Abstract
It has long been suspected, but never directly shown, that bone formed to accommodate an increase in mechanical loading is related to the creation of osteoblasts from skeletal stem cells. Indeed, biophysical stimuli potently regulate osteogenic lineage commitment in vitro. In this study, we transplanted bone marrow cells expressing green fluorescent protein, to enable lineage tracing, and subjected mice to a biophysical stimulus, to elicit a bone-forming response. We detected cells derived from transplanted progenitors embedded within the bone matrix near active bone-forming surfaces in response to loading, demonstrating for the first time, that mechanical signals enhance the homing and attachment of bone marrow cells to bone surfaces and the commitment to an osteogenic lineage of these cells in vivo. Furthermore, we used an inducible Cre/Lox recombination system to delete kinesin family member 3A (Kif3a), a gene that is essential for primary cilia formation, at will in transplanted cells and their progeny, regardless of which tissue may have incorporated them. Disruption of the mechanosensing organelle, the primary cilium in a progenitor population, significantly decreased the amount of bone formed in response to mechanical stimulation. The collective results of our study directly demonstrate that, in a novel experimental stem cell mechanobiology model, mechanical signals enhance osteogenic lineage commitment in vivo and that the primary cilium contributes to this process.—Chen, J. C., Hoey, D. A., Chua, M., Bellon, R., Jacobs, C. R. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism.
Keywords: mesenchymal stem cell, ulna loading, primary cilium, bone, homing
Bones are well known to be sensitive to mechanical loading. Whereas a decrease in mechanical loading may result in bone loss, an increase in loading promotes bone formation. Given the finite lifespan and nonproliferative state of the bone-forming osteoblast (1), continued bone formation in response to any stimulus necessitates the recruitment of osteoprogenitor cells (2). In an attempt to demonstrate this process by tracing the osteogenic lineage of marrow-derived cells, chimeric animals have been used in several studies, wherein labeled marrow was transplanted into a lethally irradiated host. Such models have demonstrated that progenitor cells home to the marrow cavity and contribute to bone maintenance and fracture repair (3–5). Although it is generally expected that the process of loading-induced bone formation involves the recruitment of progenitors (6, 7), the cellular origin of bone forming osteoblasts in this process remains surprisingly unclear. Specifically, verification through lineage tracing has never been performed in response to mechanical loading. Although in vitro studies have demonstrated that biophysical stimulation enhances osteogenic lineage commitment for stem cells (8–11), it is also possible that loading instead activates existing dormant osteoblasts and bone-lining cells in vivo (12). Identifying the source of bone-forming cells in response to physical stimuli could inform development of effective therapies for enhancing bone formation in disease.
A major challenge in mechanobiology is deciphering how cells sense a biophysical signal and transduce that signal into a biochemical response. One proposed mechanism by which stem cells may sense their mechanical environment is through the primary cilium. Primary cilia are antenna-like organelles that protrude from the cell body and serve as microdomains, concentrating and enhancing the kinetics of signaling molecules (13). Recent in vitro studies have implicated the primary cilium in mesenchymal stem cells (MSCs) as both chemosensors (14, 15) and mechanosensors (10) that are essential for osteogenic differentiation, with ∼25–90% of MSCs possessing a cilium (10, 16). Although identification of stem cell primary cilia in vivo has been challenging, recent studies have demonstrated that only 1% of marrow cells possess a primary cilium in ovine bone. However, given that only 0.01% of marrow cells constitute the MSC population (17) of marrow and that most cells derived from the hematopoietic system do not possess a cilium, it is hypothesized that MSCs in vivo demonstrate high cilia incidence similar to that demonstrated in vitro. Bone formation in response to mechanical loading has been demonstrated to be mediated by bone cell primary cilia in vivo (18). However, the role of primary cilia in progenitor cells for loading induced bone formation in vivo is unknown.
Given the importance of understanding the cellular mechanisms behind bone formation for the development of treatments for bone-loss diseases, we used a novel bone marrow transplant model to elucidate the role of the marrow progenitor cell in loading-induced bone formation and to further reveal the role of the progenitor primary cilium in this process. In this study we directly demonstrated, for the first time, marrow progenitor cell participation in mechanical-loading–driven bone anabolic responses in vivo. Furthermore, we demonstrated that the progenitor cell primary cilium mediates this loading-induced response, highlighting this organelle as a potential therapeutic target to activate the progenitor population.
MATERIALS AND METHODS
Mice
All animal protocols were approved by the Institutional Animal Care and Use Committee at Columbia University. All mice, except the Kif3afl/fl mice, were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and were maintained in the animal facility at Columbia University. Kif3afl/fl mice were recovered from a cryoarchive at the UC Davis Mutant Mice Regional Resource Center (University of California, Davis, Davis, CA, USA). Kinesin family member 3A (Kif3a) is an intraflagellar transport protein that is necessary for primary cilia formation, maintenance, and function (19, 20), and its global deletion results in embryonic lethality (21, 22). Kif3afl/fl mice were bred with a green fluorescent protein (GFP)–expressing animal to enable us to track bone marrow cells and their progeny, resulting in a Kif3afl/fl;GFP control animal, and were further crossed with a mouse that expresses an endogenous tamoxifen-inducible Cre allele driven by the ROSA26 locus (23), to obtain a Kif3afl/fl;GFP;Cre-ERT2 experimental animal. As global deletion of Kif3a results in embryonic lethality, to study the effect of deleting Kif3a in stem cells, we performed bone marrow transplants to repopulate only the bone marrow with cells in which Kif3a could be deleted at will. Wild-type mice were treated with a lethal dose of whole-body irradiation, to deplete rapidly regenerating cells, and bone marrow cells isolated from donors Kif3afl/fl;Cre-ERT2;GFP were transplanted to create experimental animals. Cells from Kif3afl/fl;GFP mice were also transplanted to create control animals.
Bone marrow transplant
Eleven-week-old wild-type male C57BL/6 host mice were subjected to whole-body irradiation with a Gammacell 40 cesium irradiator (Atomic Energy of Canada, Deep River, ON, Canada). Host mice received two lethal irradiations of 6 Gy, separated by 4 h (23). Immediately after the second dose, bone marrow cells were transplanted via tail vein injection. Fresh bone marrow cells were isolated from 6–8-wk-old control (Kif3afl/fl;GFP) or experimental (Kif3afl/fl;Cre-ERT2;GFP) male donor mice. Donor mice were euthanized, and tibias and femurs were collected. Bone marrow cells were isolated by flushing the bone marrow cavity with modified minimum essential medium (Thermo Scientific–Life Technologies, Grand Island, NY) with 27.5 gauge syringes. Cells were passed through a 70 μm cell strainer, centrifuged, and resuspended in PBS. Nucleated cells (∼20 million) were injected for transplantation. Animals were monitored daily for 2 wk after the transplant.
Fluorescence-activated cell sorting
To verify that donor cells successfully engrafted and reconstituted the hematopoietic system, we isolated blood cells and treated with them with ACK lysis buffer (Thermo Scientific–Life Technologies) for 30 s, to lyse the nonnucleated cells. The presence of GFP was detected in the cells with a flow cytometer (FACSCanto II; BD Bioscience, Franklin Lakes, NJ, USA). Data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA). Data were analyzed by using the same gate value with which >99% of cells obtained from GFP-transgenic animals were found to be positive for GFP and with which 100% of cells obtained from a wild-type animal were found to be negative.
Bone marrow culture
To verify that donor cells successfully engrafted and reconstituted the bone marrow stromal cell (BMSC) population, we isolated cells, as described above, and seeded them into tissue culture polystyrene dishes. Plastic adherence has been proposed by the International Society for Cellular Therapy to be a criterion for defining BMSCs (24), and we used this quality to separate BMSCs from the heterogeneous bone marrow population. Cells were cultured in low-glucose DMEM (Thermo Scientific–Life Technologies) supplemented with 10% fetal bovine serum (Hyclone, Pittsburgh, PA, USA) and 1% penicillin and streptomycin (Thermo Scientific–Life Technologies). The medium was changed daily for the first 5 d to remove nonadherent cells and once every 2 or 3 d afterward, until the total culture time amounted to 2 wk.
DNA isolation and PCR
Freshly isolated bone marrow cells were pelleted, and DNA was extracted with the DNeasy Tissue Kit (Qiagen, Valencia, CA, USA). PCR was performed to detect Kif3a floxed, wild-type, and deleted targets, as described elsewhere (21).
Mechanical loading
Before mechanical loading, 15-wk-old animals were injected daily for 5 d with tamoxifen (25 mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA), to induce Cre recombination. At 16 wk, skeletally mature animals were subjected to mechanical loading of the ulnae with an electromagnetic loading system (EnduraTec; Bose, Eden Prairie, MN, USA) (25). In mice under isoflurane anesthesia, the right forelimb was axially loaded at 3 N at 2 Hz for 120 cycles/d for 3 consecutive days. Left forelimbs remained nonloaded to serve as internal controls. Animals received subcutaneous injections of the fluorochrome calcium-binding label calcein (30 mg/kg body weight; Sigma-Aldrich) 4 d after the first day of loading and Alizarin Red S (70 mg/kg body weight; Sigma-Aldrich) 8 d after the first day of loading.
Immunohistochemistry
Animals were euthanized 14 d after the first day of loading. Both ulnae were carefully dissected and fixed in 10% formalin solution (Sigma-Aldrich). The samples were decalcified in RDO (Apex Engineering Products, Plainfield, NJ, USA), dehydrated through ethanol and xylene, and embedded in paraffin, and transverse sections were cut at the midshaft of the ulnae at 5 μm. The sections were deparaffinized, rehydrated, treated for antigen retrieval, blocked in goat serum, and incubated with primary antibodies against GFP (1:500; cat. no. A11122; Thermo Scientific–Life Technologies) overnight. The sections were then incubated with secondary antibodies (rabbit IgG; Vectastain ABC kit PK-4001) for 30 min, followed by ABC reagent (both from Vector Laboratories, Burlingame, CA, USA) for 30 min. Finally, sections were incubated in diaminobenzidine (Dako, Carpinteria, CA, USA) substrate solution until developed and counterstained in hematoxylin.
Dynamic histomorphometry
Both ulnae for each mouse were isolated and stored in 70% ethanol. They were then dehydrated through ethanol and xylene, infiltrated with methyl methacrylate and dibutyl phthalate, and embedded in methyl methacrylate and dibutyl phthalate in the presence of benzoyl peroxide. Transverse sections were cut at the midshaft of the ulnae with a diamond saw (IsoMet Low Speed Saw; Buehler, Lake Bluff, IL, USA) and imaged on a laser scanning confocal microscope (TCS SP5; Leica Microsystems Inc., Buffalo Grove, IL, USA). Using ImageJ software (U.S. National Institutes of Health (Bethesda, MD, USA), the following measures were made of the periosteal surface: bone perimeter (B.Pm), single-label perimeter (sL.Pm), double-label perimeter (dL.Pm), and double-label area (dL.Ar). Bone formation parameters were calculated from these measures. Mineralizing surface/bone surface [MS/BS = (1/2 sL.Pm + dL.Pm)/B.Pm × 100%], mineral apposition rate (MAR = dL.Ar/dL.Pm/4 d; in micrometers per day), and bone formation rate/bone surface (BFR/BS = MAR × MS/BS × 3.65; cubic micrometers per square micrometer per day). To determine the portion of BFR caused by mechanical loading, relative (r) measurements of rMS/BS, rMAR and rBFR/BS were calculated by subtracting the value for the nonloaded forelimb from that of the loaded forelimb in individual animals.
Statistical analysis
Data are reported as means ± sem. Student’s t test was used to calculate probabilities (Prism; GraphPad Software, Inc., La Jolla, CA, USA). Paired 2-tailed Student's t tests were used for comparing differences between loaded and nonloaded values. Unpaired 2-tailed Student's t tests with unequal variances were used for comparing differences in relative bone formation values between animals with deletion of Kif3a in bone marrow cells and controls. For all tests, α = 0.05.
RESULTS
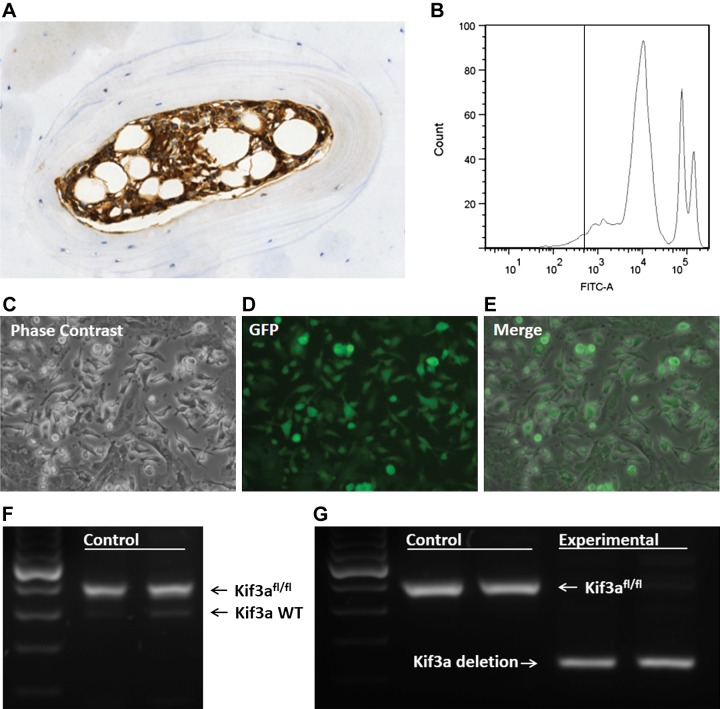
To establish the lineage of bone-forming osteoblasts in mechanically induced bone formation and determine whether primary cilia are involved, we generated chimeric mice by transplanting marrow from experimental donor animals into lethally irradiated wild-type host animals. To verify the generation of chimeric animals, we confirmed that depletion of host bone marrow cells and successful engraftment of donor cells had occurred. We verified visually that in animals receiving transplants, most bone marrow cells expressed GFP, indicating donor origin (Fig. 1A). We also used flow cytometry to observe that 95% of nucleated cells derived from whole blood were GFP+ (Fig. 1B), demonstrating that donor cells had reconstituted the hematopoietic system. This process confirmed successful engraftment of hematopoietic stem cells, but we also had to determine whether BMSCs had successfully engrafted. We cultured cells isolated from bone marrow on plastic, as proposed by the International Society for Cellular Therapy, and were able to visually detect GFP in most of the plastic-adherent cells (Fig. 1C–E), indicating that the BMSCs were primarily donor derived.
Figure 1.
Generation of chimeric animals. A) Histologic analysis of donor engraftment. GFP positive (brown) donor cells were detected in the bone marrow. B) Fluorescence activated cell sorting representative analysis in which 95.4 ± 1.2% of nucleated blood cells were positive for GFP. C–E) Cells were isolated from bone marrow, and the plastic-adherent population was analyzed for GFP. Phase contrast (C), GFP (D), and merge images (E) are shown. Nearly all of the adherent cells were positive for GFP. F) DNA was extracted from isolated bone marrow cells. PCR amplification demonstrated that the floxed allele of Kif3a was amplified in the control animal with very faint amplification of wild-type Kif3a, indicating that the marrow stromal cell population was repopulated by transplantation. G) PCR amplification of marrow stromal cells isolated from tamoxifen-treated control animals demonstrated expression of the floxed allele of Kif3a, whereas experimental animals expressed the deleted Kif3a target, indicating successful deletion of Kif3a in the experimental group.
To confirm that effective deletion of Kif3a in bone marrow cells could be achieved, we isolated DNA from bone marrow cells of tamoxifen-treated chimeric animals and used PCR to amplify Kif3a targets. In the experimental animals, most of the amplified targets were for the deleted Kif3a allele (Fig. 1G), indicating effective recombination. In control animals, the deleted Kif3a allele was not detected, and most of the amplified targets were for the Kif3a floxed allele. Furthermore, PCR bands for the wild-type version of Kif3a were very faint in both control and experimental chimeric animals, indicating that nearly all of the bone marrow cells were donor derived rather than host derived (Fig. 1F).
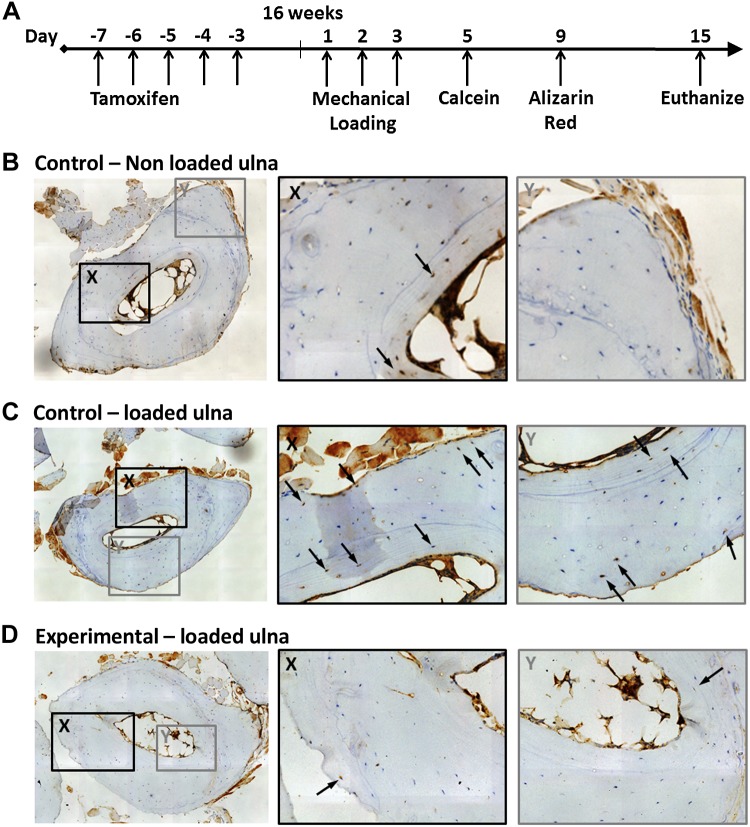
To investigate bone formation in response to mechanical loading, we loaded the forelimbs of skeletally mature 16-wk-old chimeric animals and administered fluorochrome the calcium binding labels calcein and Alizarin Red on d 5 and 9, respectively, to mark regions of new bone formation (Fig. 2A). There was no visual difference in general cage activity between groups after loading.
Figure 2.
Mechanical stimulation promotes osteogenic differentiation of bone-marrow–derived cells. A) Experimental timeline. B) Sixteen-week-old nonloaded control animal. Bone marrow–derived cells were detected only within the endocortical region (arrows). C) Control animal after mechanical loading. Bone marrow–derived cells were embedded within the endocortical and periosteal bone (arrows). D) Experimental animal after mechanical loading. Bone marrow–derived cells were also embedded in endocortical bone (X, arrow), but fewer were detected in the periosteal region (Y, arrow) than in the control animals illustrated in (C).
To determine the origin of the bone cells, we probed for GFP expression through immunohistochemistry on cross sections at the midlength of the loaded and nonloaded ulnae. We observed that mechanical loading increased the number of GFP+ cells embedded within the bone (Fig. 2C, D). Bone marrow–derived cells were detected at both the endocortical and periosteal regions of the mechanically loaded bones (Fig. 2C). In the nonloaded contralateral control ulnae, bone marrow–derived cells were also detected in the endocortical region, but their presence in the periosteal region was less than that in loaded bone (Fig. 2B). This finding demonstrates that mechanical stimulation promotes the creation of bone-forming osteoblasts and osteocytes from progenitors. In experimental animals, cells in which Kif3a was deleted were also found to be embedded within the bone (Fig. 2D), indicating that cells with disrupted primary cilia retained their inherent abilities to differentiate toward the osteogenic lineage and to form bone.
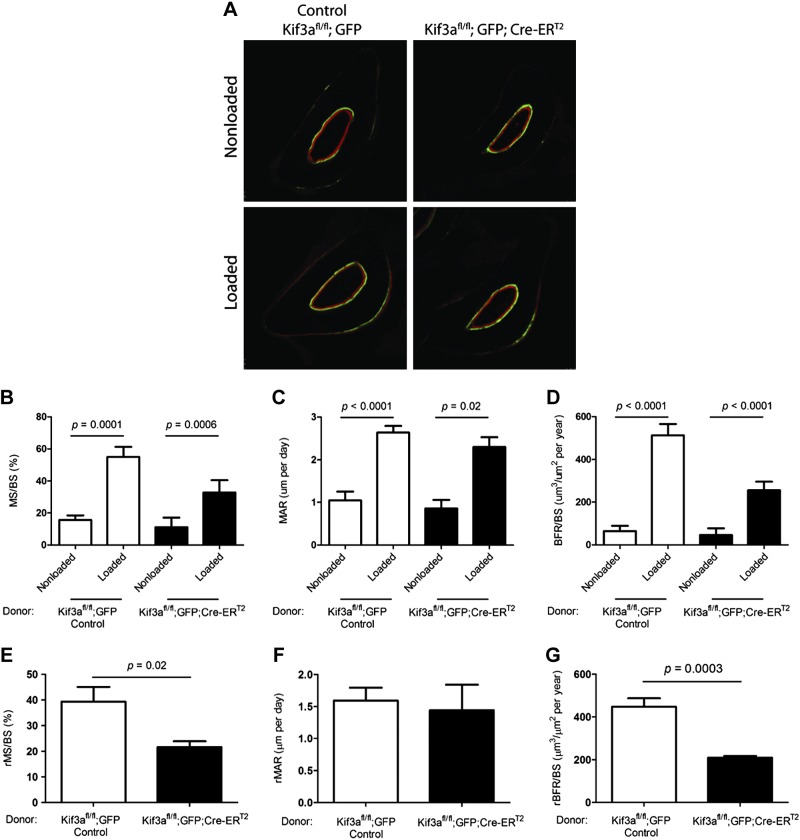
To determine whether mechanical loading would stimulate an increase in BFRs in both our control and experimental animals, we used dynamic histomorphometry to visualize areas of active bone formation (Fig. 3A) (18). In response to ulnae-compressive loading, the percentage of MS significantly increased in both the control (3.52-fold; n = 9) and experimental animals (2.93-fold; n = 5), the MAR significantly increased in both control (2.52-fold; n = 9) and experimental animals (2.68-fold; n = 5), and the BFR significantly increased in both control (7.89-fold; n = 9) and experimental animals (5.55-fold; n = 5), when compared to the nonloaded ulnae within the groups (Fig. 3B–D).
Figure 3.
Primary cilia of progenitor cells mediate mechanically induced bone formation. A) Transverse sections with fluorochrome labels marking new bone formation (green, calcein; red, alizarin). Bone formation measures were increased in loaded vs. nonloaded ulnae of control and experimental animals with primary cilia disrupted in bone marrow cells. B–D) Active mineralizing surface (MS/BS) (B), average interlabel width MAR (C), and BFR/BS (D). E–G) Relative measures (r) were obtained by subtracting nonloaded from loaded values: rMS/BS (E), rMAR (F), rBFR/BS (G). Disruption of primary cilia resulted in a diminished response to mechanical loading.
To analyze the effect of primary cilia on loading-induced bone formation, we compared relative rates of bone formation between control and experimental animals. We calculated relative (r) rates by subtracting the value of the nonloaded ulna from that of the loaded ulna. Therefore, the relative rate did not include the baseline BFR and captured the proportion of bone formation that was a response to mechanical loading. This response was attenuated in animals where primary cilia were disrupted in bone marrow–derived cells. The percentage of the periosteal surface that was activated for bone formation decreased by nearly half (45%; P < 0.05), compared to that in animals with intact primary cilia (Fig. 3E). However, within activated areas, the BFR was not affected by the absence of primary cilia (Fig. 3F). The total BFR was the product of the previous measures (percentage of the activated periosteal surface × BFR in these areas), and because of the decrease in activated surface area, the rate decreased overall (Fig. 3G).
DISCUSSION
Bone diseases resulting from low bone mass, such as osteoporosis, are a severe health threat. One avenue for combating these diseases is to encourage an increase in bone formation. Although mechanical loading has long been known to be a potent stimulus, the origin of bone-forming cells involved in this process and how these cells sense mechanical signals is poorly understood. Progenitor cells differentiate into osteoblasts during development and have long been thought to be a natural source of new active osteoblasts during loading-induced bone formation. In this study, we directly demonstrated that mechanical stimulation promotes osteogenic lineage commitment and migration of progenitor cells in vivo, leading to enhanced bone formation, and furthermore demonstrated that this osteogenic response is mediated in part by the primary cilium in the progenitor population. Our findings therefore highlight not only the osteoprogenitor, but the primary cilium as well, as novel therapeutic targets for bone loss diseases such as osteoporosis.
Mechanical loading was shown to enhance the number of bone marrow–derived cells embedded within bone, demonstrating that, in response to a physical stimulus, engrafted progenitor cells home to the bone surface and undergo osteogenic lineage commitment. An important finding is that bone marrow–derived cells were detected in both the endocortical and periosteal regions of mechanically loaded bones. Because the endocortical region is in intimate contact with bone marrow, we were not surprised to detect bone marrow–derived cells in this region. However, there are many intriguing possibilities for how bone marrow–derived cells may have arrived in the periosteal region. It is possible that cells that had migrated from the bone marrow, were circulating, or were present in the periosteum or muscle before mechanical loading. Possible mechanisms for activation of the periosteal bone surface in response to loading include recruitment and subsequent osteogenic differentiation of progenitor cells or activation of previously dormant osteoblasts that are already present on the bone surface. We observed a decrease in loading-induced periosteal activation in bone, where the cilium was deleted in the progenitor population and not the osteoblast, which suggests that loading-induced periosteal activation is caused by the recruitment and differentiation of osteogenic progenitors, not dormant osteoblasts. One potential mechanism for recruitment and differentiation is that osteocytes, previously thought to be essential in bone mechanotransduction (26), detect mechanical stimulation and then release paracrine signals, which are detected by progenitor cells (27–29). Recent work by our group has demonstrated that soluble factors secreted by mechanically stimulated osteocytes can significantly enhance MSC migration in vitro (28); however, an alternative mechanism may be that progenitor cells directly sense these biophysical signals. It is clear from these findings that progenitor cells are essential for loading-induced bone formation, but whether their contribution is regulated directly by physical stimulus or indirectly through a coordinating cell, such as the osteocyte, remains unclear.
Mechanical-loading–induced bone formation was inhibited in animals with defective marrow cell primary cilia, demonstrating an important role for the cilium in mediating skeletal adaptation. Within an activated area, the rate of bone formation was not affected by the absence of primary cilia (Fig. 3F). Rather, the higher total bone formation we observed in the control group was the result of the activation of a larger portion of the surface. This finding suggests that once cells with disrupted primary cilia differentiate into osteoblasts, the absence of primary cilia does not influence osteoblast activity. In contrast, Tummala et al. (14) demonstrated reduced RUNX2 expression in hMSCs with defective cilia, which may indicate that MSC osteogenic differentiation is delayed but can recover with time. Qiu et al. (30), using an osteoblast-specific primary cilia–deletion model, discovered that mice at 6 wk were osteopenic yet recovered at 24 wk. Furthermore, Temiyasathit et al. (18) found no decrease in activated area with loading, but osteoblast activity decreased when primary cilia were disrupted in osteocytes and osteoblasts, suggesting that primary cilia in osteocytes and osteoblasts influence how quickly bone is laid down in active areas, whereas primary cilia in progenitor cells influence the amount of area that is activated, potentially by regulating stem cell homing. The primary cilium has been shown to coordinate PDGFα-induced directional migration in fibroblasts by acting as a signaling microdomain for this pathway (31). Although a significant inhibition of loading-induced bone formation was demonstrated in animals with defective cilia in marrow-derived cells, bone formation still occurred. Bone marrow stromal cells may compensate for defective cilia through activation of other mechanosensory complexes, such as at focal adhesions (32). Furthermore, this notion raises the possibility that there are other precursors that contribute to the osteoblast population in these experimental animals. For example, host progenitors that were not ablated by irradiation may still contribute to this response. Another important consideration for our study is that Kif3a was deleted not only in osteoprogenitors, but also in all bone marrow cells. Thus, it may be that the attenuated bone formation is actually the result of disrupting primary cilia in cells regulating the stem cell niche—a dynamic microenvironment that contains the stem cell and supporting cells that influence stem cell fate. Support cells may include hematopoietic stem cells, endothelial cells, osteoblasts, and adipocytes (33), all of which are present in bone marrow.
An intriguing question that remains is whether loading promotes migration of progenitors in the bone marrow to the periosteal surface or osteoblasts are derived from progenitors residing in stem cell niches in the periosteum or even the muscle. In our study, primary cilia in progenitor cells were crucial in facilitating mechanically induced skeletal adaptation. However, the specific role of progenitor cell primary cilia remains unclear. Primary cilia could be important in proliferation (10), migration from the stem cell niche (31), homing (34), initial asymmetric division (35), and differentiation (10, 14). Our study demonstrating stem cell regulation by mechanical signals and primary cilia as a mechanism for sensing these signals opens exciting avenues for future research. Understanding how stem cell differentiation and activity are regulated is applicable not only for addressing bone diseases, but for developing a broad range of regenerative therapies.
Acknowledgments
The authors thank K. Lee, A. Nguyen, H. Li, B. Shelton, S. Bhumiratana, C. Hue, K. Yamada, and K. Prestia for technical assistance, and K. Lee and A. Nguyen for helpful discussions (all from Columbia University). This work was supported by New York State Stem Cell Grant N089-210 (to C. R. J.); U.S. National Institutes of Health, Institute of Arthritis and Musculoskeletal and Skin Disease Grants AR054156, AR062177, and AR059038 (to C. R. J.); European Research Council Grant 336882 (to D.A.H.); Science Foundation Ireland European Research Council (ERC) Support Grant SFI 13/ERC/L2864 (to D.A.H.); and an Irish Reseach Council (IRCSET)–Marie Curie International Mobility Fellowship in Science, Engineering and Technology (to D.A.H). J.C.C. and D.A.H. designed and performed experiments, interpreted the data, and wrote the manuscript; M.C. and R.B. performed experiments; and C.R.J. designed experiments, interpreted the data, and wrote the manuscript. The authors declare no conflicts of interest.
Glossary
- B.Pm
bone perimeter
- BFR
bone formation rate
- BMSC
bone marrow stromal cell
- Cre-ER
tamoxifen inducible Cre-recombinase
- dL.Ar
double label area
- dL.Pm
double label perimeter
- GFP
green fluorescent protein
- Kif3a
kinesin family member 3A
- MAR
mineral apposition rate
- MSC
mesenchymal stem cell
- MS/BS
mineralizing surface/bone surface
- PDGFα
platelet-derived growth factor-α
- rBFR
relative bone formation rate
- rMAR
relative mineral apposition rate
- rMS/BS
relative mineralizing surface/bone surface
- sL.Pm
single-label perimeter
REFERENCES
- 1.Park D., Spencer J. A., Koh B. I., Kobayashi T., Fujisaki J., Clemens T. L., Lin C. P., Kronenberg H. M., Scadden D. T. (2012) Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corral D. A., Amling M., Priemel M., Loyer E., Fuchs S., Ducy P., Baron R., Karsenty G. (1998) Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc. Natl. Acad. Sci. USA 95, 13835–13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Liu Y., Kalajzic Z., Jiang X., Rowe D. W. (2005) Heterogeneity of engrafted bone-lining cells after systemic and local transplantation. Blood 106, 3650–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rombouts W. J. C., Ploemacher R. E. (2003) Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17, 160–170 [DOI] [PubMed] [Google Scholar]
- 5.Taguchi K., Ogawa R., Migita M., Hanawa H., Ito H., Orimo H. (2005) The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem. Biophys. Res. Commun. 331, 31–36 [DOI] [PubMed] [Google Scholar]
- 6.Turner C. H., Owan I., Alvey T., Hulman J., Hock J. M. (1998) Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine. Bone 22, 463–469 [DOI] [PubMed] [Google Scholar]
- 7.David V., Martin A., Lafage-Proust M. H., Malaval L., Peyroche S., Jones D. B., Vico L., Guignandon A. (2007) Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148, 2553–2562 [DOI] [PubMed] [Google Scholar]
- 8.Arnsdorf E. J., Tummala P., Castillo A. B., Zhang F., Jacobs C. R. (2010) The epigenetic mechanism of mechanically induced osteogenic differentiation. J. Biomech. 43, 2881–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y. J., Batra N. N., You L., Meier S. C., Coe I. A., Yellowley C. E., Jacobs C. R. (2004) Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J. Orthop. Res. 22, 1283–1289 [DOI] [PubMed] [Google Scholar]
- 10.Hoey D. A., Tormey S., Ramcharan S., O’Brien F. J., Jacobs C. R. (2012) Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells 30, 2561–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo A. B., Jacobs C. R. (2010) Mesenchymal stem cell mechanobiology. Curr. Osteoporos. Rep. 8, 98–104 [DOI] [PubMed] [Google Scholar]
- 12.Everts V., Delaissé J. M., Korper W., Jansen D. C., Tigchelaar-Gutter W., Saftig P., Beertsen W. (2002) The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J. Bone Miner. Res. 17, 77–90 [DOI] [PubMed] [Google Scholar]
- 13.Nachury M. V. (2014) How do cilia organize signalling cascades? Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20140465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tummala P., Arnsdorf E. J., Jacobs C. R. (2010) The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell. Mol. Bioeng. 3, 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodle J. C., Rubenstein C. D., Phillips M. E., Bernacki S. H., Qi J., Banes A. J., Loboa E. G. (2013) Primary cilia: the chemical antenna regulating human adipose-derived stem cell osteogenesis. PLoS One 8, e62554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coughlin T. R., Voisin M., Schaffler M. B., Niebur G. L., McNamara L. M. (2015) Primary cilia exist in a small fraction of cells in trabecular bone and marrow. Calcif. Tissue Int. 96, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 18.Temiyasathit S., Tang W. J., Leucht P., Anderson C. T., Monica S. D., Castillo A. B., Helms J. A., Stearns T., Jacobs C. R. (2012) Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PLoS One 7, e33368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholey J. M. (2003) Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19, 423–443 [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum J. L., Witman G. B. (2002) Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813–825 [DOI] [PubMed] [Google Scholar]
- 21.Marszalek J. R., Ruiz-Lozano P., Roberts E., Chien K. R., Goldstein L. S. (1999) Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc. Natl. Acad. Sci. USA 96, 5043–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda S., Yonekawa Y., Tanaka Y., Okada Y., Nonaka S., Hirokawa N. (1999) Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J. Cell Biol. 145, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y. Z., Hisha H., Yang G. X., Fan T. X., Jin T., Li Q., Lian Z., Ikehara S. (2002) Optimal protocol for total body irradiation for allogeneic bone marrow transplantation in mice. Bone Marrow Transplant. 30, 843–849 [DOI] [PubMed] [Google Scholar]
- 24.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj., Horwitz E. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 [DOI] [PubMed] [Google Scholar]
- 25.Robling A. G., Turner C. H. (2002) Mechanotransduction in bone: genetic effects on mechanosensitivity in mice. Bone 31, 562–569 [DOI] [PubMed] [Google Scholar]
- 26.Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., Ikeda K. (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5, 464–475 [DOI] [PubMed] [Google Scholar]
- 27.Hoey D. A., Kelly D. J., Jacobs C. R. (2011) A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem. Biophys. Res. Commun. 412, 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady R. T., O’Brien F. J., Hoey D. A. (2015) Mechanically stimulated bone cells secrete paracrine factors that regulate osteoprogenitor recruitment, proliferation, and differentiation. Biochem. Biophys. Res. Commun. 459, 118–123 [DOI] [PubMed] [Google Scholar]
- 29.Birmingham E., Niebur G. L., McHugh P. E., Shaw G., Barry F. P., McNamara L. M. (2012) Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cell. Mater. 23, 13–27 [DOI] [PubMed] [Google Scholar]
- 30.Qiu N., Xiao Z., Cao L., Buechel M. M., David V., Roan E., Quarles L. D. (2012) Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J. Cell Sci. 125, 1945–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider L., Cammer M., Lehman J., Nielsen S. K., Guerra C. F., Veland I. R., Stock C., Hoffmann E. K., Yoder B. K., Schwab A., Satir P., Christensen S. T. (2010) Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell. Physiol. Biochem. 25, 279–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J. C., Jacobs C. R. (2013) Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res. Ther. 4, 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhn N. Z., Tuan R. S. (2010) Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J. Cell. Physiol. 222, 268–277 [DOI] [PubMed] [Google Scholar]
- 34.Christensen S. T., Veland I. R., Schwab A., Cammer M., Satir P. (2013) Analysis of primary cilia in directional cell migration in fibroblasts. Methods Enzymol. 525, 45–58 [DOI] [PubMed] [Google Scholar]
- 35.Paridaen J. T., Wilsch-Bräuninger M., Huttner W. B. (2013) Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell 155, 333–344 [DOI] [PubMed] [Google Scholar]