Ogryzko V., Chung G., Nakatani Y., Wang J. Y., Kedes L. (1999) Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell 4, 725–734 [DOI] [PubMed] [Google Scholar]
- 15.Yao T. P., Oh S. P., Fuchs M., Zhou N. D., Ch’ng L. E., Newsome D., Bronson R. T., Li E., Livingston D. M., Eckner R. (1998) Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93, 361–372 [DOI] [PubMed] [Google Scholar]
- 16.Sartorelli V., Huang J., Hamamori Y., Kedes L. (1997) Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol. Cell. Biol. 17, 1010–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckner R., Ewen M. E., Newsome D., Gerdes M., DeCaprio J. A., Lawrence J. B., Livingston D. M. (1994) Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8, 869–884 [DOI] [PubMed] [Google Scholar]
- 18.Eckner R., Yao T. P., Oldread E., Livingston D. M. (1996) Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 10, 2478–2490 [DOI] [PubMed] [Google Scholar]
- 19.Polesskaya A., Duquet A., Naguibneva I., Weise C., Vervisch A., Bengal E., Hucho F., Robin P., Harel-Bellan A. (2000) CREB-binding protein/p300 activates MyoD by acetylation. J. Biol. Chem. 275, 34359–34364 [DOI] [PubMed] [Google Scholar]
- 20.Polesskaya A., Naguibneva I., Fritsch L., Duquet A., Ait-Si-Ali S., Robin P., Vervisch A., Pritchard L. L., Cole P., Harel-Bellan A. (2001) CBP/p300 and muscle differentiation: no HAT, no muscle. EMBO J. 20, 6816–6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasper L. H., Fukuyama T., Biesen M. A., Boussouar F., Tong C., de Pauw A., Murray P. J., van Deursen J. M., Brindle P. K. (2006) Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol. Cell. Biol. 26, 789–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philp A., Chen A., Lan D., Meyer G. A., Murphy A. N., Knapp A. E., Olfert I. M., McCurdy C. E., Marcotte G. R., Hogan M. C., Baar K., Schenk S. (2011) Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise. J. Biol. Chem. 286, 30561–30570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talmadge R. J., Roy R. R. (1993) Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J. Appl. Physiol. (1985) 75, 2337–2340 [DOI] [PubMed] [Google Scholar]
- 24.White A. T., Philp A., Fridolfsson H. N., Schilling J. M., Murphy A. N., Hamilton D. L., McCurdy C. E., Patel H. H., Schenk S. (2014) High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am. J. Physiol. Endocrinol. Metab. 307, E764–E772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minamoto V. B., Hulst J. B., Lim M., Peace W. J., Bremner S. N., Ward S. R., Lieber R. L. (2007) Increased efficacy and decreased systemic-effects of botulinum toxin A injection after active or passive muscle manipulation. Dev. Med. Child Neurol. 49, 907–914 [DOI] [PubMed] [Google Scholar]
- 26.Chleboun G. S., Patel T. J., Lieber R. L. (1997) Skeletal muscle architecture and fiber-type distribution with the multiple bellies of the mouse extensor digitorum longus muscle. Acta Anat. (Basel) 159, 147–155 [DOI] [PubMed] [Google Scholar]
- 27.White A. T., McCurdy C. E., Philp A., Hamilton D. L., Johnson C. D., Schenk S. (2013) Skeletal muscle-specific overexpression of SIRT1 does not enhance whole-body energy expenditure or insulin sensitivity in young mice. Diabetologia 56, 1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spinazzi M., Casarin A., Pertegato V., Ermani M., Salviati L., Angelini C. (2011) Optimization of respiratory chain enzymatic assays in muscle for the diagnosis of mitochondrial disorders. Mitochondrion 11, 893–904 [DOI] [PubMed] [Google Scholar]
- 29.Spinazzi M., Casarin A., Pertegato V., Salviati L., Angelini C. (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 7, 1235–1246 [DOI] [PubMed] [Google Scholar]
- 30.Chicco A. J., Le C. H., Schlater A., Nguyen A., Kaye S., Beals J. W., Scalzo R. L., Bell C., Gnaiger E., Costa D. P., Crocker D. E., Kanatous S. B. (2014) High fatty acid oxidation capacity and phosphorylation control despite elevated leak and reduced respiratory capacity in northern elephant seal muscle mitochondria. J. Exp. Biol. 217, 2947–2955 [DOI] [PubMed] [Google Scholar]
- 31.Gertsman I., Gangoiti J. A., Barshop B. A. (2014) Validation of a dual LC-HRMS platform for clinical metabolic diagnosis in serum, bridging quantitative analysis and untargeted metabolomics. Metabolomics 10, 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia J., Sinelnikov I. V., Han B., Wishart D. S. (2015) MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan W., Condorelli G., Caruso M., Felsani A., Giordano A. (1996) Human p300 protein is a coactivator for the transcription factor MyoD. J. Biol. Chem. 271, 9009–9013 [DOI] [PubMed] [Google Scholar]
- 34.Dowell P., Ishmael J. E., Avram D., Peterson V. J., Nevrivy D. J., Leid M. (1997) p300 functions as a coactivator for the peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 272, 33435–33443 [DOI] [PubMed] [Google Scholar]
- 35.Wallberg A. E., Yamamura S., Malik S., Spiegelman B. M., Roeder R. G. (2003) Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha. Mol. Cell 12, 1137–1149 [DOI] [PubMed] [Google Scholar]
- 36.Puigserver P., Adelmant G., Wu Z., Fan M., Xu J., O’Malley B., Spiegelman B. M. (1999) Activation of PPARgamma coactivator-1 through transcription factor docking. Science 286, 1368–1371 [DOI] [PubMed] [Google Scholar]
- 37.Virbasius J. V., Scarpulla R. C. (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA 91, 1309–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan H., Niu Y., Liu X., Fu L. (2014) Exercise increases the binding of MEF2A to the Cpt1b promoter in mouse skeletal muscle. Acta Physiol. (Oxf.) 212, 283–292 [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa Y., Kuwahara K., Takemura G., Akao M., Kato M., Arai Y., Takano M., Harada M., Murakami M., Nakanishi M., Usami S., Yasuno S., Kinoshita H., Fujiwara M., Ueshima K., Nakao K. (2009) p300 plays a critical role in maintaining cardiac mitochondrial function and cell survival in postnatal hearts. Circ. Res. 105, 746–754 [DOI] [PubMed] [Google Scholar]
- 40.McGee S. L., Fairlie E., Garnham A. P., Hargreaves M. (2009) Exercise-induced histone modifications in human skeletal muscle. J. Physiol. 587, 5951–5958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGee S. L., Hargreaves M. (2011) Histone modifications and exercise adaptations. J. Appl. Physiol. (1985) 110, 258–263 [DOI] [PubMed] [Google Scholar]
- 42.Lundby A., Lage K., Weinert B. T., Bekker-Jensen D. B., Secher A., Skovgaard T., Kelstrup C. D., Dmytriyev A., Choudhary C., Lundby C., Olsen J. V. (2012) Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Reports 2, 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q., Zhang Y., Yang C., Xiong H., Lin Y., Yao J., Li H., Xie L., Zhao W., Yao Y., Ning Z. B., Zeng R., Xiong Y., Guan K. L., Zhao S., Zhao G. P. (2010) Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327, 1004–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J., Sprung R., Pei J., Tan X., Kim S., Zhu H., Liu C. F., Grishin N. V., Zhao Y. (2009) Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol. Cell. Proteomics 8, 215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao S., Xu W., Jiang W., Yu W., Lin Y., Zhang T., Yao J., Zhou L., Zeng Y., Li H., Li Y., Shi J., An W., Hancock S. M., He F., Qin L., Chin J., Yang P., Chen X., Lei Q., Xiong Y., Guan K. L. (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsusaka T., Guo T., Yagura T., Inoue T., Yokode M., Inagaki N., Kondoh H. (2014) Deacetylation of phosphoglycerate mutase in its distinct central region by SIRT2 down-regulates its enzymatic activity. Genes Cells 19, 766–777 [DOI] [PubMed] [Google Scholar]
- 47.Hatazawa Y., Senoo N., Tadaishi M., Ogawa Y., Ezaki O., Kamei Y., Miura S. (2015) Metabolomic analysis of the skeletal muscle of mice overexpressing PGC-1α. PLoS One 10, e0129084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatazawa Y., Tadaishi M., Nagaike Y., Morita A., Ogawa Y., Ezaki O., Takai-Igarashi T., Kitaura Y., Shimomura Y., Kamei Y., Miura S. (2014) PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS One 9, e91006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyons G. E., Mühlebach S., Moser A., Masood R., Paterson B. M., Buckingham M. E., Perriard J. C. (1991) Developmental regulation of creatine kinase gene expression by myogenic factors in embryonic mouse and chick skeletal muscle. Development 113, 1017–1029 [DOI] [PubMed] [Google Scholar]
- 50.Richardson L., Venkataraman S., Stevenson P., Yang Y., Moss J., Graham L., Burton N., Hill B., Rao J., Baldock R. A., Armit C. (2014) EMAGE mouse embryo spatial gene expression database: 2014 update. Nucleic Acids Res. 42, D835–D844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S., Lee B., Lee J. W., Lee S. K. (2009) Retinoid signaling and neurogenin2 function are coupled for the specification of spinal motor neurons through a chromatin modifier CBP. Neuron 62, 641–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baar K., Esser K. (1999) Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am. J. Physiol. 276, C120–C127 [DOI] [PubMed] [Google Scholar]
- 53.Bodine S. C., Stitt T. N., Gonzalez M., Kline W. O., Stover G. L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J. C., Glass D. J., Yancopoulos G. D. (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3, 1014–1019 [DOI] [PubMed] [Google Scholar]
- 54.Aguilar V., Alliouachene S., Sotiropoulos A., Sobering A., Athea Y., Djouadi F., Miraux S., Thiaudière E., Foretz M., Viollet B., Diolez P., Bastin J., Benit P., Rustin P., Carling D., Sandri M., Ventura-Clapier R., Pende M. (2007) S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab. 5, 476–487 [DOI] [PubMed] [Google Scholar]
- 55.Fenton T. R., Gwalter J., Cramer R., Gout I. T. (2010) S6K1 is acetylated at lysine 516 in response to growth factor stimulation. Biochem. Biophys. Res. Commun. 398, 400–405 [DOI] [PubMed] [Google Scholar]
- 56.Fenton T. R., Gwalter J., Ericsson J., Gout I. T. (2010) Histone acetyltransferases interact with and acetylate p70 ribosomal S6 kinases in vitro and in vivo. Int. J. Biochem. Cell Biol. 42, 359–366 [DOI] [PubMed] [Google Scholar]
- 57.Arany Z., Sellers W. R., Livingston D. M., Eckner R. (1994) E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell 77, 799–800 [DOI] [PubMed] [Google Scholar]
- 58.Chan H. M., La Thangue N. B. (2001) p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114, 2363–2373 [DOI] [PubMed] [Google Scholar]
- 59.Yuan L. W., Giordano A. (2002) Acetyltransferase machinery conserved in p300/CBP-family proteins. Oncogene 21, 2253–2260 [DOI] [PubMed] [Google Scholar]
- 60.Valor L. M., Viosca J., Lopez-Atalaya J. P., Barco A. (2013) Lysine acetyltransferases CBP and p300 as therapeutic targets in cognitive and neurodegenerative disorders. Curr. Pharm. Des. 19, 5051–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang F., Marshall C. B., Ikura M. (2013) Transcriptional/epigenetic regulator CBP/p300 in tumorigenesis: structural and functional versatility in target recognition. Cell. Mol. Life Sci. 70, 3989–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodman R. H., Smolik S. (2000) CBP/p300 in cell growth, transformation, and development. Genes Dev. 14, 1553–1577 [PubMed] [Google Scholar]