Abstract
Inflammation plays an important role in the pathogenesis of diabetic retinopathy (DR). We have previously reported increased monocyte (Mono) trafficking into the retinas of diabetic animals. In this study, we have examined the effect of activated Monos on retinal endothelial cells (ECs). The U937 Mϕ-conditioned medium (CM) significantly decreased the transendothelial resistance of EC monolayers as measured by electric cell-substrate impedance sensing (P = 0.007). The CM was fractioned, and the effective fraction (30–100 kDa) was analyzed by liquid chromatography-mass spectrometry, and cathepsin D (CD) was identified as a major secreted product. Immunoprecipitated CD resulted in decreased resistance in ECs (P = 0.006). The specificity of CD in mediating alterations of the EC barrier was confirmed using small interfering RNA. The decreased resistance correlated with a significantly increased gap between ECs. CD altered the Ras homolog gene family, member A/Rho-associated kinase pathway with increased stress actin filament formation in the EC layer. Increased CD levels were found in the retinas of diabetic mice (3-fold) and serum samples of patients with diabetic macular edema (1.6-fold) measured by Western blot and ELISA. These findings suggest an important role for Mϕ-derived CD in altering the blood-retinal barrier and reveal a potential therapeutic target in the treatment of DR.—Monickaraj, F., McGuire, P. G., Nitta, C. F., Ghosh, K., Das, A. Cathepsin D: an Mϕ-derived factor mediating increased endothelial cell permeability with implications for alteration of the blood-retinal barrier in diabetic retinopathy.
Keywords: Rho/ROCK pathway, inflammation, DME
Diabetes causes many metabolic and physiologic abnormalities in the retina that may ultimately lead to diabetic retinopathy (DR) and loss of vision. The exact pathway through which it causes damage to the retinal blood vessels is not completely clear (1, 2). Previous studies have demonstrated a role for subclinical inflammation and leukostasis in the pathogenesis of DR (3, 4). The binding of leukocytes to the vascular endothelium via adhesion molecules present on the endothelium (intercellular adhesion molecule 1, VCAM-1, platelet/endothelial cell adhesion molecule 1, and P-selectin) triggers the release of inflammatory cytokines, growth factors, and vascular permeability factors, which subsequently alter endothelial junctions allowing for diapedesis of leukocytes into the retina and subsequent compromise of the blood-retinal barrier (BRB) (5–9).
Inflammatory cytokines such as TNF-α and VEGF in the diabetic retina have previously been shown to alter vascular permeability by decreasing the levels of tight junction proteins occludin and zona occludens-1 and the adherens junction protein VE-cadherin (vascular endothelial cadherin) (10–13). Results from our laboratory also suggest a role for endothelial cell (EC)- and/or leukocyte-derived proteinases in the breakdown of the BRB (14). We have also reported on the cytokine-mediated trafficking of monocytes (Monos) into the retinas of diabetic mice, which could also be a source of proteinases or other factors that alter the BRB (15). Although these studies have advanced our understanding of inflammation-mediated disruption of endothelial junctional barrier, they have also generated an interest in discovering additional leukocyte-derived vascular hyperpermeability factors.
Recent studies have revealed that, in addition to soluble factors, EC contractility also contributes actively to the integrity of the endothelial barrier (16–18). The mechanical control of endothelial permeability is mediated, at least in part, by the Ras homolog gene family, member A (RhoA)/Rho-associated kinase (ROCK) pathway, which generates cytoskeletal tension (cell contractility) that is transmitted to EC junctional complexes to disrupt barrier integrity. Interestingly, VEGF, which is known to enhance endothelial permeability via phosphorylation of VE-cadherin, also increases Rho/ROCK-dependent EC contractility, thereby implicating it in the mechanical (contractility-dependent) control of barrier breakdown. Whether other DR-associated vascular permeability factors promote mechanical disruption of EC junctional barrier, however, remains to be examined.
Here, we hypothesize that specific Mono-derived factors present in the diabetic retina act on vascular ECs to increase intercellular permeability during the development of DR. Results from the present study indicate that activated Monos produce a secreted factor, aspartyl proteinase cathepsin D (CD), which disrupts endothelial junctional barrier via increased Rho/ROCK-dependent cell contractility. Importantly, CD protein is increased in the retinas of diabetic mice and serum of patients with diabetic macular edema (DME). Thus, CD may play an important role in alteration of the BRB in DR.
MATERIALS AND METHODS
Animal model of diabetes
Diabetes was induced in male C57Bl6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) with 5 daily consecutive intraperitoneal injections of streptozotocin (50 mg/kg/d; Sigma-Aldrich, St. Louis, MO, USA). Age-matched nondiabetic control animals received injections of an equal volume of citrate buffer only. Animals with plasma glucose concentrations >250 mg/dl were considered diabetic and were used in the study following 4 mo of diabetes. Blood glucose levels and body weight were monitored regularly. All animal protocols were approved by the Animal Care and Use Committee (University of New Mexico Health Sciences Center, Albuquerque, New Mexico, USA).
Cell culture
Human retinal microvascular endothelial cells (HRECs; ACBRI-181) were obtained from Cell Systems (Kirkland, WA, USA). HRECs were grown on fibronectin-coated dishes and cultured in MCDB-131 supplemented with 10% fetal bovine serum (FBS), 10 ng/ml epidermal growth factor, 1 µg/ml hydrocortisone, 0.2 mg/ml EndoGro, 0.09 mg/ml heparin, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml Fungizone (VEC Technologies, Rensselaer, NY, USA). HRECs were plated in 4-well chamber slides for immunofluorescence and stress actin fiber staining. Cells were used in all experiments between passage number 5 and 8. HUVECs were grown on fibronectin-coated dishes and cultured in F-12K medium supplemented with 10% FBS, 2.5 mg/ml EC growth supplement, 0.09 mg/ml heparin, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells of passage number 3 were used in our experiment.
The human Mono cell line, U937, was grown on Petri dishes in Roswell Park Memorial Institute 1640 culture medium with 10% FBS, 1 mM l-Glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 µg/ml streptomycin without stimulation (U937 Monos). In some cases, cells were activated by treatment with 200 nM PMA (phorbol 12-myristate 13-acetate) for 48 h at 37°C in 5% CO2 (U937 Mϕ). Conditioned medium (CM) from U937 Mϕs was collected and fractioned based on molecular weight using molecular weight cutoff filters (Pall Life Sciences, Ann Arbor, MI, USA). Different molecular mass fractions of CM were used to treat HRECs as described below, and the 30 kDa fraction containing the barrier opening activity was further analyzed by mass spectrometry (ProtTech Inc., Phoenixville, PA, USA) and compared to a similar fraction from unstimulated U937 cells.
Immunoprecipitation of CD
Human CD was immunoprecipitated from 200 μl of the 30 kDa fraction of the U937 Mϕ CM using rabbit anti-CD antibody (ab75852; Abcam, Cambridge, MA, USA). This antibody was produced from a synthetic peptide corresponding to human CD amino acid 350 to the C terminus. This antibody shows no anticatalytic activity. Sample was incubated with 20 μl anti-CD antibody overnight at 4°C with agitation. Protein-A agarose beads (20 μl of a 50% slurry; Cell Signaling Technology, Danvers, MA, USA) were added, and incubation was continued for 4 h at 4°C. Beads were centrifuged and washed with cold PBS and resuspended in complete medium and used for experiments (CD beads).
Small interfering RNA transfection via the GenomOne-Neo EX Vector Kit
U937 Monos were transfected with 0.5 μg/μl CD small interfering (si)RNA or its negative control siRNA (Life Technologies, Carlsbad, CA, USA) using the GenomOne HVJ Envelope Vector Kit (Cosmo Bio, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cells were subsequently PMA stimulated to produce U937 Mϕs, and CD was immunoprecipitated from the 30 kDa fraction as described above.
Electric cell-substrate impedance sensing
Monolayer permeability was determined using the electric cell-substrate impedance sensing (ECIS) system from Applied Biophysics (Troy, NY, USA). HRECs and HUVECs (1 × 105) were plated into fibronectin-coated multiwell chambers (8W10E+) and grown for 16 h until maximum resistance was attained (∼1200 Ω). Cells were treated as described in Results, and changes in resistance were monitored for up to 18 h. Resistance values for multiple wells were normalized to an identical starting resistance value and averaged and presented as normalized resistance over time.
Western blot
Western blots were used to confirm the inhibition of CD expression in U937 Mϕs following siRNA treatment. Extracts of diabetic and nondiabetic mouse retina were also analyzed for CD expression. The retinal vascular permeability in mouse retinas was measured by Western blot for albumin in extracted whole retinas (19). Cells/tissues were lysed using RIPA buffer, sonicated, and incubated on ice for 10 min. Lysates were collected by centrifugation at 12,000 rpm for 10 min at 4°C. The protein concentration of each sample was estimated using the Bradford assay method. Protein samples were separated in precast TGX gels (Bio-Rad, Carlsbad, CA, USA) and transferred to blotting membranes and probed with anti-CD antibody, followed by incubation with fluorescently labeled secondary antibody (Li-Cor Biosciences, Lincoln, NE, USA) and images collected on the Li-Cor Odyssey. Protein levels were normalized to β-tubulin.
Cross-linking and silver staining
The CD antibody was covalently cross-linked to magnetic beads, and immunoprecipitation (IP) was performed with the 30 kDa fraction of the U937 Mϕ CM or PBS (IP no sample). Beads were eluted using Glycine and run on the SDS-PAGE. The gel was then stained with silver nitrate to visualize the bands.
VE-cadherin immunofluorescence
HRECs were treated overnight with the CD beads from untreated or siRNA-treated (CD or scrambled) U937 Mϕs in the presence or absence of the ROCK inhibitor (Y-27632; 25 µM) (Sigma-Aldrich). Differentially treated HRECs were fixed with 3.7% paraformaldehyde for 5 min, washed, blocked with 10% normal goat serum, and incubated with primary VE-cadherin antibody (Abcam) followed by fluorescently labeled secondary antibody. Slides were imaged by confocal microscopy (Leica TCS SP5; Leica Microsystems, Buffalo Grove, IL, USA), and gap width measurements were done using ImageJ software (National Institutes of Health, Bethesda, MD, USA) as described by Huynh et al. (16).
Actin stress fiber staining
HRECs were treated with identical conditions as for VE-cadherin immunofluorescence. Then, cells were washed twice with 1× PBS and fixed with 3.7% paraformaldehyde for 10 min at room temperature and permeabilized with 0.1% Triton X-100 for 5 min at room temperature. Cells were then blocked with 1% bovine serum albumin for 20 min and stained with 2 U/ml phalloidin (1:100) (Life Technologies) for 1 h. Cells were washed twice with PBS-Tween, stained with DRAQ5 nuclear stain, and coverslipped with ProLong Gold (all from Life Technologies). Images were obtained using a Leica confocal microscope and analyzed using ImageJ software. Actin filament density was determined by drawing a line across the width of the cell and counting the number of fluorescent peaks corresponding to individual actin stress fibers (20 cells).
Ras homolog gene family, member A activity assay
Active and GTP-bound Ras homolog gene family, member A (RhoA) was measured in lysates from HRECs treated with CD beads, CD beads plus 100 μM mannose-6-phosphate (M6P), or CD siRNA beads using the RhoA activation G-LISA kit (Cytoskeleton Inc., Denver, CO, USA), as per the manufacturer’s instructions. Active RhoA levels were determined by measuring absorbance at 490 nm in a microplate spectrophotometer (Tecan US, Inc. Morrisville, NC, USA).
ELISA
CD levels were measured in CD beads and beads plus CD antibody by ELISA (Abcam) according to the manufacturer’s protocol. In addition, CD was measured in human serum samples from normal subjects or subjects with type 2 diabetes or type 2 diabetes with retinopathy (BioreclamationIVT, Baltimore, MD, USA). The chemokine (C-C motif) ligand 2 (CCL2) levels in mouse retinas were measured by ELISA (Sigma-Aldrich) according to the manufacturer’s protocol.
Real-time PCR
Total RNA was isolated from mouse retinal tissue and treated cells (Direct-zol RNA MiniPrep Kit; Zymo Research, Irvine, CA, USA), converted to cDNA (RT2 First Strand Kit; Qiagen Sciences, Germantown, MD, USA), and analyzed using a ROCK2 TaqMan assay (Life Technologies) using the 7500 ABI Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Relative levels of mRNA were determined by the comparative cycle threshold method with normalization to glyceraldehyde 3-phosphate dehydrogenase mRNA.
Statistical methods
For all quantitative experiments, statistical analyses were performed with an unpaired t test or a 1-way ANOVA (Prism 4 software; GraphPad Software, La Jolla, CA, USA). Differences indicated by ANOVA were compared by the Newman-Keuls test. The value of P < 0.05 was considered significant.
RESULTS
30–100 kDa fraction of Mϕ CM causes a significant reduction of EC monolayer resistance
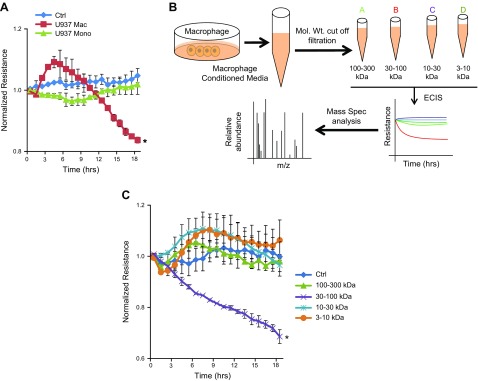
ECIS was used to determine the effect of CM from U937 Mϕs on the permeability of HRECs. U937 Mϕ CM caused a significant reduction (P = 0.007) in the resistance of the HREC monolayer after 18 h in comparison to cells treated with either normal growth medium (Ctrl) or medium from U937 Monos (Fig. 1A). We have previously reported similar results using isolated human Mϕ CM (15). CM from U937 Mϕs was subsequently fractioned using molecular mass cutoff filters, and the individual fractions were used to treat HRECs for permeability effect. These steps are illustrated in Fig. 1B. The barrier-modifying activity was found to be exclusively associated with the 30–100 kDa fraction with a significant decrease in resistance in comparison with Ctrl (P = 0.004) (Fig. 1C).
Figure 1.
Mϕs secrete a factor causing a change in EC monolayer permeability. A) Normalized resistance of HREC monolayers treated with a 1:5 dilution of CM. A significant decrease is seen in the resistance of cells treated with U937 Mϕ CM compared to HRECs treated with Ctrl, or the CM from U937 Monos. *P = 0.007, significantly less than cells in Ctrl. B) Schematic representation of the method used for the preparation of U937 Mono and Mϕ CM. Mass Spec., mass spectrometry; Mol. Wt., molecular mass. C) Normalized resistance of HREC monolayers treated with a 1:5 dilution of fractionated U937 Mϕ CM: 3–10, 10–30, 30–100, and 100–300 kDa. Data are the means ± sd. *P = 0.004, significantly less than cells in Ctrl.
CD is significantly elevated in the 30–100 kDa fraction
The 30–100 kDa CM fraction from the U937 Mϕs was further subjected to tandem mass spectrometry analysis and compared to a 30–100 kDa fraction from U937 Monos, which showed no barrier-modifying activity in the ECIS (data not shown). A number of proteins were significantly increased in the 30–100 kDa fraction from U937 Mϕs (Table 1). Compared to other proteins, CD was abundant, and its role in barrier alteration was further investigated due to the reported role of extracellular CD in the alteration of cell behavior (20).
TABLE 1.
Mass spectroscopy results identified proteins differentially regulated in the CM of PMA-stimulated U937 cell 30–100 kDa fractions compared to nonstimulated cells
| Mass (kDa) | Sequence header | Relative abundance (%) |
|---|---|---|
| 71244.31 | Serum albumin | 9.4 |
| 71082.38 | Heat shock cognate 71 kDa protein | 2.6 |
| 70814.2 | Plastin-2 | 1.0 |
| 45036.84 | CD | 2.6 |
| 46851.29 | Plasminogen activator inhibitor 2 | 0.5 |
| 42828.79 | Leukocyte elastase inhibitor | 0.4 |
| 67144.08 | Apoptosis-inducing factor 1, mitochondrial | 0.1 |
| 38759.59 | Mϕ-capping protein | 0.3 |
| 31602.26 | TNF ligand superfamily member | 0.1 |
| 82880.04 | Dipeptidyl peptidase 3 | 0.1 |
| 83554.27 | Heat shock protein 90-β | 0.1 |
The top relative abundance proteins are listed. The underlined protein and its relative abundance was studied further.
CD causes a significant decrease in resistance in the EC monolayer
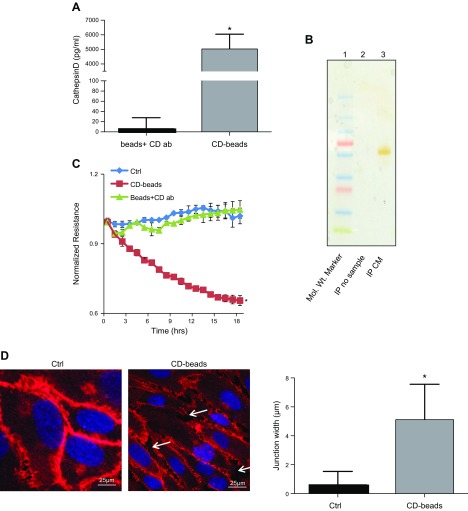
CD was immunoprecipitated from the 30–100 kDa fraction of U937 Mϕs and used to treat HRECs. The presence of CD on the immunoprecipitated agarose beads was confirmed by ELISA (Fig. 2A). The silver-stained gel of the eluted beads is represented in Fig. 2B with a single band in ∼50 kDa in IP CM, and there was no band in the lane in IP no sample. A significant decrease in HREC monolayer resistance was observed in cells treated with CD beads in comparison to HRECs treated with either Ctrl or agarose beads plus CD antibody (beads + CD ab) (P = 0.006; Fig. 2C). The decreased resistance observed in the HREC monolayer following CD bead treatment correlated with a significantly increased gap width of VE-cadherin staining between adjacent HRECs (P = 0.0001; Fig. 2D). We also performed experiments to treat HUVECs with CD beads to check the effect on permeability. However, there was no change in the resistance of the ECs, indicating that this effect was peculiar to retinal ECs only (data not shown).
Figure 2.
The 30–100 kDa fraction contains CD, which alters the EC barrier. A) CD levels associated with agarose beads as measured by ELISA following IP. *P = 0.02, significantly greater than beads plus antibody (ab) without exposure to the 30–100 kDa fraction. B) Silver-stained gel. Mol. Wt. (molecular mass) marker is in lane 1, IP no sample is in lane 2, and IP CM is in lane 3. C) Representative ECIS tracings of HRECs treated with CD beads demonstrate significantly decreased resistance in comparison to cells treated, beads plus CD antibody (ab), or Ctrl. *P = 0.006, significantly less than cells grown in Ctrl. D) Representative image of VE-cadherin immunofluorescence of HRECs grown in Ctrl and in the presence of CD beads. White arrows indicate gaps between adjacent cells treated with CD beads. Quantitation demonstrates significantly increased gap width (micrometers) between adjacent cells. Data are means ± sd. *P = 0.0001, significantly greater than cells grown in Ctrl.
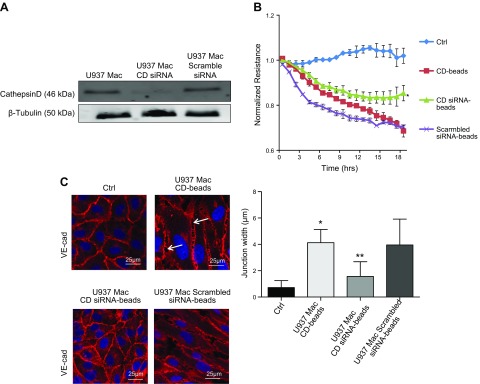
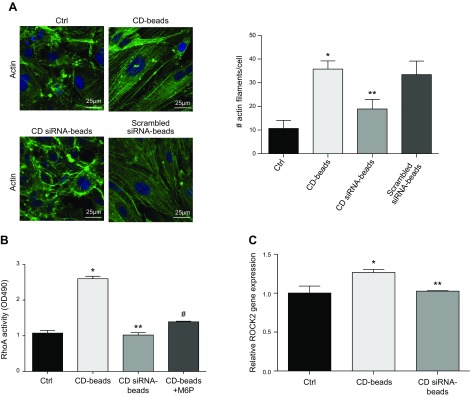
In order to confirm the unique role for CD in barrier alteration, U937 Mϕs were treated with siRNA to knock down CD expression or a scrambled siRNA (Fig. 3A). CM from these cells was immunoprecipitated, and beads were used to treat HRECs. CD beads from siRNA-treated U937 Mϕs induced significantly less barrier-altering activity as compared to those from untreated U937 Mϕs (P = 0.005) or U937 Mϕs treated with a scrambled siRNA (Fig. 3B). The gap width between cells treated with CD siRNA beads demonstrated a significant reduction as compared to CD beads from untreated U937 Mϕs (P = 0.0005), and there was also no significant difference in gap width between the CD beads from untreated U937 Mϕs and scrambled siRNA beads (Fig. 3C).
Figure 3.
CD siRNA inhibits increased permeability induced by activated U937 cells. A) Representative Western blot image of CD and β-tubulin protein in PMA-stimulated U937 cells (U937 Mϕ; Mac), U937 Mϕ treated with CD siRNA, and U937 Mϕ treated with scrambled siRNA. B) The CD beads from U937 Mϕ cells treated with siRNA (siRNA CD beads) caused a significant reduction in the degree of induced HREC barrier alteration as compared to the CD beads from untreated U937 Mϕ cells (CD beads). *P = 0.005, significantly greater than untreated U937 Mϕ cells (CD beads). C) Representative image of VE-cadherin (cad) immunofluorescence in HRECs grown in the presence of CD beads or siRNA CD beads. siRNA CD beads induced fewer gaps between adjacent cells. White arrows indicate gaps between adjacent cells treated with CD beads. *P = 0.0001, significantly greater than cells grown in Ctrl; **P = 0.0005, significantly less than HRECs grown in the presence of CD beads.
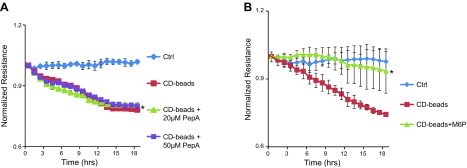
To determine if the effect of the CD beads on the reduction of EC resistance was dependent on the proteolytic activity of the enzyme, we treated cells with CD beads in the presence of the inhibitor pepstatin A (PepA; Fig. 4A). The addition of this inhibitor did not restore monolayer resistance to a normal level, suggesting that the CD beads may be functioning independently of CD enzymatic activity.
Figure 4.
Permeability effect of CD is mediated by an M6P-dependent mechanism. A) ECIS tracings of HRECs treated with CD beads, CD beads with 20 μM PepA, CD beads with 50 μM PepA, or Ctrl. *P = 0.001, significantly less than cells grown in Ctrl. B) ECIS tracings of HRECs treated with CD beads, CD beads plus 100 μM M6P, or Ctrl. *P = 0.02, significantly less than cells treated with CD beads alone.
Addition of M6P inhibits the effects of CD on EC monolayer
Previous studies have reported that CD binds to a cation-independent mannose-6-phosphate receptor (CIMPR) (21–23). To determine if this interaction is involved in the effect of CD on alteration of permeability in HRECs, cells were treated with CD beads along with excess M6P. Addition of M6P (100 μM) significantly inhibited the effect of CD beads on HRECs (P = 0.02) (Fig. 4B). Treated cells demonstrated a normalization of resistance nearly equal to cells treated with Ctrl.
CD enhances endothelial contractility via activation of Rho/ROCK
Past studies have shown that increase in cytoskeletal contractility disrupts endothelial junctions. Because CD bead-treated HRECs exhibited an increase in junctional width, we asked whether this increase in CD-induced endothelial permeability results from an increase in cytoskeletal contractility. To address this question, we stained actin stress fibers in HRECs treated with Ctrl, U937 Mϕ CD beads, or siRNA-treated U937 Mϕ CD beads. HRECs treated with U937 Mϕ CD beads demonstrated a significant increase in actin stress fiber density and thickness as compared to HRECs grown in Ctrl (P < 0.0001) (Fig. 5A). Importantly, this increase in HREC stress fiber density produced by CD beads was significantly inhibited with CD beads obtained from siRNA-treated U937 Mϕs (P = 0.0001), and there was no significant difference between the CD beads from untreated U937 Mϕs and scrambled siRNA beads (Fig. 5A).
Figure 5.
Involvement of Rho/ROCK in permeability alteration and gap formation. A) Representative immunofluorescence images of HRECs stained for stress actin filaments in cells treated with CD beads or siRNA CD beads. Right panel demonstrates the quantitation of the stress actin filaments/cells. No difference was seen in cells treated with scrambled siRNA beads in comparison with CD beads. *P < 0.0001, significantly greater than cells grown in normal medium (Ctrl); **P = 0.0001, significantly less than cells grown in the presence of U937 Mϕ CD beads. B) RhoA activity was significantly increased in HRECs treated with CD beads. This response was dampened by siRNA CD beads or CD beads along with excess of M6P. OD, optical density. *P = 0.004, significantly greater than cells grown in Ctrl; **P = 0.003, significantly less than cells grown in CD beads; #P = 0.05, significantly less than cells grown in CD beads. C) Relative gene expression levels of ROCK2 normalized to glyceraldehyde 3-phosphate dehydrogenase as measured by real-time PCR. Data are means ± sd. *P = 0.007, significantly greater than cells grown in Ctrl; **P = 0.01, significantly less than cells grown in the presence of CD beads.
Because actin stress fibers transmit contractile forces, we looked to see whether CD-induced increase in stress fiber density resulted from an increase in contractility produced by the canonical Rho/ROCK pathway. Indeed, HRECs treated with CD beads were found to exhibit significant increase in RhoA activity, which was not seen in cells treated with beads obtained from CD siRNA-treated Mϕs (Fig. 5B). Notably, this CD-induced RhoA activation in HRECs was also prevented by competitive inhibition of CD with M6P. This potent effect of CD on RhoA activation was further confirmed by measurement of the levels of its downstream effector, ROCK2. Predictably, CD bead-treated ECs exhibited the same trend in ROCK2 as that seen with RhoA activity (Fig. 5C).
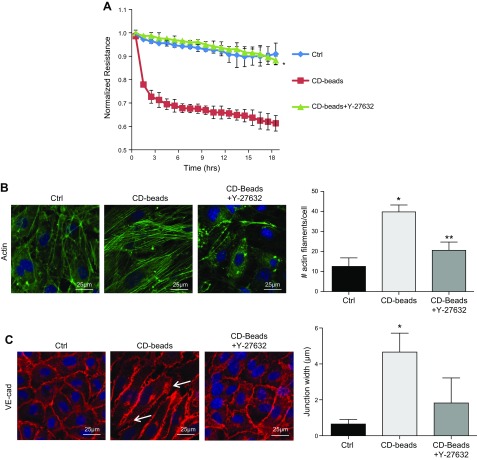
To confirm the role of RhoA/ROCK-dependent contractility in endothelial barrier disruption caused by CD, we treated CD bead-stimulated HRECs with the ROCK inhibitor Y-27632 (25 μM) and monitored barrier resistance using ECIS. Resistance was significantly restored in Y-27632–treated cells as compared to HRECs treated with CD beads alone (P = 0.01) (Fig. 6A). Predictably, this normalization of endothelial barrier resistance by Y-27632 correlated strongly with a reduction in stress actin filament formation and reestablishment of intercellular junctions (Fig. 6B, C).
Figure 6.
ROCK inhibitor Y-27632 prevents CD-induced permeability and intercellular gap formation. A) HRECs treated with CD beads along with the ROCK inhibitor, Y-27632 (25 μM), demonstrate resistance similar to cells grown in Ctrl. *P = 0.01, significantly greater than cells grown in the presence of CD beads. B) There is less stress actin fiber formation in cells treated with the ROCK inhibitor, Y-27632 (25 μM). *P = 0.0001, significantly greater than cells grown in Ctrl; **P = 0.001, significantly less than cells treated with CD beads. C) Intercellular gap formation is decreased in HRECs treated with the ROCK inhibitor, Y-27632 (25 μM). VE-cad, VE-cadherin. Data are represented as means ± sd. *P = 0.0001, significantly greater than cells grown in Ctrl; **P = 0.001, significantly less than cells treated with CD beads.
CD protein is elevated in the retinas of diabetic mice and serum of human patients with DME
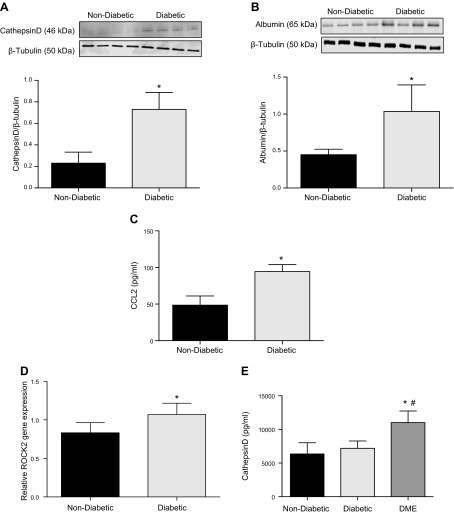
To determine whether CD has any role in vascular permeability in diabetic tissues, we checked the levels of CD in the retinas of streptozotocin diabetic mice and serum samples of human diabetic patients. Retinas of diabetic mice demonstrated significantly increased levels of CD protein as compared to retinas from nondiabetic control animals (P = 0.003) (Fig. 7A). The retinal vascular permeability in diabetic animals was significantly increased compared to nondiabetic animals (P = 0.04) (Fig. 7B). The CCL2 levels in the retinas of diabetic animals were significantly more than the levels in the nondiabetic mice (P = 0.001) (Fig. 7C). Murine diabetic retinas also showed increased mRNA expression of ROCK2 (Fig. 7D). Clinical characteristics of the patients in whom the serum CD levels were measured are included in Table 2. Serum samples from patients with DME had significantly higher levels of CD compared to diabetic patients without retinopathy (P < 0.0001) or normal subjects (P < 0.0001) when measured by ELISA (Fig. 7E). The effect of DME on the CD level still persists even after adjusting for age.
Figure 7.
CD levels are increased in the retinas of diabetic mice and serum of patients with DME. A) Albumin levels in retinal tissue as measured by Western blot in diabetic animals compared with nondiabetic animals. *P = 0.04, significantly greater than nondiabetic animals (n = 4). B) Representative Western blot image of CD and β-tubulin in the retinas of nondiabetic and 4-mo-old diabetic mice. Band density quantitation demonstrates significantly elevated levels of CD protein in the retinas of diabetic mice compared to nondiabetic animals. *P = 0.003, significantly greater than nondiabetic animals (n = 4). C) CCL2 levels measured by ELISA from diabetic mouse retinas were significantly higher than CCL2 levels in the retinas of nondiabetic mice (n = 4). *P = 0.001, significantly greater than nondiabetic mouse retinas. D) ROCK2 gene expression levels, measured by real-time PCR, show significantly increased levels in the retinas of diabetic mice compared to nondiabetic animals (n = 4). *P = 0.03, significantly greater than nondiabetic animals. E) Age-adjusted mean values of CD levels measured by ELISA from human serum samples showed significantly elevated levels in patients with DR (DME) compared to nondiabetic subjects or diabetic subjects without DME (n = 11). Data are represented as means ± sd. *P < 0.0001, significantly greater than nondiabetic subjects; #P < 0.0001, significantly greater than diabetic subjects without DME.
TABLE 2.
Clinical characteristics of the study subjects in whom the serum CD levels were measured
| Clinical parameter | Nondiabetic | Diabetic with no DR | DME |
|---|---|---|---|
| Number of subjects | 11 | 11 | 11 |
| Age (yr) | 52 ± 19 | 57 ± 7 | 61 ± 10 |
| Number with diabetes type 1/2 | 0/0 | 0/11 | 4/7 |
| Number with diabetic nephropathy | 0 | 0 | 2 |
| Number with diabetic neuropathy | 0 | 0 | 3 |
| Number with hypertension | 0 | 7 | 8 |
| Number with coronary artery disease | 0 | 1 | 4 |
DISCUSSION
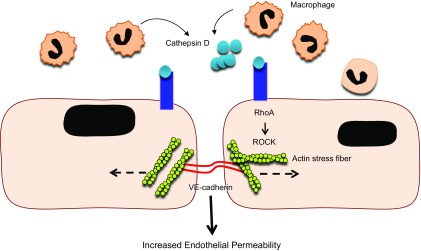
A classic sign of inflammation is recruitment of leukocytes to tissues via inflammatory chemokines and the induction of vascular endothelial activation. Studies including ours have demonstrated a cumulative and sustained increase in leukostasis and Mono trafficking into retina in the early stages of DR (7, 15). Although this local accumulation of leukocytes is implicated in retinal vascular hyperpermeability, the precise mechanism by which leukocytes achieve this effect remains poorly understood. Here, we demonstrate that activated Monos secrete aspartyl proteinase CD that causes mechanical disruption of endothelial junctional barrier through an increase in RhoA/ROCK cell contractility. A conceptual model that summarizes the micromechanical control of CD-induced endothelial hyperpermeability is shown in Fig. 8.
Figure 8.
A schematic diagram highlights the cause and effect of CD in altered vascular permeability in retinal capillaries in diabetes. In diabetes, there is increased Mono trafficking in retinal tissues, and Monos are further differentiated into Mϕs that secrete CD. The CD then binds to the CIMPR on the EC surface, activating the Rho/ROCK pathway, which results in a significant increase in actin stress fiber density and thickness. This leads to EC separation and increased endothelial permeability, and alteration of the BRB.
Activation of human U937 Monos leads to the adoption of an Mϕ phenotype (24). The protease CD is typically found in lysosomes and demonstrates maximal activity in an acidic environment (20, 25, 26). CD produced by activated U937 cells had a profound effect on the integrity of the EC barrier in this study. CD immunoprecipitated from CM of these cells reduced endothelial monolayer resistance in vitro. We were unable to block this effect using an inhibitor of CD (PepA). This suggests that the ability of CD to alter the EC barrier was independent of its proteolytic activity. Indeed, other studies have reported a role for the nonproteolytic form of CD in the progression of cancer and angiogenesis (27–29). A study by Mathieu et al. (30) reported that CD interacted with the surface of breast cancer cells via an M6P-dependent mechanism. A similar effect was not observed in experiments using recombinant-purified CD protein (data not shown). This result may reflect differences in the structure of bacterially produced recombinant protein versus the native protein produced by Mϕs. The possibility exists that additional proteins were bound to the CD and immunoprecipitated, contributing to the modification of the endothelial barrier. However, the presence of a single band following IP and elution makes this possibility unlikely.
CD transport is mediated by the CIMPR (31), otherwise called IGF-II receptor. Both CD and IGF-II can only bind to the CIMPR because only the CIMPR has 2 M6P binding sites (32–35). Our ECIS study showed that binding of CD to the CIMPR was dependent on M6P because cells treated with an excess amount of M6P along with the CD beads prevented the decrease in resistance. Our results are well supported by a previous study (36) that showed that the inhibition of IGF-II induced endothelial progenitor cell chemotaxis by M6P. Further studies are required to positively identify the receptor.
Previous studies from our lab and others have demonstrated a role for proteases in the modification of endothelial intercellular junctions and alterations of monolayer permeability (14, 37–41). In addition, junctional modification and changes in permeability can take place through nonproteolytic mechanisms involving biochemical modification of junction proteins or through physical interactions with the cell cytoskeleton (42–45). In the present study, we have shown that the interaction of CD with ECs induces intercellular gaps between adjacent cells and changes in junctional resistance. Notably, these CD-treated cells exhibited an increase in actin stress fiber formation, elevated levels of RhoA activity, and a concomitant increase in ROCK2 mRNA levels. That increased Rho/ROCK-dependent EC contractility mediates the permeability effects of CD was confirmed when addition of ROCK inhibitor (Y-27632) prevented the CD-induced changes in EC barrier integrity.
CD up-regulation is also associated with tumor growth and progression (46–48). Because tumor vessels are leaky and exhibit high Rho/ROCK activity (49, 50), it is likely that tumor vascular permeability results from CD-dependent increase in Rho/ROCK-dependent EC contractility. Various permeability factors such as TNF-α, TGF-β1, TGF-β, and CCL2 are also known to exert their effects via enhancement of Rho/ROCK-dependent EC contractility (51–55). Taken together, these findings indicate that mechanical cues play a key role in cytokine-induced endothelial barrier disruption and advocate the need to closely examine the crosstalk between biochemical and mechanical signaling pathways in vascular inflammation and hyperpermeability.
Several studies have reported the secretion of proteases from cells including Mϕs, and can be found at high levels in the plasma of patients with breast cancer and diabetes (56–59). In the present study, we detected elevated levels of CD in the retinas of diabetic animals as well as the serum of patients with DME. The level of this protein was higher in patients with DME compared to nondiabetic patients and diabetics without retinal complications. A previous study reported decreased plasma CD levels in patients with DR (60). Again, this study does not specify whether these patients had DME or not. Whether CD may be a useful prognostic factor, or biomarker for early DME, remains to be determined in larger cohort studies.
Although anti-VEGF agents are used as first-line treatment in patients with DME, targeting this molecule alone appears to have limitations. In the Diabetic Retinopathy Clinical Research Protocol I, retinal thickening (>250 μm) was seen to persist in ∼50% of patients with DME, even after 1 yr of treatment with monthly injections of ranibizumab (61). Also, the Restoring Insulin Secretion trial showed an improvement of vision (>3 lines) in only 45% of patients treated with ranibizumab, whereas 55% of patients failed to achieve a similar response (62). Because we observe increased trafficking of Monos and elevated levels of CD in diabetic retinas, it appears that CD may play an important role in alteration of the BRB in DR. Thus, targeting CD alone or in combination with anti-VEGF agents may be used as an alternative novel therapeutic strategy in the treatment of DME.
Acknowledgments
The authors thank the U.S. National Institutes of Health (NIH) National Eye Institute for grant support (RO1 EY022327) and Tamara Howard (NIH) for technical assistance in confocal microscopy and immunofluorescence. The authors declare no conflicts of interest.
Glossary
- BRB
blood-retinal barrier
- CCL2
chemokine (C-C motif) ligand 2
- CD
cathepsin D
- CIMPR
cation-independent mannose-6-phosphate receptor
- CM
conditioned medium
- Ctrl
normal growth medium
- DME
diabetic macular edema
- DR
diabetic retinopathy
- EC
endothelial cell
- ECIS
electric cell-substrate impedance sensing
- FBS
fetal bovine serum
- HREC
human retinal microvascular endothelial cell
- IP
immunoprecipitation
- M6P
mannose-6-phosphate
- Mono
monocyte
- PepA
pepstatin A
- PMA
phorbol 12-myristate 13-acetate
- RhoA
Ras homolog gene family, member A
- ROCK
Rho-associated kinase
- si
small interfering
- VE-cadherin
vascular endothelial cadherin
REFERENCES
- 1.Meta-Analysis for Eye Disease (META-EYE) Study Group (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35, 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang J., Kern T. S. (2011) Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 30, 343–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern T. S. (2007) Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp. Diabetes Res. 2007, 95103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangasamy S., McGuire P. G., Das A. (2012) Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr. J Ophthalmol. 19, 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamis A. P. (2002) Is diabetic retinopathy an inflammatory disease? Br. J. Ophthalmol. 86, 363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell E. D., Field R. A. (1964) Diabetic retinopathy and rheumatoid arthritis. Lancet 284, 17–18 [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto K., Khosrof S., Bursell S. E., Rohan R., Murata T., Clermont A. C., Aiello L. P., Ogura Y., Adamis A. P. (1999) Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. USA 96, 10836–10841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schröder S., Palinski W., Schmid-Schönbein G. W. (1991) Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am. J. Pathol. 139, 81–100 [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch F. C., Miyamoto K., Allport J. R., Fujita K., Bursell S. E., Aiello L. P., Luscinskas F. W., Adamis A. P. (2000) Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest. Ophthalmol. Vis. Sci. 41, 1153–1158 [PubMed] [Google Scholar]
- 10.Wang J., Xu X., Elliott M. H., Zhu M., Le Y. Z. (2010) Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 59, 2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Wang J. J., Chen D., Mott R., Yu Q., Ma J. X., Zhang S. X. (2009) Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp. Eye Res. 89, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami T., Felinski E. A., Antonetti D. A. (2009) Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem. 284, 21036–21046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harhaj N. S., Felinski E. A., Wolpert E. B., Sundstrom J. M., Gardner T. W., Antonetti D. A. (2006) VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest. Ophthalmol. Vis. Sci. 47, 5106–5115 [DOI] [PubMed] [Google Scholar]
- 14.Navaratna D., McGuire P. G., Menicucci G., Das A. (2007) Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 56, 2380–2387 [DOI] [PubMed] [Google Scholar]
- 15.Rangasamy S., McGuire P. G., Franco Nitta C., Monickaraj F., Oruganti S. R., Das A. (2014) Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One 9, e108508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh J., Nishimura N., Rana K., Peloquin J. M., Califano J. P., Montague C. R., King M. R., Schaffer C. B., Reinhart-King C. A. (2011) Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 3, 112ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroka K. M., Aranda-Espinoza H. (2011) Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood 118, 1632–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mammoto A., Mammoto T., Ingber D. E. (2008) Rho signaling and mechanical control of vascular development. Curr. Opin. Hematol. 15, 228–234 [DOI] [PubMed] [Google Scholar]
- 19.Tang J., Allen Lee C., Du Y., Sun Y., Pearlman E., Sheibani N., Kern T. S. (2013) MyD88-dependent pathways in leukocytes affect the retina in diabetes. PLoS One 8, e68871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liaudet-Coopman E., Beaujouin M., Derocq D., Garcia M., Glondu-Lassis M., Laurent-Matha V., Prébois C., Rochefort H., Vignon F. (2006) Cathepsin D: newly discovered functions of a long-standing aspartic protease in cancer and apoptosis. Cancer Lett. 237, 167–179 [DOI] [PubMed] [Google Scholar]
- 21.Kornfeld S. (1990) Lysosomal enzyme targeting. Biochem. Soc. Trans. 18, 367–374 [DOI] [PubMed] [Google Scholar]
- 22.Figura K. V., Hasilik A. (1986) Lysosomal enzymes and their receptors. Annu. Rev. Biochem. 55, 167–193 [DOI] [PubMed] [Google Scholar]
- 23.Benes P., Vetvicka V., Fusek M. (2008) Cathepsin D—many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 68, 12–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Reyes K., Bravo-Cuellar A., Hernández-Flores G., Lerma-Díaz J. M., Jave-Suárez L. F., Gómez-Lomelí P., de Celis R., Aguilar-Lemarroy A., Domínguez-Rodríguez J. R., Ortiz-Lazareno P. C. (2014) Cervical cancer cell supernatants induce a phenotypic switch from U937-derived macrophage-activated M1 state into M2-like suppressor phenotype with change in Toll-like receptor profile. BioMed Res. Int. 2014, 683068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper J. B. (2002) Aspartic proteinases in disease: a structural perspective. Curr. Drug Targets 3, 155–173 [DOI] [PubMed] [Google Scholar]
- 26.De Duve C. (1983) Lysosomes revisited. Eur. J. Biochem. 137, 391–397 [DOI] [PubMed] [Google Scholar]
- 27.Rochefort H., Liaudet-Coopman E. (1999) Cathepsin D in cancer metastasis: a protease and a ligand. APMIS 107, 86–95 [DOI] [PubMed] [Google Scholar]
- 28.Glondu M., Coopman P., Laurent-Matha V., Garcia M., Rochefort H., Liaudet-Coopman E. (2001) A mutated cathepsin-D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene 20, 6920–6929 [DOI] [PubMed] [Google Scholar]
- 29.Berchem G., Glondu M., Gleizes M., Brouillet J. P., Vignon F., Garcia M., Liaudet-Coopman E. (2002) Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene 21, 5951–5955 [DOI] [PubMed] [Google Scholar]
- 30.Mathieu M., Rochefort H., Barenton B., Prebois C., Vignon F. (1990) Interactions of cathepsin-D and insulin-like growth factor-II (IGF-II) on the IGF-II/mannose-6-phosphate receptor in human breast cancer cells and possible consequences on mitogenic activity of IGF-II. Mol. Endocrinol. 4, 1327–1335 [DOI] [PubMed] [Google Scholar]
- 31.Shiba Y., Kametaka S., Waguri S., Presley J. F., Randazzo P. A. (2013) ArfGAP3 regulates the transport of cation-independent mannose 6-phosphate receptor in the post-Golgi compartment. Curr. Biol. 23, 1945–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hancock M. K., Haskins D. J., Sun G., Dahms N. M. (2002) Identification of residues essential for carbohydrate recognition by the insulin-like growth factor II/mannose 6-phosphate receptor. J. Biol. Chem. 277, 11255–11264 [DOI] [PubMed] [Google Scholar]
- 33.Marron-Terada P. G., Hancock M. K., Haskins D. J., Dahms N. M. (2000) Recognition of Dictyostelium discoideum lysosomal enzymes is conferred by the amino-terminal carbohydrate binding site of the insulin-like growth factor II/mannose 6-phosphate receptor. Biochemistry 39, 2243–2253 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt B., Kiecke-Siemsen C., Waheed A., Braulke T., von Figura K. (1995) Localization of the insulin-like growth factor II binding site to amino acids 1508-1566 in repeat 11 of the mannose 6-phosphate/insulin-like growth factor II receptor. J. Biol. Chem. 270, 14975–14982 [DOI] [PubMed] [Google Scholar]
- 35.Garmroudi F., Devi G., Slentz D. H., Schaffer B. S., MacDonald R. G. (1996) Truncated forms of the insulin-like growth factor II (IGF-II)/mannose 6-phosphate receptor encompassing the IGF-II binding site: characterization of a point mutation that abolishes IGF-II binding. Mol. Endocrinol. 10, 642–651 [DOI] [PubMed] [Google Scholar]
- 36.Maeng Y. S., Choi H. J., Kwon J. Y., Park Y. W., Choi K. S., Min J. K., Kim Y. H., Suh P. G., Kang K. S., Won M. H., Kim Y. M., Kwon Y. G. (2009) Endothelial progenitor cell homing: prominent role of the IGF2-IGF2R-PLCbeta2 axis. Blood 113, 233–243 [DOI] [PubMed] [Google Scholar]
- 37.Covington M. D., Burghardt R. C., Parrish A. R. (2006) Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14). Am. J. Physiol. Renal Physiol. 290, F43–F51 [DOI] [PubMed] [Google Scholar]
- 38.Covington M. D., Bayless K. J., Burghardt R. C., Davis G. E., Parrish A. R. (2005) Ischemia-induced cleavage of cadherins in NRK cells: evidence for a role of metalloproteinases. Am. J. Physiol. Renal Physiol. 289, F280–F288 [DOI] [PubMed] [Google Scholar]
- 39.Noë V., Fingleton B., Jacobs K., Crawford H. C., Vermeulen S., Steelant W., Bruyneel E., Matrisian L. M., Mareel M. (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J. Cell Sci. 114, 111–118 [DOI] [PubMed] [Google Scholar]
- 40.Murphy F., Waung J., Collins J., Arthur M. J., Nagase H., Mann D., Benyon R. C., Iredale J. P. (2004) N-Cadherin cleavage during activated hepatic stellate cell apoptosis is inhibited by tissue inhibitor of metalloproteinase-1. Comp. Hepatol. 3(Suppl 1), S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuire J. K., Li Q., Parks W. C. (2003) Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am. J. Pathol. 162, 1831–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelhardt S., Al-Ahmad A. J., Gassmann M., Ogunshola O. O. (2014) Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J. Cell. Physiol. 229, 1096–1105 [DOI] [PubMed] [Google Scholar]
- 43.Murakami T., Frey T., Lin C., Antonetti D. A. (2012) Protein kinase cβ phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes 61, 1573–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Itallie C. M., Fanning A. S., Bridges A., Anderson J. M. (2009) ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 20, 3930–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner J. R., Rill B. K., Carlson S. L., Carnes D., Kerner R., Mrsny R. J., Madara J. L. (1997) Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 273, C1378–C1385 [DOI] [PubMed] [Google Scholar]
- 46.Allgayer H., Babic R., Grützner K. U., Beyer B. C., Tarabichi A., Wilhelm Schildberg F., Heiss M. M. (1997) An immunohistochemical assessment of cathepsin D in gastric carcinoma: its impact on clinical prognosis. Cancer 80, 179–187 [DOI] [PubMed] [Google Scholar]
- 47.Lösch A., Schindl M., Kohlberger P., Lahodny J., Breitenecker G., Horvat R., Birner P. (2004) Cathepsin D in ovarian cancer: prognostic value and correlation with p53 expression and microvessel density. Gynecol. Oncol. 92, 545–552 [DOI] [PubMed] [Google Scholar]
- 48.Masson O., Bach A. S., Derocq D., Prébois C., Laurent-Matha V., Pattingre S., Liaudet-Coopman E. (2010) Pathophysiological functions of cathepsin D: targeting its catalytic activity versus its protein binding activity? Biochimie 92, 1635–1643 [DOI] [PubMed] [Google Scholar]
- 49.Ghosh K., Thodeti C. K., Dudley A. C., Mammoto A., Klagsbrun M., Ingber D. E. (2008) Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc. Natl. Acad. Sci. USA 105, 11305–11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagy J. A., Dvorak A. M., Dvorak H. F. (2007) VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2, 251–275 [DOI] [PubMed] [Google Scholar]
- 51.Mong P. Y., Petrulio C., Kaufman H. L., Wang Q. (2008) Activation of Rho kinase by TNF-alpha is required for JNK activation in human pulmonary microvascular endothelial cells. J. Immunol. 180, 550–558 [DOI] [PubMed] [Google Scholar]
- 52.Lu Q., Harrington E. O., Jackson H., Morin N., Shannon C., Rounds S. (2006) Transforming growth factor-beta1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J. Appl. Physiol. 101, 375–384 [DOI] [PubMed] [Google Scholar]
- 53.Clements R. T., Minnear F. L., Singer H. A., Keller R. S., Vincent P. A. (2005) RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L294–L306 [DOI] [PubMed] [Google Scholar]
- 54.Stamatovic S. M., Dimitrijevic O. B., Keep R. F., Andjelkovic A. V. (2006) Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J. Biol. Chem. 281, 8379–8388 [DOI] [PubMed] [Google Scholar]
- 55.Sprague A. H., Khalil R. A. (2009) Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 78, 539–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hakala J. K., Oksjoki R., Laine P., Du H., Grabowski G. A., Kovanen P. T., Pentikäinen M. O. (2003) Lysosomal enzymes are released from cultured human macrophages, hydrolyze LDL in vitro, and are present extracellularly in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 23, 1430–1436 [DOI] [PubMed] [Google Scholar]
- 57.Brouillet J. P., Dufour F., Lemamy G., Garcia M., Schlup N., Grenier J., Mani J. C., Rochefort H. (1997) Increased cathepsin D level in the serum of patients with metastatic breast carcinoma detected with a specific pro-cathepsin D immunoassay. Cancer 79, 2132–2136 [PubMed] [Google Scholar]
- 58.Ahmad J., Zubair M., Malik A., Siddiqui M. A., Wangnoo S. K. (2012) Cathepsin-D, adiponectin, TNF-α, IL-6 and hsCRP plasma levels in subjects with diabetic foot and possible correlation with clinical variables: a multicentric study. Foot 22, 194–199 [DOI] [PubMed] [Google Scholar]
- 59.Feron D., Begu-Le Corroller A., Piot J. M., Frelicot C., Vialettes B., Fruitier-Arnaudin I. (2009) Significant lower VVH7-like immunoreactivity serum level in diabetic patients: evidence for independence from metabolic control and three key enzymes in hemorphin metabolism, cathepsin D, ACE and DPP-IV. Peptides 30, 256–261 [DOI] [PubMed] [Google Scholar]
- 60.Chen Y. H., Chou H. C., Lin S. T., Chen Y. W., Lo Y. W., Chan H. L. (2012) Effect of high glucose on secreted proteome in cultured retinal pigmented epithelium cells: its possible relevance to clinical diabetic retinopathy. J. Proteomics 77, 111–128 [DOI] [PubMed] [Google Scholar]
- 61.Diabetic Retinopathy Clinical Research Network (2010) Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology 117, 946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.RISE and RIDE Research Group (2012) Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119, 789–801 [DOI] [PubMed] [Google Scholar]