Abstract
Background:
Resection of sacral chordomas is challenging. The anatomy is complex, and there are often no bony landmarks to guide the resection. Achieving adequate surgical margins is, therefore, difficult, and the recurrence rate is high. Use of computer navigation may allow optimal preoperative planning and improve precision in tumor resection. The purpose of this study was to evaluate the safety and feasibility of computer navigation-aided resection of sacral chordomas.
Methods:
Between 2007 and 2013, a total of 26 patients with sacral chordoma underwent computer navigation-aided surgery were included and followed for a minimum of 18 months. There were 21 primary cases and 5 recurrent cases, with a mean age of 55.8 years old (range: 35–84 years old). Tumors were located above the level of the S3 neural foramen in 23 patients and below the level of the S3 neural foramen in 3 patients. Three-dimensional images were reconstructed with a computed tomography-based navigation system combined with the magnetic resonance images using the navigation software. Tumors were resected via a posterior approach assisted by the computer navigation. Mean follow-up was 38.6 months (range: 18–84 months).
Results:
Mean operative time was 307 min. Mean intraoperative blood loss was 3065 ml. For computer navigation, the mean registration deviation during surgery was 1.7 mm. There were 18 wide resections, 4 marginal resections, and 4 intralesional resections. All patients were alive at the final follow-up, with 2 (7.7%) exhibiting tumor recurrence. The other 24 patients were tumor-free. The mean Musculoskeletal Tumor Society Score was 27.3 (range: 19–30).
Conclusions:
Computer-assisted navigation can be safely applied to the resection of the sacral chordomas, allowing execution of preoperative plans, and achieving good oncological outcomes. Nevertheless, this needs to be accomplished by surgeons with adequate experience and skill.
Keywords: Chordoma, Computer-assisted Navigation, Resection, Sacral
INTRODUCTION
Chordoma is a rare malignant bone tumor that originates from the embryonic remnants of the notochord. Most diagnoses of the disease are delayed until the tumor reaches a huge size and neurological symptoms appear. Wide resection of the tumor is the treatment of choice, even though it is associated with significant complications.[1] Although malignant, this bone tumor has a low metastatic rate. The cause of death is usually related to local recurrence. Many patients die with great pain due to progressive tumor invasion despite repeated surgical intervention.
Recurrence has been noted to be related to the adequacy of surgical resection.[2] However, obtaining adequate margins is challenging due to the complex anatomy of the sacrum, and because the bony destruction from the tumor can leave the surgeon with few anatomic landmarks to refer to. The inferior edge of the sacroiliac joint is in line with the level of the posterior S3 neural foramen and is a commonly used anatomic landmark for resection. When removing tumors below the S3 level, one can usually make a clear assessment of the margins. For tumors above the S3 level, however, the bony destruction can make executing one's preoperative plan very difficult, compromising one's ability to obtain safe margins. Resecting more levels to obtain an adequate margin is also not a reasonable strategy, so it is crucial to balance the best possible oncological outcome in terms of disease control with minimizing neurological morbidity from surgery.
The need for precision in the surgical management of chordoma cannot be overstated, and the technology of computer-aided navigation is a natural choice to facilitate this need of precision. Computer navigation has been widely used in spinal, and joint surgery[3,4] but is just gaining attention in the field of bone tumor surgery.[5,6,7,8] Several case reports and small series studies have reported its usage as an aid to the removal of sacral chordomas;[9,10,11] however, there are no studies describing the combination of magnetic resonance imaging (MRI) and computed tomography (CT) images for intraoperative navigation to increase the accuracy of these resections. The aim of our study was to evaluate our center's experiences with computer navigation-aided resections of sacral chordoma with respect to the safety and feasibility, parameters including surgical margins, navigation deviation, operative time, blood loss, oncologic outcomes, postoperative function, and complications were recorded and analyzed.
METHODS
Between 2007 and 2013, a total of 26 patients underwent sacral chordoma resection with the assistance of image-guided computer navigation at our department. We identified and retrospectively reviewed the records of these patients. Informed consents were obtained from all patients. The mean age of the patients was 55.8 years old (range: 35–84 years old). In all, 21 patients had a primary tumor and 5 had a recurrent tumor. The tumor location was above S3 in 23 patients and below S3 in 3 patients [Table 1]. Imaging studies included preoperative plain radiography, CT scanning, MRI, and radionuclide bone scanning. The pathological diagnosis was confirmed by postoperative pathology or during the primary operation in recurrent cases. In all cases, the chordoma was of low-grade malignancy.
Table 1.
Clinical characteristics of patients with sacral chordoma and underwent computer navigation-aided surgery
| Characteristics | Values |
|---|---|
| Gender | |
| Male | 15 |
| Female | 11 |
| Primary or recurrence | |
| Primary | 21 |
| Recurrence | 5 |
| Levels of tumor involvement | |
| S1–S5 | 1 |
| S2–S5 | 13 |
| S3–S5 | 9 |
| S4–S5 | 3 |
| Use of preoperative embolism | |
| Yes | 12 |
| No | 14 |
| Surgical margin | |
| Intralesional | 4 |
| Marginal | 4 |
| Wide | 18 |
| Navigation error (mm) | 1.7 (1.3–1.9) |
| Age (years) | 55.8 (35.0–84.0) |
| Tumor transverse diameter (cm) | 7.1 (2.6–10.6) |
| Tumor maximum diameter (cm) | 11.6 (5.0–22.0) |
| Operative time (min) | 307 (140–495) |
| Estimated blood loss (ml) | 3065 (300–8500) |
| Volume of blood transfusion (ml) | 2720 (0–8000) |
Values are n or mean (range).
The preoperative radiographic data were obtained in digital imaging and communication in medicine format and exported to a CT-based navigation system (CT Spine, version 1.2; Stryker Navigation, Freiburg, Germany). The tumor extent was mainly determined by the MRI (Siemens, Germany) evaluation. In the navigation workstation, the MRI images were combined with the CT images so the preoperative plan could be designed using the CT images but also taking into account the extent of the tumor as shown on MRI. The CT images were used to plan the bony margins, and the MRI scan both confirmed the soft tissue extent of the tumor, as well as confirmed the level of the tumor with the bone. Tumor range was drawn layer by layer in horizon section of MRI and CT, and then fusion and correction was performed. Therefore, the three-dimensional (3D) tumor model was established in the navigation system. The fusion of these images helped the surgeons to identify the 3D margin of the tumor [Figures 1 and 2]. The mean time of preoperative planning was 41.3 min. The preoperative plan for osteotomies was designed to include a 1.5 cm margin of normal bone from the edge of the tumor. Soft tissue resection was also designed in the navigation workstation based on this 1.5 cm rule. This 1.5 cm surgical margin was not applied to the anterior margin of the tumors, which are often adjacent to visceral organs but rarely involve the viscera. If the tumor had grown transversely to involve the ischial insertion of the sacrospinous ligament, we performed intralesional resection instead of wide resection.
Figure 1.
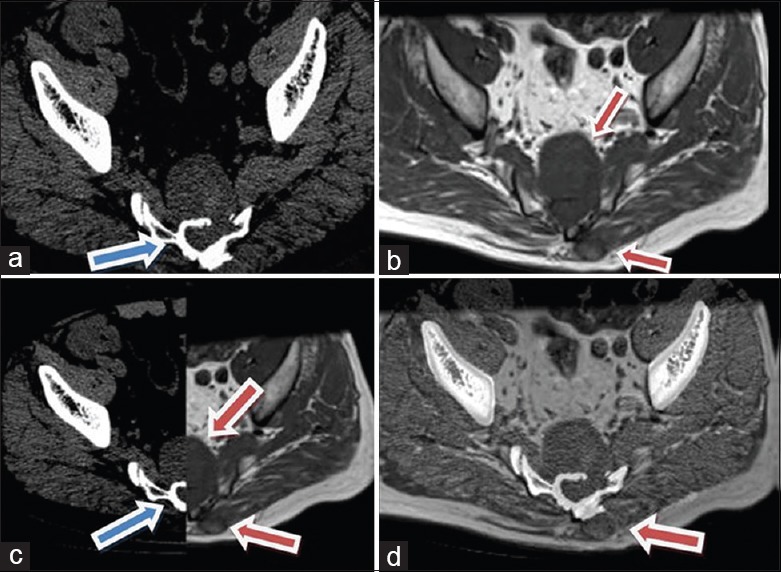
Preoperative resection planning. (a) Blue arrow showed bone margin in CT image; (b) red arrow showed soft tissue margin in MRI image; (c) CT and MRI images together; (d) fusion image of CT and MRI. CT: Computed tomography; MRI: Magnetic resonance imaging.
Figure 2.
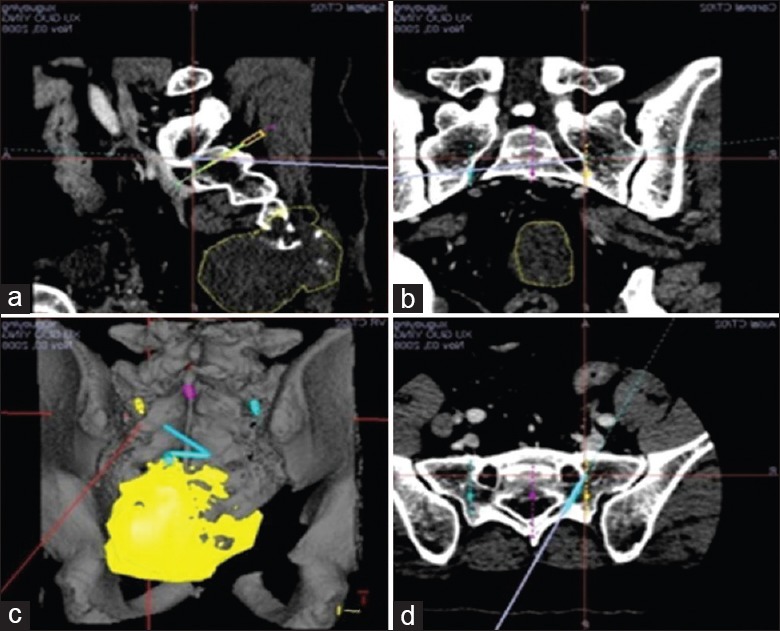
Tumor range (yellow area) was marked in three-dimensional plans, and resection range was also decided. (a) sagittal view; (b) coronal view; (c) three-dimensional view; (d) cross view.
We used a posterior approach for the operation. A median longitudinal incision was performed over the tumor mass starting at the L5 spinous process and ending at the end of the coccyx. The biopsy tract was resected together with the tumor mass. The flap with fascia was opened, and the gluteus maximus, erector spinae, and sacroiliac joint were exposed. We placed a navigation tracker on the normal bone (ilium or spinous process) at a distance from the lesion.
We then exposed the sacral spines and vertebral endplates as bony markers. Three to six bony anatomical landmarks were chosen for registration with the 3D computer model of the sacrum in the computer navigation system. Each point was registered in turn and registration was considered complete when the registration deviation was less than 4 mm. We then used indicators at multiple points on the normal bone to verify that the image on the computer screen matched the bone. The mean registration process took 19.2 min.
Computer navigation was used to confirm the level of the lesion and bone margins on both lateral sides above S3, as well as the pathway of the sacral nerve. According to the preoperative plan and navigation guidance, we then resected the tumor and protected the sacral nerve [Figure 3]. After tumor resection, we verified that the resection had been performed according to the preoperative plan with the computer navigation system. Thorough hemostasis, drainage, and wound closure were performed. If more than 50% of S1 and some of the iliac bones were removed, we would fix the unstable pelvic ring with a sacroiliac L-rod instrumentation.
Figure 3.
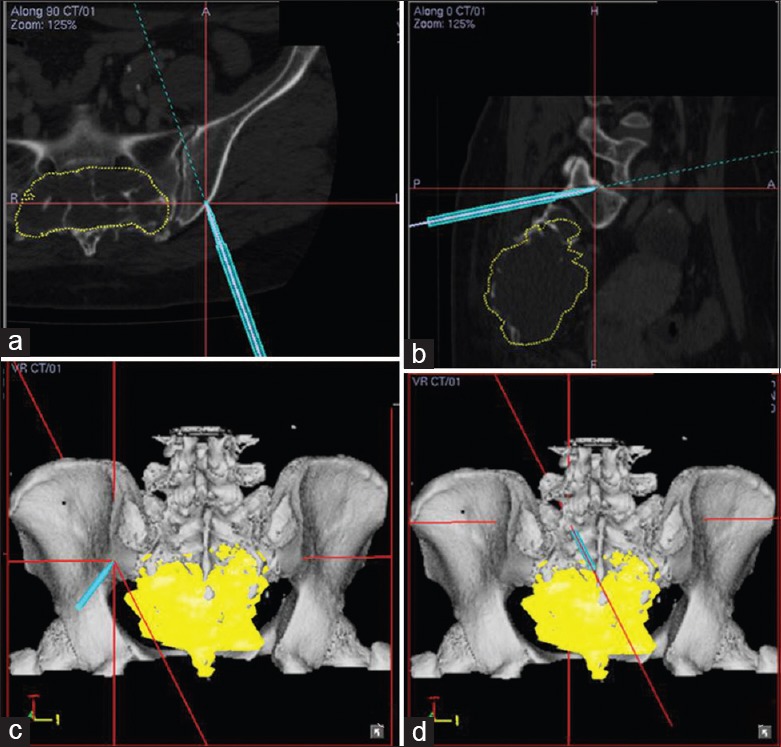
Intraoperative navigation showed visible tumor margin (yellow area). According to preoperative marking range of tumor, operation was performed with the aid of real-time computer navigation (blue line). (a) Cross view; (b) sagittal view; (c and d) three-dimensional view.
The wound was examined on postoperative day 3. Drains were removed if the drainage fluid volume was <30 ml. We took photographs of the gross specimens from the anterior, posterior, superior, inferior, and two lateral sides. The specimen was then cut in the maximum diameter of the tumor. Pathological examinations of dangerous sites were performed to reveal the surgical margins. All of the excised specimens were measured to be compared with the preoperative plan [Figure 4]. All cases with intralesional margin received radiotherapy postoperatively.
Figure 4.
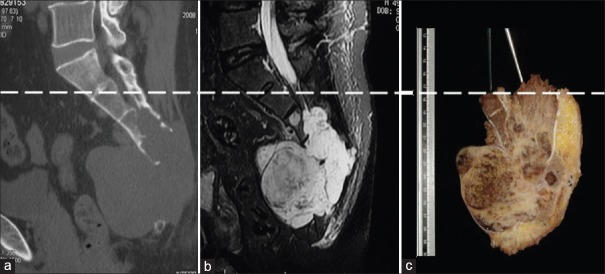
Gross specimen was evaluated with the combination of the preoperative CT and MRI images. Dash line represented the expected level of the osteotomy. (a) Preoperative CT; (b) preoperative MRI; (c) gross specimen. CT: Computed tomography; MRI: Magnetic resonance imaging.
The patients were followed every 3 months for the first 2 years postoperatively. Physical examinations (including neurological evaluation) of the lower limbs and perianal area were performed at each follow-up visit. Plain radiography of the chest, sacrum, and lower lumbar spine was also performed. Ultrasonography of the surgical site was performed at each follow-up time point to detect any possible soft tissue recurrence. If ultrasonography showed any abnormal sign, CT examination would be performed. Chest CT, lumbosacral CT, and whole-body bone scanning were performed every 6 months. After the 3rd postoperative year, patients were followed once a year.
SPSS for Windows (version 16.0, SPSS, Chicago, IL, USA) was used for the statistical analysis. All of the statistical analyses were based on the 26 cases treated in our center. The factors which may be related to intraoperative bleeding, infection/delayed healing, and local recurrence were analyzed. Pearson's correlation and Spearman's correlation were used for parametric tests and nonparametric tests, respectively. Fisher's exact test was used when comparing 2 dichotomous variables. All statistical values were considered significant at P < 0.05.
RESULTS
Computer navigation-aided operations were conducted successfully according to preoperative plans in all cases. No patient died during the perioperative period. The mean operative time was 307 min (140–495 min). The mean estimated intraoperative blood loss was 3065 ml (300–8500 ml). The mean blood transfusion during the perioperative period was 2720 ml (0–8000 ml) [Table 1]. Correlation analysis showed that the maximum tumor diameter and the operative time were significant correlative factors of intraoperative bleeding [Table 2]. Three patients required pelvic ring fixation with sacroiliac L-rod.
Table 2.
Correlation between clinical parameters and intraoperative bleeding
| Parameters | Correlation coefficient | P |
|---|---|---|
| Tumor maximum diameter* | 0.602 | 0.005 |
| Operative time* | 0.798 | 0.000 |
| Tumor level (superior vs. inferior to S3 foramen)† | −0.430 | 0.059 |
| Preoperative embolism† | −0.073 | 0.760 |
*Pearson’s correlation analysis; †Spearman’s correlation analysis.
Regarding the accuracy of the computer navigation, the mean registration deviation was 1.7 mm. The transverse diameter in the horizontal view and the maximum diameter in the reconstructive sacral images were measured with the computer navigation system. The mean transverse diameter was 7.1 cm (2.6–10.6 cm), and the mean maximum diameter was 11.6 cm (5.0–12.5 cm). Surgical margins were evaluated for all specimens. In all, there were 18 wide resections, 4 marginal resections, and 4 intralesional resections. All intralesional resections were performed as the preoperative plan. The mean difference between the bone cutting line of the preoperative plan (mean 132.5 mm) and that of the excised specimen (mean 135.7 mm) was 3.2 mm (1.1–6.8 mm). The bone cutting line of twenty-two excised specimens were longer than that of the preoperative plans (mean difference was 4.1 mm and range: 1.9–6.8 mm) and four excised specimens were shorter than that of the preoperative plans (mean difference was −2.0 mm and range: −1.1 to −3.0 mm).
The 26 patients were followed for a mean of 38.6 months (18–84 months). All patients were alive at the last follow-up. Two patients (7.7%) had a local recurrence. Both of their tumors were large and undergone intralesional resection with extensive curettage. In one of the 2 patients, the tumor extended to the ischial spine and involved nearly the whole greater sciatic notch, so wide or marginal resection was not possible. Local recurrence developed 14 months after the primary surgery, at which point resection of the recurrent tumor and an external hemipelvectomy with an anterior flap were performed. At the last follow-up, this patient was alive without disease. The second patient with local recurrence had total sacral involvement. Extensive curettage had been performed, and local recurrence appeared 48 months later. The patient refused further surgery. He underwent radiotherapy and survived (with the disease) for 78 months postoperatively. Another patient with S2 neural foramen involvement received intralesional resection because she insisted on protection of nerve root and defecation function. She received radiotherapy postoperative, and no recurrence was found 48 months later. There was no recurrence in patients who had undergone marginal or wide resections. The resection margin (intralesional or not) was significantly associated with local recurrence (P = 0.009). Neither the level of tumor (i.e., above vs. below S3 level) nor other factors were found to have a statistically significant association with local recurrence after the Fisher's exact test.
The mean Musculoskeletal Tumor Society (MSTS) score was 27.3 (19–30). The patient who underwent external hemipelvectomy after local recurrence had poor activity functional scores. MSTS scores for all of the other patients were above 26, implying good function results.
Two patients developed an infection with accompanying skin necrosis. After debridement, local flaps and hamstring muscle flaps were used for soft tissue reconstruction. Each healed satisfactorily. Three patients with delayed wound healing responded well after repeated dressings. The correlation analysis of infections and delayed healing against the factors assessed did not show any significant correlations [Table 3].
Table 3.
Correlation between clinical parameters and infection/delayed healing
| Parameters | Correlation coefficient | P |
|---|---|---|
| Tumor maximum diameter* | −0.173 | 0.431 |
| Tumor level (superior vs. inferior to S3 foramen)† | 0.321 | 0.135 |
| Operative time* | −0.251 | 0.286 |
| Intraoperative bleeding* | 0.161 | 0.497 |
*Pearson’s correlation analysis; †Spearman’s correlation analysis.
DISCUSSION
Chordomas account for 17.5% of primary malignant bone tumors of the axial skeleton, with a reported incidence of 0.5–0.8/1,000,000 population.[12,13] Although postoperative radiotherapy is useful in local control and some recent studies[14,15] suggested that microRNA-1 and signal transducer and activator of transcription-3 may be potential therapeutic targets for chordoma, wide resection is still the best treatment for sacral chordomas. Most of the patients have permanent neurological deficits after tumor resection.[16,17] The 5-year disease-free survival rate after complete resection was reported to be about 80%.[18] Patients who have gross tumor contamination during the surgery have a significantly shorter disease-free survival period than those who undergo wide resection.[19,20] Inadequate resection margins are associated with a higher risk of local recurrence and poorer survival rate.[21]
Computer-aided surgery facilitates resection and reconstruction in patients with complex bone tumors.[5,6,7,8] However, reports specifically on computer navigation-aided surgery of sacral chordomas are rare.
All patients in our study successfully underwent computer navigation-aided resection according to the preoperative plan. The mean registration deviation during the operations was 1.7 mm. CT images showed the proximal level of the tumor and sacroiliac joint involvement, while the MRI confirmed any involvement of the inferior sacrum and gluteus maximus. Together, the combination of CT and MRI images helped us to plan the required resection margin for each tumor. This is the characteristic and advantage of our study. The surgeon may obtain the visual impression of the tumor and keep it in mind. Intraoperative navigation provides excellent 3D visualization of the tumor margin.
Despite this advantage, we should be aware that the figures on the screen and the actual localization may differ. If the registration error is large or the tracker is loosened or even the system collapses, the accuracy of navigation will be destroyed. The surgeon should have an experience in dealing with these issues. Another important premise of using navigation is that one should have a certain surgical experience and related anatomical knowledge. Repeated intraoperative verification is thus necessary.[10] We chose at least three or four anatomical bony marker points that were in different planes for registration. These points were at certain distances from the tumor. We compared the postoperative specimens with the preoperatively designed images and confirmed successful resection of the tumor.
Krettek et al.[10] reported a case of sacral chordoma resection with computer navigation for the first time. Dasenbrock et al.[11] reported two cases with en bloc resection of sacral chordomas aided by frameless stereotactic image guidance. These two reports, however, used CT imaging alone, which was unable to confirm soft tissue margins. To the best of our knowledge, this study is the first to use combined CT and MRI for navigation-aided resection of sacral chordomas.
The tumor recurrence rate of our patients was 7.7% (2/26), which was lower than that in other reports. The recurrence rate of most previous reports ranged from 26% to 70%.[19,20,22,23,24,25,26,27,28] Hulen et al.[29] reported 18 patients with sacral chordomas and a recurrence rate of 75% at the 5.5-year follow-up. Most of our patients (23/26) had tumors above the S3 level. Previous studies[2,30,31] have shown that recurrence rates are significantly higher for tumors above S3 than those below S3. Considering the difficulty in intraoperative assessment of resection margins and in avoiding injury to sacral nerves when tumors are above S3, computer navigation is especially useful for high-level sacral tumors. Navigation also has the advantage in the resection of tumor with large size or indistinct structure. The computer navigation system helped us to confirm the resection margins of tumors intraoperative and allowed us to verify the accuracy of surgery and the preoperative plan had been followed. However, we must admit that our follow-up time was short compared with previous reports.
The only two recurrences in our series occurred in patients who underwent intralesional resection. These 2 patients could not undergo a wide resection because of the extensive tumor involvement. One patient was found preoperatively to have sacrotuberous ligament involvement, and another had total sacral involvement. Adequate margins can decrease the recurrence rate. Recurrence rates in literature for patients with wide margins ranged from 5% to 60%, whereas the rates for those with inadequate margins ranged from 43% to 83%.[24,25,26,27,28,29,30,31] While our study showed a statistically significant correlation between local recurrences and the resection margins. The recent review also confirmed that a wide resection margin offered the best long-term prognosis.[32]
At the last follow-up, most of our patients had satisfactory activity and functional outcomes. Only 1 patient had a poor MSTS score because of an external hemipelvectomy after local recurrence. No intolerable symptom or significant lower limb dysfunction occurred in any patient, and this was felt to be due to the accuracy of the computer navigation and the ability to minimize neurologic morbidity.
However, navigation has some other problems. It requires proprietary equipment with an expensive price, thus the operation expense increases. Navigation-aided surgery has more related steps. Therefore, the greatest concern about navigation-aided surgery is the increased operative time and blood loss from additional exposure. In our study, the point registration took a mean 19.2 min. The mean operative time was 307 min, and the mean intraoperative blood loss was 3065 ml. The latter figures suggested the relative safety of the surgeries performed in this study compared with previous reports. The mean operative time in most previous reports were 5–10 h, and the mean blood losses were 3900–5200 ml.[17,20,29,33,34] Only sufficient experience with navigation can increase the safety of surgery, otherwise the operation time and related risk may be elevated.
Our study has several limitations. Firstly, the patient population is small. This is related to the rare incidence of chordomas. Secondly, the study duration and follow-up were short. The mean follow-up of 38.6 months revealed a quite low recurrence rate among our patients. In most previous reports, however, the mean time to recurrence after surgery was 28–38 months[2,17,21] which was similar to our follow-up time. Housari et al.[34] reported 13 patients with a mean follow-up of 4.5 years and all nine recurrences occurred within the first 2 years postoperatively. Although our follow-up was relatively short compared with previous reports, satisfactory resection margin and good local control were achieved during a period of time. Thirdly, the single-group study design allows no comparative assessment with conventional surgery. Due to the different complexity in different level and nature of the sacral tumor, we did not compare navigation aid surgery with traditional surgery in our center.
In conclusion, this study illustrates our experience in using CT and MRI image fusion for preoperative planning and computer-aided navigation for the intraoperative guidance of sacral chordoma resection. It demonstrates the safety and feasibility of the technique and the favorable short-term oncological outcomes in a small population of subjects treated with this technique. However, some potential problems and risks should also be noticed. We are now performing this technique in more cases and looking forward for long-term results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Samson IR, Springfield DS, Suit HD, Mankin HJ. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am. 1993;75:1476–84. doi: 10.2106/00004623-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Zhu L, Ebraheim NA, Liu X, Castillo S, Tang T, et al. Analysis of risk factors for recurrence after the resection of sacral chordoma combined with embolization. Spine J. 2009;9:972–80. doi: 10.1016/j.spinee.2009.08.447. doi: 10.1016/j.spinee.2009.08.447. [DOI] [PubMed] [Google Scholar]
- 3.Zwingmann J, Konrad G, Kotter E, Südkamp NP, Oberst M. Computer-navigated iliosacral screw insertion reduces malposition rate and radiation exposure. Clin Orthop Relat Res. 2009;467:1833–8. doi: 10.1007/s11999-008-0632-6. doi: 10.1007/s11999-008-0632.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkler D, Vitzthum HE, Seifert V. Spinal markers: A new method for increasing accuracy in spinal navigation. Comput Aided Surg. 1999;4:101–4. doi: 10.1002/(SICI)1097-0150(1999)4:2<101::AID-IGS5>3.0.CO;2-1. doi: 10.3109/10929089909148165. [DOI] [PubMed] [Google Scholar]
- 5.Hüfner T, Kfuri M, Jr, Galanski M, Bastian L, Loss M, Pohlemann T, et al. New indications for computer-assisted surgery: Tumor resection in the pelvis. Clin Orthop Relat Res. 2004;426:219–25. doi: 10.1097/01.blo.0000138958.11939.94. [DOI] [PubMed] [Google Scholar]
- 6.Reijnders K, Coppes MH, van Hulzen AL, Gravendeel JP, van Ginkel RJ, Hoekstra HJ. Image guided surgery: New technology for surgery of soft tissue and bone sarcomas. Eur J Surg Oncol. 2007;33:390–8. doi: 10.1016/j.ejso.2006.10.030. doi: 10.1016/j.ejso. 2006.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Wong KC, Kumta SM, Antonio GE, Tse LF. Image fusion for computer-assisted bone tumor surgery. Clin Orthop Relat Res. 2008;466:2533–41. doi: 10.1007/s11999-008-0374-5. doi: 10.1007/s11999-008-0374.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision tumour resection and reconstruction using image-guided computer navigation. J Bone Joint Surg Br. 2007;89:943–7. doi: 10.1302/0301-620X.89B7.19067. doi: 10.1302/0301-620X.89B7.19067. [DOI] [PubMed] [Google Scholar]
- 9.Cho HS, Kang HG, Kim HS, Han I. Computer-assisted sacral tumor resection. A case report. J Bone Joint Surg Am. 2008;90:1561–6. doi: 10.2106/JBJS.G.00928. doi: 10.2106/JBJS.G.00928. [DOI] [PubMed] [Google Scholar]
- 10.Krettek C, Geerling J, Bastian L, Citak M, Rücker F, Kendoff D, et al. Computer aided tumor resection in the pelvis. Injury. 2004;35(Suppl 1):S–A79-83. doi: 10.1016/j.injury.2004.05.014. doi: 10.1016/j.injury.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Dasenbrock HH, Clarke MJ, Bydon A, McGirt MJ, Witham TF, Sciubba DM, et al. En bloc resection of sacral chordomas aided by frameless stereotactic image guidance: A technical note. Neurosurgery. 2012;70:ons82–88. doi: 10.1227/NEU.0b013e31822dd958. doi: 10.1227/NEU.0b013e31822dd958. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson B, Gunterberg B, Kindblom LG. Chordoma. A clinicopathologic and prognostic study of a Swedish national series. Acta Orthop Scand. 1981;52:49–58. doi: 10.3109/17453678108991758. doi.10.3109/17453678108991758. [DOI] [PubMed] [Google Scholar]
- 13.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: Incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 14.Osaka E, Yang X, Shen JK, Yang P, Feng Y, Mankin HJ, et al. MicroRNA-1 (miR-1) inhibits chordoma cell migration and invasion by targeting slug. J Orthop Res. 2014;32:1075–82. doi: 10.1002/jor.22632. doi: 10.1002/jor.22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, Hornicek FJ, Wood KB, Schwab JH, Choy E, Mankin H, et al. Blockage of Stat3 with CDDO-Me inhibits tumor cell growth in chordoma. Spine (Phila Pa 1976) 2010;35:1668–75. doi: 10.1097/BRS.0b013e3181c2d2b4. doi: 10.1097/BRS.0b013e3181c2d2b4. [DOI] [PubMed] [Google Scholar]
- 16.Wuisman P, Lieshout O, Sugihara S, van Dijk M. Total sacrectomy and reconstruction: Oncologic and functional outcome. Clin Orthop Relat Res. 2000;381:192–203. doi: 10.1097/00003086-200012000-00023. doi: 10.1097/00003086-200012000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Chen KW, Yang HL, Lu J, Liu JY, Chen XQ. Prognostic factors of sacral chordoma after surgical therapy: A study of 36 patients. Spinal Cord. 2010;48:166–71. doi: 10.1038/sc.2009.95. doi: 10.1038/sc.2009.95. [DOI] [PubMed] [Google Scholar]
- 18.Ferraresi V, Nuzzo C, Zoccali C, Marandino F, Vidiri A, Salducca N, et al. Chordoma: Clinical characteristics, management and prognosis of a case series of 25 patients. BMC Cancer. 2010;10:22. doi: 10.1186/1471-2407-10-22. doi: 10.1186/1471-2407-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sar C, Eralp L. Surgical treatment of primary tumors of the sacrum. Arch Orthop Trauma Surg. 2002;122:148–55. doi: 10.1007/s00402-001-0356-5. doi: 10.1007/s00402-001-0356-5. [DOI] [PubMed] [Google Scholar]
- 20.Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, et al. En bloc resection of primary sacral tumors: Classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3:111–22. doi: 10.3171/spi.2005.3.2.0111. doi: 10.3171/spi.2005.3.2.0111. [DOI] [PubMed] [Google Scholar]
- 21.Hanna SA, Aston S, Briggs R. Sacral chordoma: Can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466:2217–23. doi: 10.1007/s11999-008-0356-7. doi: 10.1007/s11999-008-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri A, Agarwal MG, Shah M, Srinivas CH, Shukla PJ, Shrikhande SV, et al. Decision making in primary sacral tumors. Spine J. 2009;9:396–403. doi: 10.1016/j.spinee.2008.10.001. doi: 10.1016/j.spinee.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed AR. Safety margins in resection of sacral chordoma: Analysis of 18 patients. Arch Orthop Trauma Surg. 2009;129:483–7. doi: 10.1007/s00402-008-0674-y. doi: 10.1007/s00402-008-0674-y. [DOI] [PubMed] [Google Scholar]
- 24.Baratti D, Gronchi A, Pennacchioli E, Lozza L, Colecchia M, Fiore M, et al. Chordoma: Natural history and results in 28 patients treated at a single institution. Ann Surg Oncol. 2003;10:291–6. doi: 10.1245/aso.2003.06.002. doi: 10.1245/ASO.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: A study of 39 patients. Cancer. 2000;88:2122–34. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1. doi: 10.1002/(SICI) 1097-0142 (20000501) 88:9%3C2122:AID-CNCR19%3E3.0.CO; 2-1. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–6. doi: 10.2106/JBJS.D.02693. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 27.Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer. 1999;85:878–83. doi: 10.1002/(SICI) 1097-0142 (19990215)85:4%3C878:AID-CNCR15%3E3.0.CO; 2-7. [PubMed] [Google Scholar]
- 28.York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74–9. doi: 10.1097/00006123-199901000-00041. doi: 10.1097/00006123-199901000-00041. [DOI] [PubMed] [Google Scholar]
- 29.Hulen CA, Temple HT, Fox WP, Sama AA, Green BA, Eismont FJ. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am. 2006;88:1532–9. doi: 10.2106/JBJS.D.02533. doi: 10.2106/JBJS.D.02533. [DOI] [PubMed] [Google Scholar]
- 30.Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC., Jr Lumbosacral chordoma. Prognostic factors and treatment. Spine (Phila Pa 1976) 1999;24:1639–45. doi: 10.1097/00007632-199908150-00004. doi: 10.1097/00007632-199908150.00004. [DOI] [PubMed] [Google Scholar]
- 31.Varga PP, Szövérfi Z, Fisher CG, Boriani S, Gokaslan ZL, Dekutoski MB, et al. Surgical treatment of sacral chordoma: Prognostic variables for local recurrence and overall survival. Eur Spine J. 2015;24:1092–101. doi: 10.1007/s00586-014-3728-6. doi: 10.1007/s00586-014-3728-6. [DOI] [PubMed] [Google Scholar]
- 32.Kayani B, Hanna SA, Sewell MD, Saifuddin A, Molloy S, Briggs TW. A review of the surgical management of sacral chordoma. Eur J Surg Oncol. 2014;40:1412–20. doi: 10.1016/j.ejso.2014.04.008. doi: 10.1016/j.ejso.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Asavamongkolkul A, Waikakul S. Wide resection of sacral chordoma via a posterior approach. Int Orthop. 2012;36:607–12. doi: 10.1007/s00264-011-1381-9. doi: 10.1007/s00264-011-1381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Housari G, González M, Calero P, Beni R, Lobo E. Sacral chordoma: Management of a rare disease in a tertiary hospital. Clin Transl Oncol. 2013;15:327–30. doi: 10.1007/s12094-012-0919-7. doi: 10.1007/s12094-012-0919-7. [DOI] [PubMed] [Google Scholar]