Abstract
Background:
Atopic dermatitis (AD) is characterized by defective skin barrier and imbalance in T helper 1/T helper 2 (Th1/Th2) cytokine expression. Filaggrin (FLG) is the key protein to maintaining skin barrier function. Recent studies indicated that Th1/Th2 cytokines influence FLG expression in keratinocytes. However, the role of Th1/Th2 cytokines on FLG processing is not substantially documented. Our aim was to investigate the impact of Th1/Th2 cytokines on FLG processing.
Methods:
HaCaT cells and normal human keratinocytes were cultured in low and high calcium media and stimulated by either interleukin (IL)-4, 13 or interferon-γ (IFN-γ). FLG, its major processing proteases and key protease inhibitor lymphoepithelial Kazal-type-related inhibitor (LEKTI) were measured by both real-time quantitative polymerase chain reaction and Western blotting. Their expression was also evaluated in acute and chronic AD lesions by immunohistochemistry.
Results:
IL-4/13 significantly reduced, while IFN-γ significantly up-regulated FLG expression. IL-4/13 significantly increased, whereas IFN-γ significantly decreased the expression of kallikreins 5 and 7, matriptase and channel-activating serine protease 1. On the contrary, IL-4/13 significantly decreased, while IFN-γ increased the expression of LEKTI and caspase-14. Similar trends were observed in AD lesions.
Conclusions:
Our results suggested that Th1/Th2 cytokines differentially regulated the expression of major FLG processing enzymes. The imbalance between Th1 and Th2 polarized immune response seems to extend to FLG homeostasis, through the network of FLG processing enzymes.
Keywords: Atopic Dermatitis, Cytokine, Filaggrin, Lymphoepithelial Kazal-type-related Inhibitor, Protease
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by a defective skin barrier function. The pathogenesis of AD is recognized as a complex interplay of immune, genetic, infection and neuroendocrine, etc., Filaggrin (encoded by FLG gene), the most influential genetic risk factor for AD to date,[1] is thought to be the key protein involved in epidermal barrier homeostasis. Lower FLG expression was found in acute lesions of AD than in normal controls,[2,3] the loss-of-function mutations of FLG were observed in about one-third to 42% of AD individuals, genetic predisposition does not sufficiently explain all cases with AD.[4,5,6] Recent studies indicate that T helper 1/T helper 2 (Th1/Th2) cytokines also significantly contribute to FLG expression.[2,7] However, the data are not in full accord and even controversial.[8] Mechanistic studies on the role of Th1/Th2 cytokines on FLG processing have not been explored sufficiently.
FLG has several site-specific functions governed by the program of epidermal terminal differentiation in which a number of proteases are involved, such as corneum trypsin- and chymotrypsin-like enzymes (stratum corneum tryptic enzyme/kallikrein 5 [KLK5] and stratum corneum chymotryptic enzyme/KLK7),[9,10,11] channel-activating serine protease1 (CAP1),[12] matriptase (coded by ST14),[12] and cysteine protease caspase-14[13] and their key protease inhibitor lymphoepithelial Kazal-type-related inhibitor (LEKTI) (encoded by serine protease inhibitor Kazal-type 5 gene/SPINK5).[12,13,14] All of the proteases can enhance FLG degradation processing. LEKTI inhibits FLG degradation processing through suppressing the proteases mentioned above.[12,13,14] The balance between proteases and inhibitor is the key mechanism in keeping FLG homeostasis. It has been well-characterized that IL-4 and IL-13 expression is increased in acute AD lesions whereas interferon-γ (IFN-γ) expression is increased in chronic lesions.[15,16] Since the distinctive imbalance of Th1and Th2 immune response exists in AD, we hypothesized that Th1/Th2 cytokines might regulate the proteases involved in FLG metabolic processes.
Here, we examined the expression of FLG and related serine proteases (KLK5, KLK7, matriptase, and CAP1), cysteine protease caspase-14, and LEKTI by immunohistochemical staining in acute and chronic lesions of AD. We also examined their expression in HaCaT cells and normal human keratinocytes (NHK) in culture. High calcium (HC) condition induces keratinocyte differentiation.[17] We stimulated the cultured keratinocytes with interleukin (IL)-4/13 or IFN-γ, at different Ca2+ levels, simulating the inflammatory state of human AD.
METHODS
Atopic dermatitis patients and skin samples
The adult patients from Northern China were diagnosed with AD according to the diagnostic criteria proposed by Hanifin and Rajka.[18] All enrolled patients had not undergone any treatments for the last 4 weeks. Biopsies were obtained from six patients (three on acute and three on chronic lesions). Normal adult skin specimens (n = 3) as normal controls were obtained from patients undergoing plastic surgery. Informed consents were obtained from all the participants.
This study was approved by the Ethics Committee of China Medical University.
Immunohistochemical staining
Frozen sections of skin samples were cut at 6 µm. Endogenous peroxidase was blocked by incubating slides with 3% H2O2 for 5 min, and tissue sections were then blocked with serum blocking reagent (Zhongshan Goldenbridge Biotechnology, Beijing, China) for 15 min at room temperature. Slides were incubated with anti-LEKTI, anti-caspase14, anti-FLG, anti-matriptase, anti-CAP1, and anti-KLK7 (Santa Cruz Biotechnology, Inc., CA, USA), anti-KLK5 (Abcam Ltd., CA, USA) for 3 h at 37°C. The secondary antibody and 3 3,-diaminobenzidine stain were added according to the manufacturer's protocol (Zhongshan Goldenbridge Biotechnology, Beijing, China). The sections were counterstained with hematoxylin.
Keratinocytes culture
HaCaT cells, an immortalized human keratinocyte line, were grown in Dulbecco's modified Eagle's Medium (HyClone, USA) containing a low calcium (LC) concentration (1.3 mmol/L calcium), supplemented with 10% fetal calf serum and 1% penicillin/streptomycin at 37°C in 5% CO2. Cells were seeded at 2 × 105 cells/ml. Cells grown at 60–80% confluence were then stimulated with 50 ng/ml of recombinant human IL-4 and IL-13 (Pepro Tech Inc., New Jersey, USA) or 20 ng/ml of recombinant human IFN-γ (Pepro Tech Inc., New Jersey, USA) with LC (1.3 mmol/L) and a HC concentration (10 mmol/L calcium), respectively.[2,7] Cells were harvested 24 h after adding cytokines. We chose this time point to avoid contact inhibition due to the fast growth rate of HaCaT cells.
Normal foreskin specimens were obtained from subjects (n = 3) undergoing genital plastic surgery after informed consents. Human primary keratinocytes were isolated as described by Barlow and Pye[19] and cultured in serum-free defined keratinocyte-serum free medium (SFM) (Gibco Invitrogen Corporation, USA) containing a LC concentration (0.09 mmol/L calcium) supplemented with defined keratinocyte-SFM growth supplement (Gibco Invitrogen Corporation, USA) and 1% penicillin/streptomycin at 37°C in 5% CO2. Aliquot of cultured cells were histochemically stained with anti-human cytokeratin monoclonal antibody (clone number: AE1/AE3) (Fuzhou Maixin Biotech Co., Ltd., China) showing that more than 99% of the cultured cells were keratinocytes. Third or fourth passage keratinocytes were used in subsequent experiments. Cells were seeded at 1 × 105 cells/ml and cultured for 3–5 days (reaching 60–80% confluence) before performing cytokine stimulation with 50 ng/ml IL-4 and IL-13, or 20 ng/ml IFN-γ, in LC (0.09 mmol/L calcium) and HC (1.3 mmol/L calcium) condition, respectively.[2,7] Cells were harvested 5 days after cytokine stimulation.[2,7,20]
RNA isolation and fluorescent real-time reverse transcription-polymerase chain reaction of keratinocytes
Total RNA was isolated from HaCaT cell and NHK using RNA prep pure Cell Kit (Tiangen Biotech Co., Ltd., Beijing, China) following the manufacturer's protocols and reverse-transcribed with Prime Script™ RT reagent kit (TaKaRa Biotechnology Co., Ltd., Dalian, China). Real-time polymerase chain reaction analyses were performed under the protocols of SYBR® Premix Ex TaqTM II (TaKaRa Biotech Co., Ltd., Dalian, China) and the primers used in this study were designed as shown in Table 1. Relative quantities of all targets in test samples were normalized to their corresponding β-actin levels. Relative quantification using the 2 standard curves or delta-delta computed tomography method was performed.
Table 1.
The primer sequences of target and housekeeping genes for quantitative RT-PCR
| Genes | Sequences of primers | Size of amplification fragments (bp) |
|---|---|---|
| β-actin | FW: 5’-TGGCACCCAGCACAATGAA-3’ | 186 |
| Rev: 5’-CTAAGTCATAGTCCGCCTAGAAGCA-3’ | ||
| FLG | FW: 5’-AGTCACGTGGCAGTCCTCACA-3’ | 143 |
| Rev: 5’-TCTAAACCCGGATTCACCATAATCA-3’ | ||
| Matriptase | FW: 5’-TCTGAGCAGTGGGTGCTGTC-3’ | 98 |
| Rev: 5’-CTCGGAGTAGGAGTCTAGCTGGTG-3’ | ||
| CAP1 | FW: 5’-AAGGGCAACCCTGAGTGTGA-3’ | 105 |
| Rev: 5’-CGAGCCTGTCTCGTGAATGA-3’ | ||
| SPINK5 | FW: 5’-GAATTTCGGGACCAAGTGAGGA-3’ | 113 |
| Rev: 5’-ACACTGGCACACATGGCACA-3’ | ||
| KLK5 | FW: 5’-TGCTAAGGCCCAACCAGCTC-3’ | 149 |
| Rev: 5’-GAACATCTGCTGCCCAGATTCA-3’ | ||
| KLK7 | FW: 5’-CTCTGACCTCATGTGCGTGGA-3’ | 166 |
| Rev: 5’-CAGGGTACCTCTGCACACCAAC-3’ | ||
| Caspase14 | FW: 5’-GACCTGGATGCTCTGGAACACA-3’ | 119 |
| Rev: 5’-GAATCGATGGCCTGCTGGA-3’ |
FW: Forward primer; Rev: Reverse primer; FLG: Filaggrin coding gene; CAP1: Channel-activating serine protease 1; SPINK5: Serine protease inhibitor Kazal-type 5 gene; KLK: Kallikrein; RT-PCR: Real-time polymerase chain reaction.
Western blotting of keratinocytes
Expression of FLG, LEKTI, caspase14, KLK7, KLK5, matriptase, and CAP1 proteins was evaluated by Western blotting. Total protein extracts were prepared as described by kit KGP250 supplier (Keygen Biotech Co., Ltd., Nanjing, China). Protein concentration was measured using a protein assay kit (Bio-Rad, Hercules, CA, USA). Aliquots of protein fractions were incubated for 5 min at 99°C with loading buffer. A total of 100 μg of protein mixture was separated by 8–12% SDS-PAGE according to the molecular weight of protein, transferred to a polyvinylidene difluoride membrane (Millipore, CA, USA). Membranes were then blocked with 0.1% Tween 20 and 5% nonfat milk at room temperature for 2 h. The immunoblots were incubated with anti-LEKTI, anti-Caspase14, anti-FLG, anti-matriptase, anti-CAP1, anti-KLK7 (diluted 1:50–100, Santa Cruz Biotechnology Inc., CA, USA), and anti-KLK5 (diluted 1:250, Abcam Ltd., CA, USA) monoclonal antibodies in TBS with 1% bovine serum albumin at 4°C overnight. Anti-human β-actin antibody (ZSGB-Bio Corporation, Beijing, China) was used as a control. The membranes were washed 3 times with 0.1% Tween 20 in TBS and incubated with horseradish peroxidase-conjugated secondary antibody (ZSGB-Bio Co., Beijing, China). All immunoblots were detected by the Western blotting luminol reagent (Pierce, USA) according to the manufacturer's instructions by MF-Chemi BIS 3.2 (DNR Bio-Imaging Systems Ltd., Israel). Results from the representative triplicate experiment are shown.
Statistical analysis
Data of the messenger RNA (mRNA) expressions was analyzed with SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Normality of data distribution was examined by Kolmogorov–Smirnov test and data variance equality by Levene's test. Quantitative results were expressed as a mean ± standard deviation (SD). A minimum of three independent experiments (double wells in each experiment) was conducted for statistical comparison. Comparison of multiple independent variables was analyzed by Kruskal–Wallis test after determining the normality of data distribution. For comparison of two independent variables, corresponding significant results were further assessed by Mann–Whitney U-test, and Bonferroni method was used for adjustment alpha. A P < 0.05 was considered statistically significant.
RESULTS
The expression of filaggrin and its processing proteases in acute and chronic atopic dermatitis lesions
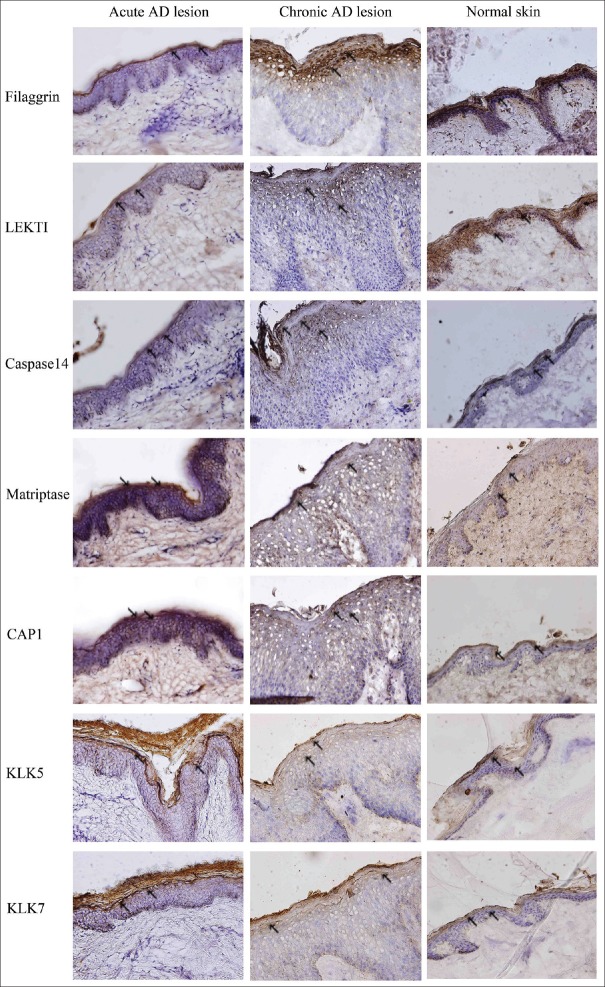
As shown in Figure 1, FLG, LEKTI, and caspase14 expressions were significantly decreased, whereas matriptase, CAP1, KLK5, and KLK7 expressions were increased in acute AD lesions, as compared to normal controls. Similar trends were observed in chronic AD lesions. However, higher levels of FLG, LEKTI, caspase14, while lower levels of matriptase, CAP1, KLK5, and KLK7 were present in chronic AD lesions, as compared to the acute lesions.
Figure 1.
Expression models of filaggrin and proteases involved in filaggrin processing in acute and chronic lesions of atopic dermatitis patients. Skin sections were stained for filaggrin, LEKTI, caspase14, matriptase, CAP1, KLK5, and KLK7. Filaggrin, LEKTI, and caspase14 expressions were significantly decreased, whereas matriptase, CAP1, KLK5, and KLK7 expressions were increased in AD lesions, as compared to normal controls. This phenomenon was more pronounced in the skin from patients with acute lesions (DAB staining, Original magnification ×100, the arrows point to the stained positive cells), (n = 3/three experiments in duplicate). AD: Atopic dermatitis; LEKTI: Lymphoepithelialkazal-type-related inhibitor; CAP1: Channel-activating serine protease 1; KLK5: Kallikrein 5; KLK7: Kallikrein 7.
Interleukin-4/13 down-regulates, while interferon-γ up-regulates, filaggrin expression
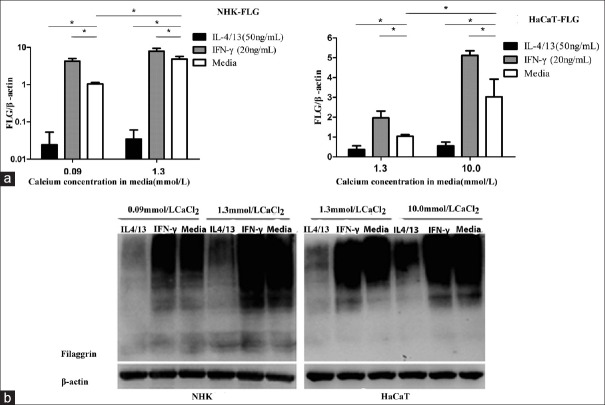
In LC culture condition, as shown in Figure 2a, IL-4 and IL-13 significantly reduced, whereas IFN-γ significantly increased FLG mRNA expression, in both NHK and HaCaT cells as compared with normal controls.
Figure 2.
IL-4/13 and IFN-γ regulate FLG expression in cultured keratinocytes. (a) Relative FLG mRNA was measured by real-time (quantitative) PCR and calculated as fold increase versus untreated control. *P < 0.05. (b) FLG expression was evaluated by Western blotting. HaCaT in LC (1.3 mmol/L CaCl2) or HC (10.0 mmol/L CaCl2) culture medium; NHK in LC (0.09 mmol/L CaCl2) or HC (1.3 mmol/L CaCl2), (n = 3/three experiments in duplicate). IL-4/13: Interleukin-4/13; IFN-γ: Interferon-γ; FLG: Filaggrin; LC: Low calcium; HC: High calcium; NHK: Normal human keratinocyte; PCR: Polymerase chain reaction.
In HC culture media, keratinocytes showed increased FLG mRNA expression, as compared with that in LC culture media. Incubation of differentiated keratinocytes with IL-4 and IL-13 in HC media down-regulated FLG mRNA expression as compared to control cells, whereas IFN-γ significantly increased FLG mRNA expression. In parallel with the above findings, Western blotting detected similar changes in FLG expression, as shown in Figure 2b.
Effects of interleukin-4/13 and interferon-γ on filaggrin degradation related proteases
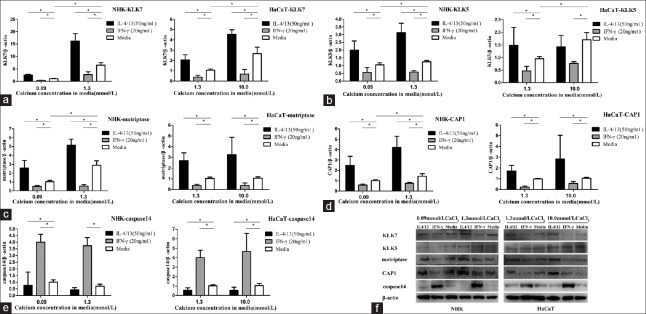
Proteases, such as KLK5 and 7,[9,10,11] matriptase,[12] CAP1,[12] and caspase-14[13] can enhance FLG degradation process. We next screened whether the test cytokines had any effects on the expression of these proteases in keratinocytes. As shown in Figure 3a, IL-4/13 significantly induced KLK7 expression in both NHK and HaCaT cells. On the contrary, IFN-γ significantly reduced KLK7 mRNA expression. Keratinocytes in HC media showed increased KLK7 mRNA expression versus that in LC media. As shown in Figure 3b, IL-4/13 significantly affected KLK5 expression in both NHK and HaCaT cells. In LC culture media, KLK5 mRNA expression levels were significantly higher than in the respective controls. In HC media, KLK5 mRNA expression level in NHK was significantly higher than the control cells; however, there was no difference of KLK5 mRNA expression in HaCaT versus control cells (P > 0.05). On the contrary, IFN-γ significantly reduced KLK5 mRNA expression in both cell types. Keratinocytes showed increased KLK5 mRNA expression in HC media but not in LC media. As shown in Figure 3c, IL-4/13 significantly induced matriptase expression in both NHK and HaCaT cells. On the other hand, IFN-γ significantly reduced matriptase mRNA expression in both cell types. In NHK, the mRNA expression level was higher in HC than in LC culture media. Figure 3d shows that IL-4/13 significantly induced CAP1 in both NHK and HaCaT cells. On the contrary, IFN-γ significantly reduced CAP1 mRNA expression in both cell types. In NHK, the mRNA expression level was higher in HC than in LC culture media. Figure 3e shows that IL-4/13 reduced caspase14 expression in both NHK and HaCaT cells. However, IFN-γ significantly induced caspase 14 mRNA expression in both cell types in LC culture and HC media. The calcium level in culture medium had no effect on caspase 14 expression.
Figure 3.
IL-4/13 and IFN-γ regulate the expression of proteases involved in filaggrin processing in cultured keratinocytes. KLK7 (a), KLK5 (b), matriptase (c), CAP1 (d), and caspase 14 (e) messenger RNA were measured by real-time (quantitative) PCR, *P < 0.05. (f) Protein expression was evaluated by Western blotting. HaCaT in LC (1.3 mmol/L CaCl2) or HC (10.0 mmol/L CaCl2) culture medium; NHK in LC (0.09 mmol/LCaCl2) or HC (1.3 mmol/L CaCl2) culture medium (n =/three experiments in duplicate). IL-4/13: Interleukin-4/13; IFN-γ: Interferon-γ; LC: Low calcium; HC: High calcium; NHK: Normal human keratinocyte; KLK7: Kllikrein 7; KLK5: Kllikrein 5; CAP1: Channel-activating serine protease 1; PCR: Polymerase chain reaction.
The protein expression levels of the above FLG degradation proteases evaluated by Western blotting were consistent with the respective mRNA changes, especially in NHK, as shown in Figure 3f.
Interleukin-4/13 down-regulates, but interferon-γ up-regulates the expression of lymphoepithelial Kazal-type-related inhibitor in keratinocytes
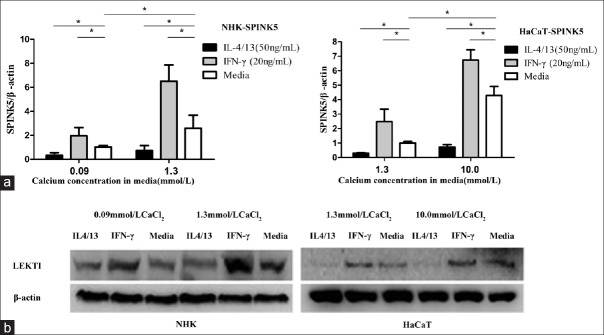
As shown in Figure 4a, IL-4/13 significantly reduced LEKTI mRNA in both NHK and HaCaT cells. On the contrary, IFN-γ significantly induced LEKTI mRNA expression. Data showed a higher increase in LEKTI mRNA expression by keratinocytes in HC than in LC culture media.
Figure 4.
IL-4/13 and IFN-γ regulate LEKTI expression in cultured keratinocytes. (a) LEKTI messenger RNA was measured by real-time (quantitative) PCR. *P < 0.05. (b) Protein expression was evaluated by Western blotting. HaCaT in LC (1.3 mmol/L CaCl2) or HC (10.0 mmol/L CaCl2) culture medium; NHK in LC (0.09 mmol/L CaCl2) or HC (1.3 mmol/L CaCl2) culture medium (n =/three experiments in duplicate). IL-4/13: Interleukin-4/13; IFN-γ: Interferon-γ; LC: Low calcium; HC: High calcium; NHK: Normal human keratinocyte; LEKTI: Lymphoepithelial Kazal-type-related inhibitor; PCR: Polymerase chain reaction.
The protein expression levels of LEKTI by Western blotting analysis were consistent with respective mRNA changes in HC and LC culture conditions, as shown in Figure 4b.
DISCUSSION
FLG is the key protein for maintaining normal epidermal barrier integrity and stratum corneum hydration.[21] Many proteases are involved in this complex processing.[14] The precursor pro-FLG is dephosphorylated with increasing calcium concentration and proteolytically cleaved by proteases, such as matriptase,[22] CAP1,[23] KLK7, and KLK5[24] to release FLG protein during keratinocyte differentiation. Further processing is triggered by cysteine protease caspase-14,[25] which produces the hygroscopic degradation products urocanic acid and pyrrolidone carboxylic acid (known as essential components of the natural moisturizing factors, NMF). LEKTI can globally inhibit FLG degradation processing through suppressing the proteases mentioned above.[10,11,12,13,14,26] The balance between proteolytic activity and its inhibitor is pivotal in keeping FLG homeostasis. While loss-of-function mutations in FLG is a strong genetic factor in the pathogenesis of AD. This study confirms the recent report that FLG expression is significantly decreased by Th2 cytokines, such as IL4/13, whereas it is up-regulated by Th1 cytokine IFN-γ.[2] Contrary to our findings, Hvid et al.[8] stated IFN-γ down-regulated FLG expression. We argued the difference may be due to the LC level of the culture media in Hvid's study.
Increased Th2 cytokines and reduced IFN-γ was reported in acute AD lesions, whereas a markedly increased lesional expression of IFN-γ was found in the chronic phase of AD.[15,16] This study confirms the biphasic role of Th1/Th2 cytokines in FLG expression in keratinocytes, either in undifferentiated or calcium-induced differentiated state in vitro, as well as in lesional skin. The imbalance between Th1 and Th2 polarized immune response seems to extend to FLG homeostasis as well.
KLK5 and KLK7 are essential epidermal desquamation-related serine protease in the skin.[27,28] KLK5 and KLK7 activity have previously been shown to be up-regulated in the skin of AD patients,[29,30,31] especially in acute lesions.[29,30] Our results showed that IL-4/13 can increase KLK5 and KLK7 expression in NHK, with a stronger effect on KLK7 than on KLK5, in both undifferentiated and differentiated cell states. In HaCaT cells, the effect was less significant, especially for KLK5 expression. The discrepant KLK5 expression in NHK and HaCaT cells may be due to their inherent different responses to Th2 cytokines, as exemplified by Morizane et al.[32] that IL-4 or IL-13 had no influence to KLK5 expression in his study. We detected that IL-4/13 up-regulated the expression of matriptase and CAP1. It is presumed that high level of these enzymes may disrupt skin barrier through increasing FLG degradation and decreasing intercellular adhesion and accelerating desquamation. Similarly, some researches demonstrated that barrier dysfunction also leads to increased serine protease activity.[33,34] Therefore, the high expression level of serine proteinases (SPs) and Th2 cytokine may interact with each other and form a vicious circle to disrupt the skin barrier in AD lesion.
On the other hand, IL4/13 moderately down-regulated caspase14 expression in both NHK and HaCaT cells, similar with the result of Hvid et al.[8] These results suggest that IL4/13 could speed up the proFLG proteolysis and at the last step of FLG proteolysis, prohibit NMF formation through down-regulated caspase14. The decreased level of NMF results in a change of pH in stratum corneum, which may affect the activity of KLK5, 7 and injure the skin barrier further.[35]
In contrast with IL-4/13, our study indicated that IFN-γ decreased KLK5, KLK7, matriptase, and CAP1 expression, while significantly increased caspase14 expression in both LC and HC culture conditions and tissue sections of AD. This scenario would give rise to increased FLG and NMF level in the skin.
LEKTI is expressed in the most differentiated epidermis. Many proteases are the substrate of LEKTI in upper SC.[13,14,36] LEKTI buffers proteases hyperactivity, so it is the key protease inhibitor during terminal differentiation and maintenance of permeability barrier homeostasis. The absence of LEKTI causes stratum corneum detachment secondary to the epidermal excessive reaction of SPs. Defects of the LEKTI-encoding gene (SPINK5) result in the lack of LEKTI and lead to Netherton syndrome which mimics AD.[37] Only a proportion of AD is susceptible to Spink5 polymorphisms,[38,39,40,41] which suggest that other factors may influence LEKTI expression in AD. Our results showed, on the contrary to proteinase, IL-4/13 can decrease the expression of LEKTI in human keratinocyte. This result was confirmed by cultured keratinocytes from AD patients[42] and that from the noninvolved skin of AD patients.[43] Our immunohistochemical staining also revealed the decrease of LEKTI in AD, especially in acute AD lesion. We argued that the LEKTI deficient expression and the increased KLK5, 7 expressions in the keratinocytes under imbalance Th1/Th2 cytokines may lead desmosome cleavage and abnormal FLG expression and processing and induce the impairment the skin barrier, though Hatano et al.[32] suggested that neither IL-4 nor calcium level influence the expression of LEKTI,[44] and Morizane et al. found that neither IL-4 nor IL-13 can affect the expression of LEKTI.
It was reported that changes in the proteolytic balance of epidermis could result in inflammation as well.[9] Our results indicated the Th1/Th2 cytokines might regulate FLG expression through the proteases and their inhibitor involved in FLG metabolic processes in keratinocyte. The SPs expression (such as KLK5, 7, matriptase, and CAP1) was higher in acute lesions than chronic lesions, while caspase14, LEKTI expression was lower than in chronic lesions. The difference in expression in FLG and its degradation-related proteases was in accorded with Th1 and Th2 immune response. AD seems to entail a vicious circle between inflammatory response and skin barrier defect through FLG and its processes proteases. Breaking this vicious circle may be attempted through administering recombinant Th1 cytokines or specific blockers or inhibitors of Th2 cytokines or LEKTI directly. Indeed, due to its inhibition effect on Th2 and Th2 cytokines, IFN-γ was successfully applied to treat AD, especially in patients with acute erosive lesions and erythema.[45,46,47] We presume one of the therapeutic effects of IFN-γ may be through regulating the expression of FLG and its processing proteases. While the ineffectiveness of IFN-γ in chronic stages of AD might be explained partly through its effect on the induction of LEKTI and inhibition of SPs expression, which slow down the desquamation of stratum corneum.
The study further disclosed the necessity of immunity, in addition to genetic mutation, to the expression of FLG in AD. Although FLG is the key protein involved in epidermal barrier homeostasis, gene therapy is complex for FLG due to the bulk of FLG protein sequences with many kinds of mutations. More recently, it was reported that restoration of LEKT1 in deficient cells of Netherton patients recovered FLG homeostasis.[48] The results presented here provide the initial basis that local protease inhibitors LEKTI therapy might be a potential therapeutic approach for AD through regulating the FLG proteolytic balance.
Financial support and sponsorship
This study was supported by the public welfare research fund for healthcare (No. 201202013) and the National Natural Science Foundation of China (No. 81301366).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Claude Bouillon of L’Oreal Company for his critical reading in preparing the manuscript.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Irvine AD. Fleshing out filaggrin phenotypes. J Invest Dermatol. 2007;127:504–7. doi: 10.1038/sj.jid.5700695. doi: 10.1038/sj.jid.5700695. [DOI] [PubMed] [Google Scholar]
- 2.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5. doi: 10.1016/j.jaci.2007.04.031. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tintle S, Shemer A, Suárez-Fariñas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583–93. doi: 10.1016/j.jaci.2011.05.042. e1-4 doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Guo Y, Wang W, Shi M, Chen X, Yao Z. Mutations in the filaggrin gene in Han Chinese patients with atopic dermatitis. Allergy. 2011;66:420–7. doi: 10.1111/j.1398-9995.2010.02493.x. doi: 10.1111/j.1398-9995.2010.02493.x. [DOI] [PubMed] [Google Scholar]
- 5.Ma L, Zhang L, Di ZH, Zhao LP, Lu YN, Xu J, et al. Association analysis of filaggrin gene mutations and atopic dermatitis in Northern China. Br J Dermatol. 2010;162:225–7. doi: 10.1111/j.1365-2133.2009.09539.x. doi: 10.1111/j.1365-2133.2009.09539.x. [DOI] [PubMed] [Google Scholar]
- 6.Barker JN, Palmer CN, Zhao Y, Liao H, Hull PR, Lee SP, et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol. 2007;127:564–7. doi: 10.1038/sj.jid.5700587. doi: 10.1038/sj.jid.5700587c. [DOI] [PubMed] [Google Scholar]
- 7.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–58. doi: 10.1038/jid.2008.74. doi: 10.1038/jid.2008.74/ [DOI] [PubMed] [Google Scholar]
- 8.Hvid M, Johansen C, Deleuran B, Kemp K, Deleuran M, Vestergaard C. Regulation of caspase 14 expression in keratinocytes by inflammatory cytokines – A possible link between reduced skin barrier function and inflammation. Exp Dermatol. 2011;20:633–6. doi: 10.1111/j.1600-0625.2011.01280.x. doi: 10.1111/j1600-0625201101280x. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Hoffert U. Reddish, scaly, and itchy: How proteases and their inhibitors contribute to inflammatory skin diseases. Arch Immunol Ther Exp (Warsz) 2009;57:345–54. doi: 10.1007/s00005-009-0045-6. doi: 10.1007/s00005-009-0045-6. [DOI] [PubMed] [Google Scholar]
- 10.Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. doi: 10.1038/ng1493. doi: 10.1038/ng1493. [DOI] [PubMed] [Google Scholar]
- 11.Resing KA, Thulin C, Whiting K, al-Alawi N, Mostad S. Characterization of profilaggrin endoproteinase 1. A regulated cytoplasmic endoproteinase of epidermis. J Biol Chem. 1995;270:28193–8. doi: 10.1074/jbc.270.47.28193. doi: 10.1074/jbc.270.47.28193. [DOI] [PubMed] [Google Scholar]
- 12.Ovaere P, Lippens S, Vandenabeele P, Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci. 2009;34:453–63. doi: 10.1016/j.tibs.2009.08.001. doi: 10.1016/j.tibs.2009.08001. [DOI] [PubMed] [Google Scholar]
- 13.Bennett K, Callard R, Heywood W, Harper J, Jayakumar A, Clayman GL, et al. New role for LEKTI in skin barrier formation: Label-free quantitative proteomic identification of caspase 14 as a novel target for the protease inhibitor LEKTI. J Proteome Res. 2010;9:4289–94. doi: 10.1021/pr1003467. doi: 10.1021/pr1003467. [DOI] [PubMed] [Google Scholar]
- 14.O’Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–93. doi: 10.1016/j.jaci.2008.08.002. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–31. doi: 10.1016/s0091-6749(96)70246-4. doi: S0091-6749(96)70246-4[pii] [DOI] [PubMed] [Google Scholar]
- 17.Micallef L, Belaubre F, Pinon A, Jayat-Vignoles C, Delage C, Charveron M, et al. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp Dermatol. 2009;18:143–51. doi: 10.1111/j.1600-0625.2008.00775.x. doi: 10.1111/j.1600-0625.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- 18.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatol Venereol (Stockh) 1980;(Suppl 92):44–7. doi: 10.2340/00015555924447. [Google Scholar]
- 19.Barlow Y, Pye RJ. Keratinocyte culture. Methods Mol Biol. 1997;75:117–29. doi: 10.1385/0-89603-441-0:117. doi: 10.1385/0-89603-441-0:117. [DOI] [PubMed] [Google Scholar]
- 20.Morizane S, Yamasaki K, Kabigting FD, Gallo RL. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol. 2010;130:1297–306. doi: 10.1038/jid.2009.435. doi: 10.1038/jid.2009.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: Role in skin barrier function and disease. J Cell Sci. 2009;122(Pt 9):1285–94. doi: 10.1242/jcs.033969. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.List K, Szabo R, Wertz PW, Segre J, Haudenschild CC, Kim SY, et al. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1. J Cell Biol. 2003;163:901–10. doi: 10.1083/jcb.200304161. doi: 10.1083/jcb.200304161/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, et al. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005;170:487–96. doi: 10.1083/jcb.200501038. doi: 10.1083/jcb.200501038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, D’Alessio M, et al. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J Invest Dermatol. 2006;126:1622–32. doi: 10.1038/sj.jid.5700284. doi: 10.1038/sjjid.5700284. [DOI] [PubMed] [Google Scholar]
- 25.Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–74. doi: 10.1038/ncb1597. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- 26.Mägert HJ, Ständker L, Kreutzmann P, Zucht HD, Reinecke M, Sommerhoff CP, et al. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J Biol Chem. 1999;274:21499–502. doi: 10.1074/jbc.274.31.21499. doi: 10.1074/jbc.274.31.21499. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y, Koyama J, Moro O, Horii I, Kikuchi K, Tanida M, et al. The role of two endogenous proteases of the stratum corneum in degradation of desmoglein-1 and their reduced activity in the skin of ichthyotic patients. Br J Dermatol. 1996;134:460–4. doi: 10.1111/j.1365-2133.1996.tb16230.x. [PubMed] [Google Scholar]
- 28.Ekholm IE, Brattsand M, Egelrud T. Stratum corneum tryptic enzyme in normal epidermis: A missing link in the desquamation process. J Invest Dermatol. 2000;114:56–63. doi: 10.1046/j.1523-1747.2000.00820.x. doi: 10.1046/j.1523-1747.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 29.Voegeli R, Rawlings AV, Breternitz M, Doppler S, Schreier T, Fluhr JW. Increased stratum corneum serine protease activity in acute eczematous atopic skin. Br J Dermatol. 2009;161:70–7. doi: 10.1111/j.1365-2133.2009.09142.x. doi: 10.1111/j.1365-2133.2009.09142.x. [DOI] [PubMed] [Google Scholar]
- 30.Komatsu N, Saijoh K, Kuk C, Liu AC, Khan S, Shirasaki F, et al. Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Exp Dermatol. 2007;16:513–9. doi: 10.1111/j.1600-0625.2007.00562.x. doi: 10.1111/j.1600-0625.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu N, Saijoh K, Toyama T, Ohka R, Otsuki N, Hussack G, et al. Multiple tissue kallikrein mRNA and protein expression in normal skin and skin diseases. Br J Dermatol. 2005;153:274–81. doi: 10.1111/j.1365-2133.2005.06754.x. doi: 10.1111/j.1365-2133.2005.06754.x. [DOI] [PubMed] [Google Scholar]
- 32.Morizane S, Yamasaki K, Kajita A, Ikeda K, Zhan M, Aoyama Y, et al. TH2 cytokines increase kallikrein 7 expression and function in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;130:259–61.e1. doi: 10.1016/j.jaci.2012.03.006. doi: 10.1016/j.jaci.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, et al. Acute modulations in permeability barrier function regulate epidermal cornification: Role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008;172:86–97. doi: 10.2353/ajpath.2008.070161. doi: 10.2353/ajpath.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126:2074–86. doi: 10.1038/sj.jid.5700351. doi: 10.1038/sj.jid.5700351. [DOI] [PubMed] [Google Scholar]
- 35.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–908. doi: 10.1038/jid.2009.133. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 36.Borgoño CA, Michael IP, Komatsu N, Jayakumar A, Kapadia R, Clayman GL, et al. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem. 2007;282:3640–52. doi: 10.1074/jbc.M607567200. doi: 10.1074/jbc.M607567200. [DOI] [PubMed] [Google Scholar]
- 37.Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–2. doi: 10.1038/75977. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 38.Zhao LP, Di Z, Zhang L, Wang L, Ma L, Lv Y, et al. Association of SPINK5 gene polymorphisms with atopic dermatitis in Northeast China. J Eur Acad Dermatol Venereol. 2012;26:572–7. doi: 10.1111/j.1468-3083.2011.04120.x. doi: 10.1111/j.1468-3083.2011.04120.x. [DOI] [PubMed] [Google Scholar]
- 39.Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, Lawrence R, et al. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29:175–8. doi: 10.1038/ng728. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- 40.Kato A, Fukai K, Oiso N, Hosomi N, Murakami T, Ishii M. Association of SPINK5 gene polymorphisms with atopic dermatitis in the Japanese population. Br J Dermatol. 2003;148:665–9. doi: 10.1046/j.1365-2133.2003.05243.x. doi: 10.1046/j.1365-2133.2003.05243.x. [DOI] [PubMed] [Google Scholar]
- 41.Fölster-Holst R, Stoll M, Koch WA, Hampe J, Christophers E, Schreiber S. Lack of association of SPINK5 polymorphisms with nonsyndromic atopic dermatitis in the population of Northern Germany. Br J Dermatol. 2005;152:1365–7. doi: 10.1111/j.1365-2133.2005.06602.x. doi: 10.1111/j.1365-2133.2005.06602.x. [DOI] [PubMed] [Google Scholar]
- 42.Roedl D, Traidl-Hoffmann C, Ring J, Behrendt H, Braun-Falco M. Serine protease inhibitor lymphoepithelial Kazal type-related inhibitor tends to be decreased in atopic dermatitis. J Eur Acad Dermatol Venereol. 2009;23:1263–6. doi: 10.1111/j.1468-3083.2009.03302.x. doi: 10.1111/j.1468-3083.2009.03302.x. [DOI] [PubMed] [Google Scholar]
- 43.Roelandt T, Thys B, Heughebaert C, De Vroede A, De Paepe K, Roseeuw D, et al. LEKTI-1 in sickness and in health. Int J Cosmet Sci. 2009;31:247–54. doi: 10.1111/j.1468-2494.2009.00516.x. doi: 10.1111/j.1468-2494.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 44.Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, et al. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: Implications for pathogenesis of atopic dermatitis. Exp Dermatol. 2013;22:30–5. doi: 10.1111/exd.12047. doi: 10.1111/exd.12047. [DOI] [PubMed] [Google Scholar]
- 45.Chang TT, Stevens SR. Atopic dermatitis: The role of recombinant interferon-gamma therapy. Am J Clin Dermatol. 2002;3:175–83. doi: 10.2165/00128071-200203030-00004. doi: 030304[pii] [DOI] [PubMed] [Google Scholar]
- 46.Hanifin JM, Schneider LC, Leung DY, Ellis CN, Jaffe HS, Izu AE, et al. Recombinant interferon gamma therapy for atopic dermatitis. J Am Acad Dermatol. 1993;28(2 Pt 1):189–97. doi: 10.1016/0190-9622(93)70026-p. doi: 10.1016/0190-9622(93)70026-P. [DOI] [PubMed] [Google Scholar]
- 47.Stevens SR, Hanifin JM, Hamilton T, Tofte SJ, Cooper KD. Long-term effectiveness and safety of recombinant human interferon gamma therapy for atopic dermatitis despite unchanged serum IgE levels. Arch Dermatol. 1998;134:799–804. doi: 10.1001/archderm.134.7.799. doi: 10.1001/archderm.134.7.799. [DOI] [PubMed] [Google Scholar]
- 48.Roedl D, Oji V, Buters JT, Behrendt H, Braun-Falco M. rAAV2-mediated restoration of LEKTI in LEKTI-deficient cells from Netherton patients. J Dermatol Sci. 2011;61:194–8. doi: 10.1016/j.jdermsci.2010.12.004. doi: 10.1016/j.jdermsci.2010.12.004. [DOI] [PubMed] [Google Scholar]