Abstract
Background:
Adenomyosis (AM) has impaired contraction. This study aimed to explore the expression of potassium channels related to contraction in myometrial smooth muscle cells (MSMCs) of AM.
Methods:
Uterine tissue samples from 22 patients (cases) with histologically confirmed AM and 12 (controls) with cervical intraepithelial neoplasia were collected for both immunohistochemistry and real-time polymerase chain reaction to detect the expression of large conductance calcium- and voltage-sensitive K+ channel (BKCa)-α/β subunits, voltage-gated potassium channel (Kv) 4.2, and Kv4.3. Student's t-test was used to compare the expression.
Results:
The BKCa-α/β subunits, Kv4.2, and Kv4.3 were located in smooth muscle cells, glandular epithelium, and stromal cells. However, BKCa-β subunit expression in endometrial glands of the controls was weak, and Kv4.3 was almost undetectable in the controls. The expression of BKCa-α messenger RNA (mRNA) (0.62 ± 0.19-fold decrease, P < 0.05) and Kv4.3 mRNA (0.67 ± 0.20-fold decrease, P < 0.05) decreased significantly in the MSMCs of the control group compared with the AM group. However, there were no significant differences in BKCa-β subunit mRNA or Kv4.2 mRNA.
Conclusions:
The BKCa-α mRNA and the Kv4.3 mRNA are expressed significantly higher in AM than those in the control group, that might cause the abnormal uterus smooth muscle contractility, change the microcirculation of uterus to accumulate the inflammatory factors, impair the endometrium further, and aggravate the pain.
Keywords: Adenomyosis, Potassium Channels, Uterine Contraction, Uterus Smooth Muscle Cells
INTRODUCTION
Adenomyosis (AM) is characterized by invasion of the myometrium by endometrial glands and stroma. It affects women mainly in their reproductive period. Patients with AM suffer from dysmenorrhea (30%), menorrhagia (50%), metrorrhagia (20%), and dyspareunia, as well as infertility. The pathogenesis of AM is unknown. A new understanding of the pathophysiology of such cases became possible when the cyclic peristaltic activity of the nonpregnant uterus was discovered.[1] Uterine peristalsis in women of reproductive age was demonstrated by transvaginal ultrasound, magnetic resonance imaging (MRI), and hysterosalpingoscintigraphy. Women with symptomatic AM exhibited increased uterine contractility associated with dysmenorrhea.[2] It was also documented by MRI that sperm transport was disturbed by hyperperistalsis when at least one focus of AM could be detected, and dysperistalsis when diffuse AM. This unusual peristalsis is also considered to be associated with the pathogenesis of AM.[3] It is well known that the changes in the expression or activity of K+ channels in myometrial smooth muscle cells (MSMCs) can cause inadequate membrane repolarization, resulting in abnormal uterine activity. It has been reported that the expression levels of oxytocin, prostaglandins, their receptors, and other cytokines in MSMCs in women with AM were related with nonpregnant uterine contractility. However, how ion channels of MSCMs are affected in cases of AM is unknown. Several types of potassium channels have been identified in the myometrium, mainly including the large conductance calcium- and voltage-sensitive K+ channel (BKCa channel), the ATP-sensitive K+ channel, voltage-gated potassium channels (Kvs), and small-conductance calcium-sensitive potassium channels.[4] Here, we aimed to measure the expression levels of the BKCa-α and -β subunits, Kv4.2, and Kv4.3 in MSCMs from women with AM to explore the pathogenesis of AM with the aim of developing new therapies to prevent or treat it.
METHODS
Patients and samples
This study was approved by the Human Ethics Committees of Peking Union Medical College Hospital, and all women gave their informed consent for participation. Thirty-four specimens from women who had undergone a hysterectomy (22 with AM and 12 controls) without using exogenous hormones were selected for the study. Clinical characteristics such as age, proliferative phase/secretory phase, hypermenorrhea, dysmenorrhea, fibroids, and any history of intrauterine manipulation were studied. All participants were parous. There was no statistically significant difference between the mean age of the women in the AM group (40.7 ± 4.6 years) and the control group (41.7 ± 5.8 years). Full-thickness histological sections including the serosa were obtained from the posterior uterine wall near the fundus. AM was defined by the presence of endometrial glands and stroma through >25% of the myometrial thickness.
Immunohistochemistry
Serial 4-mm sections were immunostained for BKCa-α, BKCa-β, Kv4.2, and Kv4.3 using standard techniques. Briefly, all excised lesions were fixed immediately in buffered 4% formalin for 24 h and then embedded in paraffin wax. The first two sections were stained with hematoxylin and eosin for identifying AM, and the remaining four sections of each case were immunostained with peptide-specific antibodies against BKCa-α, BKCa-β, Kv4.2, and Kv4.3 (all from Abcam, Cambridge, UK).
A heat-induced epitope retrieval procedure was performed with the sections in 10 mmol/L citrate buffer (pH 6.0) for 20 min. Endogenous peroxidase and nonspecific binding were blocked with hydrogen peroxide and 3% bovine serum albumin, respectively (Zhongshan Goldbridge Biotechnology, Beijing, China). Sections were then incubated overnight at 4°C with primary polyclonal antibodies against BKCa-α (1:100 v/v). BKCa-β (1:300 v/v) Kv4.2 (1:300 v/v) or Kv4.3 (1:200 v/v), all diluted in phosphate-buffered saline (PBS). After three washes in PBS, sections were incubated with biotinylated goat anti-rabbit antibodies (1:400 v/v) (Dako, Cambridge, UK) for 15 min at 37°C. After three washes in PBS, sections were incubated with a horseradish peroxidase-conjugated streptavidin complex. Staining was completed by a 3-min incubation with 3,3′ diaminobenzidine tetrahydrochloride (Maixin-Bio, Fuzhou, China) producing a brown precipitate at the antigen site. Finally, sections were lightly counterstained with Mayer's hematoxylin (Sigma-Aldrich, St. Louis, MO, USA), dehydrated, cleared, and mounted with Permount mounting medium (Beijing Biosynthesis Biotechnology Co., Beijing, China). Negative control slides were run in parallel using PBS alone.
Laser-microdissection and RNA extraction
The myometrium was embedded in optimal cutting temperature compound, cut at 10 μm, and stored frozen at −80°C. Frozen sections were fixed in 100% methanol for 3 min and stained with 1% toluidine blue. The section was laser-microdissected using a Leica AS LMD System (Leica Camera Ag, Wetzlar, Germany) for epithelial and stromal areas, which were collected in small tubes, while the remaining tissue on the slice was used for studying the myometrium. Approximately, 8–10 sections were laser-microdissected for each case. Contamination with nontarget components was monitored morphologically. Total RNA was extracted from the tissue section using RNeasy Plus Micro kits (Qiagen, Hilden, Germany).
Real-time reverse-transcription polymerase chain reaction
RNA was reverse transcribed using oligo (dT) primers using PrimeScript® RT reagent kits (Takara Bio Inc., Otsu, Japan) according to the manufacturer's instructions. Reverse-transcription polymerase chain reaction (RT-PCR) was performed using the ABI PRISM 7500 Real-Time PCR System (Applied Biosystems Inc., Waltham, MA, USA). SYBR® Premix Ex Taq™ was from Takara Bio Inc. Gene-specific primers sequences are listed in Table 1, and values were normalized against those for the control, human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Amplification conditions were 95°C for 10 min and 40 cycles of 95°C for 5 s and 60°C for 34 s.
Table 1.
Primers used for real-time RT-PCR
| Subtype | Product size (bp) | Primer sequence (forward/reverse) | Annealing temperature (°C) |
|---|---|---|---|
| Kv4.2 | 154 | 5’-ATgCAgAgCAAACggAATggT-3’ | 60 |
| 5’CgTCCACAAACTCgTgATTCg-3’ | |||
| Kv4.3 | 230 | 5’-AgCTgATTgTCCTCAACgTgA-3’ | 60 |
| 5’-gTCgTCgTAggCAgAgATgC-3’ | |||
| BKCa-α | 191 | 5’-AgCAATATCCACgCgAACCAT-3’ | 60 |
| 5’-AAAgCCCACCACATgCgTT-3’ | |||
| BKCa-β1 | 80 | 5’-AAggTCAgAgCCAAATTCCAAg-3’ | 60 |
| 5’-AATAggACgCTggTTTCgTTC-3’ | |||
| GAPDH | 119 | 5’-CTCTgCTCCTCCTgTTCgAC-3’ | 60 |
| 5’-ACgACCAAATCCgTTgACTC-3’ |
RT-PCR: Reverse-transcription polymerase chain reaction; BKCa: large conductance calcium- and voltage-sensitive K+ channel; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; Kv: Voltage-gated potassium channel.
Statistical analysis
Data were expressed as n or mean ± standard error (SE). All statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Comparisons of the means of paired quantitative data means were performed using Student's t-tests. The Chi-square test was applied for statistical analysis of qualitative data. Statistical significance was defined as P < 0.05.
RESULTS
Clinical characteristics
The clinical and histopathological characteristics of the cases are summarized in Table 2. The two groups were similar in terms of age, menstrual phase, and history of any intrauterine manipulation. Among the 22 patients with AM, 13 had a focal AM, and 9 had a diffusely distributed tumor. However, the AM group had significantly more cases with a history of menorrhagia (83% vs. 9%), and with more severe dysmenorrhea (estimated using a subjective visual analog score).
Table 2.
Characteristics of patients with and without AM
| Characteristics | Patients with AM (n = 22) | Controls (n = 12) | χ2/t | P |
|---|---|---|---|---|
| Age (years) | 40.7 ± 4.6 | 41.7 ± 5.8 | 1.260* | >0.05 |
| Proliferative/secretory phase | 10/12 | 7/5 | 0.515† | >0.05 |
| AM distribution (focal/diffuse) | 13/9 | – | ||
| Menorrhagia (%) | 10/12 (83) | 1/11 (9) | 4.889† | <0.05 |
| Dysmenorrhea (VAS) | 7.3 ± 2.9 | 0 | ||
| Intrauterine manipulations (n) | 3.2 ± 2.1 | 2.3 ± 1.9 | 0.600* | >0.05 |
Values were shown as n/N or mean ± SE. *t; †χ2 value. “–”: Data not applicable; SE: Standard error; VAS: Visual analog scale; AM: Adenomyosis.
Detection of BKCa-α, BKCa-β, kv4.2, and kv4.3 in uterine tissues with and without adenomyosis
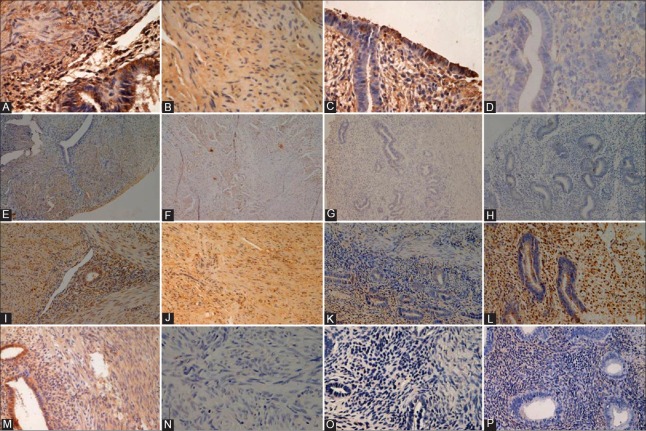
The BKCa-α subunit was found both in ectopic [Figure 1A] and eutopic [Figure 1C] endometrial tissues in the AM group, as well as in the controls [Figure 1D]. It was also detectable in MSMCs in both groups [Figure 1A and 1B]. The BKCa-β subunit was present in MSMCs of the AM [Figure 1E] and control groups [Figure 1F], and in the ectopic [Figure 1E] and eutopic [Figure 1G] endometrial tissues of the AM group. Its expression in the endometrium from patients without AM [Figure 1H] was weak. The distribution of kv4.2 [Figure 1I–L] was similar to that of the BKCa-α subunit. The intensity of kv4.3 was strong in MSMCs and ectopic endometrium of the AM group [Figure 1M]. In contrast, the eutopic endometrium in the AM group was mostly negative for this channel [Figure 1O]. Little kv4.3 expression was detectable from patients without AM [Figure 1N and 1P].
Figure 1.
Immunohistochemistry staining of paraffin-embedded sections. (A-D) Strong BKCa-α expression in smooth muscle cells with adenomyosis or not, ectopic or eutopic glandular epithelial cells and stromal cells (original magnification, ×400). So was the expression of Kv 4.2 (I-L) (original magnification, ×200). Moderate BKCa-β expression in smooth muscle cells of the adenomyotic lesion and the control myometrium (E-H) (original magnification, ×100). Weak Kv4.3 expression in myometrium without adenomyosis, while strong stained immunoreactive smooth muscle cell, ectopic glands, and stromas of adenomyosis (M-P) (original magnification, ×200).
Microdissection and real-time RT-polymerase chain reaction
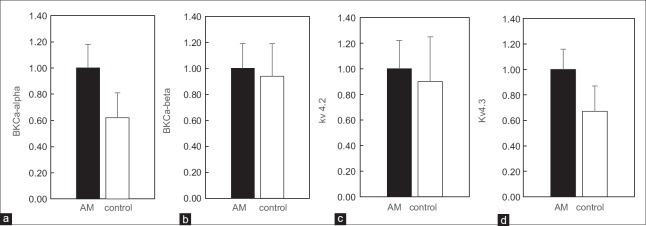
Human endometrial epithelia and stromal areas were laser-microdissected, and myometrium were collected for RNA extraction [Figure 2]. Total RNA was extracted and reverse transcribed into complementary DNA. Real-time RT-PCR was used to verify the changes in messenger RNA (mRNA) expression levels of BKCa-α, BKCa-β, Kv4.2, and Kv4.3. Each value was corrected for differences in loading relative to GAPDH mRNA expression. The mRNA levels of BKCa-α (0.62 ± 0.19-fold decrease, P < 0.05) and Kv4.3 mRNA (0.67 ± 0.20-fold decrease, P < 0.05) decreased significantly in the MSMCs of the control group compared with the AM group. However, no statistically significant changes in the expression levels of BKCa-β and Kv4.2 mRNA were observed between the two groups [Figure 3a–3d].
Figure 2.
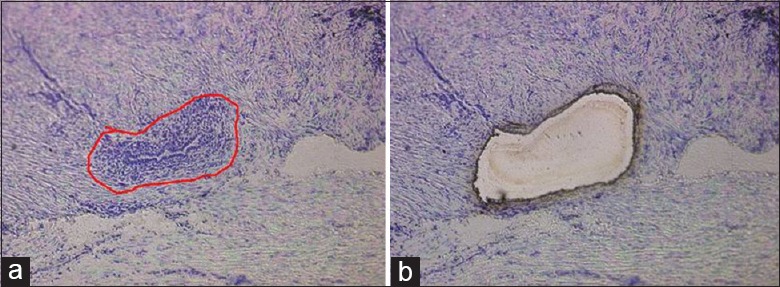
Human endometrial epithelia and stromal areas were laser-microdissected (a → b), and myometrium was left for RNA extraction (original magnification ×100).
Figure 3.
The relative mRNA expression in adenomyosis (AM) and the control group. The expression of BKCa-alpha (a) was significantly increased in myometrium of AM compared to the control group (P < 0.05), so was Kv4.3 (d). The relative expression of mRNA for BKCa-beta (b) and Kv4.2 (c) showed no statistically significant difference.
DISCUSSION
Irregular uterine contractility in the nonpregnant state of women with adenomyosis
Although there is growing awareness of the potential importance of the abnormal function of uterine smooth muscle, there has been little research concerning the role of the myometrium in the pathogenesis of AM. Uterine contractions (UCs) were observed throughout the menstrual cycle in nonpregnant women with the development of medical imaging techniques such as three-dimensional ultrasonography and MRI.[5] The frequency, amplitude, duration, and direction of muscle contractions were observed to change during the menstrual cycle in hormone-dependent ways.[6] Bulletti et al.[7] compared the UC patterns of women with or without endometriosis (EM) and found that patients with EM had UCs with higher frequency and greater basal muscle tone. A thicker junctional zone (exceeding 12 mm)[8] and hyperperistalsis can be detected via MRI when at least one focus of AM is present, and dysperistalsis is seen when it is diffuse. Leyendecker et al.[9] suggested that such permanent hyperperistalsis might lead to microtraumatization with activation of mechanisms of tissue injury and repair in the local and basal endometrial layers resulting in AM.
Potassium channel expressions in adenomyosis
We found that the BKCa-α subunit and Kv4.3 in the MSMCs of patients with AM were expressed significantly more strongly than in the control group. In another study of ours (unpublished), we found that expression of the BKCa-α subunit was slightly lower in the proliferative phase than in the secretary phase, but there were no statistically significant differences. How do these changes affect AM? Ion channels are well known to mediate uterine excitability during gestation and potassium channels have widespread distributions and maintain the uterus in a state of quiescence. They were shown to have an effect on contractility of the uterus in the nonpregnant rat.[10] The BKCa channel consists of four α subunits and an accessory β subunit, and the Kv4.x channels in human include three subtypes (Kv4.1, Kv4.2, and Kv4.3). Most studies on BKCa channels in MSMCs have concentrated on the pregnant state. It has been reported that at different stages of pregnancy, the BKCa channel varies its distribution density, and voltage-dependent/calcium-sensitivity to regulate the tension of the myometrium.[11] In the pregnant nonlabor state, increased intracellular Ca2+ levels lead to activation of the BKCa channel, effectively counteracting the depolarizing effects of Ca2+ entry while promoting K+ efflux, thereby maintaining myometrial quiescence. However with the onset of labor, BKCa-generated currents are almost completely blocked.[12] Hormonal regulation of myometrial tone during gestation might act through the activation or expression of ion channels. BKCa channels mediate uterine relaxation by modulating nuclear translocation of nuclear factor kappa B.[13] In animal models, BKCa channels contribute approximately 35% of the total myocyte repolarizing K+ currents in the nonpregnant state.
Different subtypes of Kv4 are distributed in different tissues, and different subtypes can be coexpressed in the same cell. In human myometrium, the most important subtype during pregnancy has been identified as the Kv4 subfamily.[4] Suzuki and Takimoto[14] found that Kv4.2 levels increased before labor, whereas Kv4.1 and Kv4.3 declined, suggesting that these ion channels might be involved in controlling the contractility of MSMCs during gestation. In an animal experiment, the Kv channel could be blocked by 4-aminopyridine and cause contractions in the nonpregnant mouse myometrium. However, this phenomenon disappeared after conception. Another study showed that the Kv4.2/Kv4.3 blocker phrixotoxin-2 could also lead to UCs in the nonpregnant, but not a pregnant mouse.[15] In ovariectomized rats, estrogen reduced currents in Kv4.3 channels and 17β-estradiol inhibited myometrial K+ channel activity in cultured cells, whereas progesterone had the opposite role.[16] These findings suggest that Kv4.2/4.3 channels might play an important roles in the regulation of uterine contractility both in pregnant and nonpregnant MSMCs.
Overexpression of BKCa and Kv4.3 channels in AM might suppress coordinated myometrial activity and maintain a low tone in the uterine smooth muscle layers. This would maintain the myometrium in a relaxed state. This might slow down the blood flow in the microcirculation in the endometrium, resulting in the accumulation of prostaglandins and other pain-related factors, and retard the elimination of acidic metabolites. These factors could well explain the key symptoms of AM such as menorrhagia, dysmenorrhea, and subfertility. Mehasseb et al. found that women with AM showed altered contractility and ultrastructural features in the inner myometrium.[17]
Technical difficulty of this experiment
We found that cells in the myometrium and endometrium in the endometriotic lesions in AM tissues played specific roles, and all expressed the BKCa, Kv4.2, and Kv4.3 channels. However, it is very difficult to eliminate interference of other cell components accurately even with the most advanced technologies such as next generation sequencing and proteomics. In vitro cell culture is widely accepted in cell biology; however, isolation and culture of the cells changes their composition, as well as the environment. As early as 1997, Zhang et al.[18] reported differences in gene expression between the original colon cancer cells and colon cancer cell lines. How to isolate the MSMCs from the AM lesions is important in such experiments.
In this study, we obtained myometrium from AM tissues using laser microdissection and applied real-time RT-PCR. A rapid and harmless staining method was used to differentiate the target from surrounding tissues. However, section dehydration and RNA degradation were the most difficult problems in this experiment. We improved the experimental program as follows. (1) The frozen block of tissue was sectioned using a cryomicrotome at 10 μm thickness. This protected the inner layer of cells, and also increased the amount of tissue that could be obtained. (2) Tissues were stained with 1% toluidine blue instead of hematoxylin.[19] As toluidine blue is a quick biological stain for nuclei, we could greatly reduce the time of exposure to the environment and minimize the damage to ion channels expressed in the membrane and cytoplasm. (3) Sections were dehydrated in alcohol diluted with RNase-free water and then incubated further,[20] so that the laser-microdissected endometrium could be detached from the coated glass slides rapidly.
To summarize, we found that mRNAs for the BKCa-α subunit and Kv4.3 were expressed significantly more in the myometrium with AM than in normal myometrium. The elevated levels of these ion channels might lead to a relatively relaxed smooth muscle layer. This in turn might change the microcirculation of the uterus and facilitate the accumulation of inflammatory factors in the uterine wall, thereby aggravating abnormal UCs and pain. This study will help us to understand the pathogenesis of AM from a new perspective, and explain its symptoms such as menorrhagia and dysmenorrhea. Moreover, inhibition of abnormal UCs with progesterone or oral contraceptives might be effective in protecting women against AM. We also hope to find new ways to cure AM from an ion channel perspective. Therefore, we plan more studies to explore this field further.
Financial support and sponsorship
This study was supported by a grant of National Natural Science Foundation of China (No. 30872747).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Nakai A, Koyama T, Fujimoto K, Togashi K. Functional MR imaging of the uterus. Magn Reson Imaging Clin N Am. 2008;16:673–84. doi: 10.1016/j.mric.2008.07.010. ix. doi: 10.1016/j.mric.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Guo SW, Mao X, Ma Q, Liu X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril. 2013;99:231–40. doi: 10.1016/j.fertnstert.2012.08.038. doi: 10.1016/j.fertnstert.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G, et al. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet. 2015;291:917–32. doi: 10.1007/s00404-014-3437-8. doi: 10.1007/s00404-014-3437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol. 2007;18:332–9. doi: 10.1016/j.semcdb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulletti C, de Ziegler D, Polli V, Diotallevi L, Del Ferro E, Flamigni C. Uterine contractility during the menstrual cycle. Hum Reprod. 2000;15(Suppl 1):81–9. doi: 10.1093/humrep/15.suppl_1.81. [DOI] [PubMed] [Google Scholar]
- 6.Shaked S, Jaffa AJ, Grisaru D, Elad D. Uterine peristalsis-induced stresses within the uterine wall may sprout adenomyosis. Biomech Model Mechanobiol. 2015;14:437–44. doi: 10.1007/s10237-014-0614-4. doi: 10.1007/s10237-014-0614-4. [DOI] [PubMed] [Google Scholar]
- 7.Bulletti C, De Ziegler D, Polli V, Del Ferro E, Palini S, Flamigni C. Characteristics of uterine contractility during menses in women with mild to moderate endometriosis. Fertil Steril. 2002;77:1156–61. doi: 10.1016/s0015-0282(02)03087-x. [DOI] [PubMed] [Google Scholar]
- 8.Novellas S, Chassang M, Delotte J, Toullalan O, Chevallier A, Bouaziz J, et al. MRI characteristics of the uterine junctional zone: From normal to the diagnosis of adenomyosis. AJR Am J Roentgenol. 2011;196:1206–13. doi: 10.2214/AJR.10.4877. doi: 10.2214/AJR.10.4877. [DOI] [PubMed] [Google Scholar]
- 9.Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch Gynecol Obstet. 2009;280:529–38. doi: 10.1007/s00404-009-1191-0. doi: 10.1007/s00404-009-1191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novakovic R, Ilic B, Beleslin-Cokic B, Radunovic N, Heinle H, Scepanovic R, et al. The effect of resveratrol on contractility of non-pregnant rat uterus: The contribution of K(+) channels. J Physiol Pharmacol. 2013;64:795–805. [PubMed] [Google Scholar]
- 11.Khan RN, Matharoo-Ball B, Arulkumaran S, Ashford ML. Potassium channels in the human myometrium. Exp Physiol. 2001;86:255–64. doi: 10.1113/eph8602181. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Cong B, Zhang L, Ni X. Expression of the calcium-activated potassium channel in upper and lower segment human myometrium during pregnancy and parturition. Reprod Biol Endocrinol. 2009;7:27. doi: 10.1186/1477-7827-7-27. doi: 10.1186/1477-7827-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Lorca RA, Ma X, Rhodes A, England SK. BK channels regulate myometrial contraction by modulating nuclear translocation of NF-κB. Endocrinology. 2014;155:3112–22. doi: 10.1210/en.2014-1152. doi: 10.1210/en.2014-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T, Takimoto K. Differential expression of Kv4 pore-forming and KChIP auxiliary subunits in rat uterus during pregnancy. Am J Physiol Endocrinol Metab. 2005;288:E335–41. doi: 10.1152/ajpendo.00250.2004. [DOI] [PubMed] [Google Scholar]
- 15.Smith RC, McClure MC, Smith MA, Abel PW, Bradley ME. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol. 2007;5:41. doi: 10.1186/1477-7827-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knock GA, Tribe RM, Hassoni AA, Aaronson PI. Modulation of potassium current characteristics in human myometrial smooth muscle by 17beta-estradiol and progesterone. Biol Reprod. 2001;64:1526–34. doi: 10.1095/biolreprod64.5.1526. [DOI] [PubMed] [Google Scholar]
- 17.Mehasseb MK, Bell SC, Pringle JH, Habiba MA. Uterine adenomyosis is associated with ultrastructural features of altered contractility in the inner myometrium. Fertil Steril. 2010;93:2130–6. doi: 10.1016/j.fertnstert.2009.01.097. doi: 10.1016/j.fertnstert.2009.01.097. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–72. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 19.Yanaihara A, Otsuka Y, Iwasaki S, Koide K, Aida T, Okai T. Comparison in gene expression of secretory human endometrium using laser microdissection. Reprod Biol Endocrinol. 2004;2:66. doi: 10.1186/1477-7827-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuzaki S, Canis M, Mage G. Use of laser capture microdissection in studying hormone-dependent diseases: Endometriosis. Methods Mol Biol. 2009;590:295–306. doi: 10.1007/978-1-60327-378-7_19. doi: 10.1007/978-1-60327-378-7_19. [DOI] [PubMed] [Google Scholar]