Abstract
Background:
Coronary computed tomographic angiography (CCTA) has been widely used in patients who are at intermediate risk for having stable coronary artery disease (SCAD), and 2013 European Society of Cardiology Guidelines on the Management of SCAD (2013G) recommended the appropriate application of CCTA. However, 2013G has not been subjected to systematic analyses for subsequent impact on clinical practice.
Methods:
A total of 5320 patients suspected with SCAD were enrolled and scheduled for CCTA from March 2013 to September 2014. For each patient, pretest probability of SCAD was calculated according to updated Diamond-Forrester model (UDFM). Appropriate CCTA or appropriate stress test was determined as described in the 2013G. A generalized estimating equation model was used to determine the trends in the half-monthly rate of appropriate CCTA.
Results:
Overall, only 61.37% of patients received appropriate CCTA, and there was insignificant change over time (P = 0.8701). The application of CCTA in patients who should have had a stress test accounted for most of the inappropriate CCTA before (22.29%) or after (19.98%) the publication of the 2013G. In all patients or any subgroup, no significant change in the adjusted half-monthly rate of appropriate CCTA was found after the publication of the 2013G (odds ratio, 1.002; 95% confidence interval, 0.982–1.021; P = 0.8678).
Conclusions:
These findings suggest that the 2013G have not, to date, been fully incorporated into clinical practice, and the clinical utilization of CCTA remains unreasonable to some extent.
Keywords: Clinical Practice, Coronary Computed Tomographic Angiography, Coronary Artery Disease, Guideline Recommendations
INTRODUCTION
It has been widely accepted that the application of coronary computed tomographic angiography (CCTA) is most likely to benefit patients who are at intermediate risk of having stable coronary artery disease (SCAD).[1,2,3,4,5,6] As a result, in 2013 European Society of Cardiology Guidelines on the Management of SCAD (2013G),[7] importance was given to systematical testing with the consideration of pretest probability and the updated Diamond-Forrester model (UDFM)[8] was recommended as the clinical algorithm. According to 2013G, CCTA should be considered as an alternative to stress test in patients with ejection fraction (EF) ≥50% and pretest probability between 15% and 50%, or a complement to stress tests in patients with EF ≥50% and pretest probability between 50% and 85% or with EF <50% and without typical angina.
The major expectation of the clinical guidelines was to establish a scientific basis for clinical practice. However, 2013G has not been subjected to systematic analyses on its impact on clinical practice. Therefore, the purpose of this study was to investigate the utilization of CCTA in clinical practice and to determine the impact of 2013G on the use of CCTA.
METHODS
Study population and data collection
From March 2013 to September 2014, 5320 consecutive patients were eligible for the analysis. Inclusion criteria were patients suspected of SCAD and scheduled for CCTA. The exclusion criteria were acute coronary syndrome, previous coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), impaired renal function (serum creatinine >120 μmol/L), New York Heart Association class III or IV heart failure, atrial fibrillation, aortic disease, or >90 years old. Patients were considered to have diabetes mellitus, hyperlipidemia, or hypertension if they were taking corresponding treatment or had been diagnosed as such according to their medical records. Typical chest pain was defined as having (1) substernal chest pain or discomfort, which was (2) provoked by exertion or emotional stress and (3) relieved by rest and/or nitroglycerin. Atypical chest pain was defined as having two of these three criteria. If one or none of the criteria was present, the patient was classified as having nonangina associated chest pain.[8]
Coronary computed tomographic angiography
All scans were performed with a second-generation dual-source CCTA scanner (Somatom Definition Flash, Siemens Medical Solutions, Forchheim, Germany). Patients received heart rate control, as well as sublingual nitroglycerin before CCTA, and underwent prospectively electrocardiogram (ECG)-triggered high-pitch spiral scan, step-on sequence, or retrospective spiral scan as appropriate. Two experienced observers, a radiologist and a cardiologist, evaluated the CCTA data on a Syngo Multimodality Workstation (Siemens Medical Solutions, Forchheim, Germany) using volume rendering, multiplanar reformation and maximum intensity projection. Interobserver disagreements were resolved by a consultation. In image analyses, all patients’ vessels were identified and analyzed using a modified classification.[9] Each segment was assessed for diameter, location, presence of atherosclerotic changes (calcified and noncalcified), and concomitant coronary artery stenosis. A significant stenosis was defined as an obstructive luminal reduction (≥50%) compared with normal reference segment, while nonsignificance referred to mild stenosis (<50%).
Data analysis
For each patient, pretest probability was determined using UDFM as previously described.[8] Appropriate CCTA or appropriate stress test (exercise ECG, stress echo, cardiac magnetic resonance, single photon emission computed tomography, and positron emission tomography) was chosen as described in the 2013G:[7] (1) appropriate CCTA - an alternative to stress tests for patients with EF ≥50% and pretest probability between 15% and 50% or a complement to stress tests for patients with EF ≥50% and pretest probability between 50% and 85% or with EF <50% and without typical angina and (2) appropriate stress test - a preferred test for patients with EF ≥50% and pretest probability between 50% and 85% or patients with EF <50% and without typical angina.
The trends in the half-monthly rate of appropriate CCTA were examined across the following time periods: (1) prior to publication of the 2013G (March 1, 2013 to September 1, 2013) and (2) after the publication of the 2013G (September 1, 2013 to September 1, 2014). Baseline differences between the time periods, as well as the conventional risk factors for SCAD, were adjusted using covariate analysis. The classified trends in subgroups were also examined by these factors.
Statistical analysis
Continuous variables were compared using Students’ t-tests or Mann–Whitney U-tests as appropriate and were expressed as a mean ± standard deviation (SD). Count variables were expressed as frequencies with percentages and differences in the percentages were assessed using Chi-square test or Fisher's exact test as appropriate. Analysis of the trends in the half-monthly rate of appropriate CCTA within the time periods was performed using a generalized estimating equation model. The model was adjusted for gender, age, the number of other risk factors, pretest probability, EF, appropriate stress test, patients (admitted or out), and symptom. Wald Chi-square tests were used to test the significance of the time trends. All statistical analysis was performed by SAS version 9.2 (SAS Institute Inc., Cary, North Carolina, USA). Two-tailed P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of patients before and after 2013G
The baseline characteristics before and after the publication of 2013G were compared [Table 1]. The mean age of the patients and the proportion of women increased over time (P = 0.0042 and 0.0096, respectively). More patients presented with angina pectoris (43.01% atypical angina and 23.66% typical angina), and this ratio was also elevated over time (P = 0.0096). The number of patients receiving appropriate stress tests was small, although it increased markedly (P < 0.0001). Overall, 61.37% of patients received appropriate CCTA, but there was no significant change over time (P = 0.8701).
Table 1.
The baseline clinical characteristics before and after the publication of 2013G
| Characteristics | Total (n=5320) | Prior to guideline (n=1871) | After guideline (n=3449) | χ2 or t | P |
|---|---|---|---|---|---|
| Age (years) | 57.18 ± 10.59 | 56.60 ± 10.51 | 57.50 ± 10.63* | 2.960 | 0.0042 |
| Men | 2756 (51.97) | 1018 (54.38) | 1747 (50.65)* | 6.612 | 0.0096 |
| Diabetes mellitus | 889 (16.71) | 296 (15.81) | 593 (17.19) | 1.562 | 0.9981 |
| Hypertension | 2626 (49.36) | 924 (49.36) | 1702 (49.35) | 0.0005 | 0.9981 |
| Hyperlipidemia | 1899 (35.70) | 709 (37.87) | 1190 (34.50)† | 5.856 | 0.0149 |
| Smoking | 1418 (26.65) | 531 (28.37) | 887 (25.72)† | 4.223 | 0.0389 |
| Family history of CAD | 1139 (21.41) | 410 (21.90) | 729 (21.14) | 0.372 | 0.5322 |
| Symptom | |||||
| Asymptomatic patient | 964 (18.12) | 299 (15.97) | 665 (19.28)* | 7.301 | 0.0069 |
| Nonanginal chest pain | 809 (15.21) | 280 (14.95) | 529 (15.34) | ||
| Atypical angina | 2288 (43.01) | 838 (44.76) | 1450 (42.04) | ||
| Typical angina | 1259 (23.66) | 454 (24.25) | 805 (23.34) | ||
| Pretest probability§ | |||||
| <15 | 839 (15.77) | 266 (14.21) | 573 (16.61) | 0.226 | 0.6344 |
| 15≤, <50 | 3161 (59.42) | 1155 (61.73) | 2006 (58.16) | ||
| 50≤, <85 | 1238 (23.27) | 425 (22.72) | 813 (23.57) | ||
| ≥85 | 82 (1.54) | 25 (1.34) | 57 (1.65) | ||
| EF ≥50% | 5130 (96.43) | 1728 (95.19) | 3348 (97.07)‡ | 11.846 | 0.0008 |
| Appropriate stress tests | 222 (4.17) | 49 (2.62) | 173 (5.02)‡ | 16.866 | <0.0001 |
| Outpatient | 4154 (78.08) | 1453 (77.62) | 2701 (78.31) | 0.298 | 0.6062 |
| Appropriate CCTA | 3265 (61.37) | 1145 (61.16) | 2120 (61.46) | 0.034 | 0.8701 |
Values are presented as mean ± SD or n (%). *P<0.01; †P<0.05; ‡P<0.001; §Pretest probability for each patient was calculated using Updated Diamond-Forrester method; CAD: Coronary artery disease; EF: Ejection fraction; CCTA: Coronary computed tomographic angiography; SD: Standard deviation.
Details of the CCTA procedural characteristics are summarized in Table 2. Nearly, one-third (32.5%) of the 5320 patients had at least one significant stenosis. Moreover, the multivessel disease was found in 15.9% of patients (2 vessels: 9.3% and >2 vessels: 6.6%). This proportion did not change over time (P = 0.3451), but a significant (P < 0.05) decline was observed in complications, as well as radiation exposure.
Table 2.
CCTA procedural characteristics before and after the publication of 2013G
| Procedural characteristic | Total (n=5320) | Prior to guidelines (n=1871) | After guidelines (n=3449) | χ2 or t | P |
|---|---|---|---|---|---|
| Positive CCTA | 1729 (32.50) | 586 (31.30) | 1143 (33.14) | 1.789 | 0.1860 |
| Site of stenosis | |||||
| LM‡ | 52 (0.98) | 21 (1.12) | 31 (0.90) | 2.877 | 0.8241 |
| Proximal LAD§ | 1261 (23.71) | 429 (22.93) | 831 (24.09) | ||
| Other LAD|| | 424 (7.97) | 153 (8.81) | 271 (7.86) | ||
| Proximal LCX¶ | 476 (8.95) | 158 (8.44) | 318 (9.22) | ||
| Other LCX** | 263 (4.94) | 97 (5.18) | 166 (4.81) | ||
| Proximal RCA†† | 543 (10.21) | 197 (10.53) | 346 (10.03) | ||
| Other RCA‡‡ | 228 (4.29) | 79 (4.22) | 149 (4.32) | ||
| Diseased vessels | |||||
| 1 | 882 (16.58) | 282 (15.07) | 600 (17.40) | 0.891 | 0.3451 |
| 2 | 495 (9.30) | 187 (9.99) | 308 (8.93) | ||
| >2 | 352 (6.62) | 117 (6.25) | 235 (6.81) | ||
| Radiation dose (mSv) | 2.23 ± 4.47 | 2.34 ± 4.55 | 2.04 ± 4.30* | −2.380 | 0.0145 |
| Complications | |||||
| Contrast reaction | 26 (0.48) | 16 (0.86) | 10 (0.28)† | 7.311 | 0.0069 |
| Impaired renal function | 12 (0.22) | 8 (0.42) | 3 (0.09)* | 4.919 | 0.0266 |
| Contrast extravasation | 18 (0.34) | 11 (0.59) | 7 (0.20)* | 4.91 | 0.0361 |
| Other complications | 14 (0.26) | 9 (0.48) | 5 (0.14)* | 4.182 | 0.0408 |
Values are presented as mean ± SD or n (%).*P<0.05, †P<0.01;. ‡LM: Left main; §LAD: Left anterior descending; ||Other LAD included ramus intermedius, diagonal branch and middle and distal of LAD; ¶LCX: Left circumflex; **Other LCX included posterior descending artery from LCX, posterior-lateral branch from LCX and middle and distal of LCX; ††RCA: Right coronary artery; ‡‡Other RCA included posterior descending artery from RCA, posterior-lateral branch from RCA and middle and distal of RCA. CCTA: Coronary computed tomographic angiography; SD: Standard deviation.
Coronary computed tomographic angiography application in patients before and after 2013G
To investigate the rate of inappropriate CCTA application, enrolled patients were sorted and compared according to different clinical risk levels [Figure 1]. Patients with EF ≥50% and pretest probability ≤15%, who were devoid of other complications, received more inappropriate CCTAs after 2013G in comparison to those before 2013G (16.3% vs. 13.9%, P = 0.0243). We observed a decrease in inappropriate use in patients with EF <50% and without typical angina who should receive stress tests before CCTA (2% vs. 3.3%, P = 0.0047) although there was no significant difference between these 2 time periods (P = 0.0821).
Figure 1.
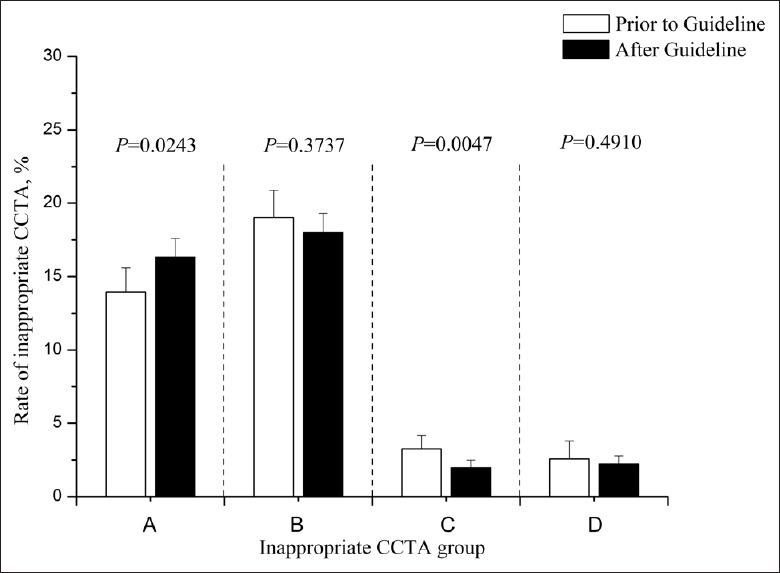
Rate of inappropriate coronary computed tomographic angiography before or after guideline publication. (A) Patients with ejection fraction ≥50% and pretest probability ≤15% should have other diseases excluded. (B) Patients with ejection fraction ≥50% and pretest probability between 50% and 85% should take stress tests before coronary computed tomographic angiography. (C) Patients with ejection fraction <50% and without typical angina should take stress tests before coronary computed tomographic angiography. (D) For patients with ejection fraction <50% and typical angina or ejection fraction ≥50% and pretest probability ≥85%, invasive coronary angiography was necessary. The bars indicate the 95% confidence interval.
In an analysis of appropriate CCTA applications continuously, a nadir was found in the rate of appropriate CCTA in May 2013, 3 months prior to the publication of the 2013G [Figure 2]. To avoid influencing the examination of trends in CCTA within the time periods, the first time period was divided into two stages at the nadir (March 1, 2013 to May 1, 2013 and May 1, 2013 to September 1, 2013).
Figure 2.
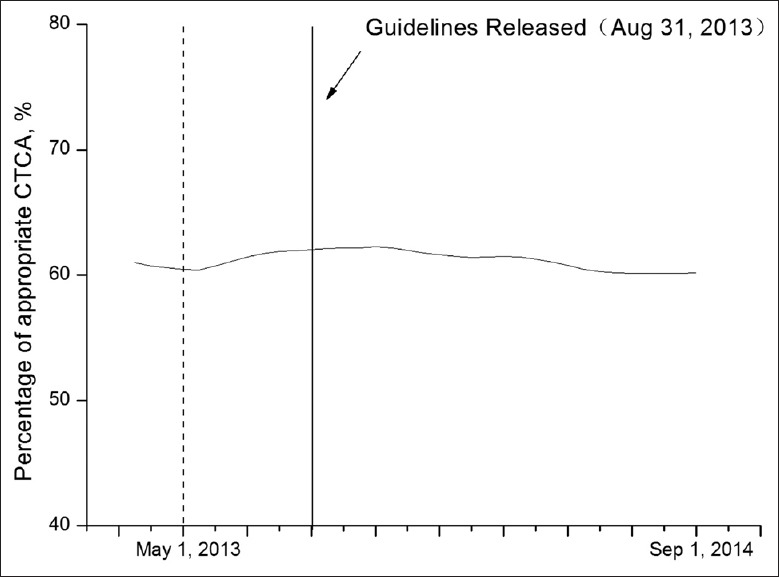
Rate of appropriate coronary computed tomographic angiography over time. The rate of appropriate coronary computed tomographic angiography over the study period is shown for the overall study population.
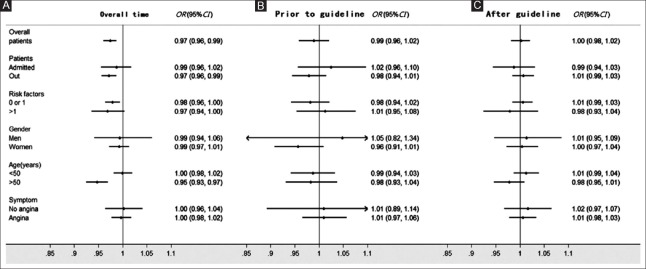
Furthermore, a significant decline was demonstrated in the adjusted rate of appropriate CCTA [Figure 3] from the nadir on May 1, 2013 to September 1, 2014 (odds ratio [OR] for appropriate CCTA per 15-day increase in time, 0.974; 95% confidence interval [CI], 0.960–0.987; P = 0.0002). However, there was no significant change before (OR, 0.989; 95% CI, 0.958–1.021; P = 0.9683) or after (OR, 1.002; 95% CI, 0.982–1.021; P = 0.8678) the publication of the 2013G. The ORs referred to the adjusted odds of appropriate CCTA in the interval of 15 days. When subgroups were analyzed on the basis of gender, age, other risk factors, patients (in-patient or out-patient), or symptom, no significant change was found in the adjusted rate of appropriate CCTA following the publication of the 2013G.
Figure 3.
Odds ratios for change in the rate of appropriate coronary computed tomographic angiography in different subgroups, per 15-day period. (A) Overall time. (B) Prior to the guideline. (C) After guideline. The final model was adjusted for gender, age, number of other risk factors, pretest probability, ejection fraction, appropriate stress test, patients (admitted and out), and symptom. CI: Confidence interval; OR: Odds ratio.
DISCUSSION
In this study, in patients subjected to CCTA due to suspected SCAD, the rate of appropriate CCTA was slightly more than 60%, and no significant change was found in this rate following the publication of the 2013G. Our study indicated that there was a trend toward a decrease over time in the rate of CCTA in some subgroups. Despite the follow-up for 12 months following the publication of the 2013G, inappropriate CCTA continued to be performed in a considerable proportion of patients suspected to have SCAD. These findings suggested that the evidence provided by recent studies[1,2,3,4,5,6] and the guideline recommendations[7] have not been widely incorporated into clinical practice.
Although reasons for the lack of impact of the 2013G on clinical practice are multifactorial, the following one should emerge as a particularly strong candidate. The recommendations in the 2013G were based on pretest probability estimated by UDFM. It has been investigated extensively that a pretest probability model developed in one population may overestimate or underestimate the probability in another population with different characteristics.[10,11,12,13,14] Moreover, discrepancies also existed between the different models.[15,16,17] For example, several observations[15,16,17] indicated a superior predictive accuracy of Duke clinical score,[18] but significant overestimations were observed by others.[10,13] UDFM was the most efficient and operational model in the study of Jensen et al.,[16] only revealing a moderate predictive accuracy. Taking the limitations of the models into consideration, physicians were mainly concerned regarding the overestimation, which might lead to overuse of downstream tests and therapies.[19] To improve acceptability of the pretest probability models in clinical practice, more rigorous external validations for the existing models are needed. What is more, a standardized, reliable and easily usable model is more likely to contribute to promoting the application of guidelines in clinical practice.
Except for the belief that the model may overestimate the pretest probability, this study provided additional possible explanations about hindrances to the application of 2013G. Actually, nearly one in two inappropriate CCTAs resulted from the lack of appropriate stress tests, regardless of whether they were before or after the publication of 2013G. Neither the class I recommendation (“should be considered”) for stress tests as an initial test nor the class IIa recommendation (“is recommended/is indicated”) for CCTA as a complement to stress tests in patients (1) with EF ≥50% and pretest probability between 50% and 85% or (2) with EF <50% and without typical angina[7] was widely incorporated into clinical practice. Although there is a general consensus that stress tests are more cost-effective than CCTA in patients with a higher rate of intermediate risk,[20,21,22] several speculative reasons accounted for the unsatisfactory use of stress tests, such as the lack of awareness, resources and reimbursement, and fear about the increase in malpractice liability.[23,24] The other major reason for inappropriate CCTA was the overuse of CCTA in patients with EF ≥50% and pretest probability ≤15%. According to the class III recommendation (“is not recommendeds”) in 2013G,[7] CCTA should not be recommended as a “screening” test. However, physicians are not inclined to alter their practice based on a negative recommendation, especially when it contradicts traditional beliefs and external influences, such as conflicting patient expectations, financial incentives, and fear of missed diagnosis.[23,24,25] All of these may play an important role in the development of the barriers between evidence and practice, but future studies may provide a more valuable explanation.
It is noteworthy that there are two different types of barriers: “overuse” and “underuse.”[26] This study only retrospectively included patients referred for CCTA and focused on the “overuse” of CCTA. According to the guideline recommendation,[7,27] some patients who should have undergone CCTA after an inconclusive stress test were referred directly to invasive angiography.[28,29] More investigations are required to determine the “underuse” of CCTA, which lead to “overuse” of invasive angiography in actual clinical practice.
The absolute rate of appropriate CCTA in this study should be interpreted with caution. First, while we strived to define a population that reflected the inclusion and exclusion criteria of UDFM and 2013G, there are considerable differences between the cohort in this study and others previously described.[7,8] The population in this study consisted of asymptomatic patients at risk for SCAD, such as patients with palpitation (caused by arrhythmia rather than SCAD according to ECG) and dizziness. Instead of estimation of pretest probability, assessment of cardiovascular risk using “screening” tests (such as ECG, carotid ultrasound, and coronary calcium) was recommended (IIa or IIb) and led to CCTA overuse for these patients. However, after sending them into the estimation of pretest probability, it resulted in an increase in the amount of appropriate CCTA. Then, as a complement to stress tests in patients with a higher rate of intermediate risk, CCTA was recommended after an inconclusive stress test. In this subgroup, we defined an appropriate CCTA as one with a prior stress test, regardless of the conclusion. As a result, an unnecessary CCTA after a conclusive stress test was defined as an appropriate one. Finally, the influence of coronary artery calcification on CCTA was not taken into consideration in this study. Although all of the limitations may lead to overestimation of the actual rate of appropriate CCTA, they would have had no influence on the examination of trends in the rate of appropriate CCTA.
Another important limitation to this study was the generalization of the cohort. Although we collected data from a large, complex, and professional center, the impact of 2013G in different centers, regions, and countries with different models of health care delivery and practice environments has not been assessed. Thus, a multicenter study with a large sample size will be needed in the future.
In conclusion, this study found very little evidence that 2013G has influenced practice in the subsequent 12 months. The use of CCTA as a “screening” test or an inappropriate alternative to a stress test remains commonplace despite little evidence to support it and new clinical practice guidelines recommending against it.[27] This study causes two main concerns: On one hand, it demonstrated that many patients suspected of SCAD continue to undergo a costly and ineffective CCTA. On another hand, a huge investment in the generation of clinical evidence has yet to effectively influence clinical practice. According to this study, a widely accepted method to estimate pretest probability and interventions in education, media campaigns, technical innovation, finance, multidisciplinary collaboration, and quality management may fundamentally improve this incomplete knowledge transfer.[23,24,26]
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81400229) and Capital Special Clinical Application Grants (No. Z141107002514103).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Redberg RF, Walsh J. Pay now, benefits may follow – The case of cardiac computed tomographic angiography. N Engl J Med. 2008;359:2309–11. doi: 10.1056/NEJMp0805920. doi: 10.1056/NEJMp0805920. [DOI] [PubMed] [Google Scholar]
- 2.Voros S. What are the potential advantages and disadvantages of volumetric CT scanning? J Cardiovasc Comput Tomogr. 2009;3:67–70. doi: 10.1016/j.jcct.2008.12.010. doi: 10.1016/j.jcct.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Min JK, Gilmore A, Budoff MJ, Berman DS, O’Day K. Cost-effectiveness of coronary CT angiography versus myocardial perfusion SPECT for evaluation of patients with chest pain and no known coronary artery disease. Radiology. 2010;254:801–8. doi: 10.1148/radiol.09090349. doi: 10.1148/radiol.09090349. [DOI] [PubMed] [Google Scholar]
- 4.Cheneau E, Vahdat B, Bernard L, Molon A, Panagides D. Routine use of coronary computed tomography as initial diagnostic test for angina pectoris. Arch Cardiovasc Dis. 2011;104:29–34. doi: 10.1016/j.acvd.2010.11.007. doi: 10.1016/j.acvd.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: A systematic review and meta-analysis. J Am Coll Cardiol. 2011;57:1237–47. doi: 10.1016/j.jacc.2010.10.011. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Meijboom WB, van Mieghem CA, Mollet NR, Pugliese F, Weustink AC, van Pelt N, et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol. 2007;50:1469–75. doi: 10.1016/j.jacc.2007.07.007. doi: 10.1016/j.jacc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 8.Genders TS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, et al. A clinical prediction rule for the diagnosis of coronary artery disease: Validation, updating, and extension. Eur Heart J. 2011;32:1316–30. doi: 10.1093/eurheartj/ehr014. doi: 10.1093/eurheartj/ehr014. [DOI] [PubMed] [Google Scholar]
- 9.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–36. doi: 10.1016/j.jcct.2009.01.001. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Kumamaru KK, Arai T, Morita H, Sekine T, Takamura K, Takase S, et al. Overestimation of pretest probability of coronary artery disease by Duke clinical score in patients undergoing coronary CT angiography in a Japanese population. J Cardiovasc Comput Tomogr. 2014;8:198–204. doi: 10.1016/j.jcct.2014.02.002. doi: 10.1016/j.jcct.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Diamond GA. Right answer, wrong question: On the clinical relevance of the cardiovascular history. Circulation. 2011;124:2377–9. doi: 10.1161/CIRCULATIONAHA.111.068718. doi: 10.1161/CIRCULATIONAHA.111.068718. [DOI] [PubMed] [Google Scholar]
- 12.Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: Results from the multinational coronary CT angiography evaluation for clinical outcomes: An international multicenter registry (CONFIRM) (1-8).Circulation. 2011;124:2423–32. doi: 10.1161/CIRCULATIONAHA.111.039255. doi: 10.1161/CIRCULATIONAHA.111.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genders TS, Steyerberg EW, Hunink MG, Nieman K, Galema TW, Mollet NR, et al. Prediction model to estimate presence of coronary artery disease: Retrospective pooled analysis of existing cohorts. BMJ. 2012;344:e3485. doi: 10.1136/bmj.e3485. doi: 10.1136/bmj.e3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickett CA, Hulten EA, Goyal M, Surry L, Villines TC. Accuracy of traditional age, gender and symptom based pre-test estimation of angiographically significant coronary artery disease in patients referred for coronary computed tomographic angiography. Am J Cardiol. 2013;112:208–11. doi: 10.1016/j.amjcard.2013.03.015. doi: 10.1016/j.amjcard.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Jensen JM, Ovrehus KA, Nielsen LH, Jensen JK, Larsen HM, Nørgaard BL. Paradigm of pretest risk stratification before coronary computed tomography. J Cardiovasc Comput Tomogr. 2009;3:386–91. doi: 10.1016/j.jcct.2009.10.006. doi: 10.1016/j.jcct.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Jensen JM, Voss M, Hansen VB, Andersen LK, Johansen PB, Munkholm H, et al. Risk stratification of patients suspected of coronary artery disease: Comparison of five different models. Atherosclerosis. 2012;220:557–62. doi: 10.1016/j.atherosclerosis.2011.11.027. doi: 10.1016/j.atherosclerosis.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Wasfy MM, Brady TJ, Abbara S, Nasir K, Ghoshhajra BB, Truong QA, et al. Comparison of the Diamond-Forrester method and Duke clinical score to predict obstructive coronary artery disease by computed tomographic angiography. Am J Cardiol. 2012;109:998–1004. doi: 10.1016/j.amjcard.2011.11.028. doi: 10.1016/j.amjcard.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE, Jr, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. doi: 10.7326/0003-4819-118-2-199301150-s00001. [DOI] [PubMed] [Google Scholar]
- 19.Eichler K, Zoller M, Tschudi P, Steurer J. Barriers to apply cardiovascular prediction rules in primary care: A postal survey. BMC Fam Pract. 2007;8:1. doi: 10.1186/1471-2296-8-1. doi: 10.1186/1471-2296-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewey M, Hamm B. Cost effectiveness of coronary angiography and calcium scoring using CT and stress MRI for diagnosis of coronary artery disease. Eur Radiol. 2007;17:1301–9. doi: 10.1007/s00330-006-0439-3. doi: 10.1007/s00330-006-0439-3. [DOI] [PubMed] [Google Scholar]
- 21.Genders TS, Meijboom WB, Meijs MF, Schuijf JD, Mollet NR, Weustink AC, et al. CT coronary angiography in patients suspected of having coronary artery disease: Decision making from various perspectives in the face of uncertainty. Radiology. 2009;253:734–44. doi: 10.1148/radiol.2533090507. doi: 10.1148/radiol.2533090507. [DOI] [PubMed] [Google Scholar]
- 22.Zeb I, Abbas N, Nasir K, Budoff MJ. Coronary computed tomography as a cost-effective test strategy for coronary artery disease assessment – A systematic review. Atherosclerosis. 2014;234:426–35. doi: 10.1016/j.atherosclerosis.2014.02.011. doi: 10.1016/j.atherosclerosis.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 24.Grol R, Grimshaw J. From best evidence to best practice: Effective implementation of change in patients’ care. Lancet. 2003;362:1225–30. doi: 10.1016/S0140-6736(03)14546-1. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 25.Deyell MW, Buller CE, Miller LH, Wang TY, Dai D, Lamas GA, et al. Impact of national clinical guideline recommendations for revascularization of persistently occluded infarct-related arteries on clinical practice in the United States. Arch Intern Med. 2011;171:1636–43. doi: 10.1001/archinternmed.2011.315. doi: 10.1001/archinternmed.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease? Eur Heart J. 2013;34:1262–9. doi: 10.1093/eurheartj/ehs481. doi: 10.1093/eurheartj/ehs481. [DOI] [PubMed] [Google Scholar]
- 27.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. doi: 10.1016/j.cardfail.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Bittencourt MS, Christman MP, Hulten E, Divakaran S, Skali H, Kwong RY, et al. Comparison of the use of downstream tests after exercise treadmill testing by cardiologists versus noncardiologists. Am J Cardiol. 2014;114:305–11. doi: 10.1016/j.amjcard.2014.04.040. doi: 10.1016/j.amjcard.2014.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christman MP, Bittencourt MS, Hulten E, Saksena E, Hainer J, Skali H, et al. Yield of downstream tests after exercise treadmill testing: A prospective cohort study. J Am Coll Cardiol. 2014;63:1264–74. doi: 10.1016/j.jacc.2013.11.052. doi: 10.1016/j.jacc.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]