INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide and contributes considerably to morbidity.[1,2,3] The underlying cause is atherosclerosis.[4] The development of new preventive therapies is one of the steps to control the CVD epidemic. It is increasingly demanded that promising therapies be evaluated in trials using cardiovascular (CV) morbidity and mortality (M and M) as a primary outcome.[5] M and M trials, however, are very costly, often multicenter studies requiring thousands of participants, and with a long follow-up period. There is great interest in alternative endpoints that can be used as a valid alternative or proxy for CV M and M alternative endpoints (surrogate endpoints) allow for the evaluation of novel therapies in randomized controlled trials within a shorter timeframe, fewer participants, at lower costs, and with a shorter time to availability of trial results when compared to an M and M trial. These studies may show a direct effect on atherosclerosis progression and in the same time may serve to direct or exclude subsequent large M and M trials. A measure of atherosclerosis is intuitively a suitable alternative endpoint for CVD events as atherosclerosis is the disease between exposure to risk factors and the majority of CV events. Atherosclerosis can be noninvasively assessed from early to late stages of the disease process using different imaging techniques.[6,7,8,9]
B-mode ultrasound is one of those imaging techniques and has been used to obtain quantitative measurements of the carotid intima-media thickness (CIMT) and as such provides estimates for an individual on the absolute value (presence) and its change over time.[10,11] CIMT has been suggested to be an adequate alternative measurement for CV events (surrogate endpoint) in intervention studies.[10,11] Prentice and Boissel have proposed several criteria [Supplementary Table 1] for a surrogate endpoint that should have been met before it could be validly used.[12] We set out to review literature and provide evidence for the validity of CIMT measurements as an alternative measurement for atherosclerosis elsewhere in the arterial system and for CV events.
Supplementary Table 1.
Criteria that markers must meet to be considered as valid surrogates for clinical endpoints according to Boissel and Prentice (12)
| Boissel (clinical criteria) | Prentice (statistical criteria)* |
|---|---|
| B1: (Efficiency) The surrogate marker should be relatively easy to evaluate, preferably by noninvasive means, and more readily available than the gold standard. The time course of the surrogate should precede that of the endpoints so that disease and/or disease progression may be established more quickly via the surrogate. Clinical trials based on surrogates should require fewer resources, less participant burden, and a shorter time frame | P1: The intervention should affect the distribution of T |
| B2: (Linkage) The quantitative and qualitative relationship between the surrogate marker and the clinical endpoint should be established based on epidemiological and clinical studies. The nature of this relationship may be understood in terms of its pathophysiology or in terms of an expression of joint risk | P2: The intervention should affect the distribution of S |
| B3: (Congruency) The surrogate should produce parallel estimates of risk and benefit as endpoints. Individuals with and without vascular disease should exhibit differences in surrogate marker measurements. In intervention studies, anticipated clinical benefits should be deducible from the observed changes in the surrogate marker | P3: The distribution of T should be dependent on S |
| P4: Endpoint T should be conditionally independent of Z given S, i.e., S should fully account for the impact of Z on T |
*Prentice views surrogacy as a statistical property and defines it with mathematical expressions. Four criteria are required for S to serve as a surrogate for endpoint T with respect to intervention Z.
VALIDITY, CONCEPT AND REPRODUCIBILITY OF THE MEASUREMENT
Validity
In 1986, Italian investigators reported for the first time the results of an in vitro study which compared direct measurements of arterial wall thickness by gross and microscopic examination with B-mode real-time imaging of those same specimens.[13] Several studies followed. The overall conclusion was that CIMT measurements of the far wall closely relate to the true biological thickness of the vessel wall, whereas near wall CIMT measurements are an approximation of the true wall thickness.[14,15,16,17,18] Since then, the number of scientific publication has increased steadily [Figure 1] and CIMT is currently one of the most widely used noninvasive measures of atherosclerosis employed by clinicians and clinical investigators, both to quantify the extent of subclinical disease and to monitor change over time.
Figure 1.
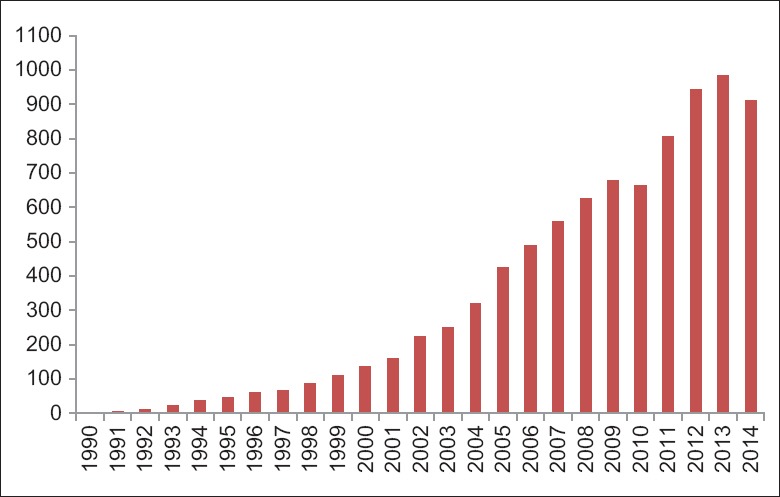
The number of publications (y-axis) using “carotid intima-media thickness (not animal)” in the title or abstract as assessed using PubMed database (http://www.ncbi.nlm.nih.gov/sites/entrez), by year of publication (x-axis), (December 16, 2014).
Acquisition of the images
Typically, the carotid artery is classified into three segments, each approximately 10 mm in length [Figure 2].[19] The most proximal segment, the 1 cm straight segment of the carotid artery immediately prior to the bifurcation, is the common carotid artery (CCA). Its distal boundary is identified by a divergence of the near and far walls as the artery begins to divide into its internal and external branches. This focal widening of the bifurcation extends over approximately 1 cm and is labeled the carotid bulb or bifurcation (CB). The distal margin is defined by the tip of the flow divider separating the diverging internal carotid artery (ICA) and external carotid artery. The final segment that is frequently examined is the proximal 1 cm of the ICA.
Figure 2.
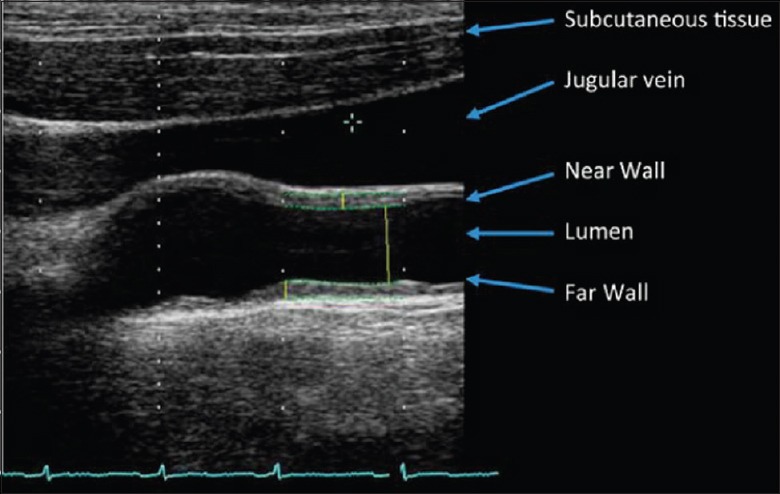
A typical B-mode ultrasound image from the carotid artery.[19]
As indicated in Figure 3, CIMT has been assessed in a several ways, varying in side (left carotid artery, right carotid artery, or both), segment (common, bifurcation, and internal), wall (near wall and far wall on the image), and in angles (60, 90, 120, 150, 180, 210, 240, 270, and 300) by using an external arc for positioning (Meijer Carotid ArcR).[20] Some studies measure CIMT only once and choose an image in which the interfaces are most clear (i.e., single optimal B-mode image).[21] Others searches for the point with the thickest CIMT (e.g., the highest burden of atherosclerosis).[22] Others choose from multiple optimal B-mode images,[23] or measure the CIMT from multiple images that were obtained from various standardized angles of interrogation [Figure 4]. The latter, using the Meijer Carotid Arc approach, allows for measurement at exactly the same location over time.[23] It is important to realize that each measurement approach has its own specific characteristics. Since atherosclerosis is asymmetrically distributed across the carotid artery, selectively measuring only one angle is likely to ignore the asymmetric nature of the disease.[24,25] Furthermore, each measurement approach has its own characteristics with respect to the assessment of atherosclerosis progression, as previously shown.[26] Finally, also measurement error or missing values tend to vary across the measurement approaches.[27,28]
Figure 3.
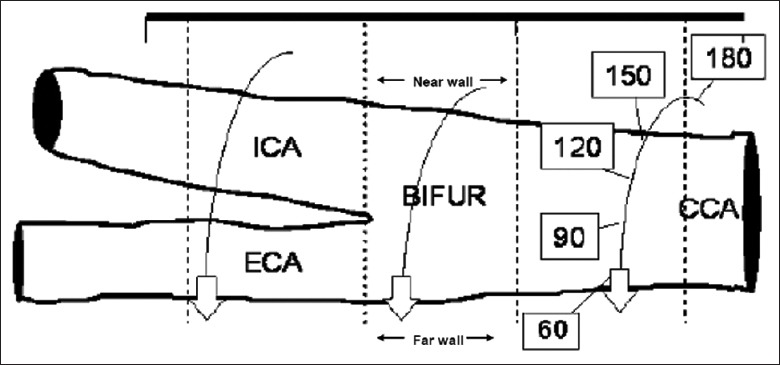
Graphical representation of the circumferential assessment of the artery sites. The angle values from 60 to 180 represent the standardized angles of interrogation. BIFUR: Carotid bifurcation; CCA: Common carotid artery; ECA: External carotid artery; ICA: Internal carotid artery.[29]
Figure 4.
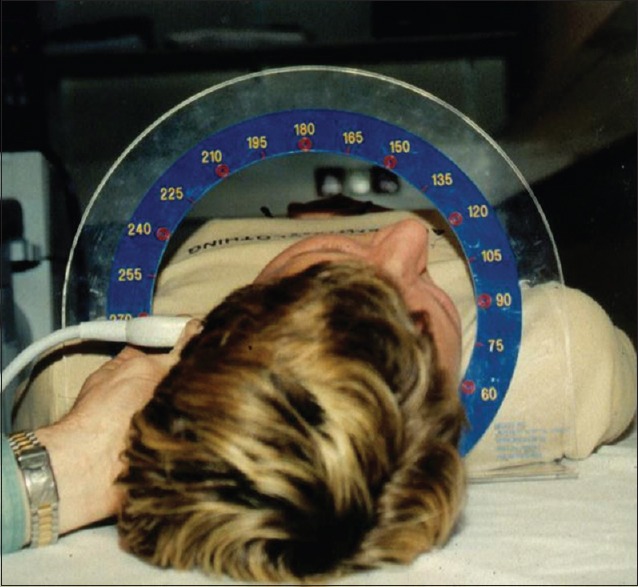
The Meijer Carotid Arc that allows for assessment of angles specific images.[23]
Actual measurement of the images
Ultrasound images in CIMT studies are typically acquired at the study site, stored digitally, and send to a reading laboratory for offline reading. At the core lab, typically quality control and quality assurance typically takes place first before the actual readings can start. These actual readings can be performed using several different edge detection methods (semi-automatic or manual).[29] Semi-automated edge detection is more often applied in settings where only the CCA is examined while manual edge detection is usually applied in settings where the carotid bifurcation and the ICA are also measured.[30]
The main difference between semi-automated edge detection and manual edge detection, however, is the actual manual drawing of the lines on the interfaces with manual edge detection. With semi-automatic edge detection, the reader still may adjust or modify the automatically drawn lines when the reader judges that the lines were incorrectly placed. A major advantage of semi-automated edge detection programs, besides being less resource intensive and time-consuming, may be the reduction in variability in CIMT readings as a result of reduction in the variability between readers and reduction of change in reading behavior over time (reader drift).[30] Many investigators have a clear view on this topic, mostly based on personal experience. Yet, there is little published evidence on this topic. Two recent studies indicated that manual and semi-automated edge detection of far wall common CIMT both result in high reproducibility, and largely show similar relations to CV risk factors, rates of change, and treatment effects.[30,31] Hence, choices between semi-automated and manual reading software for CIMT studies likely should be based on logistical and cost considerations rather than differences in expected data quality in populations with a low burden of atherosclerotic disease.
Reproducibility of the measurement for an individual
With reproducibility is meant that when an individual is measured today, the obtained value should be similar to that obtained tomorrow or next week. Between visit, reproducibility covers all sources that may affect the CIMT measurement: Position of the patient, image acquisition, reader variability, and within-patient variability in, for example, blood pressure or heart rate.
Although difficult to quantify, due to a wide variation in reporting of reproducibility results, it seems that the reproducibility of the CIMT measurements has improved considerably over the years.[32] Studies reporting on the intraclass correlation coefficient (ICC), showed that the ICC ranged from 0.60 to 0.75 in studies conducted during the late eighties.[33,34,35,36,37] whereas, more recent studies reported an ICC between 0.80 and 0.95.[38,39,40] Of note, it seems that in studies that started as an observational study the reproducibility was less than in studies focuses on measuring progression.[41,42]
A number of reports from randomized controlled trials recently addressed the reproducibility of CIMT measurements based on various ultrasound protocols.[28,43,44,45,46] In these trials, the ultrasound protocols were based on an assessment of both sides, all three segments, both walls and at least eight angles. With those data points, the interest was in providing the best balance between reproducibility, magnitude of CIMT change over time and its associated precision, and magnitude of effect of the intervention on CIMT change over time and its associated precision.
RELATIONS WITH ESTABLISHED CARDIOVASCULAR RISK FACTORS
There is a wealth of evidence on the relation between unfavorable level of risk factors and increased CIMT. We made no attempt to refer to all the available publications on that issue. Most of the evidence comes from cross-sectional studies. Traditional risk factors such as ageing, male gender, elevated blood pressure, increased body mass index, high low-density lipoprotein (LDL) cholesterol, low high-density lipoprotein (HDL) cholesterol, diabetes mellitus, and smoking have shown to be related to increased CIMT.[47,48,49,50,51,52,53,54,55,56] These relations also hold for individuals with an Asian ancestry.[57,58,59,60,61,62,63] In addition, increased CIMT has been associated with abnormalities in other organ systems such as the presence of white matter lesions in the brain,[64] left ventricular hypertrophy.[65,66,67] renal disease,[68] and endothelial dysfunction measured at the level of the brachial artery.[69]
Data on risk factors and change in CIMT or change in risk factors and change in CIMT is less readily available.[70,71,72,73] The Atherosclerosis Risk In Communities study in one of the earliest reports showed that baseline levels of established risk factors (such as diabetes, hypertension, LDL, and HDL cholesterol) were related to increased progression on CIMT.[70]
RELATION WITH ATHEROSCLEROSIS ELSEWHERE IN THE ARTERIAL SYSTEM
A variety of studies evaluated the relation between CIMT and presence of atherosclerotic abnormalities elsewhere in the arterial system. Relations were shown for the presence of atherosclerotic abnormalities in the carotid bifurcation and the ICA,[74,75,76] the abdominal aorta.[77] the arteries of the lower extremities,[78,79] and the coronary arteries.[80,81]
In a recent systematic review, most of the studies (29 out of 33) showed a graded positive relationship between CIMT and angiographically assessed coronary atherosclerosis with correlation coefficients in the order of 0.3–0.4.[82] Of importance, is to realize that these reported associations between carotid atherosclerosis and coronary atherosclerosis are of similar magnitude to those shown in autopsy studies.
Several studies looking at the relation between CIMT and coronary calcium showed similar kind of results.[83,84,85] In studies using intravascular ultrasound, generally significant positive relations are found between angiographic left main coronary atherosclerosis and CIMT with correlation coefficients between 0.26 and 0.55.[86]
In summary, the relationship found between CIMT and coronary atherosclerosis in various studies support the notion that CIMT measurements are reflecting atherosclerosis elsewhere.
CAROTID INTIMA-MEDIA THICKNESS AND THE RELATION WITH FUTURE CARDIOVASCULAR EVENTS
Several large observational studies studied the relation of CIMT with future events.[87,88,89] In 2007, Lorenz et al. published a systematic review and meta-analysis of eight relevant general population-based studies that had reported on the ability of CIMT to predict future CV end points, including the three above, involving a total of 37,197 subjects followed for a mean of 5.5 years.[90] They reported that for an absolute CIMT difference of 0.1 mm, the future risk of myocardial infarction increases by 10–15%, and the stroke risk increases by 13–18%. There is a number of studies performed in participants with an Asian ancestry showing results consistent with those found in Caucasians.[91,92,93,94,95]
Currently, over 20 cohort studies that were performed among subjects with or without previous vascular disease, and with and without CVD risk factors, showed consistently that increased CIMT relates to increased CV risk, independently of established vascular risk factors.[96,97]
LIPID LOWERING AND RATE OF CHANGE IN CAROTID INTIMA-MEDIA THICKNESS
In Supplementary Table 2, an overview is provided of several trials that have been performed to evaluate the effects of therapeutic interventions on the rate of change in CIMT. In those trials, the rate of change in CIMT over time was the primary endpoint. The majority have evaluated the efficacy of lipid-level modifying therapies, primarily statins. These trials exemplify the ability to measure change over time in CIMT, which is a sensitive enough marker to detect differences across treatment arms.[98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142]
Supplementary Table 2.
Characteristics and results of CIMT trials on different drug therapies and agreement with results from mortality and mortality trials from reference 26 and 157
| Study | Comparison | Condition | N | Fu, years | CIMT sites | Outcome | Treatment change | Control change | P value | Efficacy M&M trial | Agreement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipidlevel modifying therapy | |||||||||||
| ACAPS (37) | Lovastatin 20–40 mg vs. placebo | Asymptomatic, moderately elevated LDLC | 919 | 3 | Nfw CCA, BIF, and ICA | Mnmx, mm | −0.0090 | 0.0060 | 0.001 | 9 | Yes |
| ARBITER (105) | Atorvastatin 80 mg vs. Pravastatin 40 mg | Meeting NCEP II criteria for lipidlowering therapy | 161 | 1 | Fw CCA | Mn, mm | −0.0340 | 0.0250 | 0.030 | 9 | Yes |
| ASAP (106) | Atorvastatin 80 mg vs. Simvastatin 40 mg | FH | 325 | 2 | Nfw CCA and BIF, fw ICA | Mn, mm | −0.0310 | 0.0360 | <0.001 | 9 | Yes |
| BCAPS (107) | Fluvastatin 40 mg vs. placebo | Asymptomatic | 793 | 3 | Fw CCA and BIF | Mn, mm | 0.0110 | 0.0360 | 0.002 | 9 | Yes |
| CAIUS (108) | Pravastatin 40 mg vs. placebo | Asymptomatic, moderately elevated LDLC | 305 | 3 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | −0.0043 | 0.0089 | 0.001 | 9 | Yes |
| CERDIA (109) | Simvastatin 20 mg vs. placebo | DM2, no CAD | 250 | 4 | Nfw CCA, BIF, and ICA | Mn, mm | 0.0020 | −0.0060 | 0.480 | 9 | No |
| HYRIM (110) | Fluvastatin 40 mg vs. placebo | Treated hypertension | 568 | 4 | Fw CCA | Mxmn, mm | 0.0490 | 0.0760 | 0.030 | 9 | Yes |
| INDIA (111) | Atorvastatin 10 mg vs. placebo | CAD, normal LDLC | 150 | 1 | CCA, BIF, and ICA | Mnmn, mm | −0.0130 | 0.0090 | 0.001 | 9 | Yes |
| KAPS (112) | Pravastatin 40 mg vs. placebo | Asymptomatic, elevated LDLC | 447 | 3 | Fw CCA and BIF | Mnmx, mm/y | 0.0168 | 0.0309 | 0.005 | 9 | Yes |
| LIPID (113) | Pravastatin 40 mg vs. placebo | CAD, moderately elevated TC | 522 | 4 | Fw CCA | Mn, mm | −0.0140 | 0.0480 | <0.001 | 9 | Yes |
| MARS (114) | Lovastatin 80 mg vs. placebo | CAD, moderately elevated TC | 188 | 2 | Fw CCA | Mn, mm/y | −0.0280 | 0.0150 | <0.001 | 9 | Yes |
| METEOR (115) | Rosuvastatin 40 mg vs. placebo | Asymptomatic, elevated LDLC | 984 | 2 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | −0.0014 | 0.0131 | <0.001 | 9 | Yes |
| PLAC II (116) | Pravastatin 10–40 mg vs. Placebo | CAD, elevated LDLC | 151 | 3 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | 0.0593 | 0.0675 | 0.001 | 9 | Yes |
| REGRESS (117) | Pravastatin 40 mg vs. Placebo | CAD, normal to moderately elevated TC | 225 | 2 | Nfw CCA | Mn, mm | −0.0500 | 0.0000 | 0.009 | 9 | Yes |
| SANDS (118) | Aggressive vs. standard | DM2 | 499 | 3 | Fw CCA | Mn, mm | −0.0120 | 0.0380 | <0.001 | 9 | Yes |
| ARBITER 2 (99) | Simvastatin + Niacin 1000 mg vs. Simvastatin | CAD, low HDLC | 167 | 1 | Fw CCA | Mn, mm | 0.0140 | 0.0400 | 0.080 | 53 | Yes |
| FIELD (100) | Fenofibrate 200 mg vs. placebo | DM2 | 170 | 5 | Nfw CCA, BIF, and ICA | Mnmn, mm | 0.0540 | 0.0690 | 0.987 | 14, 54 | Yes |
| ENHANCE (98) | Simvastatin 80 mg + Ezetimibe 10 mg vs. Simvastatin 80 mg | FH | 720 | 2 | Fw CCA, BIF, and ICA | Mn, mm | 0.0111 | 0.0058 | 0.290 | 55 | Yes |
| RADIANCE 1 (102) | Atorvastatin 56.5 mg + torcetrapib 60 mg vs. Atorvastatin 56.5 mg | FH | 904 | 2 | Nfw of CCA, BIF, and ICA | Mnmx, mm/y | 0.0047 | 0.0053 | 0.870 | 17 | Yes |
| RADIANCE 2 (73) | Atorvastatin 13.5 mg + torcetrapib 60 mg vs. Atorvastatin 13.5 mg | Mixed dyslipidaemia | 752 | 2 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | 0.0250 | 0.0300 | 0.460 | 17 | Yes |
| CAPTIVATE (101) | Pactimibe 100 mg vs. placebo | FH | 892 | 2 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | 0.0170 | 0.0130 | 0.640 | 15 | Yes |
| Antihypertensive therapy | |||||||||||
| PHYLLIS (119) | Fosinopril 20 mg vs. hydrochlorothiazide 25 mg | Hypertension and hypercholesterolemia | 508 | 2.6 | Nfw CCA and BIF | Mnmx, mm | −0.0020 | 0.0100 | 0.010 | 57 | Yes |
| SECURE (120) | Ramipril 10 mg vs. Placebo | Vascular disease or DM | 732 | 4.5 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | 0.0137 | 0.0217 | 0.033 | 58 | Yes |
| STARR (121) | Ramipril 15 mg vs. placebo | Impaired glucose tolerance and/or impaired fasting glucose | 1425 | 3 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | 0.0083 | 0.0069 | 0.37 | 57 | No |
| PART2 (122) | Ramipril 5–10 mg vs. Placebo | CAD | 617 | 4 | Fw CCA | Mn, mm | 0.0300 | 0.0200 | 0.58 | 57 | No |
| BCAPS (107) | Metoprolol 25 mg vs. placebo | Asymptomatic | 794 | 3 | Fw CCA and BIF | Mn, mm | 0.1540 | 0.2270 | 0.014 | 61 | Yes |
| ELVA (103) | Metoprolol 100 mg vs. placebo | Primary hypercholesterolemia | 129 | 3 | Fw CCA and BIF | Mn | −0.06 | 0.0300 | 0.0110 | 61 | Yes |
| ELSA (123) | Lacidipine 4 mg vs. atenolol 50 mg | Hypertension | 2334 | 4 | Fw CCA and BIF | Mnmx, mm | 0.0087 | 0.0145 | <0.001 | 57 | Yes |
| INSIGHTIMT (124) | Nifedipine 30 mg or Amiloride 2.5 mg and HCL 25 mg | Hypertension | 439 | 4 | Fw CCA | Mn, mm | −0.0007 | 0.0077 | 0.003 | 57 | Yes |
| MIDAS (125) | Isradipine 2.5–5 mg vs. hydrochlorothiazide 12.5–25 mg | Hypertension | 883 | 3 | Nfw CCA, BIF, and ICA | Mnmx, mm | 0.1210 | 0.1490 | 0.680 | 64 | Yes |
| PREVENT (126) | Amlodipine 5–10 mg vs. placebo | CAD | 377 | 3 | Nfw of CCA, BIF, and ICA | Mnmx, mm/y | −0.0126 | 0.0330 | 0.007 | 57 | Yes |
| Stanton et al. (127) | Amlodipine 5–10 mg vs. Lisinopril 5–20 mg | Hypertension | 69 | 1 | Fw CCA | Mn, mm | −0.0480 | −0.0270 | 0.044 | 57 | Yes |
| DAPHNE (128) | Doxazosin 1–16 mg vs. diuretic hydrochlorothiazide 12.5100 mg | Hypertension | 80 | 3 | Nfw CCA, BIF, and ICA | Mnmx, mm | −0.1500 | −0.1800 | 0.850 | 68 | Yes |
| LAARS (129) | Losartan 50 mg vs. Atenolol 50 mg | Hypertension | 280 | 2 | Fw CCA | Mean CCA | −0.038 | −0.0370 | NS | 70 | No |
| Antioxidants | |||||||||||
| SECURE (120) | Vitamin E vs. Placebo | Vascular disease or DM | 732 | 4.5 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | 0.0180 | 0.0174 | NS | 71 | Yes |
| VEAPS (130) | Vitamin E vs. Placebo | Asymptomatic | 332 | 3 | Fw CCA | Mn, mm/y | 0.0040 | 0.0023 | 0.08 | 71 | Yes |
| MAVET (131) | Vitamin E vs. Placebo | Smoking | 409 | 4 | Nfw CCA, fw BIF and ICA | Mn, mm | 0.0035 | −0.0005 | 0.20 | 71 | Yes |
| FACIT (132) | Folic acid 800 ug vs. placebo | Asymptomatic | 819 | 3 | Nfw CCA | Mn, mm/y | 0.0019 | 0.0013 | 0.59 | 75 | Yes |
| ASFAST (133) | Folic acid 15 mg vs. placebo | Chronic renal failure | 315 | 3.6 | Fw CCA | Mnmx, mm | −0.0200 | 0.0300 | 0.43 | 75 | Yes |
| BVAIT (134) | Folic acid 5 mg + vitamin B12 0.4 mg + vitamin B6 50 mg vs. placebo | Asymptomatic | 506 | 3.1 | Fw CCA | Mn, mm | 0.0022 | 0.0029 | 0.31 | 75 | Yes |
| Hormone replacement therapy | |||||||||||
| EPAT (135) | Estradiol 1 mg vs. placebo | Asymptomatic, postmenopausal | 222 | 2 | Fw CCA | Mn, mm | −0.0017 | 0.0036 | 0.046 | 78 | No |
| OPAL (136) | Tibolone 2.5 mg vs. CEE/MPA (0.625 + 2.5 mg vs. placebo | Asymptomatic, postmenopausal | 866 | 3 | Nfw CCA, BIF, and ICA | Mn | 0.0077/0.0074 | 0.0035 | 0.03/0.04 | 78 | Yes |
| Colacurci (138) | Raloxifene 60 mg vs. placebo | Asymptomatic and postmenopausal | 155 | 1.5 | Nfw CCA, fw BIF and ICA | Mn, mm | 0.0112 | 0.0857 | 0.0040 | 83 | Yes |
| Glucoselowering therapy | |||||||||||
| CHICAGO (139) | Pioglitazone hydrochloride 15–45 mg vs. glimepiride 14 mg | DM2 | 462 | 1.5 | Fw CCA | Mn, mm | −0.0010 | 0.0120 | 0.020 | 85 | Yes |
| Langenfeld (140) | Pioglitazone 45 mg vs. glimepiride | DM2 | 179 | 0.5 | Nfw CCA | Mn | −0.033 | −0.0020 | 0.01 | 85 | Yes |
| RAS (141) | Rosiglitazone 48 mg vs. placebo | DM2 or insulin resistance syndrome | 555 | 1 | Fw CCA and BIF | Mn, mm | 0.0490 | 0.0600 | 0.310 | 88 | Yes |
| STARR (121) | Rosiglitazone 8 mg vs. placebo | Impaired glucose tolerance and/or impaired fasting glucose | 1425 | 3 | Nfw CCA, BIF, and ICA | Mnmx, mm/y | 0.0063 | 0.0090 | 0.0800 | 88 | Yes |
| Antiobesity therapy | |||||||||||
| AUDITOR (142) | Rimonabant 20 mg vs. placebo | Abdominal obesity and metabolic syndrome | 661 | 2.5 | Fw CCA, BIF, and ICA | Mn, mm/y | 0.005 | 0.007 | 0.45 | 90 | Yes |
CCA: Common carotid artery; ICA: internal carotid artery; BIF: Bifurcation; CEE: Conjugated equine estrogen; MPA: Medroxyprogesterone acetate; CHD: Coronary heart disease; HCL: Hydrochloride; CAD: Coronary artery disease; LDL-C: Low-density lipoprotein cholesterol; TC: Total cholesterol; FH: Familial hypercholesterolemia; NCEP: National Cholesterol Education Program;
When the trials in which the efficacy of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) are compared to placebo are reviewed, all trials, except for one,[109] reported a statistically significant beneficial effect of statin therapy on rate of change in CIMT. In a meta-analysis of seven statin trials on different regimens, statin therapy was associated with a mean annual change in CIMT of −0.012 mm (95% confidence interval [CI]: −0.016 to −0.007).[143] Another meta-analysis of 11 statin trials showed that the difference in the rate of change in CIMT between statin therapy and placebo was −0.040 mm (95% CI: −0.052 to −0.028).[144] A systematic review by Huang et al. up to December 2011 identified 21 randomized controlled trials using different statins with a minimum follow-up of 6 months. This meta-analysis involving 6317 individuals showed that the pooled weighted mean difference in progression rate between statin therapy and placebo or usual care for the common CIMT was −0.029 mm (95% CI: −0.045, −0.013).[145] Feng et al. performed a systematic search of the regular databases and a Chinese database up to January 2013 to studies comparing Rosuvastatin with a placebo or other statins on CIMT.[146]
The statin trials indicate that statin therapy inhibits atherosclerotic disease at the subclinical stage and provide evidence that CIMT measurements are able to show the beneficial effects of statins. A point of interest and importance is that the effect of statins on rate of change in CIMT appeared to be different across different carotid walls and segments, which calls for assessment of information from different carotid segments in trials.[26]
RATE OF CHANGE IN CAROTID INTIMA-MEDIA THICKNESS AND FUTURE CARDIOVASCULAR EVENTS
Investigators have argued that data should become available that assesses whether a change in CIMT relates to change in risk of CV events. In particular in the clinical trial world, such an observation is regarded as a final part of the evidence chain to support the CIMT measurement for use in trials.[147] Yet, studies to demonstrate that are difficult to conduct as they first need to show the impact of an intervention on rate of change in CIMT and next have sufficient sample size and follow-up time after this demonstration to assess the ability of measured CIMT change to account for subsequent changes in CVD risk. Importantly, most trials with excellent CIMT data on the rate of change in CIMT did not follow participants for the occurrence of events after the trial was finished. Moreover, these CIMT trials were not designed (too small sample size) for evaluation of vascular events. And thus data on change in CIMT induced by lipid-level modifying or blood pressure lowering therapies and change risk for CV events is very limited.
The only published paper is the Cholesterol Lowering Atherosclerosis Study (CLAS) trial.[148] The 2-year CLAS trial demonstrated that colestipol-niacin therapy reduced rate of change in CIMT. The trial cohort subsequently was surveyed over an average of 8.8 years after the conclusion of CLAS to evaluate the posttrial incidence of coronary events. The trial showed statistically significant favorable results: That is, a lower rate of change in common CIMT over time was related to a lower risk of an event. Those with an annual common CIMT progression rate of 0.034 mm/year had a 2.9-fold higher CVD risk compared to those with a common CIMT progression rate of 0.011 mm or less. The risk for those with progression rates between 0.011 and 0.017 mm was increased 1.8-fold, and the risk for those with progression rates between 0.018 and 0.033 mm was increased 2.3-fold. In addition to this paper, Espeland et al. performed a meta-analysis showing that across the trials, statin therapy was associated with an average decrease of CIMT progression of 0.012 mm/year (95% CI, −0.016 to −0.007).[143] Using the same studies, they performed a meta-analysis which yielded a risk reduction of 52% for CVD events. In this approach, the authors linked the CIMT benefit to the reduction of events.
In addition, the PROG-IMT initiative recently published their findings on the rate of change in CIMT and future events.[41] PROG-IMT was based on a change in CIMT found in observational studies, so natural history in common CIMT rather than the pharmaceutically induced rate of change. No relation between the rate of change in common CIMT and risk of CV events was detected. The reproducibility between the first and the second CIMT measurement was surprisingly low (correlation coefficient <0.10). The IMPROVE study, a recent observational initiative performed in 7 centers in 5 European countries enrolled 3482 subjects, (median 64 years; 47.8% men) with 3 or more vascular risk factors, and was designed to assess CIMT progression.[42] An increase in mean common CIMT of 0.058 mm was related to an increased risk of CV events of 11% (95% CI: 8%, 34%). The CIMT estimate based on the fastest change in any segment showed a progression rate of 0.27 mm/year. An increase of one standard deviation, that is, 0.26, was related to an increase in risk of 26% (95% CI: 8%, 44%).[42] In a cohort of 342 Japanese patients with type 2 diabetes mellitus without history of CV events whose CIMT was assessed more than twice by ultrasonography were recruited and followed up for CV events, Okayama et al. showed that the change in CIMT was significantly associated with CV events, with a hazard ratio of 2.24 (95% CI, 1.25–4.03, using the median of CIMT change of 0.011,14 mm/year as cut point.[149]
Recently, two meta-analyses were published trying to address this issue using aggregated data from published reports.[150,151] The two meta-analyses have been criticized because of flaws in the design and analyses.[152,153] Flaws include the misuse of the concept of atherosclerosis, pooling of trials carried out with treatments having heterogeneous efficacy and among patients, who had very different risk profiles; pooling of measurements from a wide variety of methodologies that shared a common name, “CIMT;” lack of power for detecting relationships using meta-regression techniques, and lastly, the ecologic fallacy. Hence, the conclusions of these two meta-analyses should not be considered appropriate.[153]
At present, direct quantitative evidence to translate the reduction in CIMT progression rates in a reduction in clinical outcome is modestly available.[148] Yet, the lack of such information does not invalidate the CIMT measurement for use in trials on atherosclerosis regression and reduction of CV risk.
CRITERIA OF PRENTICE AND BOISSEL TO SUPPORT SURROGACY
Prentice and Boissel have proposed several criteria [Supplementary Table 1] for a surrogate marker that should have been met before it could be validly used. In the previous paragraphs, we have addressed in detail the criteria by Prentice (P1, P2 and P3 from the Supplementary Table 1). The criteria P4 is more difficult to address since it mandates studies in which data on the intervention, on the CIMT change and on clinical events are all present in one study. This type of data is only present from meta-analyses as performed by Espeland et al.[143] They showed a pooled estimate between statin use and CV events of 0.48 (0.30, 0.78). When in the analyses they adjusted for the rate of change in CIMT, the pooled estimate was attenuated to 0.64 and no longer statistically significant (P = 0.13). This suggests that changes in CIMT may account for some, but not all, of the effect of statins on CV events.
With respect to the evidence fitting the Boissel criteria:
B1: Efficiency. CIMT measurements are relatively easy to obtain using noninvasive means and are can be obtained in nearly every individual. It is clear that M and M trials on lipid-level modifying therapies generally were conducted in thousands of participants with 5 years of follow-up, whereas CIMT trials have been performed in hundreds of participants who were followed for 24–36 months.[26]
B2: Linkage. There is abundant evidence from several observational studies to suggest that increased CIMT is related to an increased risk of CV events.[41,96] The linkage between pharmaceutical induced rate of change in CIMT and future CV events has not been firmly established
B3: Congruency. The congruency argument is important and has a number of aspects that should be addressed. First, individuals with and without vascular disease should exhibit differences in CIMT, which has been clearly demonstrated.[29] Moreover, evidence from randomized controlled trials suggests that CIMT change over time are larger in individual with CVD as compared to those without. The pooled annual common CIMT progression rate observed in the control arm of trials in patients with coronary heart disease was 0.0170 mm (SD 0.06 [median]). The reported estimate for the annual mean maximum CIMT progression rate was 0.0258 mm (SD 0.068 [median]).[154] The second aspect is that anticipated clinical benefits should be deducible from the observed changes in the surrogate marker in the trials. As indicated under B2: Linkage, this type of evidence is not readily available yet. The third aspect deals with the notion that the surrogate should produce parallel estimates of risk and benefit as endpoints. This has been made likely by Espeland et al.[143] Recently, the approach by Espeland has been substantiated and extended by a systematic review in which the agreement between the results from CIMT and M and M trials was assessed, and positive and negative predictive values were calculated.[155] Forty-eight CIMT trials were included. CIMT trials (n = 20) on lipid-level modifying therapies were all, except one, in agreement with the M and M trial findings. These results demonstrate a strong congruency between results from a CIMT trial and an M and M trial using the same compound.
THE BEST APPROACH FOR CAROTID INTIMA-MEDIA THICKNESS TRIALS
Based on the experience in previous large-scale trials there is a number of aspects that one may consider in designing a multicenter randomized controlled trial with CIMT as primary outcome parameter.[29]
How to choose the best carotid intima-media thickness measurement: Side, segments, walls, and angles?
Guidelines on how to measure CIMT have been published.[156,157] Nevertheless, there are still no accepted standards on the most optimal ultrasound protocol for either single nor repeated CIMT assessments. Hence, choices on the CIMT ultrasound protocol to be used are generally based on experience and expert opinion rather than on solid evidence from methodological studies. Even though some methodological issues are being addressed, there are many outstanding topics that need to be further evaluated. The debate is about simple protocols versus extensive protocols. Important to realize is the setting for which a protocol is used.[20] For protocols used in randomized controlled trials, emphasis is on assessment of the rate of change. A number of reports have provided evidence on the best balance between reproducibility, completeness of the data, magnitude of CIMT change over time and its associated precision, and magnitude of effect of intervention on CIMT change over time, and its associated precision.[27,28,44,45,46] Trials evaluating the effect of lipid lowering on CIMT progression have shown that CIMT measurements of both the near and far wall measurements are superior to trials only having only far wall measurements. With respect the use of angle specific measurement, analyses from studies indicated that extensive ultrasound protocols including near and far wall measurements from two or more angles provide a better balance between high reproducibility, large progression rates, and large and precise intervention effects when compared to single angle protocols from the far wall alone. This may especially be beneficial in settings where sample sizes and effect sizes are small. With respect to segments (common versus all three segments), there are trials showing a beneficial response to an intervention on the rate of change in CIMT measured using a single angle far wall common CIMT measurement.[26] The issue is that it remains unknown whether trials showing an effect on the CCA alone would have found a similar or improved effect if an extensive protocol has been used. There are trials that failed to show an intervention effect on the common CIMT, whereas a beneficial effect was found on the aggregate CIMT measure and on clinical events. These findings underscore our viewpoint that an ideal ultrasound protocol does not exist and that the choice for an ultrasound protocol always should depend on a well-considered evaluation of the expected rates of change and associated precision at the different carotid segments. However, as it remains impossible to predict at which carotid segment drug therapies will have their effect,[26,158] extensive protocols may be preferred.
Although studies with extensive ultrasound protocols may be considered the most precise and most comprehensive studies, there are disadvantages in terms of cost and logistics as well. Extensive ultrasound protocols take more time for acquisition and quantification. Moreover, extensive ultrasound protocols require more extensive training of sonographers than ultrasound protocols measuring the CCA alone. While the current evidence indicates that extensive ultrasound protocols do provide the highest quality data in intervention studies, the choice of the ultrasound protocol should always be based on the specific questions that one wants to address and the resources available. It should be noted that a less extensive protocol with careful quality control is always preferable to a more extensive protocol with inadequate quality control.
Missing data when having an extensive ultrasound protocol?
We showed that high levels of complete data can be obtained with extensive ultrasound protocols that include measurement from the carotid bifurcation and ICA.[44,45,158] For example, in the METEOR study, the percentage of CIMT measurements at the baseline examinations was 94% for the near wall of the right ICA, and 96% for the near wall of the left ICA. Completeness on the other carotid artery sites, including the carotid bifurcation, was >99%. A high body mass index contributes to the incompleteness of CIMT measurements.[158]
Sample size consideration for a trial
Sample size calculations for l trials generally use expected changes between groups, and variances obtained from literature. However, this approach neglects the impact of differences in trial design. Designs with a shorter duration of follow-up increased within-individual variance and required larger sample sizes to detect the same treatment effect.[159,160] In addition, reducing the number of scans at the end of the study from two to one increased and reducing the number of baseline scans from two to one further increased the required sample size.[160]
What to do with implausible biological values?
Implausible CIMT values may refer to an observation within an individual that is far from the values observed in the remainder of the individuals. These implausible values are observed at a single time point and reflect extreme values of an individual relative to other individuals. However, in longitudinal datasets, implausible values may also occur within an individual with repeated measurements, that is, one of the repeated measurements is temporally far distant from the previous and/or subsequent measurements. No established cut-off values or rigid mathematical definition exists of what constitutes a biologically implausible value either between or within individuals. Hence, determining whether or not an observation is biologically implausible has subjective components. There are two options to deal with biologically implausible values. The first is to accept that they are a genuine part of the outcome of the study, and the second is to delete these values from the dataset. It is currently unclear to what extent implausible values affect the assessment of treatment effects. In METEOR, the percentage of biologically implausible CIMT values ranged from 0.6% to 9.7%, depending on the definition used. Across all definitions, removal of biologically implausible CIMT values marginally reduced standard errors and did not change the primary outcome. Given the relative subjectivity involved in defining biologically implausible CIMT values, removal of data should be discouraged in situations in which there is no immediate concern about the plausibility of the data.[161]
Imputation needed or not?
Missing endpoint data are a common and severe problem in clinical trials in which the endpoint is repeatedly measured over time. Missing data may lead to bias in the point estimates or may affect precision. Several techniques have been described to deal with the impact of missing data. Multiple imputation (MI) has shown to be the preferred method for incomplete data situations where information on determinants or outcomes is missing. We empirically showed that MI of missing endpoint data prior to linear effects model analyses does not increase precision in the estimated rate of change in the endpoint.[162] Hence, MI had no added value in this setting, and standard LME modeling remains the method of choice.
Batch reading or not?
In CIMT trials, carotid ultrasound scans are collected in central core laboratories (specialized vascular imaging centers) where CIMT is measured in a later stage. Typically, there are two approaches to read CIMT from images: Random continuous readings (nonbatch) and batch readings.[154] In the nonbatch approach, CIMT measurements are performed continuously over the course of the study, by randomly allocating a reader to a scan that is received at the core laboratory. In batch reading, one reader reads all the scans of a certain participant in a short time period after collection of the last scan. A logistic advantage of nonbatch reading is efficiency and short lag time between data availability and completion of the trial. A disadvantage of nonbatch reading, however, may be the temporal component. In studies that last several years between the first CIMT measurements and the last CIMT measurements, theoretically a drift may occur in the estimates of the rate of change, due to change over time in measuring habits of reading personnel of the core laboratory. Drift may affect rates of change in theory. Drift should not affect treatment effects, as readers are blinded for assignment of the intervention, and thus potential drift is likely to affect both treatment arms and thereby the estimated difference between the treatment arms should remain equal. In batch reading, all images of one individual are collected at the end of the trial and CIMT is quantified from all images in a short time window by one “reader.” Empirical data on this issue is, however, scarce.[132]
SUMMARY
Advances in the field of carotid ultrasound have been incremental, resulting in a steady decrease in measurement variability. Improvements in edge detection algorithms point toward increasing automation of CIMT measurements. The major advantage of CIMT is that it is completely noninvasive and can be repeated as often as required. It provides a continuous measure since all subjects have a measurable carotid wall. It is also relatively inexpensive to perform, and the technology is widely available. A graded relation between raising LDL cholesterol and increased CIMT is apparent. Increased CIMT has been shown consistently to relate the atherosclerotic abnormalities elsewhere in the arterial system. Moreover, increased CIMT predicts future vascular events in both populations from Caucasian ancestry and those from Asian ancestry. Furthermore, lipid-lowering therapy has been shown to affect CIMT progression within 12–18 months in properly designed trials with results congruent with clinical events trials. In conclusion, when one wants to evaluate the effect of a pharmaceutical intervention that is to be expected to beneficially affect atherosclerosis progression and to reduce CV event risk, the use of CIMT measurements over time is a valid, suitable, and evidence-based choice.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This review has been made possible by an unrestricted grant from Astra Zeneca.
Conflicts of interest
Dr. Bots previously received study grants for studies on carotid intima-media thickness and/or honoraria for professional input regarding issues on carotid intima-media thickness from Astra-Zeneca, Icelandic Heart Foundation, Organon, Pfizer, Dutch Foundation, Netherlands Organisation for Health Research and Development, Servier and Unilever. Dr. Bots runs the Vascular Imaging Center, a core laboratory for the quantification of noninvasively assessed atherosclerosis in observational and intervention studies. Mr. Evans has received honoraria, consulting fees and grant support for professional input on CIMT issues from Astra-Zeneca, Organon, and Pfizer.
Acknowledgements
We thank Eur Heart J, Eur J Epidemiol, and J Stroke for the permission of using their published figures in the present paper.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.World Health Organization. The World Health Report. 2008. [Last accessed on 2009 Jun 07]. Available from: http://www.who.int/whr/en/
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 5.Revkin JH, Shear CL, Pouleur HG, Ryder SW, Orloff DG. Biomarkers in the prevention and treatment of atherosclerosis: Need, validation, and future. Pharmacol Rev. 2007;59:40–53. doi: 10.1124/pr.59.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Geerts CC, Bots ML, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in young adult offspring: Is early life exposure critical? The atherosclerosis risk in young adults study. Arterioscler Thromb Vasc Biol. 2008;28:2296–302. doi: 10.1161/ATVBAHA.108.173229. doi: 10.1161/ATVBAHA.108.173229. [DOI] [PubMed] [Google Scholar]
- 7.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: A systematic review and meta-analysis of observational studies. Heart. 2009;95:1826–35. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 8.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403–10. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 9.Janssen I, Powell LH, Matthews KA, Cursio JF, Hollenberg SM, Sutton-Tyrrell K, et al. Depressive symptoms are related to progression of coronary calcium in midlife women: The Study of Women's Health Across the Nation (SWAN) Heart Study. Am Heart J. 2011;161:1186–91. doi: 10.1016/j.ahj.2011.03.017. doi: 10.1016/j.ahj.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bots ML, Sutton-Tyrrell K. Lessons from the past and promises for the future for carotid intima-media thickness. J Am Coll Cardiol. 2012;60:1599–604. doi: 10.1016/j.jacc.2011.12.061. doi: 10.1016/j.jacc.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 11.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: Recommendations for standard assessment for clinical research: A scientific statement from the American Heart Association. Hypertension. 2009;54:919–50. doi: 10.1161/HYPERTENSIONAHA.109.192639. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 12.Prentice RL. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med. 1989;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 13.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation. 1986;74:1399–406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 14.Wong M, Edelstein J, Wollman J, Bond MG. Ultrasonic-pathological comparison of the human arterial wall. Verification of intima-media thickness. Arterioscler Thromb. 1993;13:482–6. doi: 10.1161/01.atv.13.4.482. [DOI] [PubMed] [Google Scholar]
- 15.Gamble G, Beaumont B, Smith H, Zorn J, Sanders G, Merrilees M, et al. B-mode ultrasound images of the carotid artery wall: Correlation of ultrasound with histological measurements. Atherosclerosis. 1993;102:163–73. doi: 10.1016/0021-9150(93)90158-q. [DOI] [PubMed] [Google Scholar]
- 16.Wendelhag I, Gustavsson T, Suurküla M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: Fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11:565–77. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 17.Persson J, Formgren J, Israelsson B, Berglund G. Ultrasound-determined intima-media thickness and atherosclerosis. Direct and indirect validation. Arterioscler Thromb. 1994;14:261–4. doi: 10.1161/01.atv.14.2.261. [DOI] [PubMed] [Google Scholar]
- 18.Gussenhoven EJ, Essed CE, Lancée CT, Mastik F, Frietman P, van Egmond FC, et al. Arterial wall characteristics determined by intravascular ultrasound imaging: An in vitro study. J Am Coll Cardiol. 1989;14:947–52. doi: 10.1016/0735-1097(89)90471-3. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary DH, Bots ML. Imaging of atherosclerosis: Carotid intima-media thickness. Eur Heart J. 2010;31:1682–9. doi: 10.1093/eurheartj/ehq185. [DOI] [PubMed] [Google Scholar]
- 20.Peters SA, den Ruijter HM, Bots ML METEOR Study Group. Measuring carotid intima-media thickness: Extensive ultrasound protocols have value. J Am Soc Echocardiogr. 2012;25:1128–30. doi: 10.1016/j.echo.2012.04.017. doi: 10.1016/j.echo.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Salonen R, Salonen JT. Determinants of carotid intima-media thickness: A population-based ultrasonography study in eastern Finnish men. J Intern Med. 1991;229:225–31. doi: 10.1111/j.1365-2796.1991.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 22.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96:1432–7. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 23.Oren A, Vos LE, Uiterwaal CS, Bak AA, Gorissen WH, Grobbee DE, Bots ML. The Atherosclerosis Risk in Young Adults (ARYA) study: rationale and design. Eur J Epidemiol. 2003;18:715–27. doi: 10.1023/a:1024898900106. [DOI] [PubMed] [Google Scholar]
- 24.Tajik P, Meijer R, Duivenvoorden R, Peters SA, Kastelein JJ, Visseren FJ, et al. Asymmetrical distribution of atherosclerosis in the carotid artery: Identical patterns across age, race, and gender. Eur J Prev Cardiol. 2012;19:687–97. doi: 10.1177/1741826711410821. doi: 10.1177/1741826711410821. [DOI] [PubMed] [Google Scholar]
- 25.Espeland MA, Hoen H, Byington R, Howard G, Riley WA, Furberg CD. Spatial distribution of carotid intimal-medial thickness as measured by B-mode ultrasonography. Stroke. 1994;25:1812–9. doi: 10.1161/01.str.25.9.1812. [DOI] [PubMed] [Google Scholar]
- 26.Peters SA, den Ruijter HM, Bots ML. Attenuation of rate of change in carotid intima-media thickness by lipid-modifying drugs: Impact on clinical outcomes. Am J Cardiovasc Drugs. 2011;11:253–63. doi: 10.2165/11591960-000000000-00000. doi: 10.2165/11591960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Peters SA, den Ruijter HM, Bots ML. Ultrasound protocols to measure carotid intima-media thickness: One size does not fit all. J Am Soc Echocardiogr. 2012;25:1135–7. doi: 10.1016/j.echo.2012.08.003. doi: 10.1016/j.echo.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Dogan S, Plantinga Y, Crouse JR, 3rd, Evans GW, Raichlen JS, O’Leary DH, et al. Algorithms to measure carotid intima-media thickness in trials: A comparison of reproducibility, rate of progression and treatment effect. J Hypertens. 2011;29:2181–93. doi: 10.1097/HJH.0b013e32834b0eba. doi: 10.1097/HJH.0b013e32834b0eba. [DOI] [PubMed] [Google Scholar]
- 29.Peters SA, Bots ML. Carotid intima-media thickness studies: Study design and data analysis. J Stroke. 2013;15:38–48. doi: 10.5853/jos.2013.15.1.38. doi: 10.5853/jos.2013.15.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters SA, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR, 3rd, O’Leary DH, et al. Manual or semi-automated edge detection of the maximal far wall common carotid intima-media thickness: A direct comparison. J Intern Med. 2012;271:247–56. doi: 10.1111/j.1365-2796.2011.02422.x. doi: 10.1111/j.1365-2796.2011.02422.x. [DOI] [PubMed] [Google Scholar]
- 31.Freire CM, Ribeiro AL, Barbosa FB, Nogueira AI, de Almeida MC, Barbosa MM, et al. Comparison between automated and manual measurements of carotid intima-media thickness in clinical practice. Vasc Health Risk Manag. 2009;5:811–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Kanters SD, Algra A, van Leeuwen MS, Banga JD. Reproducibility of in vivo carotid intima-media thickness measurements: A review. Stroke. 1997;28:665–71. doi: 10.1161/01.str.28.3.665. [DOI] [PubMed] [Google Scholar]
- 33.Bots ML, Mulder PG, Hofman A, van Es GA, Grobbee DE. Reproducibility of carotid vessel wall thickness measurements. The Rotterdam Study. J Clin Epidemiol. 1994;47:921–30. doi: 10.1016/0895-4356(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 34.O’Leary DH, Polak JF, Wolfson SK, Jr, Bond MG, Bommer W, Sheth S, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–63. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 35.Riley WA, Barnes RW, Applegate WB, Dempsey R, Hartwell T, Davis VG, et al. Reproducibility of noninvasive ultrasonic measurement of carotid atherosclerosis. The Asymptomatic Carotid Artery Plaque Study. Stroke. 1992;23:1062–8. doi: 10.1161/01.str.23.8.1062. [DOI] [PubMed] [Google Scholar]
- 36.Salonen R, Haapanen A, Salonen JT. Measurement of intima-media thickness of common carotid arteries with high-resolution B-mode ultrasonography: Inter-and intra-observer variability. Ultrasound Med Biol. 1991;17:225–30. doi: 10.1016/0301-5629(91)90043-v. [DOI] [PubMed] [Google Scholar]
- 37.Furberg CD, Adams HP, Jr, Applegate WB, Byington RP, Espeland MA, Hartwell T, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90:1679–87. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 38.Bots ML, Evans GW, Riley W, Meijer R, McBride KH, Paskett ED, et al. The Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study: Design and baseline characteristics. Control Clin Trials. 2003;24:752–75. doi: 10.1016/s0197-2456(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 39.Crouse JR, 3rd, Grobbee DE, O’Leary DH, Bots ML, Evans GW, Palmer MK, et al. Carotid intima-media thickness in low-risk individuals with asymptomatic atherosclerosis: Baseline data from the METEOR study. Curr Med Res Opin. 2007;23:641–8. doi: 10.1185/030079907X178711. [DOI] [PubMed] [Google Scholar]
- 40.Kastelein JJ, van Leuven SI, Evans GW, Riley WA, Revkin JH, Shear CL, et al. Designs of RADIANCE 1 and 2: Carotid ultrasound studies comparing the effects of torcetrapib/atorvastatin with atorvastatin alone on atherosclerosis. Curr Med Res Opin. 2007;23:885–94. doi: 10.1185/030079907x182121. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): A meta-analysis of individual participant data. Lancet. 2012;379:2053–62. doi: 10.1016/S0140-6736(12)60441-3. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldassarre D, Hamsten A, Veglia F, de Faire U, Humphries SE, Smit AJ, et al. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: Results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) study. J Am Coll Cardiol. 2012;60:1489–99. doi: 10.1016/j.jacc.2012.06.034. doi: 10.1016/j.jacc.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 43.Dogan S, Plantinga Y, Dijk JM, van der GY, Grobbee DE, Bots ML. Manual B-mode versus automated radio-frequency carotid intima-media thickness measurements. J Am Soc Echocardiogr. 2009;22:1137–44. doi: 10.1016/j.echo.2009.07.008. doi: 10.1016/j.echo.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Dogan S, Duivenvoorden R, Grobbee DE, Kastelein JJ, Shear CL, Evans GW, et al. Ultrasound protocols to measure carotid intima-media thickness in trials; comparison of reproducibility, rate of progression, and effect of intervention in subjects with familial hypercholesterolemia and subjects with mixed dyslipidemia. Ann Med. 2010;42:447–64. doi: 10.3109/07853890.2010.499132. doi: 10.3109/07853890.2010.499132. [DOI] [PubMed] [Google Scholar]
- 45.Dogan S, Duivenvoorden R, Grobbee DE, Kastelein JJ, Shear CL, Evans GW, et al. Completeness of carotid intima media thickness measurements depends on body composition: The RADIANCE 1 and 2 trials. J Atheroscler Thromb. 2010;17:526–35. doi: 10.5551/jat.3269. [DOI] [PubMed] [Google Scholar]
- 46.Dogan S, Plantinga Y, Evans GW, Meijer R, Grobbee DE, Bots ML. OPAL investigators. Ultrasound protocols to measure carotid intima-media thickness: A post-hoc analysis of the OPAL study. Curr Med Res Opin. 2009;25:109–22. doi: 10.1185/03007990802589727. doi: 10.1185/03007990802589727. [DOI] [PubMed] [Google Scholar]
- 47.Handa N, Matsumoto M, Maeda H, Hougaku H, Ogawa S, Fukunaga R, et al. Ultrasonic evaluation of early carotid atherosclerosis. Stroke. 1990;21:1567–72. doi: 10.1161/01.str.21.11.1567. [DOI] [PubMed] [Google Scholar]
- 48.Dempsey RJ, Moore RW. Amount of smoking independently predicts carotid artery atherosclerosis severity. Stroke. 1992;23:693–6. doi: 10.1161/01.str.23.5.693. [DOI] [PubMed] [Google Scholar]
- 49.Bots ML, Hofman A, de Bruyn AM, de Jong PT, Grobbee DE. Isolated systolic hypertension and vessel wall thickness of the carotid artery. The Rotterdam Elderly Study. Arterioscler Thromb. 1993;13:64–9. doi: 10.1161/01.atv.13.1.64. [DOI] [PubMed] [Google Scholar]
- 50.Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O’Leary DH, et al. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA. 1992;268:1287–91. [PubMed] [Google Scholar]
- 51.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: Associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134:250–6. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 52.Bonithon-Kopp C, Scarabin PY, Taquet A, Touboul PJ, Malmejac A, Guize L. Risk factors for early carotid atherosclerosis in middle-aged French women. Arterioscler Thromb. 1991;11:966–72. doi: 10.1161/01.atv.11.4.966. [DOI] [PubMed] [Google Scholar]
- 53.Prati P, Vanuzzo D, Casaroli M, Di Chiara A, De Biasi F, Feruglio GA, et al. Prevalence and determinants of carotid atherosclerosis in a general population. Stroke. 1992;23:1705–11. doi: 10.1161/01.str.23.12.1705. [DOI] [PubMed] [Google Scholar]
- 54.O’Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, et al. Distribution and correlates of sonographically detected carotid artery disease in theCardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23:1752–60. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 55.el-Barghouti N, Elkeles R, Nicolaides A, Geroulakos G, Dhanjil S, Diamond J. The ultrasonic evaluation of the carotid intima-media thickness and its relation to risk factors of atherosclerosis in normal and diabetic population. Int Angiol. 1997;16:50–4. [PubMed] [Google Scholar]
- 56.den Ruijter HM, Peters SA, Groenewegen KA, Anderson TJ, Britton AR, Dekker JM, et al. Common carotid intima-media thickness does not add to Framingham risk score in individuals with diabetes mellitus: The USE-IMT initiative. Diabetologia. 2013;56:1494–502. doi: 10.1007/s00125-013-2898-9. doi: 10.1007/s00125-013-2898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang Y, Yan Z, Sun B, Cai C, Jiang H, Song A, Qiu C. Cardiovascular risk factor profiles for peripheral artery disease and carotid atherosclerosis among Chinese older people: A population-based study. PLoS One. 2014;9:e85927. doi: 10.1371/journal.pone.0085927. doi: 10.1371/journal.pone.0085927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geroulakos G, O’Gorman DJ, Kalodiki E, Sheridan DJ, Nicolaides AN. The carotid intima-media thickness as a marker of the presence of severe symptomatic coronary artery disease. Eur Heart J. 1994;15:781–5. doi: 10.1093/oxfordjournals.eurheartj.a060585. [DOI] [PubMed] [Google Scholar]
- 59.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: The British Regional Heart Study. Stroke. 1999;30:841–50. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 60.Oren A, Vos LE, Uiterwaal CS, Grobbee DE, Bots ML. Cardiovascular risk factors and increased carotid intima-media thickness in healthy young adults: The Atherosclerosis Risk in Young Adults (ARYA) Study. Arch Intern Med. 2003;163:1787–92. doi: 10.1001/archinte.163.15.1787. [DOI] [PubMed] [Google Scholar]
- 61.Geroulakos G, O’Gorman D, Nicolaides A, Sheridan D, Elkeles R, Shaper AG. Carotid intima-media thickness: Correlation with the British Regional Heart Study risk score. J Intern Med. 1994;235:431–3. doi: 10.1111/j.1365-2796.1994.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 62.Sakurai S, Kitamura A, Cui R, Yamagishi K, Tanigawa T, Iso H. Relationships of soluble E-selectin and high-sensitivity C-reactive protein with carotid atherosclerosis in Japanese men. J Atheroscler Thromb. 2009;16:339–45. doi: 10.5551/jat.no182. [DOI] [PubMed] [Google Scholar]
- 63.Baldassarre D, De Jong A, Amato M, Werba JP, Castelnuovo S, Frigerio B, et al. Carotid intima-media thickness and markers of inflammation, endothelial damage and hemostasis. Ann Med. 2008;40:21–44. doi: 10.1080/07853890701645399. [DOI] [PubMed] [Google Scholar]
- 64.Bots ML, van Swieten JC, Breteler MM, de Jong PT, van Gijn J, Hofman A, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet. 1993;341:1232–7. doi: 10.1016/0140-6736(93)91144-b. [DOI] [PubMed] [Google Scholar]
- 65.Linhart A, Gariepy J, Giral P, Levenson J, Simon A. Carotid artery and left ventricular structural relationship in asymptomatic men at risk for cardiovascular disease. Atherosclerosis. 1996;127:103–12. doi: 10.1016/s0021-9150(96)05940-0. [DOI] [PubMed] [Google Scholar]
- 66.Cuspidi C, Lonati L, Sampieri L, Leonetti G, Zanchetti A. Similarities and differences in structural and functional changes of left ventricle and carotid arteries in young borderline hypertensives and in athletes. J Hypertens. 1996;14:759–64. doi: 10.1097/00004872-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 67.Cuspidi C, Lonati L, Sampieri L, Pelizzoli S, Pontiggia G, Leonetti G, et al. Left ventricular concentric remodelling and carotid structural changes in essential hypertension. J Hypertens. 1996;14:1441–6. doi: 10.1097/00004872-199612000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Mykkänen L, Zaccaro DJ, O’Leary DH, Howard G, Robbins DC, Haffner SM. Microalbuminuria and carotid artery intima-media thickness in nondiabetic and NIDDM subjects. The Insulin Resistance Atherosclerosis Study (IRAS) Stroke. 1997;28:1710–6. doi: 10.1161/01.str.28.9.1710. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto M, Eto M, Akishita M, Kozaki K, Ako J, Iijima K, et al. Correlation between flow-mediated vasodilatation of the brachial artery and intima-media thickness in the carotid artery in men. Arterioscler Thromb Vasc Biol. 1999;19:2795–800. doi: 10.1161/01.atv.19.11.2795. [DOI] [PubMed] [Google Scholar]
- 70.Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, et al. Risk factors for progression of common carotid atherosclerosis: The Atherosclerosis Risk in Communities Study, 1987-1998. Am J Epidemiol. 2002;155:38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- 71.Lorenz MW, Karbstein P, Markus HS, Sitzer M. High-sensitivity C-reactive protein is not associated with carotid intima-media progression: The carotid atherosclerosis progression study. Stroke. 2007;38:1774–9. doi: 10.1161/STROKEAHA.106.476135. [DOI] [PubMed] [Google Scholar]
- 72.Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, et al. Predictors of carotid thickness and plaque progression during a decade: The Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:3257–62. doi: 10.1161/STROKEAHA.114.005669. doi: 10.1161/STROKEAHA.114.005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): A randomised, double-blind trial. Lancet. 2007;370:153–60. doi: 10.1016/S0140-6736(07)61088-5. [DOI] [PubMed] [Google Scholar]
- 74.Polak JF, O’Leary DH, Kronmal RA, Wolfson SK, Bond MG, Tracy RP, et al. Sonographic evaluation of carotid artery atherosclerosis in the elderly: Relationship of disease severity to stroke and transient ischemic attack. Radiology. 1993;188:363–70. doi: 10.1148/radiology.188.2.8327679. [DOI] [PubMed] [Google Scholar]
- 75.Howard G, Burke GL, Evans GW, Crouse JR, 3rd, Riley W, Arnett D, et al. Relations of intimal-medial thickness among sites within the carotid artery as evaluated by B-mode ultrasound. ARIC Investigators. Atherosclerosis Risk in Communities. Stroke. 1994;25:1581–7. doi: 10.1161/01.str.25.8.1581. [DOI] [PubMed] [Google Scholar]
- 76.Bots ML, Hofman A, De Jong PT, Grobbee DE. Common carotid intima-media thickness as an indicator of atherosclerosis at other sites of the carotid artery. The Rotterdam Study. Ann Epidemiol. 1996;6:147–53. doi: 10.1016/1047-2797(96)00001-4. [DOI] [PubMed] [Google Scholar]
- 77.Bots ML, Witteman JC, Grobbee DE. Carotid intima-media wall thickness in elderly women with and without atherosclerosis of the abdominal aorta. Atherosclerosis. 1993;102:99–105. doi: 10.1016/0021-9150(93)90088-c. [DOI] [PubMed] [Google Scholar]
- 78.Bots ML, Hofman A, Grobbee DE. Common carotid intima-media thickness and lower extremity arterial atherosclerosis. The Rotterdam Study. Arterioscler Thromb. 1994;14:1885–91. doi: 10.1161/01.atv.14.12.1885. [DOI] [PubMed] [Google Scholar]
- 79.Allan PL, Mowbray PI, Lee AJ, Fowkes FG. Relationship between carotid intima-media thickness and symptomatic and asymptomatic peripheral arterial disease. The Edinburgh Artery Study. Stroke. 1997;28:348–53. doi: 10.1161/01.str.28.2.348. [DOI] [PubMed] [Google Scholar]
- 80.Cohen GI, Aboufakher R, Bess R, Frank J, Othman M, Doan D, et al. Relationship between carotid disease on ultrasound and coronary disease on CT angiography. JACC Cardiovasc Imaging. 2013;6:1160–7. doi: 10.1016/j.jcmg.2013.06.007. doi: 10.1016/j.jcmg.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Ogata T, Yasaka M, Yamagishi M, Seguchi O, Nagatsuka K, Minematsu K. Atherosclerosis found on carotid ultrasonography is associated with atherosclerosis on coronary intravascular ultrasonography. J Ultrasound Med. 2005;24:469–74. doi: 10.7863/jum.2005.24.4.469. [DOI] [PubMed] [Google Scholar]
- 82.Bots ML, Baldassarre D, Simon A, de Groot E, O’Leary DH, Riley W, et al. Carotid intima-media thickness and coronary atherosclerosis: Weak or strong relations? Eur Heart J. 2007;28:398–406. doi: 10.1093/eurheartj/ehl482. [DOI] [PubMed] [Google Scholar]
- 83.Taylor AJ, Bindeman J, Le TP, Bauer K, Byrd C, Feuerstein IM, et al. Progression of calcified coronary atherosclerosis: Relationship to coronary risk factors and carotid intima-media thickness. Atherosclerosis. 2008;197:339–45. doi: 10.1016/j.atherosclerosis.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 84.Gronewold J, Bauer M, Lehmann N, Mahabadi AA, Kälsch H, Weimar C, et al. Coronary artery calcification, intima-media thickness, and ankle-brachial index are complementary stroke predictors. Stroke. 2014;45:2702–9. doi: 10.1161/STROKEAHA.114.005626. doi: 10.1161/STROKEAHA.114.005626. [DOI] [PubMed] [Google Scholar]
- 85.Amato M, Montorsi P, Ravani A, Oldani E, Galli S, Ravagnani PM, et al. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: Correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur Heart J. 2007;28:2094–101. doi: 10.1093/eurheartj/ehm244. [DOI] [PubMed] [Google Scholar]
- 86.Oh BH, Kaligis RW, Wang Y, Punzalan FE, Suwanwela NC, Nguyen VL, et al. Survey of atherosclerotic disease in Asian subjects with cardiovascular disease risk factors who were not receiving lipid-lowering agents. Int J Cardiol. 2013;168:2761–6. doi: 10.1016/j.ijcard.2013.03.132. doi: 10.1016/j.ijcard.2013.03.132. [DOI] [PubMed] [Google Scholar]
- 87.Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb. 1991;11:1245–9. doi: 10.1161/01.atv.11.5.1245. [DOI] [PubMed] [Google Scholar]
- 88.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 89.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 90.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 91.Lee S, Cho GY, Kim HS, Yoon YE, Lee SP, Kim HK, et al. Common carotid intima-media thickness as a risk factor for outcomes in Asian patients with acute ST-elevation myocardial infarction. Can J Cardiol. 2014;30:1620–6. doi: 10.1016/j.cjca.2014.06.026. doi: 10.1016/j.cjca.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 92.Chuang SY, Bai CH, Chen JR, Yeh WT, Chen HJ, Chiu HC, et al. Common carotid end-diastolic velocity and intima-media thickness jointly predict ischemic stroke in Taiwan. Stroke. 2011;42:1338–44. doi: 10.1161/STROKEAHA.110.605477. doi: 10.1161/STROKEAHA.110.605477. [DOI] [PubMed] [Google Scholar]
- 93.Kitamura A, Iso H, Imano H, Ohira T, Okada T, Sato S, et al. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke. 2004;35:2788–94. doi: 10.1161/01.STR.0000147723.52033.9e. [DOI] [PubMed] [Google Scholar]
- 94.Okazaki S, Furukado S, Abe Y, Tanaka M, Miwa K, Yamagami H, et al. Association of inflammatory markers and carotid intima-media thickness with the risk of cardiovascular events in high-risk patients. Cerebrovasc Dis. 2010;30:180–7. doi: 10.1159/000317106. [DOI] [PubMed] [Google Scholar]
- 95.Xie W, Liang L, Zhao L, Shi P, Yang Y, Xie G, et al. Combination of carotid intima-media thickness and plaque for better predicting risk of ischaemic cardiovascular events. Heart. 2011;97:1326–31. doi: 10.1136/hrt.2011.223032. doi: 10.1136/hrt.2011.223032. [DOI] [PubMed] [Google Scholar]
- 96.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 97.Lorenz MW, Bickel H, Bots ML, Breteler MM, Catapano AL, Desvarieux M, et al. Individual progression of carotid intima media thickness as a surrogate for vascular risk (PROG-IMT): Rationale and design of a meta-analysis project. Am Heart J. 2010;159:730–6.e2. doi: 10.1016/j.ahj.2010.02.008. doi: 10.1016/j.ahj.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 99.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial biology for the investigation of the treatment effects of reducing cholesterol (ARBITER) 2: A double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–7. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 100.Hiukka A, Westerbacka J, Leinonen ES, Watanabe H, Wiklund O, Hulten LM, et al. Long-term effects of fenofibrate on carotid intima-media thickness and augmentation index in subjects with type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:2190–7. doi: 10.1016/j.jacc.2008.09.049. doi: 10.1016/j.jacc.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 101.Meuwese MC, de Groot E, Duivenvoorden R, Trip MD, Ose L, Maritz FJ, et al. ACAT inhibition and progression of carotid atherosclerosis in patients with familial hypercholesterolemia: The CAPTIVATE randomized trial. JAMA. 2009;301:1131–9. doi: 10.1001/jama.301.11.1131. doi: 10.1001/jama.301.11.1131. [DOI] [PubMed] [Google Scholar]
- 102.Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620–30. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 103.Wiklund O, Hulthe J, Wikstrand J, Schmidt C, Olofsson SO, Bondjers G. Effect of controlled release/extended release metoprolol on carotid intima-media thickness in patients with hypercholesterolemia: A 3-year randomized study. Stroke. 2002;33:572–7. doi: 10.1161/hs0202.102332. [DOI] [PubMed] [Google Scholar]
- 104.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 105.Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: A randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106:2055–60. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- 106.Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): A prospective, randomised, double-blind trial. Lancet. 2001;357:577–81. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 107.Hedblad B, Wikstrand J, Janzon L, Wedel H, Berglund G. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: Main results from the Beta-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS) Circulation. 2001;103:1721–6. doi: 10.1161/01.cir.103.13.1721. [DOI] [PubMed] [Google Scholar]
- 108.Mercuri M, Bond MG, Sirtori CR, Veglia F, Crepaldi G, Feruglio FS, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population: The Carotid Atherosclerosis Italian Ultrasound Study. Am J Med. 1996;101:627–34. doi: 10.1016/s0002-9343(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 109.Beishuizen ED, van de Ree MA, Jukema JW, Tamsma JT, van der Vijver JC, Meinders AE, et al. Two-year statin therapy does not alter the progression of intima-media thickness in patients with type 2 diabetes without manifest cardiovascular disease. Diabetes Care. 2004;27:2887–92. doi: 10.2337/diacare.27.12.2887. [DOI] [PubMed] [Google Scholar]
- 110.Anderssen SA, Hjelstuen AK, Hjermann I, Bjerkan K, Holme I. Fluvastatin and lifestyle modification for reduction of carotid intima-media thickness and left ventricular mass progression in drug-treated hypertensives. Atherosclerosis. 2005;178:387–97. doi: 10.1016/j.atherosclerosis.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 111.Shukla A, Sharma MK, Jain A, Goel PK. Prevention of atherosclerosis progression using atorvastatin in normolipidemic coronary artery disease patients – A controlled randomized trial. Indian Heart J. 2005;57:675–80. [PubMed] [Google Scholar]
- 112.Salonen R, Nyyssonen K, Porkkala E, Rummukainen J, Belder R, Park JS, et al. Kuopio Atherosclerosis Prevention Study (KAPS).A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92:1758–64. doi: 10.1161/01.cir.92.7.1758. [DOI] [PubMed] [Google Scholar]
- 113.MacMahon S, Sharpe N, Gamble G, Hart H, Scott J, Simes J, et al. Effects of lowering average of below-average cholesterol levels on the progression of carotid atherosclerosis: Results of the LIPID Atherosclerosis Substudy. LIPID Trial Research Group. Circulation. 1998;97:1784–90. doi: 10.1161/01.cir.97.18.1784. [DOI] [PubMed] [Google Scholar]
- 114.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu C, Liu C, et al. Reduction in carotid arterial wall thickness using lovastatin and dietary therapy: A randomized controlled clinical trial. Ann Intern Med. 1996;124:548–56. doi: 10.7326/0003-4819-124-6-199603150-00002. [DOI] [PubMed] [Google Scholar]
- 115.Crouse JR, 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O’Leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–53. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 116.Crouse JR, 3rd, Byington RP, Bond MG, Espeland MA, Craven TE, Sprinkle JW, et al. Pravastatin, lipids, and atherosclerosis in the carotid arteries (PLAC-II) Am J Cardiol. 1995;75:455–9. doi: 10.1016/s0002-9149(99)80580-3. [DOI] [PubMed] [Google Scholar]
- 117.de Groot E, Jukema JW, Montauban van Swijndregt AD, Zwinderman AH, Ackerstaff RG, van der Steen AF, et al. B-mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: A report of the Regression Growth Evaluation Statin Study (REGRESS) J Am Coll Cardiol. 1998;31:1561–7. doi: 10.1016/s0735-1097(98)00170-3. [DOI] [PubMed] [Google Scholar]
- 118.Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: The SANDS randomized trial. JAMA. 2008;299:1678–89. doi: 10.1001/jama.299.14.1678. doi: 10.1001/jama.299.14.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zanchetti A, Crepaldi G, Bond MG, Gallus G, Veglia F, Mancia G, et al. Different effects of antihypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering by pravastatin on progression of asymptomatic carotid atherosclerosis: Principal results of PHYLLIS – A randomized double-blind trial. Stroke. 2004;35:2807–12. doi: 10.1161/01.STR.0000147041.00840.59. [DOI] [PubMed] [Google Scholar]
- 120.Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, et al. Effects of ramipril and Vitamin E on atherosclerosis: The study to evaluate carotid ultrasound changes in patients treated with ramipril and Vitamin E (SECURE) Circulation. 2001;103:919–25. doi: 10.1161/01.cir.103.7.919. [DOI] [PubMed] [Google Scholar]
- 121.Lonn EM, Gerstein HC, Sheridan P, Smith S, Diaz R, Mohan V, et al. Effect of ramipril and of rosiglitazone on carotid intima-media thickness in people with impaired glucose tolerance or impaired fasting glucose: STARR (STudy of Atherosclerosis with Ramipril and Rosiglitazone) J Am Coll Cardiol. 2009;53:2028–35. doi: 10.1016/j.jacc.2008.12.072. doi: 10.1016/j.jacc.2008.12.072. [DOI] [PubMed] [Google Scholar]
- 122.MacMahon S, Sharpe N, Gamble G, Clague A, Mhurchu CN, Clark T, et al. Randomized, placebo-controlled trial of the angiotensin-converting enzyme inhibitor, Ramipril, in patients with coronary or other occlusive arterial disease. PART-2 Collaborative Research Group. Prevention of Atherosclerosis with Ramipril. J Am Coll Cardiol. 2000;36:438–43. doi: 10.1016/s0735-1097(00)00736-1. [DOI] [PubMed] [Google Scholar]
- 123.Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palù C, et al. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: Principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation. 2002;106:2422–7. doi: 10.1161/01.cir.0000039288.86470.dd. [DOI] [PubMed] [Google Scholar]
- 124.Simon A, Gariépy J, Moyse D, Levenson J. Differential effects of nifedipine and co-amilozide on the progression of early carotid wall changes. Circulation. 2001;103:2949–54. doi: 10.1161/01.cir.103.24.2949. [DOI] [PubMed] [Google Scholar]
- 125.Borhani NO, Mercuri M, Borhani PA, Buckalew VM, Canossa-Terris M, Carr AA, et al. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). A randomized controlled trial. JAMA. 1996;276:785–91. [PubMed] [Google Scholar]
- 126.Pitt B, Byington RP, Furberg CD, Hunninghake DB, Mancini GB, Miller ME, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102:1503–10. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]
- 127.Stanton AV, Chapman JN, Mayet J, Sever PS, Poulter NR, Hughes AD, et al. Effects of blood pressure lowering with amlodipine or lisinopril on vascular structure of the common carotid artery. Clin Sci (Lond) 2001;101:455–64. [PubMed] [Google Scholar]
- 128.Hoogerbrugge N, de Groot E, de Heide LH, de Ridder MA, Birkenhägeri JC, Stijnen T, et al. Doxazosin and hydrochlorothiazide equally affect arterial wall thickness in hypertensive males with hypercholesterolaemia (the DAPHNE study). Doxazosin Atherosclerosis Progression Study in Hypertensives in the Netherlands. Neth J Med. 2002;60:354–61. [PubMed] [Google Scholar]
- 129.Ludwig M, Stapff M, Ribeiro A, Fritschka E, Tholl U, Smith RD, et al. Comparison of the effects of losartan and atenolol on common carotid artery intima-media thickness in patients with hypertension: Results of a 2-year, double-blind, randomized, controlled study. Clin Ther. 2002;24:1175–93. doi: 10.1016/s0149-2918(02)80028-5. [DOI] [PubMed] [Google Scholar]
- 130.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: The Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–9. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 131.Magliano D, McNeil J, Branley P, Shiel L, Demos L, Wolfe R, et al. The melbourne atherosclerosis Vitamin E trial (MAVET): A study of high dose Vitamin E in smokers. Eur J Cardiovasc Prev Rehabil. 2006;13:341–7. doi: 10.1097/01.hjr.0000219108.10167.46. [DOI] [PubMed] [Google Scholar]
- 132.Durga J, Bots ML, Schouten EG, Grobbee DE, Kok FJ, Verhoef P. Effect of 3 y of folic acid supplementation on the progression of carotid intima-media thickness and carotid arterial stiffness in older adults. Am J Clin Nutr. 2011;93:941–9. doi: 10.3945/ajcn.110.006429. doi: 10.3945/ajcn.110.006429. [DOI] [PubMed] [Google Scholar]
- 133.Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: A multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–16. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 134.Hodis HN, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, et al. High-dose B Vitamin supplementation and progression of subclinical atherosclerosis: A randomized controlled trial. Stroke. 2009;40:730–6. doi: 10.1161/STROKEAHA.108.526798. doi: 10.1161/STROKEAHA.108.526798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 136.Bots ML, Evans GW, Riley W, McBride KH, Paskett ED, Helmond FA, et al. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima-media thickness: The Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. Eur Heart J. 2006;27:746–55. doi: 10.1093/eurheartj/ehi695. [DOI] [PubMed] [Google Scholar]
- 137.Byington RP, Furberg CD, Herrington DM, Herd JA, Hunninghake D, Lowery M, et al. Effect of estrogen plus progestin on progression of carotid atherosclerosis in postmenopausal women with heart disease: HERS B-mode substudy. Arterioscler Thromb Vasc Biol. 2002;22:1692–7. doi: 10.1161/01.atv.0000033514.79653.04. [DOI] [PubMed] [Google Scholar]
- 138.Colacurci N, Fornaro F, Cobellis L, De FP, Torella M, Sepe E, et al. Raloxifene slows down the progression of intima-media thickness in postmenopausal women. Menopause. 2007;14:879–84. doi: 10.1097/gme.0b013e3180577893. [DOI] [PubMed] [Google Scholar]
- 139.Wilcox R, Kupfer S, Erdmann E. PROactive Study investigators. Effects of pioglitazone on major adverse cardiovascular events in high-risk patients with type 2 diabetes: Results from PROspective pioglitAzone Clinical Trial In macro Vascular Events (PROactive 10) Am Heart J. 2008;155:712–7. doi: 10.1016/j.ahj.2007.11.029. doi: 10.1016/j.ahj.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 140.Langenfeld MR, Forst T, Hohberg C, Kann P, Lübben G, Konrad T, et al. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: Results from a controlled randomized study. Circulation. 2005;111:2525–31. doi: 10.1161/01.CIR.0000165072.01672.21. [DOI] [PubMed] [Google Scholar]
- 141.Hedblad B, Zambanini A, Nilsson P, Janzon L, Berglund G. Rosiglitazone and carotid IMT progression rate in a mixed cohort of patients with type 2 diabetes and the insulin resistance syndrome: Main results from the Rosiglitazone Atherosclerosis Study. J Intern Med. 2007;261:293–305. doi: 10.1111/j.1365-2796.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- 142.O’Leary DH, Reuwer AQ, Nissen SE, Després JP, Deanfield JE, Brown MW, et al. Effect of rimonabant on carotid intima-media thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: The AUDITOR Trial. Heart. 2011;97:1143–50. doi: 10.1136/hrt.2011.223446. doi: 10.1136/hrt.2011.223446. [DOI] [PubMed] [Google Scholar]
- 143.Espeland MA, O’leary DH, Terry JG, Morgan T, Evans G, Mudra H. Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr Control Trials Cardiovasc Med. 2005;6:3. doi: 10.1186/1468-6708-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amarenco P, Labreuche J, Lavallée P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: Systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902–9. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 145.Huang Y, Li W, Dong L, Li R, Wu Y. Effect of statin therapy on the progression of common carotid artery intima-media thickness: An updated systematic review and meta-analysis of randomized controlled trials. J Atheroscler Thromb. 2013;20:108–21. doi: 10.5551/jat.14001. [DOI] [PubMed] [Google Scholar]
- 146.Feng X, Zhang J, Liu M, Li X. Impact on the carotid intima-medial thickness and safety of rosuvastatin in Chinese patients with carotid atherosclerosis: A meta-analysis (in Chinese) Chin J Cardiol. 2014;42:247–53. [PubMed] [Google Scholar]
- 147.Domanski M, Pocock S, Bernaud C, Borer J, Geller N, Revkin J, et al. Surrogate endpoints in randomized cardiovascular clinical trials. Fundam Clin Pharmacol. 2011;25:411–3. doi: 10.1111/j.1472-8206.2010.00865.x. doi: 10.1111/j.1472-8206.2010.00865.x. [DOI] [PubMed] [Google Scholar]
- 148.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 149.Okayama KI, Mita T, Gosho M, Yamamoto R, Yoshida M, Kanazawa A, et al. Carotid intima-media thickness progression predicts cardiovascular events in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2013;101:286–92. doi: 10.1016/j.diabres.2013.06.008. doi: 10.1016/j.diabres.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 150.Goldberger ZD, Valle JA, Dandekar VK, Chan PS, Ko DT, Nallamothu BK. Are changes in carotid intima-media thickness related to risk of nonfatal myocardial infarction? A critical review and meta-regression analysis. Am Heart J. 2010;160:701–14. doi: 10.1016/j.ahj.2010.06.029. doi: 10.1016/j.ahj.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 151.Costanzo P, Perrone-Filardi P, Vassallo E, Paolillo S, Cesarano P, Brevetti G, et al. Does carotid intima-media thickness regression predict reduction of cardiovascular events? A meta-analysis of 41 randomized trials. J Am Coll Cardiol. 2010;56:2006–20. doi: 10.1016/j.jacc.2010.05.059. doi: 10.1016/j.jacc.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 152.Bots ML, Taylor AJ, Kastelein JJ, Peters SA, den Ruijter HM, Tegeler CH, et al. Rate of change in carotid intima-media thickness and vascular events: Meta-analyses can not solve all the issues. A point of view. J Hypertens. 2012;30:1690–6. doi: 10.1097/HJH.0b013e32835644dc. [DOI] [PubMed] [Google Scholar]
- 153.Taylor AJ, Bots ML, Kastelein JJ. Vascular disease: Meta-regression of CIMT trials-data in, garbage out. Nat Rev Cardiol. 2011;8:128–30. doi: 10.1038/nrcardio.2011.12. doi: 10.1038/nrcardio.2011.12. [DOI] [PubMed] [Google Scholar]
- 154.Bots ML, Evans GW, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies: Design options, progression rates, and sample size considerations: A point of view. Stroke. 2003;34:2985–94. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 155.Peters SA, den Ruijter HM, Grobbee DE, Bots ML. Results from a carotid intima-media thickness trial as a decision tool for launching a large-scale morbidity and mortality trial. Circ Cardiovasc Imaging. 2013;6:20–5. doi: 10.1161/CIRCIMAGING.112.978114. doi: 10.1161/CIRCIMAGING.112.978114. [DOI] [PubMed] [Google Scholar]
- 156.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–6. doi: 10.1159/000343145. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. doi:10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 158.Peters SA, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR, 3rd, O’Leary DH, et al. Extensive or restricted ultrasound protocols to measure carotid intima-media thickness: Analysis of completeness rates and impact on observed rates of change over time. J Am Soc Echocardiogr. 2012;25:91–100. doi: 10.1016/j.echo.2011.09.009. doi: 10.1016/j.echo.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 159.Peters SA, Palmer MK, Grobbee DE, Crouse JR, 3rd, Evans GW, Raichlen JS, et al. Effect of number of ultrasound examinations on the assessment of carotid intima-media thickness changes over time: The example of the METEOR study. J Hypertens. 2011;29:1145–54. doi: 10.1097/HJH.0b013e328345d85e. doi: 10.1097/HJH.0b013e328345d85e. [DOI] [PubMed] [Google Scholar]
- 160.Peters SA, Palmer MK, den Ruijter HM, Grobbee DE, Crouse JR, 3rd, O’Leary DH, et al. Sample size requirements in trials using repeated measurements and the impact of trial design. Curr Med Res Opin. 2012;28:681–8. doi: 10.1185/03007995.2012.678937. doi: 10.1185/03007995.2012.678937. [DOI] [PubMed] [Google Scholar]
- 161.Peters SA, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR, 3rd, O’Leary DH, et al. Biologically implausible carotid intima-media thickness measurement values: Effects on rate of change over time. Curr Med Res Opin. 2012;28:891–9. doi: 10.1185/03007995.2012.689255. doi: 10.1185/03007995.2012.689255. [DOI] [PubMed] [Google Scholar]
- 162.Peters SA, Bots ML, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR, 3rd, et al. Multiple imputation of missing repeated outcome measurements did not add to linear mixed-effects models. J Clin Epidemiol. 2012;65:686–95. doi: 10.1016/j.jclinepi.2011.11.012. doi: 10.1016/j.jclinepi.2011.11.012. [DOI] [PubMed] [Google Scholar]


