Abstract
Background:
Rheumatic diseases involve multiple organs that are affected by immunological mechanisms. Treatment with corticosteroids and immunosuppressive agents may also increase the frequency of infection. Cytomegalovirus (CMV) is a widespread herpes virus and a well-recognized pathogen, which causes an opportunistic and potentially fatal infection in immunocompromised patients. This retrospective study aimed to investigate the clinical and laboratory characteristics of CMV pneumonia in patients with rheumatic diseases after immunosuppressive therapy in a single center in Shanghai, China.
Methods:
Eight hundred and thirty-four patients with rheumatic diseases who had undergone CMV-DNA viral load tests were included, and the medical records of 142 patients who were positive for CMV-DNA in plasma samples were evaluated. GraphPad Prism version 5.013 (San Diego, CA, USA) was used to conduct statistical analysis. The correlation between CMV-DNA viral loads and lymphocyte counts was assessed using the Spearman rank correlation coefficient test. Significance between qualitative data was analyzed using Pearson's Chi-squared test. The cut-off thresholds for CMV-DNA viral load and lymphocyte count were determined by receiver operating characteristic (ROC) curve analysis.
Results:
One hundred and forty-two patients had positive CMV viral load tests. Of these 142 patients, 73 patients with CMV pneumonia were regarded as symptomatic, and the other 69 were asymptomatic. The symptomatic group received higher doses of prednisolone (PSL) and more frequently immunosuppressants than the asymptomatic group (P < 0.01). The symptomatic group had lower lymphocyte counts, especially CD4+ T-cells, than the asymptomatic group (P < 0.01). By ROC curve analysis, when CD4+ T-cell count was <0.39 × 109/L, patients with rheumatic diseases were at high risk for symptomatic CMV infection. The CMV-DNA load was significantly higher in the symptomatic patients than that in asymptomatic patients (P < 0.01; threshold viral loads: 1.75 × 104 copies/ml). Seven patients had a fatal outcome, and they had lower peripheral lymphocyte counts (P < 0.01), including CD4+ and CD8+ T-cells (P < 0.01).
Conclusions:
When CD4+ T-cell count is <0.39 × 109/L, patients are at high risk for pulmonary CMV infection. Patients are prone to be symptomatic with CMV-DNA load >1.75 × 104 copies/ml. Lymphopenia (especially CD4+ T-cells), presence of symptoms, and other infections, especially fungal infection, are significant risk factors for poor outcome, and a higher PSL dosage combined with immunosuppressants may predict CMV pneumonia.
Keywords: Cytomegalovirus, Cytomegalovirus Pneumonia, Polymerase Chain Reaction, Rheumatic Disease, Viral Load
INTRODUCTION
Rheumatic diseases involve multiple organs that are affected by immunological mechanisms. Treatment with corticosteroids and immunosuppressive agents can improve the prognosis of rheumatic diseases, but may also increase the frequency of infection. Infectious disease is one of the major life-threatening complications in patients with rheumatic diseases. Although community-acquired infections are common, patients are also prone to opportunistic infections.[1,2,3,4,5,6]
Cytomegalovirus (CMV) is a widespread herpes virus and a well-recognized pathogen, which causes an opportunistic and potentially fatal infection in immunocompromised patients with acquired immune deficiency syndrome (AIDS).[7] The seroprevalence of prior CMV infection ranges from 40% to 100% in adults worldwide,[8] and most of the recent survey in 2010 reported that the prevalence of CMV infection was >95% in Mainland China. CMV reactivation may occur depending on host immune status.[9,10] CMV infection in this immunocompromised population is associated with end-organ disease, such as colitis, retinitis, pneumonia, hepatitis, and encephalitis. Although the widespread use of antiretroviral therapy has had a positive impact on the course and long-term outcome of CMV infection, the prevalence of CMV infection has shown no sign of abating in these patients. Early detection and preemptive treatment may help to reduce morbidity and mortality.
Attempts to improve outcomes have focused on developing new diagnostic methods for early detection of CMV. Molecular methods such as polymerase chain reaction (PCR) have become widely available and are used increasingly in clinical practice. CMV viral load test based on quantitative PCR can be used for direct detection of virus in the whole blood, peripheral blood lymphocytes, and plasma with quantitative measurement of viremia. It is one of the well-established assays for CMV, and several studies have indicated that PCR allows for sensitive and rapid detection of CMV-DNA in clinical samples. It is more useful and beneficial for diagnosing CMV infection than CMV antigenemia assay or histological examination and is correlated with the clinical course or severity of CMV disease in organ transplantation or AIDS. The CMV viral load test is now widely used as a marker of viral reactivation or a threshold to start antiviral therapy.[7,11,12,13,14]
There have been few studies on CMV infection in patients with rheumatic disease and the incidence, clinical characteristics and prognosis of CMV infection complications in rheumatic disease have not been clarified. In the present retrospective study, we investigated the prognosis and clinical and laboratory characteristics of CMV infection in patients with rheumatic diseases, with a focus on CMV pneumonia.
METHODS
Patients
A retrospective study was performed in a single center, Huashan Hospital, Fudan University, between January 2006 and February 2013. We retrospectively included 834 patients with rheumatic diseases who had undergone CMV-DNA viral load tests. The medical records of 142 patients with rheumatic diseases who were positive for CMV-DNA in plasma samples were reviewed, and 73 patients with CMV pneumonia were further investigated.
CMV infection was diagnosed by CMV PCR. Plasma CMV-DNA viral load was determined using the COBAS Amplicor CMV Monitor Quantitative PCR (Roche Diagnostics, Indianapolis, IN, USA). The range of quantification of these assays was 600–100,000 copies/ml for CMV. CMV pneumonia was defined as detection of ground glass opacity by chest X-ray film or computed tomography, in addition to clinical signs such as fever, cough, dyspnea, and hypoxemia.
Because the forms of glucocorticoids we used in treating patients were different, we converted different types of glucocorticoids to equivalent prednisolone (PSL) for the statistical requirement.
This study was approved by the Ethics Committee of Huashan Hospital, Fudan University, and written informed consent was obtained from all patients.
Statistical analysis
GraphPad Prism version 5.013 (San Diego, CA, USA) was used to conduct statistical analysis. Nonparametric measurement data are presented as median (range), parametric measurement data are presented as the mean ± standard deviation (SD), and enumerative data are shown as percentages and numbers of cases. The significance of quantitative, parametric data was analyzed using t-test. The significance of quantitative, nonparametric data was analyzed using the Mann–Whitney U-test. The correlation between CMV-DNA viral loads and lymphocyte counts was assessed using the Spearman rank correlation coefficient test. Significance between qualitative data was analyzed using Pearson's Chi-squared test. For the latter method, the numerical variables, that is, age, CMV-DNA viral load, and peripheral blood lymphocyte count, were divided into two nominal variables using the cut-off lines for each parameter. The cut-off thresholds for CMV-DNA viral load and lymphocyte count were determined by receiver operating characteristic (ROC) curve analysis. A P < 0.05 was considered statistically significant.
RESULTS
Patient profiles and treatment for underlying diseases
During the past 7 years, 834 patients with rheumatic diseases underwent the CMV PCR test, and 142 were regarded as having CMV infection. The median age of infected patients was 49.0 years (range: 16.0–87.0 years), and the ratio of male to female was 1.0:4.3. CMV-infected patients had a wide range of underlying diseases, but the dominant ones were systemic lupus erythematosus (SLE; n = 52), dermatomyositis (n = 52) and antineutrophil cytoplasmic antibody-associated arteritis (n = 15). The median duration of underlying diseases was 31.00 months (0.25–360.00 months). All the patients received corticosteroid therapy. The initial dose was 0.5–1.0 mg·kg−1·d−1 of PSL and patients with severe illness received 0.5 g intravenous methylprednisolone for 3 consecutive days in each course. Almost all patients continued with the initial dose of PSL for 4 weeks, and then the dose was decreased by 10% per week. In addition to PSL, immunosuppressants were also administered. The commonly used immunosuppressants were cyclophosphamide (CTX) 0.6–1.0 g/month, azathioprine 50–100 mg/d, cyclosporine (CsA) 150–250 mg/d, leflunomide 20 mg/d, mycophenolate mofetil (MMF) 0.5–2.0 g/d, and methotrexate 7.5–15.0 mg/week. The average dose of PSL in the past 3 months was 2.8 g (0.1–9.0 g), daily dose of PSL was 32 mg/d (1–100 mg/d), and percentage of immunosuppressant use was 69%.
Clinical characteristics in Cytomegalovirus-infected patients
Of the 834 patients, 142 were positive for CMV viral load tests. In new-onset cases, CMV was detected at a median of 14.0 days and 93.5 days after combined therapy with corticosteroids and immunosuppressive drugs and corticosteroid therapy alone, respectively. Seventy-three of the 142 patients showed CMV pneumonia on chest X-ray film or high-resolution computed tomography and had related symptoms.
The 73 patients with CMV pneumonia were regarded as symptomatic, and the other 69 were asymptomatic. As shown in Table 1, no significant differences were found in age and duration of PSL therapy between the two groups. Compared with the asymptomatic group, the symptomatic group had more male patients, shorter disease duration and the dose of daily or recent 3 months PSL intake was significantly higher. The median dose of PSL was 32 mg/d (range: 4–100 mg/d). The dose of PSL was positively correlated with CMV viral load (Spearman coefficient = 0.315, P < 0.01). The percentages of patients using immunosuppressants showed a significant difference between the two groups (79% vs. 58%, P < 0.01), and the use of MMF, CsA or CTX was significantly more frequently observed in symptomatic patients than asymptomatic patients (all P < 0.05). There was also an increase in hepatic enzyme levels and mild renal insufficiency in the symptomatic group.
Table 1.
Comparison of patients with and without symptoms in CMV-infected group
| Items | Asymptomatic patients (n = 69) | Symptomatic patients (n = 73) | Statistical values | P |
|---|---|---|---|---|
| Male:female, n | 6:63 | 21:52 | 9.280* | <0.01 |
| Age (years) | 52.0 ± 14.7 | 48.5 ± 16.7 | 0.963† | 0.34 |
| Disease duration (months) | 8.00 (0.03–360.00) | 3.00 (0.25–156.00) | −2.571‡ | <0.05 |
| Duration of PSL therapy (months) | 8.00 (0.50–360.00) | 3.00 (0.03–156.00) | −1.017‡ | 0.31 |
| PSL for recent 3 months (g) | 1.8 (0.1–4.6) | 2.8 (0.1–9.0) | −3.069‡ | <0.01 |
| Average dose of PSL (mg/d) | 20 (1–50) | 32 (4–100) | −3.865‡ | <0.01 |
| Immunosuppressants, n | 40 | 58 | 7.654* | <0.01 |
| CsA, n | 9 | 31 | 15.176* | <0.01 |
| CTX, n | 24 | 14 | 4.407* | <0.05 |
| MMF, n | 4 | 13 | 4.856* | <0.05 |
| WBC (×109/L) | 5.8 (2.6–16.4) | 7.8 (1.0–28.7) | −0.609‡ | 0.54 |
| Neutrophil (×109/L) | 4.4 (0.9–13.8) | 6.8 (0.9–27.5) | −1.464‡ | 0.14 |
| Lymphocyte (×109/L) | 1.2 (0.1–5.7) | 0.6 (0.1–4.0) | −2.314‡ | <0.05 |
| ALT (U/L) | 28 (7–177) | 60 (7–1357) | −2.923‡ | <0.01 |
| AST (U/L) | 25 (9–315) | 32 (9–886) | −1.262‡ | 0.21 |
| ALP (U/L) | 60 (24–111) | 88 (30–892) | −2.877‡ | <0.01 |
| γ-GT (U/L) | 30 (5–200) | 105 (6–1335) | −3.704‡ | <0.01 |
| LDH (U/L) | 321 (48–674) | 509 (50–4488) | −3.174‡ | <0.01 |
| BUN (mmol/L) | 5.9 (3.5–20.3) | 9.0 (7.3–36.0) | −2.450‡ | <0.05 |
| Cr (μmol/L) | 50 (20–264) | 43 (19–798) | −2.072‡ | <0.05 |
| CD4+ T-cell (×109/L) | 0.51 (0.10–1.75) | 0.18 (0.02–1.72) | −3.216‡ | <0.01 |
| CD8+ T-cell (×109/L) | 0.25 (0.11–3.28) | 0.26 (0.02–3.15) | −0.533‡ | 0.59 |
| Co-infection patients, n | 7 | 25 | 11.845* | <0.01 |
| Bacterial pneumonia, n | 5 | 20 | 9.929* | <0.01 |
| Fungal infection, n | 5 | 14 | 4.357* | <0.05 |
Data are presented as mean ± SD, median (range) or numbers. *Chisquare values; †t values; ‡Z values. SD: Standard deviation; PSL: Prednisolone; CsA: Cyclosporine; CTX: Cyclophosphamide; MMF: Mycophenolate mofetil; WBC: White cell count; ALT: Alanine aminotransferase; AST: Aspartate transaminase; ALP: Alkaline phosphatase; γ-GT: Gamma-glutamyl transpeptidase; LDH: Lactate dehydrogenase; BUN: Blood urea nitrogen; Cr: Creatinine; CMV: Cytomegalovirus.
Other infections were also observed in 32 (23.2%) patients based on the detection of bacteria and fungi in sputum, urine, stools, and body fluid: 7 in the asymptomatic group and 25 in the symptomatic group (P < 0.01). The major bacteria pathogens were Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus. Of 19 patients with fungal infection, 5 were in an asymptomatic group and 14 were in the symptomatic group (P < 0.05). The most common fungi were Candida albicans, aspergillosis, and Candida tropicalis. Seven patients had urinary tract infection, three had an intestinal infection, two had septicemia, and one each had infections of ascites fluid, pleural effusion, and renal cyst. Multiple infections were uncontrollable and fatal, but antibiotics and antifungal agents resolved the other infection. The average dose of PSL used in these 32 patients was 32 mg/d. Compared to the patients without co-infections, these 32 patients showed lower lymphocyte counts (0.9 [0.1–4.0] × 109/L vs. 1.2 [0.6–5.7] × 109/L, P < 0.01).
Peripheral blood lymphocyte counts and Cytomegalovirus loads
As shown in Table 1, the median peripheral blood lymphocyte counts in the symptomatic and asymptomatic patients were 0.6 × 109/L and 1.2 × 109/L, respectively (P < 0.05). The median CD4+ T-cell counts in the symptomatic and asymptomatic patients were 0.18 × 109/L and 0.51 × 109/L, respectively (P < 0.01). In particular, the number of CD4+ T-cells was associated with CMV load (relative risk [RR] =1.95, 95% confidence interval [CI]: 1.15–2.66). ROC curve analysis indicated that when CD4+ T-cell count was <0.39 × 109/L, patients with rheumatic diseases were at high risk for symptomatic CMV infection, with a sensitivity of 77.5% and specificity of 87.5% [Figure 1].
Figure 1.

Receiver operating characteristic curve of CD4+ T-cell numbers.
The CMV loads at the time of diagnosis were analyzed in terms of whether they were correlated with the presence of clinical symptoms or patient outcome. The mean value of symptomatic patients was (3.68 ± 1.90) × 104 copies/ml, whereas that of asymptomatic patients was (0.60 ± 0.35) ×104 copies/ml. Statistical significance was observed between two groups (P < 0.01). ROC curve analysis showed that a level of 1.75 × 104 copies/ml was the optimal threshold for prediction of CMV-associated symptoms, with a sensitivity of 84.9% and specificity of 98.6% [Figure 2].
Figure 2.
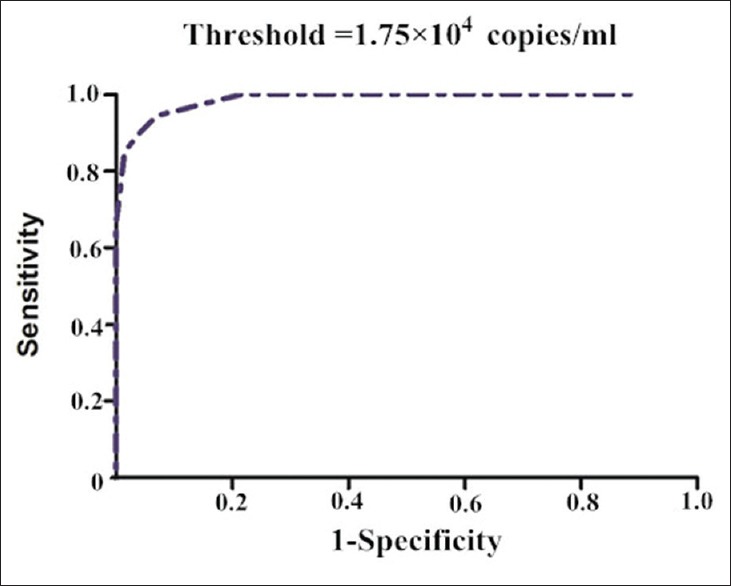
Receiver operating characteristic curve of Cytomegalovirus-DNA viral loads.
Clinical characteristics in patients with a fatal outcome
Seven patients (five women and two men) died in the CMV infection group during admission: Six of them had dermatomyositis, and one had SLE. The average age was 54.0 years (range: 24.0–75.0 years); the duration of disease was 9.70 months (range: 2.00–36.00 months); and the average dose of PSL was 20 mg/d (range: 8–64 mg/d). Dermatomyositis was more frequently observed in deceased patients (P < 0.01). The clinical characteristics of deceased and living patients were compared, particularly in terms of age, dose of PSL, immunosuppressants, underlying disorders, and laboratory data. As shown in Table 2, the deceased patients showed significantly lower median peripheral lymphocyte counts, as well as CD4+ T-cells and CD8+ T-cells (all P < 0.01). The dose of PSL and administration of immunosuppressants did not differ significantly between the two groups. The deceased patients showed a higher level of lactate dehydrogenase than those who survived (P < 0.01). A higher rate of fungal infection was observed in the deceased group (42.9% vs. 11.9%, P < 0.05).
Table 2.
The profile and survival outcomes of CMV-infected patients
| Items | Deceased patients (n = 7) | Surviving patients (n = 135) | Statistical values | P |
|---|---|---|---|---|
| Lymphocyte (×109/L) | 0.6 (0.1–1.1) | 1.2 (0.1–5.7) | −2.874* | <0.01 |
| LDH (U/L) | 1264 (509–4488) | 532 (48–1335) | −3.704* | <0.01 |
| CD4+ Tcell (×109/L) | 0.18 (0.02–0.72) | 0.51 (0.10–1.75) | −3.316* | <0.01 |
| CD8+ Tcell (×109/L) | 0.16 (0.02–0.32) | 0.32 (0.24–3.28) | −2.963* | <0.01 |
| Fungal infection, n | 3 | 16 | 5.520† | <0.05 |
Data are presented as median (range) or numbers. *Z values; †Chi-square values. LDH: Lactate dehydrogenase; CMV: Cytomegalovirus.
DISCUSSION
Rheumatic diseases are characterized by inflammation, often related to a disordered immune response. The role of CMV infection in rheumatic diseases has long been debated because of its ability to interact with the immune system in several ways, as well as its ability to establish latent infection. Although CMV can induce a primary immune response capable of controlling viral replication, it can still establish a latent infection in cell reservoirs.[15,16] CMV may cause opportunistic infections as a complication of immunosuppressive therapy in rheumatic diseases.[17,18,19,20,21] Even without systemic and active infection, local CMV reactivation may directly induce excessive extracellular matrix deposition in cutaneous tissues of patients with systemic sclerosis.[22] Opportunistic infection complicating rheumatic diseases is generally due to a compromised immune function caused by high-dose corticosteroids and treatment with immunosuppressive agents, rather than the disease itself or an immune function abnormality.[23,24,25,26,27,28,29] In this study, CMV-DNA was detected in 142 (17.0%) of 834 patients received corticosteroid therapy for rheumatic diseases. In new-onset cases, CMV was detected at a median of 14.0 days and 93.5 days after combined therapy with corticosteroids and immunosuppressive drugs and corticosteroids therapy alone, respectively, suggesting that combination immunosuppressive therapy may shorten the time of CMV reactivation. The dose of PSL and/or the use of immunosuppressants, especially, MMF, CsA or CTX may precipitate CMV infection. The patients with rheumatoid arthritis on ≥7.5 mg/d PSL had more than 6 times the rate of infection requiring hospitalization.[30] In some other rheumatic diseases, there was also an increased likelihood of CMV infection if immunosuppressive therapy such as corticosteroids or CTX were prescribed.[31,32]
Corticosteroids can impair lymphocyte proliferation, inhibit T-cell function and block the production of inflammatory cytokines.[26] In the case of lymphopenia in SLE, primary infection or reactivation of latent CMV infection is more likely to occur in patients receiving corticosteroids. Finally, corticosteroids can impair other immune cells and alter inflammatory responses in a dose-dependent manner.[33,34] Consistent with other conclusions, our study showed that the dose of PSL was correlated with CMV-positivity (Spearman coefficient = 0.315, P < 0.01). When comparing the laboratory characteristics of patients with or without the symptoms of CMV infection, the former patients had significantly lower lymphocyte counts, especially for CD4+ T-cells. An effective immune response is crucial for controlling CMV replication and attack. Sester et al.[35] have investigated the CMV-specific T-cell responses (CD8+ and CD4+ cells) in controlling virus replication. Bieniek et al.[36] showed that HIV patients with CD4+ cell counts <75 cells/μl and high HIV loads were at high risk for CMV end-organ disease. In HIV patients, CMV viremia was more likely to occur when CD4+ cell counts were <175 cells/μl. However, Takizawa et al.[26] have shown that lymphopenia was a significant risk factor of poor outcome in patients with rheumatic disease and CMV infection. The median (range) lymphocyte count of deceased patients was 492 (0–1778)/mm3, whereas that of surviving patients was 762 (144–3256)/mm3. By ROC curve analysis, 600/mm3 was determined as the optimal threshold value to predict poor outcome. In our recent study, the peripheral blood lymphocyte counts in patients with CMV pneumonia were 0.6 (0.1–4.0) × 109/L and the CD4+ T-cell counts were 0.18 (0.02–1.72) × 109/L. The number of CD4+ T-cells was associated with CMV loads (RR = 1.95, 95% CI: 1.15–2.66). ROC curve analysis indicated that when CD4+ T-cell count was <0.39 × 109/L, patients with rheumatic diseases were at high risk for pulmonary CMV infection, with a sensitivity of 77.5% and specificity of 87.5%. Hence, the use of corticosteroids and lymphopenia in patients with rheumatic disease may lead to a lower immune response, which may increase the risk for CMV infection.
The most important effect of CMV infection is that it predisposes patients to other opportunistic infections with a variety of pathogenic agents. In our observations, 32 patients developed other infections based on detection of bacteria and fungi in sputum, urine, stools and body fluids, especially in the symptomatic group. We demonstrated that a moderate dose of PSL (average 32 mg/d) and lower lymphocyte counts might precipitate co-infections. Hence, the use of corticosteroids and lymphopenia in patients with rheumatic disease and CMV infection may lead to weak humoral and cellular immune responses, which increase the risk of co-infection with other pathogens.
Sometimes symptoms of CMV infection may be similar to those due to exacerbation of rheumatic diseases.[18] Ground glass opacity by chest X-ray film or computed tomography would be also found in connective tissue disease related interstitial pneumonia as well as other infections such as Pneumocystis carinii pneumonia. Clinical signs including fever, cough, dyspnea, and hypoxemia were not specific to distinguish CMV pneumonia from other pneumonia. Lung biopsy examination has been considered as the gold standard for the diagnosis in these patients, whereas it is defective in its invasiveness and is hard to use in clinical experiences. In this study, 142 CMV-positive patients underwent lung imaging, and 73 showed interstitial pneumonia. We are regret that none of our patients was diagnosed via lung biopsy. Many of the manifestations were nonspecific and could not be clearly differentiated. It is important to identify and manage CMV infection as early as possible because prognosis of CMV pneumonia could be unsatisfactory even with administration of anti-CMV agents.[18] In this point of view, we would consider these CMV-DNA positive patients with immune compromised background might have had CMV pneumonia or CMV pneumonia combined pulmonary lesions due to rheumatic diseases or other infection rather than exclude the diagnosis of CMV pneumonia. All 73 symptomatic patients received ganciclovir treatment and rapidly became negative for CMV-DNA. Seven patients died despite the change of biological marker after ganciclovir treatment. One patient with SLE died of multiple organ failure, and six patients with dermatomyositis died from exacerbation of interstitial pneumonia.
Sometimes it was difficult to judge CMV infection in rheumatic diseases. Considering the use of steroids and immunosuppressive therapy, CMV infection should be aggressively diagnosed. Antiviral treatment at an early stage of virus reactivation has been established as a beneficial option in many clinical situations. Preemptive therapy has proved that ganciclovir is useful in reducing risks for late CMV disease, neutropenia, and fungal disease.[37] In our study, 33 asymptomatic patients received oral ganciclovir therapy and rapidly became CMV-DNA negative. The remaining 36 asymptomatic patients who were receiving corticosteroid tapering were monitored without ganciclovir therapy until the change of biological marker. They may have derived benefit from the rebuilding of their immune system. Administration of ganciclovir-based on monitoring CMV-DNA positivity may have resulted in overzealous treatment in these patients, and more evidence is needed to confirm this conclusion.
In conclusion, CMV-DNA-positive viremia is not rare in patients with rheumatic diseases. Patients were prone to be symptomatic with CMV-DNA loads >1.75 × 104 copies/ml. Lymphopenia (especially CD4+ T-cell count), and the presence of symptoms and other infections, especially fungal infection, are significant risk factors for poor outcome. A high-dose of PSL and combined use of immunosuppressants may predict such outcome. During high-dose PSL and immunosuppressive therapy for rheumatic diseases, attention should be paid to CMV infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Staples PJ, Gerding DN, Decker JL, Gordon RS., Jr Incidence of infection in systemic lupus erythematosus. Arthritis Rheum. 1974;17:1–10. doi: 10.1002/art.1780170102. [DOI] [PubMed] [Google Scholar]
- 2.Pryor BD, Bologna SG, Kahl LE. Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus. Arthritis Rheum. 1996;39:1475–82. doi: 10.1002/art.1780390906. [DOI] [PubMed] [Google Scholar]
- 3.Kadoya A, Okada J, Iikuni Y, Kondo H. Risk factors for Pneumocystis carinii pneumonia in patients with polymyositis/dermatomyositis or systemic lupus erythematosus. J Rheumatol. 1996;23:1186–8. [PubMed] [Google Scholar]
- 4.Godeau B, Mainardi JL, Roudot-Thoraval F, Hachulla E, Guillevin L, Huong Du LT, et al. Factors associated with Pneumocystis carinii pneumonia in Wegener's granulomatosis. Ann Rheum Dis. 1995;54:991–4. doi: 10.1136/ard.54.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz A, Ehrenfeld M, Livneh A, Bank I, Gur H, Pauzner R, et al. Aspergillosis in systemic lupus erythematosus. Semin Arthritis Rheum. 1996;26:635–40. doi: 10.1016/s0049-0172(96)80014-x. doi: 10.1016/S0049-0172(96)80014-X. [DOI] [PubMed] [Google Scholar]
- 6.Manzi S, Kuller LH, Kutzer J, Pazin GJ, Sinacore J, Medsger TA, Jr, et al. Herpes zoster in systemic lupus erythematosus. J Rheumatol. 1995;22:1254–8. [PubMed] [Google Scholar]
- 7.Preiksaitis JK, Brennan DC, Fishman J, Allen U. Canadian society of transplantation consensus workshop on Cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218–27. doi: 10.1111/j.1600-6143.2004.00692.x. doi: 10.1111/j.1600-6143.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- 8.Krech U. Complement-fixing antibodies againstCytomegalovirus in different parts of the world. Bull World Health Organ. 1973;49:103–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Radha R, Jordan S, Puliyanda D, Bunnapradist S, Petrosyan A, Amet N, et al. Cellular immune responses to Cytomegalovirus in renal transplant recipients. Am J Transplant. 2005;5:110–7. doi: 10.1111/j.1600-6143.2003.00647.x. doi: 10.1111/j.1600-6143.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- 10.von Muller L, Klemm A, Durmus N, Weiss M, Suger-Wiedeck H, Schneider M, et al. Cellular immunity and active human Cytomegalovirus infection in patients with septic shock. J Infect Dis. 2007;196:1288–95. doi: 10.1086/522429. doi: 10.1086/522429. [DOI] [PubMed] [Google Scholar]
- 11.Boeckh M, Bowden RA, Goodrich JM, Pettinger M, Meyers JD. Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood. 1992;80:1358–64. [PubMed] [Google Scholar]
- 12.Bek B, Boeckh M, Lepenies J, Bieniek B, Arasteh K, Heise W, et al. High-level sensitivity of quantitative pp65 Cytomegalovirus (CMV) antigenemia assay for diagnosis of CMV disease in AIDS patients and follow-up. J Clin Microbiol. 1996;34:457–9. doi: 10.1128/jcm.34.2.457-459.1996. doi: 10.1016/S0042-6989(00)00253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machida U, Kami M, Fukui T, Kazuyama Y, Kinoshita M, Tanaka Y, et al. Real-time automated PCR for early diagnosis and monitoring of Cytomegalovirus infection after bone marrow transplantation. J Clin Microbiol. 2000;38:2536–42. doi: 10.1128/jcm.38.7.2536-2542.2000. doi: 10.1088/0256-307X/21/6/035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishore J, Ghoshal U, Ghoshal UC, Krishnani N, Kumar S, Singh M, et al. Infection with Cytomegalovirus in patients with inflammatory bowel disease: Prevalence, clinical significance and outcome. J Med Microbiol. 2004;53(Pt 11):1155–60. doi: 10.1099/jmm.0.45629-0. doi: 10.1099/jmm.0.45629-0. [DOI] [PubMed] [Google Scholar]
- 15.Vossen MT, Westerhout EM, Söderberg-Nauclér C, Wiertz EJ. Viral immune evasion: A masterpiece of evolution. Immunogenetics. 2002;54:527–42. doi: 10.1007/s00251-002-0493-1. doi: 10.1007/s00251-002-0493-1. [DOI] [PubMed] [Google Scholar]
- 16.Jackson SE, Mason GM, Wills MR. Human Cytomegalovirus immunity and immune evasion. Virus Res. 2011;157:151–60. doi: 10.1016/j.virusres.2010.10.031. doi: 10.1016/j.virusres.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Aringer M, Smolen JS, Graninger WB. Severe infections in plasmapheresis-treated systemic lupus erythematosus. Arthritis Rheum. 1998;41:414–20. doi: 10.1002/1529-0131(199803)41:3<414::AID-ART6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Weiss DJ, Greenfield JW, Jr, O’Rourke KS, McCune WJ. Systemic Cytomegalovirus infection mimicking an exacerbation of Wegener's granulomatosis. J Rheumatol. 1993;20:155–7. [PubMed] [Google Scholar]
- 19.Zhang C, Shen K, Jiang Z, He X. Early diagnosis and monitoring of active HCMV infection in children with systemic lupus erythematosus. Chin Med J. 2001;114:1309–12. [PubMed] [Google Scholar]
- 20.Shahnaz S, Choksi MT, Tan IJ. Bilateral Cytomegalovirus retinitis in a patient with systemic lupus erythematosus and end-stage renal disease. Mayo Clin Proc. 2003;78:1412–5. doi: 10.4065/78.11.1412. doi: 10.4065/78111412. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto O, Ando M, Yoshimatsu S, Kohrogi H, Suga M, Ando M. Systemic lupus erythematosus complicated by Cytomegalovirus-induced hemophagocytic syndrome and colitis. Intern Med. 2002;41:151–5. doi: 10.2169/internalmedicine.41.151. [DOI] [PubMed] [Google Scholar]
- 22.Markiewicz M, Smith EA, Rubinchik S, Dong JY, Trojanowska M, LeRoy EC. The 72-kilodalton IE-1 protein of human Cytomegalovirus (HCMV) is a potent inducer of connective tissue growth factor (CTGF) in human dermal fibroblasts. Clin Exp Rheumatol. 2004;22(3 Suppl 33):S31–4. [PubMed] [Google Scholar]
- 23.Sekigawa I, Nawata M, Seta N, Yamada M, Iida N, Hashimoto H. Cytomegalovirus infection in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2002;20:559–64. [PubMed] [Google Scholar]
- 24.Mori T, Kameda H, Ogawa H, Iizuka A, Sekiguchi N, Takei H, et al. Incidence of Cytomegalovirus reactivation in patients with inflammatory connective tissue diseases who are under immunosuppressive therapy. J Rheumatol. 2004;31:1349–51. doi: 10.1016/j.ejso.2006.03.035. [PubMed] [Google Scholar]
- 25.Bulpitt KJ, Brahn E. Systemic lupus erythematosus and concurrent Cytomegalovirus vasculitis: diagnosis by antemortem skin biopsy. J Rheumatol. 1989;16:677–80. [PubMed] [Google Scholar]
- 26.Takizawa Y, Inokuma S, Tanaka Y, Saito K, Atsumi T, Hirakata M, et al. Clinical characteristics of Cytomegalovirus infection in rheumatic diseases: Multicentre survey in a large patient population. Rheumatology (Oxford) 2008;47:1373–8. doi: 10.1093/rheumatology/ken231. doi: 10.1093/rheumatology/ken231. [DOI] [PubMed] [Google Scholar]
- 27.Aglas F, Rainer F, Hermann J, Gretler J, Hüttl E, Domej W, et al. Interstitial pneumonia due to Cytomegalovirus following low-dose methotrexate treatment for rheumatoid arthritis. Arthritis Rheum. 1995;38:291–2. doi: 10.1002/art.1780380222. [DOI] [PubMed] [Google Scholar]
- 28.Ikura Y, Matsuo T, Ogami M, Yamazaki S, Okamura M, Yoshikawa J, et al. Cytomegalovirus associated pancreatitis in a patient with systemic lupus erythematosus. J Rheumatol. 2000;27:2715–7. [PubMed] [Google Scholar]
- 29.Fraenkel G, Ross B, Wong HC. Cytomegalovirus retinitis in a patient with rheumatoid arthritis being treated with combination immunosuppressive therapy. Retina. 1995;15:169–70. doi: 10.1097/00006982-199515020-00015. [DOI] [PubMed] [Google Scholar]
- 30.Au K, Reed G, Curtis JR, Kremer JM, Greenberg JD, Strand V, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:785–91. doi: 10.1136/ard.2010.128637. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 31.Yoda Y, Hanaoka R, Ide H, Isozaki T, Matsunawa M, Yajima N, et al. Clinical evaluation of patients with inflammatory connective tissue diseases complicated byCytomegalovirus antigenemia. Mod Rheumatol. 2006;16:137–42. doi: 10.1007/s10165-006-0470-x. doi: 10.1007/s10165-006-0470-x. [DOI] [PubMed] [Google Scholar]
- 32.Palafox Sánchez CA, Satoh M, Chan EK, Carcamo WC, Muñoz Valle JF, Orozco Barocio G, et al. Reduced IgG anti-small nuclear ribonucleoprotein autoantibody production in systemic lupus erythematosus patients with positive IgM anti-Cytomegalovirus antibodies. Arthritis Res Ther. 2009;11:R27. doi: 10.1186/ar2621. doi: 10.1186/ar2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doria A, Zampieri S, Sarzi-Puttini P. Exploring the complex relationships between infections and autoimmunity. Autoimmun Rev. 2008;8:89–91. doi: 10.1016/j.autrev.2008.07.036. doi: 10.1016/j.autrev.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 34.Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, et al. Infections and autoimmunity: The multifaceted relationship. J Leukoc Biol. 2010;87:385–95. doi: 10.1189/jlb.0709517. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 35.Sester M, Sester U, Gärtner BC, Girndt M, Meyerhans A, Köhler H. Dominance of virus-specific CD8 T cells in human primary Cytomegalovirus infection. J Am Soc Nephrol. 2002;13:2577–84. doi: 10.1097/01.asn.0000030141.41726.52. doi: 10.1097/01ASN0000030141.41726.52. [DOI] [PubMed] [Google Scholar]
- 36.Bieniek R, Kirby JE, Cheng A, Eichelberger K, Qian Q. Effective use of PCR for the detection of Cytomegalovirus viremia and monitoring therapy in immunocompromised patients. J Lab Med. 2011;6:339–43. doi: 10.1309/LMP4IL3XGU6MQJCE. [Google Scholar]
- 37.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–71. [PubMed] [Google Scholar]


