Abstract
Objective:
This paper aimed to review the current literature on the surface modification of intraocular lenses (IOLs).
Data Sources:
All articles about surface modification of IOLs published up to 2015 were identified through a literature search on both PubMed and ScienceDirect.
Study Selection:
The articles on the surface modification of IOLs were included, but those on design modification and surface coating were excluded.
Results:
Technology of surface modification included plasma, ion beam, layer-by-layer self-assembly, ultraviolet radiation, and ozone. The main molecules introduced into IOLs surface were poly (ethylene glycol), polyhedral oligomeric silsesquioxane, 2-methacryloyloxyethyl phosphorylcholine, TiO2, heparin, F-heparin, titanium, titanium nitride, vinyl pyrrolidone, and inhibitors of cytokines. The surface modification either resulted in a more hydrophobic lens, a more hydrophilic lens, or a lens with a hydrophilic anterior and hydrophobic posterior surface. Advances in research regarding surface modification of IOLs had led to a better biocompatibility in both in vitro and animal experiments.
Conclusion:
The surface modification is an efficient, convenient, economic and promising method to improve the biocompatibility of IOLs.
Keywords: Biocompatibility, Capsule Biocompatibility, Cataract, Intraocular Lenses, Surface Modification, Uveal Biocompatibility
INTRODUCTION
Surgical intervention is the main treatment option for symptomatic cataract and phacoemulsification combined with intraocular lenses (IOLs) implantation is the main method. Surgical complications such as endophthalmitis, cystoid macular edema (CME), or posterior capsule opacification (PCO) could affect the visual outcome and even potential blinding. The reported incidence of these complications were 0.1–0.2% for endophthalmitis,[1] 0.1–2.35% for CME,[2] and 20–40% for PCO.[3,4] The exact etiologies of all these complications remain unclear, and association with the IOL implantation have been suggested. Endophthalmitis could have resulted from bacteria adhering to the implanted IOLs with subsequent biofilm formation;[5] leukotrienes and prostaglandins stimulated by IOLs could increase the retinal vessel permeability and lead to CME;[6,7] and PCO resulted from lens epithelial cells (LEC) adherence, proliferation and epithelial-mesenchymal transition (EMT) on IOLs.[8,9] Although various treatment options are available for these complications, we believe prevention is the best cure, and choosing the most biocompatible IOL is an essential prevention measure.
INTRODUCTION FOR BIOCOMPATIBILITY
Biocompatibility refers to materials that can function well and is compatible with surrounding tissues. The first original IOL was made of polymethylmethacrylate (PMMA) and was first described by Ridley in 1949s.[10] Until date, there are mainly four types of IOL materials: Hydrophobic acrylic, hydrophilic acrylic, silicone, and PMMA.[11] Currently, the biocompatibility of IOL does not have a uniform definition. Amon[12] classified biocompatibility into uveal biocompatibility and capsule biocompatibility. Uveal biocompatibility referred to the foreign body inflammatory response of the uveal tissue to the IOL, while capsule biocompatibility referred to the wound healing response of the LEC to the IOL. Miyake et al.[13] proposed contact angle, aqueous flare, and anterior capsule opacification as standards for evaluating biocompatibility. However, whether an IOL material is biocompatible or not also depends on the clinical scenario. For example, the hydrophobic acrylic has the lowest PCO rate. However, it is unsuitable for uveitis patients due to its strong adherence to foreign body cell. A hydrophilic acrylic IOL would be more biocompatible in these cases;[14,15] an ultraviolet (UV)-absorbing IOL is superior for diabetic patients;[16,17] the silicone IOL is out of alternative to the patients with high myopia at high-risk vitrectomy.[18] No single type of IOLs is suitable for all cases.
FACTORS DETERMINING BIOCOMPATIBILITY
Many factors affect the IOL biocompatibility. These include patient demographics (for example, younger the patients, higher the rate of PCO[19,20]), surgeons’ technique (a smaller capsulorhexis is preferable to a large capsulorhexis in reducing PCO[21,22]), the surgical approach (femtosecond laser-assisted cataract operation cause less damage to peripheral ocular tissue ocular and are more uveal-biocompatible[23]), and the IOL itself, which is often the determining factor.[24,25] IOL factors include the design and the material, which include intrinsic properties of the material and the surface properties. In 1999s, Nishi et al.[26] found that lens with the square edges inhibited LEC migration and PCO formation, and this was later verified in other studies.[27,28] In addition to changes in the lens design, IOL surface materials may also improve the overall IOL biocompatibility as the surface is directly in contact with the ocular tissues, cells, proteins, and inflammation mediators.[29] Surface modification of IOL can modify the contact stimulus of the IOL material. Several studies have shown that surface modification with physical or chemical methods (without altering the main IOL bulk) is a promising way to alter the surface characteristics.[30,31]
TECHNOLOGY OF SURFACE MODIFICATION
Surface modification methods include the surface coating technique,[32,33] surface grafted modification,[34,35] plasma surface modification,[36] photochemical immobilization,[37] and layer-by-layer-self-assembly.[38,39] These techniques could be employed alone or combined with others.
POLYMETHYLMETHACRYLATE
PMMA was the first IOL material introduced by Ridley in 1949s,[10] and is being implanted for almost 66 years.[40] PMMA has many advantages such as excellent tissue tolerance, less foreign body inflammatory response, higher uveal biocompatibility, relatively higher refractive index (1.49), better optical clarity, light-weighted, and stable in nature.[41] However, in view of their rigidity and intolerance to high pressure and high temperature, PMMA has almost been completely replaced by modern foldable lenses. Nevertheless, PMMA is still available from various companies due to its low cost.
HEPARIN-SURFACE-MODIFIED POLYMETHYLMETHACRYLATE INTRAOCULAR LENSE
Since Boffa et al.[42] compounded the first heparin-surface-modified (HSM) PMMA by a radical polymerization of methyl methacrylate (MMA) from oxidative reaction initiated by Ce4+ ions in the presence of heparin in 1979s, it has been in use for nearly 36 years. Heparin is a type of anticoagulant, which has an effect on the expression of adhesion molecules and matrix degrading enzymes. The heparin modification of PMMA IOL changed the surface property from hydrophobic to hydrophilic, which resulted in a reduced inflammatory cell adhesion and, therefore, a better uveal biocompatibility. Roesel et al.[14] performed a prospective randomized study on patients with endogenous uveitis and concluded that the more hydrophilic IOLs such as HSM PMMA had a lower rate of uveitis after cataract surgeries. Other studies confirmed that HSM PMMA have good uveal biocompatibility in uveitic patients.[43,44] However, Rønbeck and Kugelberg[45] made a 12-year prospective study on PCO rate of HSM PMMA, silicone, and hydrophobic acrylic IOL and concluded that HSM PMMA IOL had a significantly higher PCO rate. The different PCO rates with different IOL materials showed that both “material surface factor” and “design factor” were important. A more hydrophilic IOL surface such as HSM PMMA had a lower inflammatory rate.
HYDROXYPROPYL-POLY (ETHYLENE GLYCOL)-POLYMETHYLMETHACRYLATE
Zhang and Wu[46] prepared a hydroxypropyl-poly (ethylene glycol) (Hp-PEG)-PMMA IOL with Ar plasma and both Hp and PEG in a plasma atmosphere. Heparin is ordinarily used as anticoagulant, PEG is nontoxic, nonimmunogenic, and nonantigenic polymer and may decrease the attractive forces between surfaces and proteins, so the new Hp-PEG-PMMA IOL was expected to have a remarkable anti-thrombogenicity and better uveal biocompatibility. They also performed a platelet adhesion experiment, and the result showed that the surface platelet was distinctly reduced compared with the pristine PMMA.[46] The contact angle of Hp-PEG-PMMA was 61.68° compared with 75.6° of PMMA, which meant that the hydrophilic property had increased. This study made Hp-PEG-PMMA conceivable, although further assessment is needed.
ALLYL POLYHEDRAL OLIGOMERIC SILSESQUIOXANE-POLYMETHYLMETHACRYLATE
In contrary to IOL surface modification, polyhedral oligomeric silsesquioxane (POSS)-PMMA changes the IOL properties by a bulk modification. POSS consists of a cage-like inorganic core based on Si-O-Si bonds and the shell composed of eight surrounded organic groups, resulting in a regular structure with good biocompatibility, small scale, and large surface area, thus making POSS one of the most promising biomaterials.[47] Wang et al.[48] tried to synthesize POSS-PMMA by dissolving in a 50 ml round bottom flask, allyl POSS (0.34 g, 0.40 mmol), MMA (2.0 g, 20.0 mmol) and azobisisobutyronitrile (AIBN; 0.025 g, 0.15 mmol) in ethyl acetate (16 ml) and tetrahydrofuran(THF; 4 ml) under a nitrogen atmosphere. The water contact angle (WCA) of allyl POSS-PMMA was higher than PMMA by 12°, which meant that allyl POSS-PMMA was more hydrophobic. Human lens epithelial cells on the surfaces of allyl POSS-PMMA copolymer films had a better morphology than that on the surface of PMMA, which meant allyl POSS-PMMA copolymer had a better capsule biocompatibility. This new IOL still requires more in vivo study to support its use.
TIO2 MODIFICATION POLYMETHYLMETHACRYLATE
TiO2, a photocatalyst, can produce electrons and holes pairs when activated by light energy.[49] Electrons and holes pairs generate hydroxy radical and oxygen radical while reacting with H2 O and the oxygen soluble in H2 O. Hydroxy radical and oxygen radical have a strong antioxidant ability, which may cause protein denaturation and lipid degradation, thus possessing a variation of the bacterial films and inhibition of cell proliferation.[50] Yang et al.[51] proposed amazing composite films of TiO2 and PMMA. Generally, the contact angle of this PMMA/TiO2 composite films reached 140° at 50 vol% of TiO2, which kept a state of high hydrophobicity; however, when exposed to UV light, PMMA on the surface of the mixture film turned to be super-hydrophilic, and this state could maintain several months. The proposed mechanisms were that the water affinity was very sensitive to surface states of the TiO2, and TiO2 was the alignment of a self-assembled monolayer (SAM) on its hydrophilic surface, which has been made for creating the hydrophobic or super hydrophobic surface on SAM treated TiO2. When exposed to UV light, the coated polymer layer was removed, and the surface returned to the hydrophilic state. As a result, the former more hydrophilic IOL surface of IOLs leads to less postoperative inflammation, and the later more hydrophobic surface means a lower PCO rate.
F-HEPARIN SURFACE MODIFICATION PMMA INTRAOCULAR LENSES
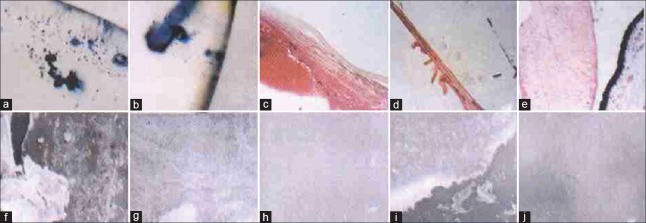
Fluoride (F) is a hydrophobic molecule. IOLs treated with F had a higher contact angle and became more hydrophobic. On the other hand, heparin is hydrophilic, and when grafted on a substrate, the heparin could make it more hydrophilic. Yuan et al.'s team[52] modified PMMA with F combined with heparin by carrier energy ion beam combined low temperature and low-pressure plasma. When PMMA was treated with F, part of the hydroxyl on PMMA surface cracked and chemical bond dissociated, which made PMMA easier to integrate with heparin.[53] The F-HSM PMMA passed the transmittance, resolution and diopter tests, which meant that the modification did not change the functions as IOLs. This F-HSM PMMA possessed a composition of hydrophilic radical and hydrophobic groups phase separation, which made PMMA a balanced surface of hydrophilic and hydrophobic. Platelet and macrophages in rat abdominal cavity were less adhesive on F-heparin PMMA compared with the PMMA. And the standard error of mean (SEM) results of PMMA implanted for 180 days and 360 days showed that granulated matters and protein films on the margin of F-HSM PMMA, but much meshed fibers in PMMA groups, which showed a better uveal and capsule biocompatibility of F-heparin PMMA.[53] Wang et al.'s[54] study in monkeys had the same conclusion. Furthermore, Wang et al. made a first and unique biocompatibility evaluation of the modified IOL in monkeys’ eyes. The results are shown in Figure 1.[53] The monkey is the nearest to human beings in terms of organic evolution, anatomical structure, and physiological functions, so the experiment in monkeys’ eyes should be fairly convincing. The F-HSM PMMA had obtained government approval and was to proceed to clinical studies.
Figure 1.
(a) Many cells adhered to the surface of polymethylmethacrylate intraocular lense; (b) scanty cells on the F-heparin-surface-modified intraocular lenses; (c) fibrous cataract; (d) elschningæs body; (e) soemmeringæs ring; (f) surface membrane on the polymethylmethacrylate intraocular lense 180 days after implantation; (g) surface membrane on the heparin-surface-modified intraocular lense 180 days after implantation; (h) surface membrane on the F-heparin-surface-modified intraocular lense 180 days after implantation; (i) surface m embrane on the polymethylmethacrylate intraocular lense 360 days after implantation; (j) surface membrane on the F-heparin-surface-modified intraocular lense 360 days after implantation.
SILICONE
Mehta et al.[55] and Epstein[56] implanted the silicone IOLs in the mild-1970s and Epstein et al. tried to make foldable silicone IOLs in the early 1980s. Until date, the silicone IOLs has widespread clinical applications,[57] as it possess inertness, chemical stability, autoclavability, flexibility, thermostability, breakdown voltage, low interface energy and water content of 0.38% and the refractive index of 1.43.[58] Silicone IOL, however, may have a higher incidence of endophthalmitis, posterior synechiae formation and giant-cell adhesion due to its tendency to adhere bacteria, air particle and inflammatory cells, and a relatively higher incidence of anterior capsule contraction.[59,60]
METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE GRAFTED ON SILICONE INTRAOCULAR LENSES
2-methacryloyloxyethyl phosphorylcholine (MPC) imitates the phosphatide of the cellular member and with no antigenicity, and is super-hydrophilic.[61] Huang et al.'s team[62] pretreated silicone IOLs with air plasma (air pressure 0.4 Torr) for 5 min, then dropped the 5 ml MPC aqueous solution on IOL surface and air plasma again, thus making the MPC-modification silicone IOL, which possesses a hydrophilic anterior surface and a hydrophobic posterior surface. The MPC-modified IOL had a WCA of 36° compared with 110° of untreated silicone at 25°C. Compared with 2 peaks of flare value in control lens, the flare level in modified silicone was stable, and less small round cells, fibroblast-like cells, and epithelial cells adhesion. With a posterior Miyake-Apple view, the central PCO in this modified silicone IOL was the lowest. The modified silicone IOL showed superior uveal biocompatibility and capsule biocompatibility. Wang et al.'s[63] paper confirmed the MPC-modified silicone hydrogels repelled the proteins sufficiently. Huang et al.[62] also reported that the MPC modified silicone IOL reduced the silicone Oil adhesion effectively. Shimizu et al.[64] utilized MPC and bis (trimethylsilyloxy) methylsilylpropyl glycerol methacrylate to prepare a new SiMA-co-MPC contact lens.
HEPARIN-SURFACE-MODIFIED SILICONE INTRAOCULAR LENSE
HSM silicone IOL became more hydrophilic with mean contact angle of 40°, about 65° lower than the standard silicone. Arthur et al.[65] made a silicone oil test on HSM silicone IOL. The HSM silicone IOL revealed a mean silicone oil coverage of 6.7 ± 3.2% (ranging from 1.6% to 11.8%), while the standard silicone IOL 98.2 ± 3.2% (ranging from 90.0% to 100%), showing the significant difference (P = 0.00001). The HSM silicone IOL solved the problem of implanting silicone IOL in patients needing vitrectomy with silicone oil.
TITANIUM AND TITANIUM NITRIDE MODIFICATION SILICONE INTRAOCULAR LENSE
Titanium (Ti) is a metal with high mechanical strength and corrosion resistance and is widely used as a biomedical engineering material. On the other hand, nitrogen ion injected with polymer would improve corrosion resistance and abrasion resistance. Guiqin et al.[66] prepared a new type silicone soft IOLs using ion beam combined with low temperature and low-pressure plasma to make Ti and titanium nitride (TiN) modification. The Ti-modification and TiN-modification shared contact angle of 95.75° and 92.25° respectively while the silicone had a contact angle of 102.39°. The Ti and TiN modification silicone IOL had a good morphology passing the tests of UV/visible (VIS) scanning spectrophotometer, electron spectroscopy for chemical analysis and SEM. Wang's another paper about the rabbits’ response to the modification IOL showed a lighter anterior chamber exudation and PCO.[67] The platelet and macrophages adhesion experiment reported by Wang et al. showed that the Ti modification silicone IOL had a significantly less affinity to platelet and macrophages compared with the pure silicone IOL (P < 0.01). This meant that the Ti modification silicone IOL may have a lighter postoperative inflammation and better uveal biocompatibility. Moreover, the adhesion of Staphylococcus epidermidis and Pseudomonas aeruginosa on Ti modification were less than that on pristine IOL (P < 0.01). Thus, the endophthalmitis risk was smaller.
ACRYLIC INTRAOCULAR LENSE
Acrylic includes hydrophobic acrylic and hydrophilic acrylic. Since hydrophobic acrylic lenses was introduced in 1990s by Alcon company.[68] it has been the preferred material for the manufacture of IOL optics due to its foldable property.[69,70] Hydrophobic acrylic lenses have excellent properties, such as superb chemical inertness, optically transparent, a relatively high refractive index, and a special viscoelasticity availing IOL to unfold within 3–5 s which can be safely implanted and has the lowest PCO incidence.[71] Unfortunately, hydrophobic acrylic lenses have the heaviest surgically-induced inflammation, which limits its use in uveitis patients.[72,73] Hydrophilic acrylic IOL shares similar advantages, such as foldability, controlled unfolding behavior, less glistening and lower refractive index, which gives less glare. Hydrophilic IOL is mainly used for uveitis cases due to less adhesion of protein and cells, but because of the soft physical property of the hydrophilic acrylic material, the posterior square edge design is not as sharp as compared to the hydrophobic acrylic IOL, leading to a higher PCO rates.
A NEW HYDROPHOBIC ACRYLIC MATERIAL
Advanced Vision Science, a subsidiary of Santen Pharmaceutical Co. Ltd., in Japan, introduced a copolymer of hydroxylethyl methacrylate, PEG phenyl ether acrylate, and styrene crosslinked with ethylene glycol dimethacrylate, producing a new hydrophobic acrylic material. This IOL material was named Eternity-Uni W-60 and had been approved for use in the United Stated in February 2009 and in Japan in 2008.[74,75] Compared with the conventional hydrophobic acrylic IOLs, this material has a water content of nearly 4% and the contact angle of 76°. Ollerton et al.[76] made a short-term evaluation at 4-week after implanting IOL in rabbits and concluded that the new hydrophobic acrylic has the potential to enhance PCO prevention.
POLY (ETHYLENE GLYCOL) MODIFIED HYDROPHOBIC ACRYLIC INTRAOCULAR LENSE
Lina et al.[77] grafted the PEG on hydrophobic acrylic with the process of immobilizing PEGs with argon as the discharge gas by atmospheric pressure glow discharge (APGD). The PEG-grafted IOL gained a good hydrophilicity with a WCA of 43.3°. As a result, platelet adhesion and macrophage adhesion were scarcely seen (P < 0.01), and appeared to be in an inactive state, which showed perfect uveal biocompatibility. Moreover, when LECs planted into the IOL surface, small-sized spherical or ellipsoid-shaped LECs were the typical forms in an overwhelming majority on PEG-grafted IOL surfaces. Even after incubated for 72 h, only a few individual cells flattened into hexagonal or elliptical shapes and attached to the IOLs surface, along with a small increase in cell quantity and slightly increased proliferation, indicating great capsule biocompatibility.
PYRROLIDONE-MODIFICATION HYDROPHOBIC ACRYLIC INTRAOCULAR LENSE
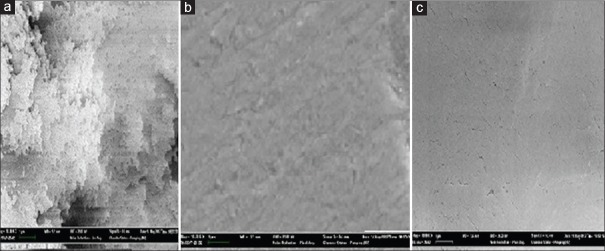
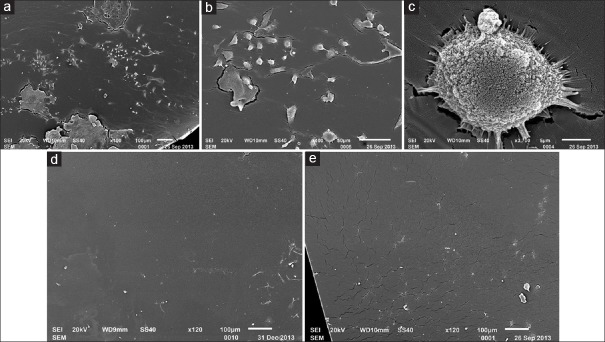
N-vinyl pyrrolidone (NVP) is a hydrophilic monomer, which compose of nitrogen atoms five-membered ring. The nitrogen bone with a vinyl monomer made NVP apt to polymerization and hydrophilic. Wang et al.[78] made the hydrophobic acrylic IOL surfaces with free radicals by 400 eV argon ion bombardment and then chemical grafted with vinyl pyrrolidone monomer on it to form a super hydrophilic film. The modification IOL containing 50% NVP showed flatter, more smooth and homogeneous. The SEM, WCA, and UV-vis spectrophotometer showed the modification IOL had a perfect morphology with a hydrophilic anterior surface and a hydrophobic posterior surface. The NVP modification IOL absorbed a small amount of protein particles and scattered inactivated round cells compared with geographic protein and forms of tufted cells on the control IOL surfaces in rabbits’ experiment. The rabbits’ corneal edema with NVP grafted hydrophobic acrylic IOL were lighter, and the exudation in the anterior chamber were less compared with the control (P < 0.05). The NVP modification on the anterior surface of hydrophobic acrylic IOL exhibited good uveal biocompatibility. The maintained hydrophobic posterior surface of NVP-modification showed the good capsule biocompatibility as the control. The results are shown in Figures 2 and 3.[79]
Figure 2.
Intraocular lense under scanning electron microscope: (a) nonmodified hydrophobic acrylic intraocular lense; (b) TiO2 modified hydrophobic acrylic intraocular lense; (c) N-vinyl pyrrolidone modified hydrophobic acrylic intraocular lense.
Figure 3.
(a) Cells and protein membrane adhered to the surface of nonmodified intraocular lense; (b) kinds of cells adhered to the surface of nonmodified intraocular lense; (c) adhered macrophage had many long and thin cytoplasmic synapsis; (d) less macrophages and membrane remnants adhered to the surface of TiO2 modified intraocular lense; (e) few cells and proteinic granularity adhered to the surface of N-vinyl pyrrolidone modified intraocular lense.
HYDROXYPROPYL-MODIFICATION HYDROPHOBIC ACRYLIC INTRAOCULAR LENSE
Krall et al.[80] made a comparison between HSM hydrophobic acrylic (EC-1YH PAL) and the same IOL without heparin coating (EC-1Y-PAL) by the flare and cell intensity in the anterior chamber after cataract surgery. The mean flare value was statistically significantly lower in the HSM IOL group than in the uncoated IOL group 1 day postoperatively (P = 0.04) and a greater and quicker decrease reaching statistical significance 1 month postoperatively (P = 0.01). The HSM hydrophobic acrylic exhibited a better uveal biocompatibility.
FUNCTIONALIZED HYDROPHOBIC ACRYLIC INTRAOCULAR LENSE
Tissue growth factor beta 2 (TGF-β2) is an important factor of the lens epithelial-mesenchymal transformation (EMT) signaling cascade that promotes PCO. Thus, blocking the TGF-β2 will decrease the PCO growth rate. Sun's et al. team[81] prepared anti-TGF-β2 antibody functionalized hydrophobic acrylic IOL as follows: APGD plasma to produce negatively charged chemical groups, then depositing polyethylenimine onto IOL surfaces as a precursor monolayer and then anti-TGF-β2 (anti-T) antibody and poly-l-lysine sequentially onto the surface for four cycles followed by another upmost monolayer of anti-T antibody via layer-by-layer self-assembly technique. Quartz crystal microbalance revealed that the anti-T antibody immobilized on IOL surfaces was nearly 800 ng, enough to in situ capture and neutralization of the aqueous TGF-β2, compared with hundreds of pg in active TGF-β2in the aqueous humor. Anti-T antibody functionalized IOL surface possessed WCA of 73.2°, which was an evident increase in surface hydrophility (P < 0.01). Anti-T antibody multilayers on IOL surfaces had good stability, which maintained at least 3 months. Compared with the untreated IOLs, the anti-T antibody functionalized IOL greatly restrained LECs from migration and EMT, yet anti-T antibody multilayers did not manage to the optical and physical properties of IOLs. The anti-T antibody functionalized IOL improve the uveal and capsule biocompatibility.
RGD SURFACE FUNCTIONALIZATION OF THE HYDROPHILIC ACRYLIC INTRAOCULAR LENSE
RGD (Arg-Gly-Asp sequence) is a cell adhesion peptide with the sequence of the functional motif of fibronectin. RGD can be recognized by numerous integrins in various cell types, thus can absorb different cells. Huang et al.[82] grafted reticulated polymer of 25% water content (HA25) containing RGD peptide on to the surface the hydrophilic acrylic IOL. The function process was as follower. The new IOL possessed a contact angle of about 57° and MTS cytotoxicity assay resulted in above 70% of viability cells in conditional media, which meant that the RGD-functional hydrophilic IOL improved the affinity to the LECs and the LECs exhibited better morphology.
CONCLUSIONS
IOLs implantation is not only used in cataract surgery, but also in refractive surgery, such as high myopia and presbyopia. With such an extensive use, a demand for perfectly biocompatible IOLs is called upon. Understanding the biocompatibility of IOLs could better guide us on IOLs’ modification. The uveal biocompatibility not only rely on the quantities of the white cells adhesion to the surface, but also the ratios of mononuclear leucocyte being activated to the macrophage.[83] Similarly, the proportion of the LECs EMT is another criterion of assessing capsule biocompatibility.[84] Good understanding of the relationship between IOL surface and the biocompatibility benefits the proposed surface modification. With the small incision advocated, foldable soft IOLs is the most popular choice among clinicians. Nevertheless, the PMMA IOLs are still available in clinics and are still being implanted widely into eyes in developing countries because of its more economical cost and long-term stability.[85]
For most studies, more hydrophilic IOL surface meant a better uveal biocompatibility, while a more hydrophobic surface meant a better capsule biocompatibility. Recently, the surface modification focused on making the “perfect” IOL with a hydrophilic anterior surface and a hydrophobic posterior surface. New attempts include the PEG, MPC, TiO2, vinyl pyrrolidone, and special drugs self-assembled. PEG is widely used as the modification molecular. The PEG-grafted surface repels proteins due to the high dipole moment of the ethylene oxide repeats and extensive hydration.[86,87] Vinyl pyrrolidone is safety hydrophilic molecule and has been frequently used for membrane synthesis, it possesses active double bond and is easy to be initiated to produce its polymer poly-N-vinyl-pyrrolidone, which has a wide application. Therefore, biomaterials modified with phospholipid analogs have the potential for use in a wide range of medical applications, including soft contact lenses, membranes for artificial kidneys, vascular prostheses, artificial joints, and urological devices[88,89] All hydrophilic molecules makes the IOL surface hydrophilic, leading to a better uveal biocompatibility, though, the hydrophilicity is yet to reach perfection. Some studies reported more hydrophobic surface modification.
For some clinicians, the anterior surfaces hydrophilic modification makes no sense because postoperative inflammation is milder due to surgical technique improvement and widespread small incision. In fact, the uveal biocompatibility and capsular biocompatibility are not individual properties and should interact each other. It has been proposed that inflammatory cells (e.g., macrophages, giant cells) secrete cytokines, which may in turn affect the behavior of LECs, resulting in severe PCO.[90,91,92] Some reports proposed that PCO is a special form of inflammation.[93] As a result, PCO has been extensively studied. However, there is no clear mechanism to explain the cause. Medium typically results in the rapid absorption of extracellular matrix (ECM) proteins to the surface followed by cell attachment ECM proteins and different receptors on the cell membrane leads to cell adhesion, spreading, migration, proliferation and differentiation. The success or failure of biomaterials implanted in vivo depends on the initial cellular response that is mediated by the concentration, composition, and conformation of adsorbed proteins at the implant surface as well as apoptosis-programmed cell. The primary forces that drive protein adsorption to a solid surface are hydrophobic dehydration resulting from the interaction between hydrophobic patches on a protein and a hydrophobic surface and electrostatic interactions, and there are a large number of complex, interdependent, and dynamic interactions between proteins and a surface. The surface modification is promising method but needs further research.
The hydrophilic anterior surface and the hydrophobic posterior surface have great application prospects. It is possible to make the hydrophilic groups modification on the hydrophobic IOL or make the hydrophobic modification on the hydrophilic IOL. Moreover, single radical or molecule modification may not be adequate to obtain a perfect IOL surface. Two or more groups might be better.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Behrens A, McDonnell PJ, Tielsch JM, Schein OD. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112:1388–94. doi: 10.1016/j.ophtha.2005.02.028. doi: 10.1016/j.ophtha.2005.02.028ES. [DOI] [PubMed] [Google Scholar]
- 2.Loewenstein A, Zur D. Postsurgical cystoid macular edema. Dev Ophthalmol. 2010;47:148–59. doi: 10.1159/000320078. doi: 10.1159/000320078. [DOI] [PubMed] [Google Scholar]
- 3.Lundqvist B, Mönestam E. Ten-year longitudinal visual function and Nd: YAG laser capsulotomy rates in patients less than 65 years at cataract surgery. Am J Ophthalmol. 2010;149:238–244.e1. doi: 10.1016/j.ajo.2009.08.029. doi: 10.1016/j.ajo.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. 2009;88:257–69. doi: 10.1016/j.exer.2008.10.016. doi: 10.1016/j.exer.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Kodjikian L, Burillon C, Roques C, Pellon G, Freney J, Renaud FN. Bacterial adherence of Staphylococcus epidermidis to intraocular lenses: A bioluminescence and scanning electron microscopy study. Invest Ophthalmol Vis Sci. 2003;44:4388–94. doi: 10.1167/iovs.03-0186. doi: 10.1167/iovs.03-0186. [DOI] [PubMed] [Google Scholar]
- 6.Conway MD, Canakis C, Livir-Rallatos C, Peyman GA. Intravitreal triamcinolone acetonide for refractory chronic pseudophakic cystoid macular edema. J Cataract Refract Surg. 2003;29:27–33. doi: 10.1016/s0886-3350(02)01441-4. doi: 10.1016/S0886-3350(02)01441.4. [DOI] [PubMed] [Google Scholar]
- 7.Schubert HD. Cystoid macular edema: the apparent role of mechanical factors. Prog Clin Biol Res. 1989;312:277–91. [PubMed] [Google Scholar]
- 8.Astle WF, Alewenah O, Ingram AD, Paszuk A. Surgical outcomes of primary foldable intraocular lens implantation in children: Understanding posterior opacification and the absence of glaucoma. J Cataract Refract Surg. 2009;35:1216–22. doi: 10.1016/j.jcrs.2009.02.028. doi: 10.1016/j.jcrs.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Martinez G, de Iongh RU. The lens epithelium in ocular health and disease. Int J Biochem Cell Biol. 2010;42:1945–63. doi: 10.1016/j.biocel.2010.09.012. doi: 10.1016/j.biocel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Ridley H. Intra-ocular acrylic lenses; a recent development in the surgery of cataract. Br J Ophthalmol. 1952;36:113–22. doi: 10.1136/bjo.36.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue M. Wide-angle viewing system. Dev Ophthalmol. 2014;54:87–91. doi: 10.1159/000360453. doi: 10.1159/000360453. [DOI] [PubMed] [Google Scholar]
- 12.Amon M. Biocompatibility of intraocular lenses. J Cataract Refract Surg. 2001;27:178–9. doi: 10.1016/s0886-3350(01)00742-8. doi: 10.1016/S0886-3350(01)00742-8. [DOI] [PubMed] [Google Scholar]
- 13.Miyake T, Kamiya K, Amano R, Iida Y, Tsunehiro S, Shimizu K. Long-term clinical outcomes of toric intraocular lens implantation in cataract cases with preexisting astigmatism. J Cataract Refract Surg. 2014;40:1654–60. doi: 10.1016/j.jcrs.2014.01.044. doi: 10.1016/j.jcrs.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 14.Roesel M, Heinz C, Heimes B, Koch JM, Heiligenhaus A. Uveal and capsular biocompatibility of two foldable acrylic intraocular lenses in patients with endogenous uveitis – A prospective randomized study. Graefes Arch Clin Exp Ophthalmol. 2008;246:1609–15. doi: 10.1007/s00417-008-0886-4. doi: 10.1007/s00417-008-0886-4. [DOI] [PubMed] [Google Scholar]
- 15.Kohl JC, Werner L, Ford JR, Cole SC, Vasavada SA, Gardiner GL, et al. Long-term uveal and capsular biocompatibility of a new accommodating intraocular lens. J Cataract Refract Surg. 2014;40:2113–9. doi: 10.1016/j.jcrs.2014.10.011. doi: 10.1016/j.jcrs.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Henderson BA, Grimes KJ. Blue-blocking IOLs: A complete review of the literature. Surv Ophthalmol. 2010;55:284–9. doi: 10.1016/j.survophthal.2009.07.007. doi: 10.1016/j.survophthal.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Werner L, Abdel-Aziz S, Cutler Peck C, Monson B, Espandar L, Zaugg B, et al. Accelerated 20-year sunlight exposure simulation of a photochromic foldable intraocular lens in a rabbit model. J Cataract Refract Surg. 2011;37:378–85. doi: 10.1016/j.jcrs.2010.08.052. doi: 10.1016/j.jcrs.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apple DJ, Federman JL, Krolicki TJ, Sims JC, Kent DG, Hamburger HA, et al. Irreversible silicone oil adhesion to silicone intraocular lenses. A clinicopathologic analysis. Ophthalmology. 1996;103:1555–61. doi: 10.1016/s0161-6420(96)30463-6. [DOI] [PubMed] [Google Scholar]
- 19.Wilson ME, Jr, Trivedi RH. The ongoing battle against posterior capsular opacification. Arch Ophthalmol. 2007;125:555–6. doi: 10.1001/archopht.125.4.555. doi: 10.1001/archopht.125.4.555. [DOI] [PubMed] [Google Scholar]
- 20.Bao YZ, Cao XG, Li XX, Chen J, Hu JX, Zhu T. Screening of cataract surgery in four rural populations aged 50 years old and above in western China (in Chinese) Natl Med J China. 2009;89:2454–7. [PubMed] [Google Scholar]
- 21.Aykan U, Bilge AH, Karadayi K, Akin T. The effect of capsulorhexis size on development of posterior capsule opacification: small (4.5 to 5.0 mm) versus large (6.0 to 7.0 mm) Eur J Ophthalmol. 2003;13:541–5. doi: 10.1177/112067210301300606. [DOI] [PubMed] [Google Scholar]
- 22.Georgopoulos M, Menapace R, Findl O, Rainer G, Petternel V, Kiss B. Posterior continuous curvilinear capsulorhexis with hydrogel and silicone intraocular lens implantation: Development of capsulorhexis size and capsule opacification. J Cataract Refract Surg. 2001;27:825–32. doi: 10.1016/s0886-3350(00)00793-8. doi: 10.1016/S0886-3350(00)00793-8. [DOI] [PubMed] [Google Scholar]
- 23.Nagy ZZ. New technology update: Femtosecond laser in cataract surgery. Clin Ophthalmol. 2014;8:1157–67. doi: 10.2147/OPTH.S36040. doi: 10.2147/OPTH.S36040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao EC, McCanna DJ, Jones LW. Utilization of in vitro methods to determine the biocompatibility of intraocular lens materials. Toxicol In Vitro. 2011;25:1906–11. doi: 10.1016/j.tiv.2011.06.005. doi: 10.1016/j.tiv.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Spalton D. Posterior capsule opacification: Have we made a difference? Br J Ophthalmol. 2013;97:1–2. doi: 10.1136/bjophthalmol-2012-302570. doi: 10.1136/bjophthalmol.2012.302570. [DOI] [PubMed] [Google Scholar]
- 26.Nishi O, Nishi K, Wickström K. Preventing lens epithelial cell migration using intraocular lenses with sharp rectangular edges. J Cataract Refract Surg. 2000;26:1543–9. doi: 10.1016/s0886-3350(00)00426-0. doi: 10.1016/S0886-3350(00)00426-0. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi K, Hayashi H. Posterior capsule opacification in the presence of an intraocular lens with a sharp versus rounded optic edge. Ophthalmology. 2005;112:1550–6. doi: 10.1016/j.ophtha.2005.03.024. doi: 10.1016/j.ophtha.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Ness PJ, Werner L, Maddula S, Davis D, Zaugg B, Stringham J, et al. Pathology of 219 human cadaver eyes with 1-piece or 3-piece hydrophobic acrylic intraocular lenses: Capsular bag opacification and sites of square-edged barrier breach. J Cataract Refract Surg. 2011;37:923–30. doi: 10.1016/j.jcrs.2010.11.036. doi: 10.1016/j.jcrs.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Yuen C, Williams R, Batterbury M, Grierson I. Modification of the surface properties of a lens material to influence posterior capsular opacification. Clin Experiment Ophthalmol. 2006;34:568–74. doi: 10.1111/j.1442-9071.2006.01278.x. doi: 10.1111/j.1442-9071.2006.01278.x. [DOI] [PubMed] [Google Scholar]
- 30.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–8. doi: 10.1126/science.1214804. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 31.Giglio ED, Cafagna D. PHEMA-based thin hydrogel films for biomedical applications. J Bioact Compat Polym. 2011;26:420–4. doi: 10.1002/jbm.a.31316. [Google Scholar]
- 32.Davis JL, Yi NY, Salmon JH, Charlton AN, Colitz CM, Gilger BC. Sustained-release celecoxib from incubated acrylic intraocular lenses suppresses lens epithelial cell growth in an ex vivo model of posterior capsule opacity. J Ocul Pharmacol Ther. 2012;28:359–68. doi: 10.1089/jop.2011.0196. doi: 10.1089/jop.2011.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiner AL, Gilger BC. Advancements in ocular drug delivery. Vet Ophthalmol. 2010;13:395–406. doi: 10.1111/j.1463-5224.2010.00835.x. doi: 10.1111/j.1463-5224.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- 34.Chernyy S, Jensen BE, Shimizu K, Ceccato M, Pedersen SU, Zelikin AN, et al. Surface grafted glycopolymer brushes to enhance selective adhesion of HepG2 cells. J Colloid Interface Sci. 2013;404:207–14. doi: 10.1016/j.jcis.2013.04.025. doi: 10.1016/j.jcis.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Bozukova D, Pagnoulle C, De Pauw-Gillet MC, Ruth N, Jérôme R, Jérôme C. Imparting antifouling properties of poly (2-hydroxyethyl methacrylate) hydrogels by grafting poly (oligoethylene glycol methyl ether acrylate) Langmuir. 2008;24:6649–58. doi: 10.1021/la7033774. doi: 10.1021/la7033774. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Qian X, Zhang X, Xia W, Zhong L, Sun Z, et al. Plasma surface modification of rigid contact lenses decreases bacterial adhesion. Eye Contact Lens. 2013;39:376–80. doi: 10.1097/ICL.0b013e31829e8f73. doi: 10.1097/ICL.0b013e31829e8f73. [DOI] [PubMed] [Google Scholar]
- 37.Parsons C, McCoy CP, Gorman SP, Jones DS, Bell SE, Brady C, et al. Anti-infective photodynamic biomaterials for the prevention of intraocular lens-associated infectious endophthalmitis. Biomaterials. 2009;30:597–602. doi: 10.1016/j.biomaterials.2008.10.015. doi: 10.1016/j.biomaterials.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 38.McCoy CP, Craig RA, McGlinchey SM, Carson L, Jones DS, Gorman SP. Surface localisation of photosensitisers on intraocular lens biomaterials for prevention of infectious endophthalmitis and retinal protection. Biomaterials. 2012;33:7952–8. doi: 10.1016/j.biomaterials.2012.07.052. doi: 10.1016/j.biomaterials.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 39.Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS. N-halamine biocidal coatings via a layer-by-layer assembly technique. Langmuir. 2011;27:4091–7. doi: 10.1021/la104923x. doi: 10.1016/j.reactfunctpolym.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–92. doi: 10.1038/nature02388. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 41.Lee H, Oh HJ, Yoon KC, Tae G, Kim YH. Fast in situ enzymatic gelation of PPO-PEO block copolymer for injectable intraocular lens in vivo. J Biomater Appl. 2014;28:1247–63. doi: 10.1177/0885328213505247. doi: 10.1177/0885328213505247. [DOI] [PubMed] [Google Scholar]
- 42.Boffa MC, Labarre D, Jozefowicz M, Boffa GA. Interactions between human plasma proteins and heparin-poly(methyl methacrylate) copolymer. Thromb Haemost. 1979;41:346–56. [PubMed] [Google Scholar]
- 43.Taravati P, Lam DL, Leveque T, Van Gelder RN. Postcataract surgical inflammation. Curr Opin Ophthalmol. 2012;23:12–8. doi: 10.1097/ICU.0b013e32834cd60e. doi: 10.1097/ICU.0b013e32834cd60e. [DOI] [PubMed] [Google Scholar]
- 44.Perry LJ, Papaliodis GN. Selection of intraocular lenses in patients with uveitis. Int Ophthalmol Clin. 2010;50:61–70. doi: 10.1097/IIO.0b013e3181c55503. doi: 10.1097/IIO.0b013e3181c55503. [DOI] [PubMed] [Google Scholar]
- 45.Rønbeck M, Kugelberg M. Posterior capsule opacification with 3 intraocular lenses: 12-year prospective study. J Cataract Refract Surg. 2014;40:70–6. doi: 10.1016/j.jcrs.2013.07.039. doi: 10.1016/j.jcrs.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Wu D. Surface modification of polymethyl methacrylate intraocular lenses by plasma for improvement of antithrombogenicity and transmittance. Appl Surf Sci. 2009;255:6840–5. doi: 10.1016/j.apsusc.2009.03.029. [Google Scholar]
- 47.Kannan RY, Salacinski HJ, Butler PE, Seifalian AM. Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Acc Chem Res. 2005;38:879–84. doi: 10.1021/ar050055b. doi: 10.1021/ar050055b. [DOI] [PubMed] [Google Scholar]
- 48.Wang B, Lin Q, Shen C, Tang J, Han Y, Chen H. Hydrophobic modification of polymethyl methacrylate as intraocular lenses material to improve the cytocompatibility. J Colloid Interface Sci. 2014;431:1–7. doi: 10.1016/j.jcis.2014.05.056. doi: 10.1016/j.jcis.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 49.Majeed A, He J, Jiao L, Zhong X, Sheng Z. Surface properties and biocompatibility of nanostructured TiO2 film deposited by RF magnetron sputtering. Nanoscale Res Lett. 2015;10:56. doi: 10.1186/s11671-015-0732-7. doi: 10.1186/s11671-015-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boonprakob N, Wetchakun N, Phanichphant S, Waxler D, Sherrell P, Nattestad A, et al. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J Colloid Interface Sci. 2014;417:402–9. doi: 10.1016/j.jcis.2013.11.072. doi: 10.1016/j.jcis.2013.11.072. [DOI] [PubMed] [Google Scholar]
- 51.Yang M, Di Z, Lee JK. Facile control of surface wettability in TiO2/poly (methyl methacrylate) composite films. J Colloid Interface Sci. 2012;368:603–7. doi: 10.1016/j.jcis.2011.11.037. doi: 10.1016/j.jcis.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 52.Wang G, Yuan J, Sun H, Gu H, Xu Y, Dong Y. An analysis of adhered cells on the surface of F-heparin surface modified intraocular lenses implanted into rhesus monkeys’ eyes (in Chinese) Chin J Ophthalmol. 2002;38:154–6. [PubMed] [Google Scholar]
- 53.Yuan JQ, Sun HM, Xu YS, Guo HY, Wang GQ, Gu HQ, et al. Experimental study on fluorine-heparin surface modified intraocular lenses (in Chinese) Rec Adv Ophthalmol. 2003;3:153–6. doi: 1003-5141(2003)03-0153-04. [Google Scholar]
- 54.Wang GQ, Gu HQ, Yuan JQ, Sun HM, Xu YS. F-heparin modified intraocular lenses in Rhesus monkeys. Int J Ophthalmol. 2010;3:141–4. doi: 10.3980/j.issn.2222-3959.2010.02.11. doi: 10.3980/j.issn.2222-3959.2010.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta KR, Sathe SN, Karyekar SD. The new soft intraocular lens implant. J Am Intraocul Implant Soc. 1978;4:200–5. doi: 10.1016/s0146-2776(78)80079-2. [DOI] [PubMed] [Google Scholar]
- 56.Epstein E. History of intraocular lens implant surgery. In: Mazzocico TR, Epstein E, editors. Soft Implant Lenses in Cataract Surgery. Thorofare (NJ): Slock Inc; 1986. [Google Scholar]
- 57.Vock L, Crnej A, Findl O, Neumayer T, Buehl W, Sacu S, et al. Posterior capsule opacification in silicone and hydrophobic acrylic intraocular lenses with sharp-edge optics six years after surgery. Am J Ophthalmol. 2009;147:683–690.e2. doi: 10.1016/j.ajo.2008.11.006. doi: 10.1016/j.ajo.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Li N, Chen X, Zhang J, Zhou Y, Yao X, Du L, et al. Effect of AcrySof versus silicone or polymethyl methacrylate intraocular lens on posterior capsule opacification. Ophthalmology. 2008;115:830–8. doi: 10.1016/j.ophtha.2007.06.037. doi: 10.1016/j.ophtha.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 59.Baillif S, Ecochard R, Casoli E, Freney J, Burillon C, Kodjikian L. Adherence and kinetics of biofilm formation of Staphylococcus epidermidis to different types of intraocular lenses under dynamic flow conditions. J Cataract Refract Surg. 2008;34:153–8. doi: 10.1016/j.jcrs.2007.07.058. doi: 10.1016/j.jcrs.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi K, Hayashi H, Nakao F, Hayashi F. Reduction in the area of the anterior capsule opening after polymethylmethacrylate, silicone, and soft acrylic intraocular lens implantation. Am J Ophthalmol. 1997;123:441–7. doi: 10.1016/s0002-9394(14)70169-2. [DOI] [PubMed] [Google Scholar]
- 61.Ishihara K, Ziats NP, Tierney BP, Nakabayashi N, Anderson JM. Protein adsorption from human plasma is reduced on phospholipid polymers. J Biomed Mater Res. 1991;25:1397–407. doi: 10.1002/jbm.820251107. [DOI] [PubMed] [Google Scholar]
- 62.Huang XD, Li HY, Lin L, Yao K. Reduced silicone oil adherence to silicone intraocular lens by surface modification with 2-methacryloyloxyethyl phosphoryl-choline. Curr Eye Res. 2013;38:91–6. doi: 10.3109/02713683.2012.704477. doi: 10.3109/02713683.2012.704477. [DOI] [PubMed] [Google Scholar]
- 63.Wang JJ, Liu F. Photoinduced graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on silicone hydrogels for reducing protein adsorption. J Mater Sci Mater Med. 2011;22:2651–7. doi: 10.1007/s10856-011-4452-y. doi: 10.1007/s10856-011-4452-y. [DOI] [PubMed] [Google Scholar]
- 64.Shimizu T, Goda T, Minoura N, Takai M, Ishihara K. Super-hydrophilic silicone hydrogels with interpenetrating poly (2-methacryloyloxyethyl phosphorylcholine) networks. Biomaterials. 2010;31:3274–80. doi: 10.1016/j.biomaterials.2010.01.026. doi: 10.1016/j.biomaterials.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 65.Arthur SN, Peng Q, Apple DJ, Escobar-Gomez M, Bianchi R, Pandey SK, et al. Effect of heparin surface modification in reducing silicone oil adherence to various intraocular lenses. J Cataract Refract Surg. 2001;27:1662–9. doi: 10.1016/s0886-3350(01)00891-4. doi: 10.1016/S0886-3350(01)00891-4. [DOI] [PubMed] [Google Scholar]
- 66.Guiqin W, Pinlin H, Hanqing G, Chenxi L, Xiaohang L, Song L. Surface feature detection of the surface modification silicone intraocular lens. Acta Sci Nat Univ Nankaiensis. 2002;35:15–9. doi: 0465-7942(2002)03-0015-05. [Google Scholar]
- 67.Wang GQ, Peng XJ, Gu HQ. The intraocular lenses of surface modification implanted into rabbit eyes. Int J Ophthalmol. 2006;16:54–6. [Google Scholar]
- 68.Leaming DV. Practice styles and preferences of ASCRS members--2003 survey. J Cataract Refract Surg. 2004;30:892–900. doi: 10.1016/j.jcrs.2004.02.064. doi: 10.1016/j.jcrs.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 69.McIntyre S, Werner L, Mamalis N. Hydrophobic Acrylic IOLs: A primer. Cataract Refract Surg Today Eur. 2011. [Last accessed on 2015 Jun 10]. Available from: http://crstodayeurope.com/pdfs/CRSTE_0311_feature_werner.pdf .
- 70.Wilson ME, Jr, Bartholomew LR, Trivedi RH. Pediatric cataract surgery and intraocular lens implantation: Practice styles and preferences of the 2001 ASCRS and AAPOS memberships. J Cataract Refract Surg. 2003;29:1811–20. doi: 10.1016/s0886-3350(03)00220-7. doi: 10.1016/S0886-3350(03)00220-7. [DOI] [PubMed] [Google Scholar]
- 71.Wilson ME, Jr, Trivedi RH, Buckley EG, Granet DB, Lambert SR, Plager DA, et al. ASCRS white paper. Hydrophobic acrylic intraocular lenses in children. J Cataract Refract Surg. 2007;33:1966–73. doi: 10.1016/j.jcrs.2007.06.047. doi: 10.1016/j.jcrs.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 72.Abela-Formanek C, Amon M, Schild G, Schauersberger J, Heinze G, Kruger A. Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. J Cataract Refract Surg. 2002;28:50–61. doi: 10.1016/s0886-3350(01)01122-1. doi: 10.1016/S0886-3350(01)01122-1. [DOI] [PubMed] [Google Scholar]
- 73.Yan Q, Perdue N, Sage EH. Differential responses of human lens epithelial cells to intraocular lenses in vitro: Hydrophobic acrylic versus PMMA or silicone discs. Graefes Arch Clin Exp Ophthalmol. 2005;243:1253–62. doi: 10.1007/s00417-005-1181-2. doi: 10.1007/s00417-005-1181-2. [DOI] [PubMed] [Google Scholar]
- 74.Packer M. Phase 1 Safety and Efficacy of a Novel High Refractive Index IOL. Poster Presented at the XXII Congress of the European Society of Cataract and Refractive Surgeons, Paris, France; September. 2004 [Google Scholar]
- 75.Holland E. Phase 1 Safety and Efficacy of the Advanced Visual Science, High Refractive Index, Hydrophobic, Acrylic IOL. Presented at ASCRS Symposium on Cataract, IOL and Refractive Surgery, Washington, DC; April. 2005 [Google Scholar]
- 76.Ollerton A, Werner L, Fuller SR, Kavoussi SC, McIntyre JS, Mamalis N. Evaluation of a new single-piece 4% water content hydrophobic acrylic intraocular lens in the rabbit model. J Cataract Refract Surg. 2012;38:1827–32. doi: 10.1016/j.jcrs.2012.05.039. doi: 10.1016/j.jcrs.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 77.Lin L, Wang Y, Huang XD, Xu ZK, Ke Y. Modification of hydrophobic acrylic intraocular lens with poly(ethylene glycol) by atmospheric pressure glow discharge: A facile approach. Appl Surf Sci. 2010;256:7354–64. doi: 10.1016/j.apsusc.2010.05.068. [Google Scholar]
- 78.Wang G, Cao L, Li N, Peng X, Tang H, Wan R, et al. In vivo implantation of hydrophobic acrylic intraocular lenses with surface modification. Eye Sci. 2013;28:176–9. doi: 10.3969/j.issn.1000-4332.2013.04.002. [PubMed] [Google Scholar]
- 79.Wang GQ, Cao LQ, Li N, Peng XJ, Tang HQ, Dong YL, et al. Study on surface microstructure of surface modified hydrophobic acrylic intraocular lens (in Chinese) Rec Adv Ophthalmol. 2014;34:904–6. doi: 10.13389/j.cnki.rao.2014.0250. [Google Scholar]
- 80.Krall EM, Arlt EM, Jell G, Strohmaier C, Bachernegg A, Emesz M, et al. Intraindividual aqueous flare comparison after implantation of hydrophobic intraocular lenses with or without a heparin-coated surface. J Cataract Refract Surg. 2014;40:1363–70. doi: 10.1016/j.jcrs.2013.11.043. doi: 10.1016/j.jcrs.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 81.Sun CB, Teng WQ, Cui JT, Huang XJ, Yao K. The effect of anti-TGF-ß2 antibody functionalized intraocular lens on lens epithelial cell migration and epithelial-mesenchymal transition. Colloids Surf B Biointerfaces. 2014;113:33–42. doi: 10.1016/j.colsurfb.2013.08.024. doi: 10.1016/j.colsurfb.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 82.Huang YS, Bertrand V, Bozukova D, Pagnoulle C, Labrugère C, De Pauw E, et al. RGD surface functionalization of the hydrophilic acrylic intraocular lens material to control posterior capsular opacification. PLoS One. 2014;9:e114973. doi: 10.1371/journal.pone.0114973. doi: 10.1371/journal.pone.0114973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pintwala R, Postnikoff C, Molladavoodi S, Gorbet M. Coculture with intraocular lens material-activated macrophages induces an inflammatory phenotype in lens epithelial cells. J Biomater Appl. 2015;29:1119–32. doi: 10.1177/0885328214552711. doi: 10.1177/0885328214552711. [DOI] [PubMed] [Google Scholar]
- 84.Apple DJ, Escobar-Gomez M, Zaugg B, Kleinmann G, Borkenstein AF. Modern cataract surgery: Unfinished business and unanswered questions. Surv Ophthalmol. 2011;56(6 Suppl):S3–53. doi: 10.1016/j.survophthal.2011.10.001. doi: 10.1016/j.survophthal.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Tetz M, Jorgensen MR. New Hydrophobic IOL Materials and understanding the science of glistenings. Curr Eye Res. 2015;40:969–81. doi: 10.3109/02713683.2014.978476. doi: 10.3109/02713683.2014.978476. [DOI] [PubMed] [Google Scholar]
- 86.Nath N, Hyun J, Ma H, Chilkoti A. Surface engineering strategies for control of protein and cell interactions. Surf Sci. 2004;570:98–110. doi: 10.1177/0885328214552711. [Google Scholar]
- 87.Koupaei N, Karkhaneh A, Daliri Joupari M. Preparation and characterization of (PCL-crosslinked-PEG)/hydroxyapatite as bone tissue engineering scaffolds. J Biomed Mater Res A. 2015;103:3919–26. doi: 10.1002/jbm.a.35513. doi: 10.1002/jbm.a.35513. [DOI] [PubMed] [Google Scholar]
- 88.Petit A, Redout EM, van de Lest CH, de Grauw JC, Müller B, Meyboom R, et al. Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses. Biomaterials. 2015;53:426–36. doi: 10.1016/j.biomaterials.2015.02.109. doi: 10.1016/j.biomaterials.2015.02.109. [DOI] [PubMed] [Google Scholar]
- 89.Swartzlander MD, Barnes CA, Blakney AK, Kaar JL, Kyriakides TR, Bryant SJ. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials. 2015;41:26–36. doi: 10.1016/j.biomaterials.2014.11.026. doi: 10.1016/j.biomaterials.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barbour W, Saika S, Miyamoto T, Ohnishi Y. Biological compatibility of polymethyl methacrylate, hydrophilic acrylic and hydrophobic acrylic intraocular lenses. Ophthalmic Res. 2005;37:255–61. doi: 10.1159/000087100. doi: 10.1159/000087100. [DOI] [PubMed] [Google Scholar]
- 91.Lewis AC. Interleukin-6 in the pathogenesis of posterior capsule opacification and the potential role for interleukin-6 inhibition in the future of cataract surgery. Med Hypotheses. 2013;80:466–74. doi: 10.1016/j.mehy.2012.12.042. doi: 10.1016/j.mehy.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 92.Saika S. Relationship between posterior capsule opacification and intraocular lens biocompatibility. Prog Retin Eye Res. 2004;23:283–305. doi: 10.1016/j.preteyeres.2004.02.004. doi: 10.1016/j.preteyeres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 93.Norton JN, Kohnen T, Hackett RB, Patel A, Koch DD. Ocular biocompatibility testing of intraocular lenses: A 1 year study in pseudophakic rabbit eyes. J Cataract Refract Surg. 1999;25:1467–79. doi: 10.1016/s0886-3350(99)00237-0. doi: 10.1016/S0886-3350(99)00237-0. [DOI] [PubMed] [Google Scholar]