Abstract
Background
It remains unclear what the antiviral therapy affects disease-free survival (DFS) and overall survival (OS) of patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) at different tumor stages and baseline HBV DNA levels. In this study, we analyzed the association of antiviral treatment with DFS and OS based on the stratification of baseline HBV DNA load in early-stage (stages I and II) HCC patients.
Methods
We included 445 patients with early-stage HBV-related HCC who underwent curative resection, and then classified them into four subgroups based on baseline HBV DNA load and antiviral therapy stratification. The Kaplan–Meier and Cox regression analyses were performed to determine the association of clinical characteristics with survival.
Results
The median follow-up period was 74 months. For all patients, cumulative OS rates in the antiviral group were significantly higher than those in the non-antiviral group (log-rank test, P = 0.023), whereas no significant differences in DFS rates were observed. High baseline HBV DNA level was a risk factor associated with short DFS and OS in all patients. In patients with baseline HBV DNA levels ≥2000 IU/mL, antiviral treatment was significantly associated with prolonged DFS and OS (log-rank test, P = 0.041 and 0.001, respectively). In patients with HBV DNA levels <2000 IU/mL or undetectable, antiviral treatment did not show a significant benefit in prolonging DFS and OS.
Conclusions
High baseline HBV DNA levels are associated with poor prognosis in the patients with early-stage HCC, and the antiviral treatment could generate survival benefits for the patients. Therefore, antiviral treatment should be given for these patients. However, the effect of antiviral treatment on the patients with low viral load remains unclear, and further investigation is warranted.
Keywords: Hepatocellular carcinoma, Resection, Hepatitis B virus, Prognosis, Antiviral therapy
Background
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh most common cancer in women, and it is the third most common cause of cancer-related death worldwide [1]. The 2010 incidence and mortality of liver cancer in China were among high levels worldwide [2]. Most HCCs develop within an established background of chronic liver disease, and the most common risk factor for HCC is hepatitis B virus (HBV) infection [3]. High levels of serum HBV DNA are associated with an increased risk of developing HCC [4], and nomograms based on clinical characteristics, including serum hepatitis B e antigen (HBeAg) status and HBV DNA level, can predict the risk of developing HCC [5]. Additionally, in HBV-related HCC, high levels of serum HBV DNA appear to be associated with poor prognosis [6–8]. Therefore, careful management of HBV infection is needed in the management of HBV-related HCC [9].
Hepatic resection has been the standard curative treatment for HCC. However, a high rate of tumor recurrence has been reported after surgery [10]. So far, no effective adjuvant treatment option has been proven to reduce the risk of recurrence [11]. Recently, some studies demonstrated that antiviral therapy with nucleotide/nucleoside analogs (NAs) was associated with prolonged disease-free survival (DFS) and overall survival (OS) after hepatectomy of HBV-related HCC, suggesting that antiviral treatment may offer clinical benefits in HCC patients with HBV infection [12–15]. However, most of these studies did not stratify the patients into specific subgroups based on tumor stages or HBV DNA levels, and the results of these studies were contradictory. Early-stage tumors tend to be associated with a better prognosis than intermediate-stage tumors, and antiviral treatment may confer a significant survival benefit compared with non-antiviral treatment [16, 17]. Another issue that should be considered is the HBV DNA load. Whether it is necessary to give antiviral treatment to HCC patients with low or even undetectable HBV DNA levels remains controversial [18, 19]. So far, no consensus on this issue exists, and the indications for antiviral treatment in patients with HBV-related HCC remain uncertain.
In this retrospective study, we used strict classification of patients into subgroups, comparison of DFS and OS, and multivariate analysis to (1) evaluate the effect of HBV DNA on the prognosis of patients with early-stage [stages I and II; Union for International Cancer Control (UICC), 7th edition] HCC who underwent curative therapy and (2) analyze the effect of antiviral treatment on DFS and OS based on the stratification of baseline HBV DNA load.
Patients and methods
Patients
This retrospective cohort study in a tertiary academic hospital was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center in Guangdong, China. We included patients who were initially diagnosed with hepatitis B surface antigen (HBsAg)-positive HCC and underwent curative resection between January 2005 and December 2008 at the Hepatobiliary Department of Sun Yat-sen University Cancer Center. Prior to obtaining tissues, written informed consent was obtained from each patient.
Preoperative evaluation
As described previously [20], baseline examinations (within 10 days before surgery), including serum HBV DNA quantification, detection of HBsAg, and liver function tests, were routinely performed. Serum HBV viral loads were measured by using a quantitative fluorescence polymerase chain reaction detection kit (DaAn Gene Corporation, Guangzhou, China) with a lower detection limit of 200 IU/mL. Dynamic contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) were used to assess the intrahepatic tumor. The preoperative diagnosis of HCC was based on the criteria established by the European Association for the study of the liver [21].
Inclusion criteria
To be included in our study, patients had to meet the following criteria: (1) ≥18 years old, (2) hepatic resection for initial HCC treatment, (3) UICC TNM stage T1N0M0 or T2N0M0, (4) curative treatment (pathologic confirmation of negative resection margin) and no local recurrence within 3 months after surgery, (5) HBsAg positivity, (6) no anti-HBV treatment before surgery, (7) adequate baseline liver function (Child-Pugh grade A) and adequate renal function (serum creatinine <124 µmol/L), and (8) no tumor rupture before or during surgery.
Exclusion criteria
Patients who met any of the following criteria were excluded: (1) HBsAg negativity, (2) evidence of co-infection with other hepatotropic viruses or human immunodeficiency virus, (3) any prior treatment for HCC, (4) Child-Pugh grade B or C, (5) presence of other malignancy or concurrent non-malignant severe illness, (6) tumor stage beyond T2N0M0, (7) recurrence or metastasis within 3 months after surgery, (8) interferon administration after surgery, (9) initiation of NA administration after tumor recurrence, and (10) severe complications or adverse events (including postoperative mortality) within 3 months after surgery.
Tumor characteristics
The diagnosis of HCC was pathologically confirmed after surgery, and the histological differentiation of the tumors were graded from I to IV based on the Edmondson–Steiner classification [22]. Tumor number, maximal tumor size, tumor differentiation, tumor capsulation, the presence of microvascular invasion, and surgical margin were evaluated. Tumor characteristics and UICC TNM stage were comprehensively evaluated.
Definition of curative resection
None of the cases had major vascular invasion. Curative resection was defined as complete removal of all macroscopically evident tumors [15, 23]. The absence of tumor cells along the parenchymal transection line was confirmed histologically. The CT or MRI examination performed 3 months after surgery showed no remaining tumor.
Follow-up protocol
After discharge, each patient received regular examinations and follow-up at 3-month intervals until 3 years after resection, and at 4- to 6-month intervals thereafter. Follow-up ended on December 31, 2014. Serum liver function tests, alpha-fetoprotein (AFP) tests, chest X-ray radiography, abdominal ultrasonography, and CT/MRI scans were the routine examinations to determine possible recurrence or metastasis. Hepatic angiography, ultrasonic contrast, positron emission tomography/computed tomography, and ultrasound-guided biopsy were performed when necessary.
Statistical analysis
Demographic data [mean, standard deviation, median (P25, P75), and percentage] were calculated. Analyses were conducted using the independent Student’s t test (Mann–Whitney test for non-normal distributions), analysis of variance (Kruskal–Wallis test for non-normal distributions), and the Chi square test, as appropriate. The Kaplan–Meier method and the log-rank test were used for survival analysis. DFS was the time calculated from the date of curative surgery to HCC recurrence, death, or the last follow-up; OS was the time calculated from the date of surgery to death or the last follow-up. The Cox proportional hazards model was used in the univariate survival analysis to determine the association of individual clinical variables with OS or DFS. All variables with P < 0.1 were subsequently subjected to the multivariate Cox regression model to determine the hazard ratios (HRs) and the independence of effects. P < 0.05 were considered statistically significant. All statistical tests were two-sided. Data were analyzed using the SAS 9.1 software (SAS Institute, Cary, NC, USA).
Results
General description
During the study period, 729 HCC patients underwent hepatic resection as their initial treatment at Sun Yat-sen University Cancer Center. According to our inclusion and exclusion criteria, 284 patients were excluded, and the remaining 445 patients were enrolled. The patient flow diagram is shown in Fig. 1.
Fig. 1.
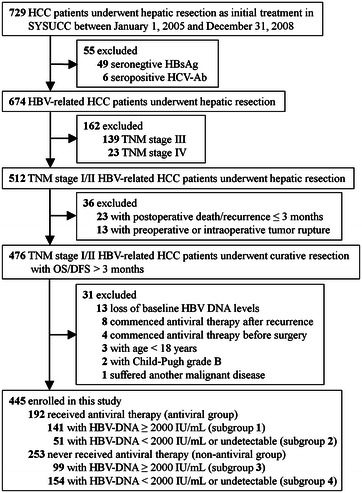
Selection of patients with early stage HBV-related HCC undergoing curative resection. HCC hepatocellular carcinoma, SYSUCC Sun Yat-sen University Cancer Center, HBsAg hepatitis B surface antigen, HCV-Ab hepatitis C virus antibody, TNM tumor-nodes-metastasis, OS overall survival time, DFS disease-free survival time, HBV hepatitis B virus
In total, 192 patients received oral administration of NAs after surgery, with a median antiviral time of 79 months (P25, 47 months; P75, 87 months). Of these, 93, 84, 13, and 2 patients took entecavir, lamivudine, adefovir dipivoxil, and telbivudine as their initial antiviral agent, respectively. During the follow-up, 13 patients received sequential treatments due to partial HBV response or HBV resistance. The common treatment regimens involved lamivudine followed by entecavir-switch (four patients) and adefovir add-on (six patients). Other regimens included adefovir followed by entecavir-switch (two patients) and entecavir add-on (one patient).
Based on the stratification of baseline HBV DNA and antiviral therapy, the patients were classified into four subgroups: (1) subgroup 1, antiviral therapy with baseline HBV DNA ≥2000 IU/mL (n = 141); (2) subgroup 2, antiviral therapy with baseline HBV DNA <2000 IU/mL or undetectable (n = 51); (3) subgroup 3, no antiviral therapy with baseline HBV DNA ≥2000 IU/mL (n = 99); and (4) subgroup 4, no antiviral therapy with baseline HBV DNA <2000 IU/mL or undetectable (n = 154).
Table 1 summarizes the characteristics of the antiviral group and non-antiviral group. Most variables were similar between these two groups. However, there were significant differences in HBeAg, HBV DNA, alanine aminotransferase (ALT), and serum total bilirubin levels, which may be explained by the fact that, in this retrospective study, patients with higher HBV replication or more severe liver damage tended to be treated with antiviral therapy. The median follow-up period was 74 months. Of the 445 patients, 235 (52.8 %) experienced tumor recurrence, and 141 (31.7 %) died during the follow-up period. The 1-, 3-, 5-, 7-, and 10-year OS rates in all study patients were 96.1 %, 82.6 %, 73.4 %, 65.6 %, and 62.8 %, respectively, whereas DFS rates were 79.8 %, 59.8 %, 51.2 %, 43.3 %, and 42.2 %, respectively (Fig. 2).
Table 1.
Comparison of clinicopathologic characteristics between antiviral and non-antiviral groups
| Characteristic | Antiviral group (n = 192) | Non-antiviral group (n = 253) | P value |
|---|---|---|---|
| Age (years) | 0.372a | ||
| Mean ± SDb | 47.9 ± 10.3 | 48.8 ± 11.8 | |
| Median and rangeb | 47 (24–76) | 49 (23–79) | |
| <50 | 111 | 128 | |
| ≥50 | 81 | 125 | |
| Gender | 0.278 | ||
| Male | 172 | 218 | |
| Female | 20 | 35 | |
| HBeAg | 0.003 | ||
| Positive | 48 | 35 | |
| Negative | 144 | 218 | |
| HBV DNA (IU/mL) | <0.001 | ||
| <2000 | 51 | 154 | |
| ≥2000 | 141 | 99 | |
| Tumor size (cm) | 0.012a | ||
| Mean ± SDb | 4.7 ± 3.1 | 5.4 ± 3.2 | |
| Rangeb | 1.0–25.0 | 1.0–18.0 | |
| <5 | 122 | 121 | |
| ≥5 | 70 | 132 | |
| Tumor number | 0.990 | ||
| Single | 176 | 232 | |
| Multiple | 16 | 21 | |
| Pathologic grade | 0.961 | ||
| I | 12 | 21 | |
| II | 121 | 150 | |
| III | 58 | 80 | |
| IV | 1 | 2 | |
| Microvascular thrombus | 0.054 | ||
| Yes | 25 | 19 | |
| No | 167 | 234 | |
| Tumor capsule | 0.271 | ||
| Complete | 87 | 112 | |
| Incomplete | 60 | 66 | |
| Without | 45 | 75 | |
| TNM stage | 0.083 | ||
| I | 151 | 215 | |
| II | 41 | 38 | |
| AFP (ng/mL) | 0.881 | ||
| ≤25 | 78 | 101 | |
| >25 | 114 | 152 | |
| ALT (IU/L) | 0.002a | ||
| Mean ± SDb | 49.8 ± 30.3 | 41.9 ± 24.6 | |
| Rangeb | 13.1–209.2 | 8.5–163.0 | |
| ≤40 | 83 | 150 | |
| >40 | 109 | 103 | |
| AST (IU/L) | 0.115a | ||
| Mean ± SDb | 41.6 ± 25.2 | 38.2 ± 20.4 | |
| Rangeb | 8.0–263.0 | 15.0–168.0 | |
| ≤45 | 138 | 189 | |
| >45 | 54 | 64 | |
| ALB (g/L) | 0.485a | ||
| Mean ± SDb | 43.1 ± 3.6 | 42.8 ± 4.0 | |
| Rangeb | 29.3–52.5 | 27.8–51.6 | |
| <35 | 3 | 8 | |
| ≥35 | 189 | 245 | |
| TBIL (μmol/L) | 0.035a | ||
| Mean ± SDb | 15.9 ± 7.3 | 14.6 ± 5.6 | |
| Rangeb | 4.6–63.3 | 5.0–47.2 | |
| ≤20.5 | 156 | 217 | |
| >20.5 | 36 | 36 | |
| Prothrombin time (s) | 0.215a | ||
| Mean ± SDb | 12.7 ± 1.5 | 12.5 ± 1.3 | |
| Rangeb | 9.6–19.1 | 9.4–17.4 | |
| ≤13.5 | 139 | 207 | |
| >13.5 | 53 | 46 | |
| Recurrence | 0.489 | ||
| Yes | 105 | 130 | |
| No | 87 | 123 | |
| Death | 0.043 | ||
| Yes | 51 | 90 | |
| No | 141 | 163 | |
Antiviral group resection plus postsurgical antiviral treatment group, Non-antiviral group resection alone group, HBV hepatitis B virus, SD standard deviation, HBeAg hepatitis B e antigen, TNM tumor-node-metastasis, AFP alpha-fetoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, ALB serum albumin, TBIL serum total bilirubin
a t test used
b Except for these values, other values are presented as the number of patients and were compared by the χ2 test
Fig. 2.
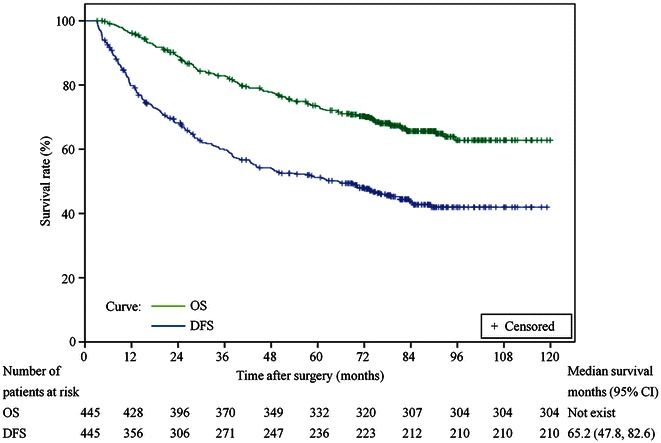
Kaplan–Meier survival curves for overall survival (OS) and disease-free survival (DFS) in patients who underwent curative resection (n = 445). CI confidence interval
OS and DFS analysis for all patients
OS
Patients in the antiviral group had higher OS rates than patients in the non-antiviral group (P = 0.023; Fig. 3a). The 1-, 3-, 5-, 7-, and 10-year OS rates in the antiviral group were 96.4 %, 86.9 %, 77.6 %, 71.3 %, and 71.3 %, respectively, whereas the corresponding rates in the non-antiviral group were 96.0 %, 78.8 %, 69.5 %, 61.4 %, and 56.9 %, respectively. Univariate analysis revealed that HBeAg, HBV DNA level, tumor size, tumor number, pathologic differentiation grade, microvascular thrombus, aspartate aminotransferase (AST) level, albumin (ALB) level, antiviral treatment, and recurrence were associated with OS (Table 2). On multivariate analysis, high baseline HBV DNA, multiple tumors, high pathologic grade, presence of microvascular thrombus, low ALB level, no antiviral treatment, and recurrence were independent risk factors that associated with short OS (Table 2).
Fig. 3.
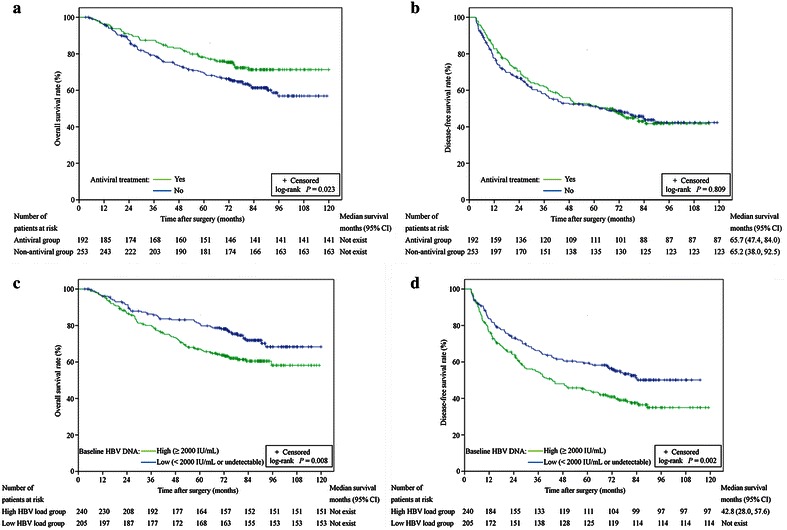
Kaplan–Meier survival curves of patients with various baseline HBV levels and use of nucleotide/nucleoside analogs (NAs). a OS rates between the antiviral and non-antiviral groups. b DFS rates between the antiviral and non-antiviral groups. c OS rates between patients with baseline serum HBV DNA levels of ≥2000 and <2000 IU/mL or undetectable. d DFS rates between patients with baseline serum HBV DNA levels of ≥2000 and <2000 IU/mL or undetectable
Table 2.
Relationship between clinical characteristics and overall survival (OS)/disease-free survival (DFS), as determined by univariate and multivariate Cox regression analysis
| Variable | OS | DFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR | P value | HR (95 % CI) | P value | HR | P value | HR (95 % CI) | P value | |
| Age (≥50 vs. <50 years) | 1.320 | 0.101 | 1.116 | 0.401 | ||||
| Gender (female vs. male) | 0.951 | 0.845 | 0.921 | 0.684 | ||||
| HBeAg (positive vs. negative) | 1.582 | 0.020 | Not significant | 1.403 | 0.034 | Not significant | ||
| HBV DNA (≥2000 vs. <2000 IU/mL) | 1.580 | 0.009 | 1.615 (1.126–2.317) | 0.009 | 1.511 | 0.002 | 1.496 (1.150–1.944) | 0.003 |
| Tumor size (≥5 vs. <5 cm) | 1.745 | 0.001 | Not significant | 1.687 | <0.001 | 1.868 (1.432–2.435) | <0.001 | |
| Tumor number (multiple vs. single) | 2.347 | <0.001 | 1.930 (1.196–3.113) | 0.007 | 1.961 | 0.001 | 2.437 (1.607–3.696) | <0.001 |
| Pathologic grade (IV/III/II/I) | 1.413 | 0.014 | 1.412 (1.064–1.875) | 0.017 | 1.172 | 0.145 | ||
| Microvascular thrombus (yes vs. no) | 2.076 | 0.002 | 2.339 (1.456–3.759) | <0.001 | 1.315 | 0.188 | ||
| Tumor capsule (without/incomplete/complete) | 1.123 | 0.244 | 0.950 | 0.520 | ||||
| AFP (>25 vs. ≤25 ng/mL) | 1.181 | 0.335 | 1.079 | 0.568 | ||||
| ALT (>40 vs. ≤40 IU/L) | 1.249 | 0.188 | 1.212 | 0.141 | ||||
| AST (>45 vs. ≤45 IU/L) | 1.510 | 0.023 | Not significant | 1.597 | 0.001 | Not significant | ||
| ALB (≥35 vs. <35 g/L) | 0.358 | 0.008 | 0.383 (0.176–0.833) | 0.015 | 0.661 | 0.250 | ||
| TBIL (>20.5 vs. ≤20.5 μmol/L) | 1.407 | 0.097 | Not significant | 1.227 | 0.222 | |||
| Prothrombin time (>13.5 vs. ≤13.5 s) | 1.051 | 0.799 | 1.011 | 0.942 | ||||
| Antiviral treatment (yes vs. no) | 0.672 | 0.024 | 0.516 (0.359–0.743) | <0.001 | 0.969 | 0.809 | ||
| Recurrence (yes vs. no) | 7.785 | <0.001 | 7.208 (4.418–11.760) | <0.001 | ||||
CI confidence interval, HR hazard ratio, Antiviral group resection plus postsurgical antiviral treatment group, Non-antiviral group resection alone group, HBV hepatitis B virus, SD standard deviation, HBeAg hepatitis B e antigen, TNM tumor-node-metastasis, AFP alpha-fetoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, ALB serum albumin, TBIL serum total bilirubin
DFS
The 1-, 3-, 5-, 7-, and 10-year DFS rates in the antiviral group were 82.8 %, 61.9 %, 51.5 %, 41.8 %, and 41.8 %, respectively, whereas the corresponding rates in the non-antiviral group were 77.6 %, 57.7 %, 51.0 %, 43.8 %, and 42.3 %, respectively. No significant difference in DFS rates were observed between these two groups (P = 0.809; Fig. 3b). Univariate analysis showed that HBeAg, HBV DNA level, tumor size, tumor number, and AST level were associated with DFS (Table 2). On multivariate analysis, high baseline HBV DNA, tumor size larger than 5 cm, and multiple tumors were independent risk factors that associated with short DFS.
Baseline HBV DNA levels as a risk factor for both OS and DFS
Kaplan–Meier analysis revealed that the OS rates in patients with baseline HBV DNA <2000 IU/mL or undetectable were significantly higher than those in patients with HBV DNA levels ≥2000 IU/ml (P = 0.008; Fig. 3c). A similar result was found in the analysis of DFS rates (P = 0.002; Fig. 3d). Univariate and multivariate analyses demonstrated that high baseline HBV DNA was a risk factor for short DFS and OS (Table 2).
Analysis based on the stratification of baseline HBV DNA and antiviral therapy
We stratified all patients by the levels of baseline HBV DNA between the antiviral and non-antiviral groups and evaluated the association of antiviral treatment with long-term prognosis in each stratum. Table 3 summarizes the characteristics of these four subgroups.
Table 3.
Comparison of clinicopathologic characteristics among the subgroups
| Variable | Subgroup 1 (n = 141) |
Subgroup 2 (n = 51) |
Subgroup 3 (n = 99) |
Subgroup 4 (n = 154) |
P 1 | P 2 | P 3 | P 4 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.290a | 0.042a | 0.108a | 0.526a | ||||
| Mean ± SDb | 48.4 ± 10.6 | 46.6 ± 9.6 | 50.7 ± 11.9 | 47.6 ± 11.7 | ||||
| <50 | 78 | 33 | 41 | 87 | ||||
| ≥50 | 63 | 18 | 58 | 67 | ||||
| Gender | 0.216 | 0.527 | 0.988 | 0.092 | ||||
| Males | 124 | 48 | 87 | 131 | ||||
| Females | 17 | 3 | 12 | 23 | ||||
| HBeAg | 0.011 | <0.001 | 0.441 | 0.360 | ||||
| Positive | 42 | 6 | 25 | 10 | ||||
| Negative | 99 | 45 | 74 | 144 | ||||
| Tumor size (cm) | 0.233a | 0.922a | 0.158a | 0.020a | ||||
| Mean ± SDb | 4.8 ± 3.1 | 4.2 ± 2.8 | 5.4 ± 3.1 | 5.5 ± 3.3 | ||||
| <5 | 86 | 36 | 45 | 76 | ||||
| ≥5 | 55 | 15 | 54 | 78 | ||||
| Tumor number | 0.460 | 0.194 | 0.278 | 0.360 | ||||
| Single | 131 | 45 | 88 | 144 | ||||
| Multiple | 10 | 6 | 11 | 10 | ||||
| Pathologic grade | 0.561 | 0.375 | 0.498 | 0.444 | ||||
| I | 10 | 2 | 12 | 9 | ||||
| II | 85 | 36 | 56 | 94 | ||||
| III | 45 | 13 | 30 | 50 | ||||
| IV | 1 | 0 | 1 | 1 | ||||
| Microvascular thrombus | 0.756 | 0.210 | 0.430 | 0.273 | ||||
| Yes | 19 | 6 | 10 | 9 | ||||
| No | 122 | 45 | 89 | 145 | ||||
| Tumor capsule (cases) | 0.083 | 0.729 | 0.056 | 0.648 | ||||
| Complete | 64 | 23 | 43 | 69 | ||||
| Incomplete | 49 | 11 | 24 | 42 | ||||
| Without | 28 | 17 | 32 | 43 | ||||
| Liver cirrhosis | 0.017 | 0.738 | 0.852 | 0.012 | ||||
| Yes | 101 | 45 | 72 | 109 | ||||
| No | 40 | 6 | 27 | 45 | ||||
| AFP (ng/mL) | 0.216 | 0.514 | 0.897 | 0.523 | ||||
| ≤25 | 61 | 17 | 42 | 59 | ||||
| >25 | 80 | 34 | 57 | 95 | ||||
| ALT (IU/L) | 0.052a | <0.001a | 0.485a | 0.220a | ||||
| Mean ± SDb | 52.4 ± 29.3 | 42.8 ± 32.1 | 49.8 ± 26.0 | 36.8 ± 22.3 | ||||
| ≤40 | 53 | 30 | 46 | 104 | ||||
| >40 | 88 | 21 | 53 | 50 | ||||
| AST (IU/L) | 0.009a | 0.008a | 0.508a | 0.596a | ||||
| Mean ± SDb | 44.4 ± 27.1 | 33.7 ± 16.8 | 42.4 ± 17.6 | 35.5 ± 21.6 | ||||
| ≤45 | 96 | 42 | 62 | 127 | ||||
| >45 | 45 | 9 | 37 | 27 | ||||
| ALB (g/L) | 0.028a | 0.092a | 0.372a | 0.168a | ||||
| Mean ± SDb | 42.8 ± 3.5 | 44.1 ± 3.7 | 42.3 ± 4.0 | 43.2 ± 3.9 | ||||
| <35 | 2 | 1 | 95 | 150 | ||||
| ≥35 | 139 | 50 | 4 | 4 | ||||
| TBIL (μmol/L) | 0.931a | 0.878a | 0.114a | 0.193a | ||||
| Mean ± SDb | 16.0 ± 7.8 | 15.9 ± 5.8 | 14.5 ± 5.4 | 14.6 ± 5.8 | ||||
| ≤20.5 | 116 | 40 | 84 | 133 | ||||
| >20.5 | 25 | 11 | 15 | 21 | ||||
| Prothrombin time (s) | 0.929a | 0.182a | 0.824a | 0.345a | ||||
| Mean ± SDb | 12.7 ± 1.5 | 12.7 ± 1.6 | 12.6 ± 1.3 | 12.4 ± 1.2 | ||||
| ≤13.5 | 101 | 38 | 76 | 131 | ||||
| >13.5 | 40 | 13 | 23 | 23 | ||||
| Recurrence | 0.535 | 0.001 | 0.180 | 0.312 | ||||
| Yes | 79 | 26 | 64 | 66 | ||||
| No | 62 | 25 | 35 | 88 | ||||
| Death | 0.189 | 0.001 | 0.731 | 0.276 | ||||
| Yes | 41 | 10 | 48 | 42 | ||||
| No | 100 | 41 | 51 | 112 | ||||
Definition of the four subgroups: (1) subgroup 1, antiviral therapy and baseline HBV DNA ≥ 2000 IU/mL (n = 141); (2) subgroup 2, antiviral therapy and baseline HBV DNA <2000 IU/mL or undetectable (n = 51); (3) subgroup 3, non-antiviral therapy and baseline HBV DNA ≥2000 IU/mL (n = 99); and (4) subgroup 4, non-antiviral therapy and baseline HBV DNA <2000 IU/mL or undetectable (n = 154)
P 1 P value calculated by comparing subgroup 1 and subgroup 2, P 2 P value calculated by comparing subgroup 3 and subgroup 4, P 3 P value calculated by comparing subgroup 1 and subgroup 3, P 4 P value calculated by comparing subgroup 2 and subgroup 4, Antiviral group resection plus postsurgical antiviral treatment group, Non-antiviral group resection alone group, HBV hepatitis B virus, SD standard deviation, HBeAg hepatitis B e antigen, TNM tumor-node-metastasis, AFP alpha-fetoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, ALB serum albumin, TBIL serum total bilirubin
a t test used
b Except for these values, other values are presented as the number of patients and were compared by the χ2 test
The association between baseline HBV DNA and the prognosis of the non-antiviral group (n = 253)
For the 253 patients in the non-antiviral group (subgroups 3 and 4), Kaplan–Meier analysis showed that higher baseline HBV DNA levels were associated with lower DFS and OS rates (Fig. 4a for OS analysis, P = 0.001; Fig. 4b for DFS analysis, P < 0.001). Univariate and multivariate Cox models showed that high baseline HBV DNA along with some tumor characteristics (multiple tumors, tumor size larger than 5 cm, and presence of microvascular thrombus) were risk factors for short DFS and OS in the non-antiviral group (Table 4).
Fig. 4.
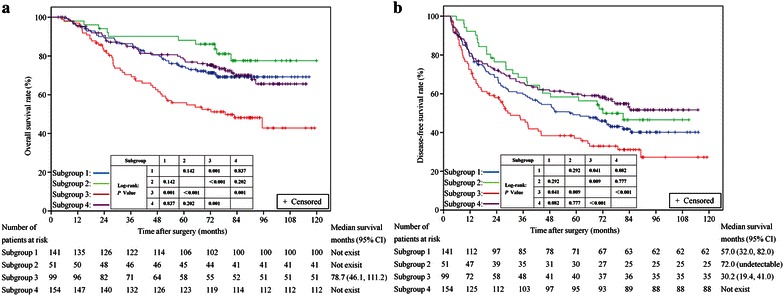
Kaplan–Meier survival curves of patients in antiviral and non-antiviral subgroups after stratification with baseline HBV DNA loads. a OS rates among the four subgroups of patients (log-rank test: subgroup 3 vs. subgroup 1, P = 0.001; subgroup 3 vs. subgroup 2, P < 0.001; subgroup 3 vs. subgroup 4, P = 0.001; subgroup 1 vs. subgroup 2, P = 0.142; subgroup 1 vs. subgroup 4, P = 0.837; subgroup 2 vs. subgroup 4, P = 0.202). b DFS rates among the four subgroups of patients (subgroup 3 vs. subgroup 1, P = 0.041; subgroup 3 vs. subgroup 2, P = 0.009; subgroup 3 vs. subgroup 4, P = 0.000; subgroup 1 vs. subgroup 2, P = 0.292; subgroup 1 vs. subgroup 4, P = 0.082; subgroup 2 vs. subgroup 4, P = 0.777). [Note: (1) Subgroup 1, antiviral treatment with baseline hepatitis B virus (HBV) DNA ≥2000 IU/mL (n = 141); (2) Subgroup 2, antiviral treatment with baseline HBV DNA <2000 IU/mL or undetectable (n = 51); (3) Subgroup 3, no antiviral treatment with baseline HBV DNA ≥2000 IU/mL (n = 99); and (4) Subgroup 4, no antiviral treatment with baseline HBV DNA <2000 IU/mL or undetectable (n = 154).]
Table 4.
Relationship between clinical characteristics and OS/DFS in patients without antiviral treatment (n = 253), as determined by univariate and multivariate Cox regression analysis
| Variable | OS | DFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR | P value | HR (95 % CI) | P value | HR | P value | HR (95 % CI) | P value | |
| Age (≥50 vs. <50 years) | 1.341 | 0.169 | 0.933 | 0.693 | ||||
| Gender (female vs. male) | 0.891 | 0.710 | 0.827 | 0.465 | ||||
| HBeAg (positive vs. negative) | 1.681 | 0.054 | Not significant | 1.716 | 0.019 | Not significant | ||
| HBV DNA (≥2000 vs. <2000 IU/mL) | 2.056 | 0.001 | 1.589 (1.042–2.422) | 0.031 | 1.840 | 0.001 | 1.705 (1.204–2.415) | 0.003 |
| Tumor size (≥5 vs. <5 cm) | 1.820 | 0.006 | 1.689 (1.087–2.624) | 0.020 | 1.655 | 0.005 | 1.713 (1.195–2.455) | 0.003 |
| Tumor number (multiple vs. single) | 2.393 | 0.004 | 2.259 (1.223–4.172) | 0.009 | 2.227 | 0.004 | 2.571 (1.470–4.499) | 0.001 |
| Pathologic grades (IV/III/II/I) | 1.357 | 0.075 | Not significant | 1.288 | 0.074 | Not significant | ||
| Microvascular thrombus (yes vs. no) | 2.915 | 0.001 | 2.587 (1.345–4.978) | 0.004 | 1.969 | 0.021 | 1.192 (1.066–3.429) | 0.030 |
| Tumor capsule (without/incomplete/complete) | 1.028 | 0.825 | 0.880 | 0.226 | ||||
| AFP (>25 vs. ≤25 ng/mL) | 1.252 | 0.302 | 1.064 | 0.728 | ||||
| ALT (>40 vs. ≤40 IU/L) | 1.539 | 0.041 | Not significant | 1.375 | 0.072 | Not significant | ||
| AST (>45 vs. ≤45 IU/L) | 1.803 | 0.009 | Not significant | 1.687 | 0.006 | Not significant | ||
| ALB (≥35 vs. <35 g/L) | 0.423 | 0.061 | Not significant | 0.643 | 0.291 | |||
| TBIL (>20.5 vs. ≤20.5 μmol/L) | 0.919 | 0.780 | 1.126 | 0.618 | ||||
| Prothrombin time (>13.5 vs. ≤13.5 s) | 1.146 | 0.593 | 1.015 | 0.945 | ||||
| Recurrence (yes vs. no) | 6.671 | <0.001 | 5.442 (3.091–9.582) | <0.001 | ||||
OS overall survival, DFS disease-free survival, CI confidence interval, HR hazard ratio, Antiviral group resection plus postsurgical antiviral treatment group, Non-antiviral group resection alone group, HBV hepatitis B virus, SD standard deviation, HBeAg hepatitis B e antigen, TNM tumor-node-metastasis, AFP alpha-fetoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, ALB serum albumin, TBIL serum total bilirubin
The effect of antiviral treatment on the prognosis of patients with high baseline HBV DNA (≥2000 IU/mL)
In the antiviral group with high baseline HBV DNA, the OS rate was significantly higher than that of the non-antiviral group (Fig. 4a, subgroup 1 vs. subgroup 3, P = 0.001). Both univariate and multivariate analyses identified antiviral treatment as a risk factor linked to OS (Table 5). As to the analysis of DFS, antiviral treatment conferred a significantly higher DFS rate (Fig. 4b, subgroup 1 vs. subgroup 3, P = 0.041). On univariate analysis, tumor size, tumor number, AST level, and antiviral treatment were significantly associated with DFS. On multivariate analysis, tumor size, tumor number, and AST level were independent risk factors linked to DFS, whereas antiviral treatment was not an independent protective factor of DFS in the model (Table 5).
Table 5.
Relationship between clinical characteristics and OS/DFS in patients with high HBV DNA level (≥2000 IU/mL), as determined by univariate and multivariate Cox regression analysis
| Variable | OS | DFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR | P value | HR (95 % CI) | P value | HR | P value | HR (95 % CI) | P value | |
| Age (≥50 vs. <50 years) | 1.329 | 0.186 | 1.384 | 0.055 | Not significant | |||
| Gender (female vs. male) | 1.087 | 0.795 | 0.914 | 0.737 | ||||
| HBeAg (positive vs. negative) | 1.407 | 0.132 | 1.216 | 0.290 | ||||
| Tumor size (≥5 vs. <5 cm) | 1.618 | 0.024 | Not significant | 1.618 | 0.004 | 1.650 (1.162–2.342) | 0.005 | |
| Tumor number (multiple vs. single) | 2.175 | 0.010 | Not significant | 1.796 | 0.028 | 2.104 (1.218–3.635) | 0.008 | |
| Pathologic grades (IV/III/II/I) | 1.365 | 0.070 | 1.613 (1.129–2.305) | 0.009 | 1.100 | 0.476 | ||
| Microvascular thrombus (yes vs. no) | 2.038 | 0.010 | 2.688 (1.534–4.710) | 0.001 | 1.128 | 0.640 | ||
| Tumor capsule (without/incomplete/complete) | 1.201 | 0.148 | 1.030 | 0.771 | ||||
| AFP (>25 vs. ≤25 ng/mL) | 1.291 | 0.240 | 1.030 | 0.863 | ||||
| ALT (>40 vs. ≤40 IU/L) | 1.064 | 0.774 | 1.088 | 0.621 | ||||
| AST (>45 vs. ≤45 IU/L) | 1.657 | 0.018 | Not significant | 1.688 | 0.002 | 1.486 (1.057–2.090) | 0.023 | |
| ALB (≥35 vs. <35 g/L) | 0.294 | 0.008 | Not significant | 0.633 | 0.316 | |||
| TBIL (>20.5 vs. ≤20.5 μmol/L) | 1.566 | 0.077 | Not significant | 1.098 | 0.672 | |||
| Prothrombin time (>13.5 vs. ≤13.5 s) | 0.867 | 0.557 | 0.814 | 0.283 | ||||
| Antiviral treatment (yes vs. no) | 0.499 | 0.001 | 0.475 (0.309–0.729) | 0.001 | 0.710 | 0.042 | Not significant | 0.120 |
| Recurrence (yes vs. no) | 9.514 | <0.001 | 9.954 (4.781–20.725) | <0.001 | ||||
OS overall survival, DFS disease-free survival, CI confidence interval, HR hazard ratio, Antiviral group resection plus postsurgical antiviral treatment group, Non-antiviral group resection alone group, HBV hepatitis B virus, SD standard deviation, HBeAg hepatitis B e antigen, TNM tumor-node-metastasis, AFP alpha-fetoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, ALB serum albumin, TBIL serum total bilirubin
The effect of antiviral treatment on the prognosis of patients with low (<2000 IU/mL) or undetectable baseline HBV DNA
Kaplan–Meier analysis showed no significant difference in OS rates between antiviral and non-antiviral treatment groups in patients with low or even undetectable baseline HBV DNA (Fig. 4a, subgroup 2 vs. subgroup 4, P = 0.202). Univariate analysis identified the following factors as significantly associated with OS: tumor size, tumor number, and recurrence. On multivariate analysis, only recurrence was an independent risk factor linked to OS (Table 6). As to the analysis of DFS, no significant difference was observed in DFS rates between the antiviral and non-antiviral groups in patients with low or even undetectable baseline HBV DNA (Fig. 4b, subgroup 2 vs. subgroup 4, P = 0.777). Both univariate and multivariate analyses showed that tumor number and tumor size were prognostic factors related to DFS (Table 6). Antiviral treatment was not a risk factor related to OS or DFS in the analysis.
Table 6.
Relationship between clinical characteristics and OS/DFS in patients with undetectable or low baseline HBV DNA levels (<2000 IU/mL), as determined by univariate and multivariate Cox regression analysis
| Variable | OS | DFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR | P value | HR (95 % CI) | P value | HR | P value | HR (95 % CI) | P value | |
| Age (≥50 vs. <50 years) | 1.173 | 0.566 | 0.750 | 0.183 | ||||
| Gender (female vs. male) | 0.819 | 0.645 | 0.963 | 0.902 | ||||
| HBeAg (positive vs. negative) | 1.321 | 0.554 | 1.407 | 0.330 | ||||
| Tumor size (≥5 or <5 cm) | 2.013 | 0.013 | Not significant | 1.772 | 0.006 | 1.997 (1.295–3.044) | 0.002 | |
| Tumor number (multiple vs. single) | 2.612 | 0.013 | Not significant | 2.194 | 0.015 | 2.761 (1.440–5.296) | 0.002 | |
| Pathologic grades (IV/III/II/I) | 1.535 | 0.073 | Not significant | 1.346 | 0.100 | Not significant | ||
| Microvascular thrombus (yes vs. no) | 1.902 | 0.140 | 1.561 | 0.205 | ||||
| Tumor capsule (without/incomplete/complete) | 1.046 | 0.784 | 0.873 | 0.284 | ||||
| AFP (>25 vs. ≤25 ng/mL) | 1.084 | 0.777 | 1.230 | 0.345 | ||||
| ALT (>40 vs. ≤40 IU/L) | 1.219 | 0.487 | 1.115 | 0.618 | ||||
| AST (>45 vs. ≤45 IU/L) | 0.820 | 0.626 | 1.107 | 0.712 | ||||
| ALB (≥35 vs. <35 g/L) | 0.490 | 0.324 | 0.712 | 0.562 | ||||
| TBIL (>20.5 vs. ≤20.5 μmol/L) | 1.167 | 0.661 | 1.439 | 0.158 | ||||
| Prothrombin time (>13.5 vs. ≤13.5 s) | 1.296 | 0.432 | 1.272 | 0.342 | ||||
| Antiviral treatment (yes vs. no) | 0.641 | 0.206 | 1.068 | 0.777 | ||||
| Recurrence (yes vs. no) | 5.859 | <0.001 | 5.859 (3.007–11.416) | <0.001 | ||||
OS overall survival, DFS disease-free survival, CI confidence interval, HR hazard ratio, Antiviral group resection plus postsurgical antiviral treatment group, Non-antiviral group resection alone group, HBV hepatitis B virus, SD standard deviation, HBeAg hepatitis B e antigen, TNM tumor-node-metastasis, AFP alpha-fetoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, ALB serum albumin, TBIL serum total bilirubin
Patients with high HBV DNA load and no antiviral treatment had the worst prognosis
Kaplan–Meier analysis showed that subgroup 3 had a significantly lower OS rate compared with the other three subgroups (subgroup 3 vs. subgroup 1, P = 0.001; subgroup 3 vs. subgroup 2, P = 0.000; subgroup 3 vs. subgroup 4, P = 0.001). Among subgroups 1, 2, and 4, the differences were not significant (Fig. 4a).
As for DFS, similarly, subgroup 3 had a significantly lower DFS rate compared with the other three subgroups (subgroup 3 vs. subgroup 1, P = 0.041; subgroup 3 vs. subgroup 2, P = 0.009; subgroup 3 vs. subgroup 4, P = 0.000). Subgroup 4 appeared to have a higher DFS rate than subgroup 1, but the difference was not significant (P = 0.082). As to the comparisons between subgroups 2 and 4 and between subgroups 1 and 2, the differences were not significant (Fig. 4b).
Discussion
In the present study, we found that baseline HBV DNA level was a significant prognostic factor that influenced both OS and DFS of early-stage HCC patients undergoing curative resection, which was consistent with the findings of most previous studies [24–27]. The precise mechanism for recurrent carcinogenesis in patients with high HBV DNA levels remains unclear. It is possible that active viral replication may contribute to hepatocarcinogenesis by direct and indirect pathways [28]. Clearly, recurrence tends to shorten OS of cancer patients. One study showed that sustained low HBV load below 2000 IU/mL was a strong protective factor for long-term DFS and OS after curative resection in HBV-related HCC [23]. In addition, the effect of NAs differs in patients with different tumor stages [16, 29]. Therefore, HBV DNA level, tumor stage, and liver function should be considered before administration of NAs [18].
In the present study, no significant differences were observed in DFS rates between the antiviral and non-antiviral groups, whereas the OS rates were higher in the antiviral group than in the non-antiviral group. Treatment was an independent prognostic factor related to OS, which is consistent with the results of a prospective-retrospective study carried out in Hong Kong [30]. However, other studies reported that antiviral therapy reduced the risk of recurrence of HCC after curative therapy [14, 15]. The discrepancy in the results regarding tumor recurrence could be due to different study populations (including tumor stage) and study designs. As to the effect of antiviral therapy on OS, there are several possible explanations. First, antiviral treatment could reduce the risk of HBV reactivation, and subsequent liver dysfunction after surgery, which would reduce perioperative liver-related mortality [20, 31]. Second, better liver function preservation at the time of recurrence could translate into a high proportion of patients receiving more aggressive curative or palliative treatment at the time of recurrence to extend OS [30, 32]. Third, although not well defined, antiviral therapy can decrease the hepatitis activity caused by HBV, leading to a reduced risk of late-phase recurrence of HCC, which can therefore increase the OS rate [33].
In the stratification analyses, antiviral treatment was associated with better prognosis (both OS rate and DFS rate) in patients with high baseline HBV DNA levels. However, in patients with low or undetectable baseline HBV DNA levels, no significant difference was found in either DFS rate or OS rate between the antiviral and non-antiviral groups. In this population, univariate and multivariate analysis did not identify antiviral treatment as a risk factor for DFS or OS. A previous study demonstrated that patients with persistently low HBV DNA levels had better survival results compared with those with high or fluctuating HBV DNA levels [23]. Although the stratification in our study was based on the evaluation of baseline HBV DNA at resection, most (135/154, 87.6 %) of the HBV DNA levels in subgroup 4 remained stable during the follow-up period (data not shown). In 33.1 % (51/154) of the patients, serum HBV DNA was even undetectable. The results indicated that HCC patients with low or undetectable HBV DNA load may not significantly benefit from antiviral therapy as patients with high HBV DNA load did.
However, some weaknesses could be noted in our study. First, the proportion of para-tumorous liver cirrhosis was higher in subgroup 2 (Table 3), which may be explained by the fact that patients with cirrhosis were more likely to be treated with NAs. Second, the mean tumor size in subgroup 2 was smaller than that in subgroup 4, which might have positively affected the outcome of the antiviral group. Third, administration of NAs was not randomized, which may have caused selection bias. In addition, for the OS curves, a small gap was observed between these two subgroups (subgroups 2 and 4), although no significant difference existed. Therefore, the results should be interpreted very carefully.
So far, no consensus exists on the indications of antiviral therapy for HCC patients with low HBV DNA load. Many guidelines have recommended NAs for patients with chronic HBV infection, evidence of active viral replication (≥2000 IU/mL), and elevated levels of ALT [18]. It remains controversial whether HCC patients with low HBV replication should be given antiviral therapy. In a study aimed to analyze the different prognosis after hepatectomy of HBV-related HCC with or without cirrhosis, the results revealed that antiviral treatment was an independent predictor for prolonged OS in HCC patients with cirrhosis [34]. However, in patients without cirrhosis, antiviral therapy did not significantly associated with either prolonged OS or prolonged DFS after radical resection, which indicated that HCC patients with cirrhosis benefit more from antiviral therapy than do those without cirrhosis. In a two-stage longitudinal clinical study, the investigators found that antiviral therapy reduced HCC recurrence in patients with a low HBV load [13]. However, that study included patients with early Barcelona clinical liver cancer (BCLC) stage (0 to A) or intermediate stage (B), and the percentage of BCLC B stage in the non-antiviral group was nearly twice that of the antiviral group. Furthermore, several imbalances were observed between the antiviral and non-antiviral groups, including tumor encapsulation, tumor differentiation, and AFP [13]. Thus, the findings are not conclusive. Most recently, several studies showed that high levels of HBsAg were associated with short survival and early recurrence after surgery for HCC patients with low HBV viral loads [35–37], which may provide a clue for selecting suitable candidates for antiviral therapy from HCC patients with low viral load. However, further investigation is needed to determine the magnitude of benefit and to clarify whether NAs should be administered to all or only a subset of patients (such as patients with cirrhosis or high levels of HBsAg) after curative treatment for HBV-related HCC [33].
Our study also showed that DFS rate tended to be higher in the non-antiviral subgroup with low or undetectable HBV DNA level than in the antiviral subgroup with high HBV DNA levels, although the OS rate was similar. Cho et al. [38] recently found that patients with active chronic hepatitis B (CHB) who were receiving long-term NA treatment had a higher incidence of HCC compared with patients with inactive CHB. One possible explanation is that the inactive CHB group may have had more intact immune response to HBV and, therefore, the HBV infection may have entered the inactive stage early in the patient’s life. However, in patients with high viral replication and active hepatitis, HBV DNA integration into hepatocytes that resulted in genomic alterations and/or chromosomal instability may have already occurred before initiation of NA treatment. This may explain why, in our study, patients in subgroup 4 had a higher DFS rate than patients in subgroup 1 who were treated with NAs after surgery. However, a more detailed investigation is needed to confirm these results.
In the present retrospective study, we included only the patients with TNM stage I and II disease who had normal liver function before surgery (Child-Pugh grade A); we excluded those who experienced severe complications shortly after surgery. We evaluated only patients who were likely to receive curative treatment and tolerate it well. Therefore, the conclusions may not be generalizable to all HCC patients undergoing surgery. During the treatment period between January 2005 and December 2008, whether it was beneficial to give antiviral therapy to HBV-related HCC patients who underwent resection was controversial at our institution. At that time, the major liver society guidelines for CHB only recommended that patients who had persistently elevated ALT levels more than two times the upper limit of normal should be considered for antiviral treatment. Decisions on antiviral treatment were made on a case-by-case basis, which may have caused selection bias. Therefore, prospective large-scale trials, with hepatitis B and cirrhotic HCC patients at various stages, will be required in the future to clarify the effects of antiviral therapy on survival and HCC recurrence [39].
In conclusion, high baseline HBV DNA levels were associated with poor prognosis. For the patients with high HBV replication, antiviral treatment significantly improved patients’ long-term prognoses, including DFS and OS. However, for the patients with low or even undetectable HBV DNA levels, whether the use of NAs is beneficial remains unclear and requires further investigation.
Authors’ contributions
JLC, XJL, and XML conceived the project. JLC and XML collected the clinical data of patients. QZ and XML performed statistical analysis. JLC and XML wrote the manuscript. MS, SPL, XML, and XJL performed surgery and analyzed the data. SPL participated in the study design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Our study was partly supported by project grants from the National Natural Science Foundation of China (no. 81201603 for Dr. XML), the Guangdong Natural Science Funds (No. S2012010009631 for Dr. XML), and the International Program for Ph.D. Candidates from Sun Yat-sen University (for Dr. JLC).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Jian-Lin Chen and Xiao-Jun Lin contributed equally to this work
Contributor Information
Jian-Lin Chen, Email: chenjianl@sysucc.org.cn.
Xiao-Jun Lin, Email: linxj@sysucc.org.cn.
Qian Zhou, Email: zhouq49@mail.sysu.edu.cn.
Ming Shi, Email: shiming@sysucc.org.cn.
Sheng-Ping Li, Email: lishp@sysucc.org.cn.
Xiang-Ming Lao, Phone: +86-20-87343828, Email: laoxming@mail.sysu.edu.cn.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Wei KR, Yu X, Zheng RS, Peng XB, Zhang SW, Ji MF, et al. Incidence and mortality of liver cancer in China, 2010. Chin J Cancer. 2014;33(8):388–394. doi: 10.5732/cjc.014.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30(1):3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Yang HI, Sherman M, Su J, Chen PJ, Liaw YF, Iloeje UH, et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Clin Oncol. 2010;28(14):2437–2444. doi: 10.1200/JCO.2009.27.4456. [DOI] [PubMed] [Google Scholar]
- 6.Shao YY, Chen PJ, Lin ZZ, Huang CC, Ding YH, Lee YH, et al. Impact of baseline hepatitis B viral DNA levels on survival of patients with advanced hepatocellular carcinoma. Anticancer Res. 2011;31(11):4007–4011. [PubMed] [Google Scholar]
- 7.Jang JW, Choi JY, Bae SH, Yoon SK, Woo HY, Chang UI, et al. The impact of hepatitis B viral load on recurrence after complete necrosis in patients with hepatocellular carcinoma who receive transarterial chemolipiodolization: implications for viral suppression to reduce the risk of cancer recurrence. Cancer. 2007;110(8):1760–1767. doi: 10.1002/cncr.22984. [DOI] [PubMed] [Google Scholar]
- 8.Yang T, Lu JH, Zhai J, Lin C, Yang GS, Zhao RH, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol. 2012;38(8):683–691. doi: 10.1016/j.ejso.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Wong GL. How does hepatitis B virus infection react to hepatocellular carcinoma treatment? J Gastroenterol Hepatol. 2012;27(1):1–2. doi: 10.1111/j.1440-1746.2011.06950.x. [DOI] [PubMed] [Google Scholar]
- 10.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi: 10.1016/S0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 11.Liccioni A, Reig M, Bruix J. Treatment of hepatocellular carcinoma. Dig Dis. 2014;32(5):554–563. doi: 10.1159/000360501. [DOI] [PubMed] [Google Scholar]
- 12.Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015;261(1):56–66. doi: 10.1097/SLA.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 13.Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31(29):3647–3655. doi: 10.1200/JCO.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 14.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 15.Chuma M, Hige S, Kamiyama T, Meguro T, Nagasaka A, Nakanishi K, et al. The influence of hepatitis B DNA level and antiviral therapy on recurrence after initial curative treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2009;44(9):991–999. doi: 10.1007/s00535-009-0093-z. [DOI] [PubMed] [Google Scholar]
- 16.Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, et al. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146(6):675–681. doi: 10.1001/archsurg.2011.125. [DOI] [PubMed] [Google Scholar]
- 17.Ke Y, Ma L, You XM, Huang SX, Liang YR, Xiang BD, et al. Antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after radical hepatectomy. Cancer Biol Med. 2013;10(3):158–164. doi: 10.7497/j.issn.2095-3941.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong JH. Nucleos(t)ide analogue therapy for HBV-related HCC after hepatic resection: clinical benefits and unanswered questions. Tumour Biol. 2014;35(12):12779–12784. doi: 10.1007/s13277-014-2881-1. [DOI] [PubMed] [Google Scholar]
- 19.Lao XM, Xia HH, Lin XJ, Li SP. Antiviral therapy in patients with hepatitis B virus-related hepatocellular carcinoma: is it ready for universal application? J Viral Hepat. 2013;20(12):e148–e149. doi: 10.1111/jvh.12147. [DOI] [PubMed] [Google Scholar]
- 20.Lao XM, Luo G, Ye LT, Luo C, Shi M, Wang D, et al. Effects of antiviral therapy on hepatitis B virus reactivation and liver function after resection or chemoembolization for hepatocellular carcinoma. Liver Int. 2013;33(4):595–604. doi: 10.1111/liv.12112. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the study of the liver. J Hepatol. 2001;35(3):421–430. doi: 10.1016/S0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 22.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi: 10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.An HJ, Jang JW, Bae SH, Choi JY, Cho SH, Yoon SK, et al. Sustained low hepatitis B viral load predicts good outcome after curative resection in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(12):1876–1882. doi: 10.1111/j.1440-1746.2010.06416.x. [DOI] [PubMed] [Google Scholar]
- 24.Qu LS, Jin F, Huang XW, Shen XZ. High hepatitis B viral load predicts recurrence of small hepatocellular carcinoma after curative resection. J Gastrointest Surg. 2010;14(7):1111–1120. doi: 10.1007/s11605-010-1211-1. [DOI] [PubMed] [Google Scholar]
- 25.Kim BK, Park JY, Kim do Y Kim JK, Kim KS, Choi JS, et al. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008;28(3):393–401. doi: 10.1111/j.1478-3231.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 26.Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103(7):1663–1673. doi: 10.1111/j.1572-0241.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 27.Kubo S, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Yamamoto T, et al. Effect of viral status on recurrence after liver resection for patients with hepatitis B virus-related hepatocellular carcinoma. Cancer. 2000;88(5):1016–1024. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1016::AID-CNCR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Qu LS, Liu JX, Kuai XL, Xu ZF, Jin F, Zhou GX. Significance of viral status on recurrence of hepatitis B-related hepatocellular carcinoma after curative therapy: a meta-analysis. Hepatol Res. 2014;44(7):750–760. doi: 10.1111/hepr.12172. [DOI] [PubMed] [Google Scholar]
- 29.Zhou ZG, Zheng XR, Zhou Q, Shi M, Zhang YJ, Guo RP, et al. Impact of oral anti-hepatitis B therapy on the survival of patients with hepatocellular carcinoma initially treated with chemoembolization. Chin J Cancer. 2015;34(5):205–216. doi: 10.1186/s40880-015-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong CC, Wong GL, Wong VW, Ip PC, Cheung YS, Wong J, et al. Antiviral therapy improves post-hepatectomy survival in patients with hepatitis B virus-related hepatocellular carcinoma: a prospective-retrospective study. Aliment Pharmacol Ther. 2015;41(2):199–208. doi: 10.1111/apt.13034. [DOI] [PubMed] [Google Scholar]
- 31.Huang L, Li J, Yan J, Sun J, Zhang X, Wu M, et al. Antiviral therapy decreases viral reactivation in patients with hepatitis B virus-related hepatocellular carcinoma undergoing hepatectomy: a randomized controlled trial. J Viral Hepat. 2013;20(5):336–342. doi: 10.1111/jvh.12036. [DOI] [PubMed] [Google Scholar]
- 32.Li N, Lai EC, Shi J, Guo WX, Xue J, Huang B, et al. A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection. Ann Surg Oncol. 2010;17(1):179–185. doi: 10.1245/s10434-009-0694-z. [DOI] [PubMed] [Google Scholar]
- 33.Lok AS. Does antiviral therapy prevent recurrence of hepatitis B virus-related hepatocellular carcinoma after curative liver resection? JAMA. 2012;308(18):1922–1924. doi: 10.1001/jama.2012.12971. [DOI] [PubMed] [Google Scholar]
- 34.Yang SL, Liu LP, Sun YF, Yang XR, Fan J, Ren JW, et al. Distinguished prognosis after hepatectomy of HBV-related hepatocellular carcinoma with or without cirrhosis: a long-term follow-up analysis. J Gastroenterol. 2015 doi: 10.1007/s00535-015-1146-0. [DOI] [PubMed] [Google Scholar]
- 35.Liu WR, Tian MX, Jin L, Yang LX, Ding ZB, Shen YH, et al. High levels of hepatitis B surface antigen are associated with poorer survival and early recurrence of hepatocellular carcinoma in patients with low hepatitis B viral loads. Ann Surg Oncol. 2015;22(3):843–850. doi: 10.1245/s10434-014-4043-5. [DOI] [PubMed] [Google Scholar]
- 36.Huang G, Lau WY, Zhou WP, Shen F, Pan ZY, Yuan SX, et al. Prediction of hepatocellular carcinoma recurrence in patients with low hepatitis B virus DNA levels and high preoperative hepatitis B surface antigen levels. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.4648. [DOI] [PubMed] [Google Scholar]
- 37.Zhou HB, Li QM, Zhong ZR, Hu JY, Jiang XL, Wang H, et al. Level of hepatitis B surface antigen might serve as a new marker to predict hepatocellular carcinoma recurrence following curative resection in patients with low viral load. Am J Cancer Res. 2015;5(2):756–771. [PMC free article] [PubMed] [Google Scholar]
- 38.Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY, Choi MS, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63(12):1943–1950. doi: 10.1136/gutjnl-2013-306409. [DOI] [PubMed] [Google Scholar]
- 39.You H, Jia JD. Hepatitis B virus DNA and hepatocellular carcinoma recurrence after resection: the lower, the better! J Gastroenterol Hepatol. 2010;25(12):1812–1814. doi: 10.1111/j.1440-1746.2010.06516.x. [DOI] [PubMed] [Google Scholar]


