Abstract
Objective:
The purpose of this review is to discuss some critical issues of isoflavones protective against the development of prostate cancer (PCa).
Data Sources:
Data cited in this review were obtained primarily from PubMed and Embase from 1975 to 2015.
Study Selection:
Articles were selected with the search terms “isoflavone”, “Phytoestrogen”, “soy”, “genistin”, and “PCa”.
Results:
Isoflavones do not play an important role on prostate-specific antigen levels reduction in PCa patients or healthy men. The effect of isoflavones on sex hormone levels and PCa risk may be determined by equol converting bacteria in the intestine, specific polymorphic variation and concentrations of isoflavones. The intake of various types of phytoestrogens with lower concentrations in the daily diet may produce synergistic effects against PCa. Moreover, prostate tissue may concentrate isoflavones to potentially anti-carcinogenic levels. In addition, it is noteworthy that isoflavones may act as an agonist in PCa.
Conclusions:
Isoflavones play a protective role against the development of PCa. However, careful consideration should be given when isoflavones are used in the prevention and treatment of PCa.
Keywords: Isoflavone, Phytoestrogen, Prostate Cancer
INTRODUCTION
Prostate cancer (PCa) is the second most common neoplasia in men worldwide, and the sixth cause of cancer-specific death worldwide. In the last 20 years, there has been a progressive increase in the global incidence of this disease.[1] In Asian countries, the incidence and mortality of PCa are low and are reported to be about 1/8 of that in Western countries.[2] However, some epidemiological investigations suggest the incidence of PCa in Asian men who migrant to the USA rapidly approaches that in western men within two generations.[3,4] Interestingly, Asian-American men retaining at least one genetic or lifestyle characteristic of Asian residents make their risk of PCa less than that of white residents of the USA.[5] Recent studies have reported that Asian men consume large quantities of isoflavones-based foods. By contrast, Western diets mainly comprise red meat-based foods with bare isoflavones.[6,7,8,9]
Isoflavone is one of the phytoestrogens with similar molecular structures to animal estrogen and has weak estrogenic effects. The major isoflavones include genistin, daidzin, glycitin, equol, and biochanin A and, in particular, genistein is the most widely investigated isoflavon [Figure 1]. Isoflavones are highly concentrated in soy products such as beans and tofu.[10,11] A food frequency questionnaire used in the Japan Collaborative Cohort Study (JACC Study) for the evaluation of cancer risk sponsored by the Ministry of Education, Science, Sports and Culture of Japan (Monbusho) in 2005 showed that serum levels of genistein and daidzein are significantly associated with the dietary intake of tofu, and slightly associated with the intake of miso soup while the consumption of fat, meat, and coffee may be associated with equol production from genistein and daidzein by intestinal microflora in this sample set.[12] There are regional differences in global isoflavones intake. The average daily intake of genistein in the oriental population is 20–80 mg/d compared with 1–3 mg/d in the USA.[13] In addition, soy consumption is different across Europe. The mean serum genistein levels of 148 nmol/L in a region with many vegetarians are much higher than mean levels of 2.6–22.6 nmol/L in a region with fewer vegetarians lived.[14] This may provide some clues for the regional difference of PCa incidence.
Figure 1.
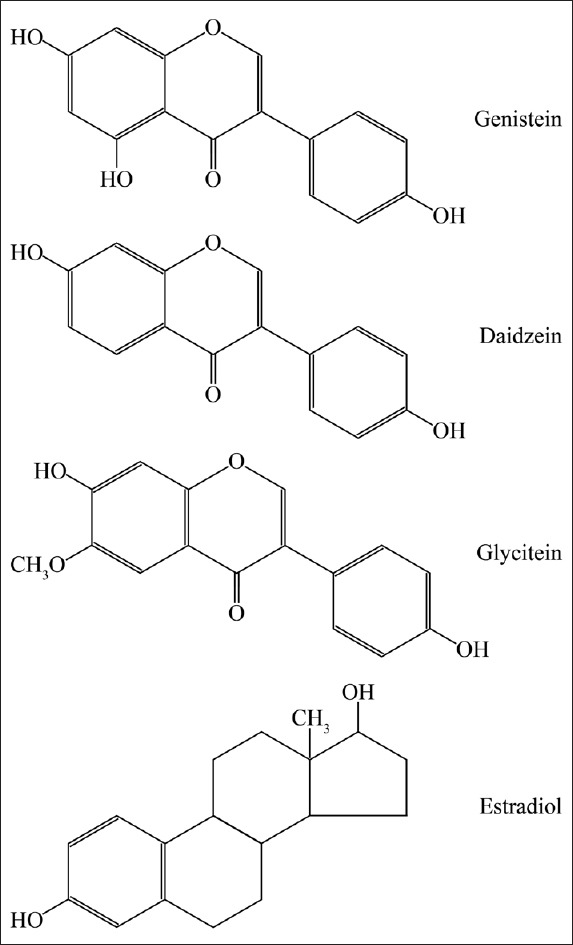
The molecular structure of isoflavones.
PCa is a hormone-dependent malignancy that develops from an androgen-dependent tissue that contains androgen receptors (ARs). Androgens are thought to be involved in the prostatic carcinogenesis and progression.[15] Further in vitro and in vivo studies have demonstrated that estrogens may be implicated as potential agents in the development and progression of PCa. This was supported by current epidemiological evidence showing that the higher incidence of PCa among African-American men is partly due to in utero exposure to maternal estrogens since African-American women have higher circulating estrogen concentrations during pregnancy than Caucasian women.[16,17] In addition, estradiol and sex hormone binding globulin (SHBG) have been found to be higher in African-American men.[18,19,20] Moreover, the correlation of increasing estrogen levels with the high incidence of PCa was observed in African-American men.[21] Estrogen may contribute to PCa through multiple mechanisms involving estrogen receptor (ER)-mediated actions, estrogenic imprinting, epigenetic modifications, direct genotoxicity, hyperprolactinemia, and inflammation and immunologic changes.[12,22]
A growing body of epidemiological studies has revealed that the incidence of PCa is reduced in population with food rich in isoflavones.[23,24] Many studies have demonstrated that isoflavones exert hormone-like effects[25] and nonhormone-like effects involving inhibiting tyrosine kinases,[26] modulation of cell proliferation, regulation of cell cycle, apoptosis, angiogenesis, and tumor cell metastasis,[27] and our recent study found isoflavones induced autophagy cell death by up-regulation of ULK1 level, especially increasing evidence has indicated that isoflavones may be protective against the development of PCa. The main mechanisms involve binding competitively ER-μ and ER-β, regulation of sex steroid hormonal synthesis and secretion,[28,29,30,31] down-regulation of AR gene expression, and inhibition of prostate-specific antigen (PSA) secretion.[32] These possible mechanisms include antioxidant defense, DNA repair, inhibition of angiogenesis and metastasis, potentiation of radio- and chemo-therapeutic agents, and antagonism of estrogen- and androgen-mediated signaling pathways. In addition, other cells in the cancer milieu, such as the fibroblastic stromal cells, endothelial cells, and immune cells, may be targeted by soy isoflavones, which may contribute to soy-mediated PCa prevention.[33]
Many randomized controlled trials (RCTs) about the role of isoflavones in men with PCa have been developed; however, these results are inconsistent. In this study, we review some critical issues involving the effects of isoflavones on PSA, sex steroid hormone levels and risk of PCa, and the difference in concentrations of isoflavones in vitro and human body. Whether isoflavones can lead to PCa progression is also discussed.
EFFECTS OF SOY ISOFLAVONS ON PROSTATE CANCER
Effects of soy isoflavons on prostate-specific antigen levels
Current results of clinical studies did not show soy isoflavones produce significant effects on PSA levels. Messina et al.[34] analyzed the results of 11 trials for examining the effects of isoflavones on serum PSA levels in PCa patients and healthy subjects. In 4 of 8 trials involving PCa patients, a delayed PSA progression was observed although, in no studies, there was an absolute decrease in PSA levels. In contrast, isoflavones did not affect serum PSA in healthy men. Another meta-analysis from eight RCTs whose subjects include PCa patients and men with clinically identified risk of PCa suggested soy isoflavones produced no effects on PSA levels.[35] In a randomized, double-blind, placebo-controlled trial, 86 men with PCa were randomized to treatment with isoflavones or placebo for up to 6 weeks before scheduled prostatectomy. The results showed changes in serum PSA in the isoflavone-treated group compared to men receiving placebo were not statistically significant.[36] Similarly, no PSA levels reduction was observed when a 12-month 83 mg/d isoflavone treatment was administered in 112 healthy men aged 50–80 years.[37] The relationship between serum PSA level and urine phytoestrogen concentration was further analyzed in 824 men of over 40 years old without PCa. The results showed that urinary isoflavones concentrations are not associated with serum PSA levels.[38] It seems that isoflavons may not play an important role on PSA levels reduction in PCa patients or men with clinically identified risk of PCa or healthy men.
Effects of soy isoflavons on sex hormone levels
Some in vitro and in vivo studies demonstrated that isoflavones might increase estrogen synthesis through inducing aromatase activity[39] and decreased the secretion of androgens.[40] Similarly, some clinical studies have demonstrated that isoflavons can affect sex hormone excretion in men. Soy protein isolates have been found to increase urinary estradiol (E2) excretion and 2-hydroxy estrogens to 16α-hydroxyestrone (2:16 OH-E1) ratio in men at high risk of progressing to advanced PCa. Lower excretion of urinary E2 and lower ratio of 2:16 OH-E1 have been reported in PCa cases compared to controls.[41] Another study found that serum estradiol and androstenedione concentrations are increased, and AR expression is suppressed after soy protein isolate supplementation in men at high risk for developing advanced PCa.[42] When healthy men aged 30–59 years are administered 60 mg of soy isoflavones for 3 months, serum free testosterone and dihydrotestosterone levels are decreased whereas SHBG levels are increased. However, no changes in the serum levels of estradiol and total testosterone are detected.[43] By contrary, there are some other studies suggesting isoflavones may not produce an effect on sex hormone level. A meta-analysis from Hamilton-Reeves et al.[44] found soy protein or isoflavone intake produced no significant effect on testosterone, free testosterone levels, SHBG, and free androgen index (FAI) in adult men. The result is consistent with another meta-analysis from van Die et al. who found no significant changes in SHBG, testosterone, and free testosterone levels after isoflavone supplementation in men with PCa or with clinically identified risk of PCa.[35] In a randomized, double-blind, placebo-controlled trial, 86 men were randomized to treatment with isoflavones or placebo for up to 6 weeks before scheduled prostatectomy. The results showed that changes in serum total testosterone, free testosterone, total estrogen, and estradiol in the isoflavone-treated group compared to men receiving placebo were not statistically significant.
Effects of soy isoflavons on risk of prostate cancer
Most of the current studies support the opinion that PCa risk is decreased by isoflavons. One meta-analysis including men with identified risk of PCa has suggested soy/soy isoflavones supplementation significantly reduce incidence of PCa of men with clinically identified risk.[35] The other meta-analyses of 15 epidemiological publications on soy consumption and nine on isoflavones found that soy/isoflavones can significantly delay progression to PCa. Moreover, the subjects were not restricted to men with PCa or identified risk of developing PCa.[9] One case–control study nested in a community-based cohort in Japan (JACC Study) demonstrated that serum genistein, daidzein, and equol dose-dependent reduce PCa risk.[45] The other case–control study nested in the European Prospective Investigation into Cancer and Nutrition also reported that higher plasma concentrations of genistein were associated with lower risk of PCa; however, the relation between plasma concentrations of daidzein, equol, enterolactone or enterodiol, and PCa risk was not observed.[46] In addition, the result from a population-based case–control study on diet, inherited susceptibility, and PCa supported the opinions that soy foods protected against PCa in older Scottish men.[47] Sugiyama et al.[48] reviewed some epidemiological studies on association of isoflavones with PCa risk. They found a significant association of isoflavones with a decreased risk of PCa, and equol-producing intestinal flora carry a significantly reduced risk of PCa.
On contrary, there are still some other studies suggested that isoflavones may not reduce PCa risk. In a case–control study nested in the European Prospective Investigation into Cancer and Nutrition, concentrations of the genistein were measured in prediagnostic plasma samples for 1605 PCa cases and 1697 matched control participants in European. The results showed that plasma genistein concentration was not associated with PCa risk.[49] Ganry analyzed epidemiological data to evaluate the effect of isoflavones on PCa risk and found no statistically significant risk reductions of PCa by isoflavones.[50]
Some factors may determine the effects of isoflavones on PCa. First, it is hypothesized that isoflavones, particularly equol, are key factors in the difference in incidence rate between Asia and the West. Therefore, it is suggested that having or not having equol converting bacteria in the intestine can determine the function of isoflavones on PCa.[51] Second, specific polymorphic variation in the ER-β gene may determine the functions of phytoestrogens. A previous study showed that high intake of phytoestrogens substantially reduces PCa risk among men with specific polymorphic variation in the promoter region of the ER-β gene. However, no association between phytoestrogens and PCa risk among carriers homozygous for the wild-type allele (TT) was found.[52] Third, polymorphisms in the CYP19 gene may affect the positive correlations between phytoestrogen levels and sex hormone levels in men. Both urinary and serum equol are associated with plasma testosterone and FAI among men with the TT genotype but not in men with the CC or CT genotypes for the CYP19 3’-untranslated region T-C polymorphism while urinary and serum enterolactone showed similar genotype-dependent associations with testosterone but not with FAI.[53] The combination of the TTTA long repeats and the minor alleles of rs10046 in CYP19A1 and rs2077647 in estrogen receptor (ESR)-alpha was a high risk for PCa despite isoflavones intakes. The combination of the TTTA short repeat and those homozygous for the major allele of rs10046 in CYP19A1 was low risk when isoflavones given.[54] In addition, the concentrations of isoflavones in serum and prostate tissue may be associated with the protective effect of PCa. Jackson et al.[55] reported that only >5 ng/ml to 10 ng/ml plasma S-equol is associated with the reduced risk of PCa.
Although most of organ systems in the fetus and neonate are highly sensitive to phytoestrogens for organogenesis, rapid growth, and extensive tissue differentiation occurring during these developmental periods, the sensitivity persists until adolescence in reproductive organ systems which continue to develop after birth.[56] Therefore, low-dose intakes of phytoestrogens during these developmental periods may have important protective consequences for carcinogenesis.[57,58] However, current clinical trials are short-term intervention studies in adult males, and the study duration is always <1 year. Therefore, long-term studies in males beginning from the developmental periods such as fetus, neonate, and adolescence may be necessary to determine the effects of phytoestrogens on PCa. In addition, progression from prostatic intraepithelial neoplasia to high-grade prostatic intraepithelial neoplasia and early latent cancer may take 10 or more years, and clinically significant carcinoma may not occur for another 3–15 years.[59] As most studies are of short duration, therefore, clinical trials with long duration are necessary to confirm the effects of phytoestrogens on PCa.
DIFFERENCE IN CONCENTRATIONS OF ISOFLAVONES IN VITRO AND HUMAN BODY
Although a growing body of evidence has indicated that isoflavones may delay PCa progression, only a few studies have emphasized on the relationship between isoflavones concentration in vitro and the serum of the human body. Isoflavone concentrations of 10 µmol/L or even as high as 50 µmol/L to 100 µmol/L in vitro studies have never been achieved in the human body with common diet.[60,61,62] A phase I study provided 20 PCa patients with 300 mg/d genistein for 28 days and then with 600 mg/d for another 56 days. The peak plasma total genistein concentrations during the study were 7.3 ± 0.8 µmol/L on day 1 and 8.1 ± 1.2 µmol/L on day 84 of treatment. Only one subject had a total genistein concentration of 27.1 µmol/L.[63] The intake doses used in this study cannot be achieved in humans by natural dietary sources. The mean intake of genistein in Asian countries is only about 50–100 mg daily.[64] Although effective serum concentrations cannot be achieved by natural diet, prostate tissue may concentrate isoflavones to potentially anti-carcinogenic levels. Short-term soy isoflavones supplementation may lead to significant elevation of intraprostatic isoflavones concentration compared to serum. One placebo-controlled study provided 40 PCa men with 240 mg of clover phytoestrogens or placebo daily for 2 weeks before their operation, and oral supplementation with phytoestrogens induced 23- and 7-fold increase in prostate tissue concentrations of genistein and daidzein, respectively. Genistein and daidzein concentrations of prostate tissue were over 2-fold higher than their plasma. Even though the placebo group did not receive phytoestrogen challenge, they also demonstrated 2-fold prostate tissue genistein and daidzein concentrations compared to their plasma values.[65] The other study had similar results. Nineteen men with PCa received either 82 mg/d total isoflavone or placebo for 2 weeks before surgery, the results showed that isoflavone concentrations in isoflavones supplement group were an average of approximately 6-fold higher in prostate tissue compared to serum.[66]
COMBINATION EFFECTS OF SOY ISOFLAVONS AND OTHER TYPES OF PHYTOESTROGENS
The intake of various types of phytoestrogens in the daily diet of men may produce synergistic effects. Kumar et al. observed the combination effects of genistein, biochanin A, and quercetin on PCa cell lines in vitro. The results showed that the combination of three phytoestrogens with low concentration (8.33 µmol/L of each) is more potent in inhibiting the growth of prostate cells than either alone (25 µmol/L) or double combinations (12.5 µmol/L of each). The study suggests that the effects are synergistic in low concentrations of various phytoestrogens, and the combination of various phytoestrogens may elicit preventive effects against PCa at their physiologically achievable concentrations.[57] Dong et al.[58] observed that combination of 25 µmol/L or 50 µmol/L of genistein and daidzein has synergistic effects on inhibiting cell proliferation and inducing apoptosis in androgen-dependent PCa cells. A in vitro study demonstrated that soy extracts containing total isoflavones induced a significantly higher percentage of cells undergoing apopotosis than genistein or daidzein alone at equal concentrations of 25 µmol/L.[67]
However, a phase II clinical trial investigated the efficacy of soy isoflavones alone or in combination with lycopene in men with PCa. The results showed that no additive or synergistic effects were observed when two phytochemicals were administered together.[68] As the intake doses of phytoestrogens in Asian men with dairy food are lower compared with that in clinical trials, various types of phytoestrogens may produce synergistic effects on the human body for decades. Therefore, more long-term invention studies will be necessary to determine the suitable dose and combination strategy of various types of phytoestrogens against PCa. In addition, for less side effects are observed in dietotherapy, instead of medication, dietotherapy of suitable dose phytoestrogens may be beneficial for PCa prevention in men.
SOY ISOFLAVONS MAY LEAD TO PROGRESSION OF PROSTATE CANCER
Although many in vitro and in vivo studies as well as clinical trials have demonstrated that isoflavones can significantly delay PCa progression, a few studies suggested that isoflavones may act as an agonist in PCa. One recent study showed daidzein can exert androgenic effects by modulating AR coactivators in PCa cells.[69] Some animal experiments further confirmed the opinion that isoflavones may lead to the progression of PCa. A study reported that genistein treatment alone increased the incidence of lymph nodes metastasis in mice orthotopic xenografts of PCa cells.[70] Another study found that physiologically achievable concentrations of genistein can increase PCa growth in the transgenic adenocarcinoma mouse prostate model. The mechanisms were associated with activated signal transducers, activation of transcription 3 (STAT3), enhanced telomerase activity, and increased STAT3 binding to the telomerase reverse transcriptase promoter.[71] In orchidectomized middle-aged rats, genistein can cause the shunting of metabolic pathways in the adrenals, supporting dehydroepiandrosterone (DHEA) secretion and inhibiting corticosterone and aldosterone secretion.[72]
The function of isoflavones on PCa may be biphasic. Concentration differences may contribute to the agonistic/antagonistic responses. Davis et al. observed low concentrations of genistein enhanced 17β-estradiol-mediated PSA expression whereas high concentrations of genistein inhibited estrogen-mediated PSA expression in PCa cells.[73] A similar result from Mahmoud et al. showed that genistein induced a biphasic effects in PCa cells with AR mutation. They found that physiological dose (0.5–5.0 µmol/L) of genistein stimulated cell growth, increased AR expression, and transcriptional activity whereas higher doses induced inhibitory effects.[74] Sex hormone levels might also determine the function of isoflavones. Pihlajamaa et al. observed that genistein is anti-androgenic in the testis, prostate, and brain, and it attenuates reporter gene activity in intact male mice. By contrast, in castrated male mice, genistein exhibits significant androgen agonistic activity in the prostate and brain by increasing reporter gene activity in both tissues.[75] In addition, isoflavones are considered endocrine disrupting compounds with weak estrogen activities. Infant exposure to these substances may lead to carcinogenesis and several anomalies of the reproductive systems.[76] Therefore, careful consideration should be given when phytoestrogens are used in the prevention and treatment of PCa.
CONCLUSIONS
Most of the clinical trials suggest isoflavones do not play an important role on PSA levels reduction in PCa patients or healthy men. The effect of isoflavones on sex hormone levels and PCa risk may be determined by some factors involving having or not having equol converting bacteria in the intestine, specific polymorphic variation in the region of the associated gene, the concentrations of isoflavones. As most studies are of short duration in adult males, therefore, long-term studies in males beginning from the developmental periods such as fetus, neonate, and adolescence may be necessary to determine the effects of phytoestrogens on PCa. The concentrations of isoflavones in vitro studies have never been achieved in the serum of human body of common diet. However, prostate tissue may concentrate isoflavones to potentially anti-carcinogenic levels. Moreover, the intake of various types of phytoestrogens with lower concentrations in the daily diet may produce synergistic effects against PCa. It is noteworthy that isoflavones may act as an agonist in PCa and lead to several anomalies of the reproductive systems for infant exposure. Therefore, careful consideration should be given when isoflavones are used in the prevention and treatment of PCa.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Plata Bello A, Concepcion Masip T. Prostate cancer epidemiology. Arch Esp Urol. 2014;67:373–82. [PubMed] [Google Scholar]
- 2.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. IX. Lyon, France: IARC Scientific Publication; 2007. Cancer Incidence in Five Continents. [Google Scholar]
- 3.Muir CS, Nectoux J, Staszewski J. The epidemiology of prostatic cancer. Geographical distribution and time-trends. Acta Oncol. 1991;30:133–40. doi: 10.3109/02841869109092336. doi: 10.3109/02841869109092336. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook LS, Goldoft M, Schwartz SM, Weiss NS. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J Urol. 1999;161:152–5. doi: 10.1016/S0022-5347(01)62086-X. [PubMed] [Google Scholar]
- 6.Adlercreutz CH, Goldin BR, Gorbach SL, Höckerstedt KA, Watanabe S, Hämäläinen EK, et al. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125(3 Suppl):757S–70S. doi: 10.1093/jn/125.3_Suppl.757S. [DOI] [PubMed] [Google Scholar]
- 7.Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61:117–31. doi: 10.1301/nr.2003.apr.117-131. doi: 10.1301/nr.2003.apr.117-131. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744–57. doi: 10.1081/cnv-120023773. doi: 10.1081/CNV-120023773. [DOI] [PubMed] [Google Scholar]
- 9.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: A revisit of a meta-analysis. Am J Clin Nutr. 2009;89:1155–63. doi: 10.3945/ajcn.2008.27029. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 10.Mazur WM, Duke JA, Wahala K, Rasku S, Adlercreutz H. Isoflavonoids and lignans in legumes: Nutritional and health aspects in humans. J Nutr Biochem. 1998;9:193–200. doi: 10.1016/S0955-2863(97)00184-8. [Google Scholar]
- 11.Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 12.Ozasa K, Nakao M, Watanabe Y, Hayashi K, Miki T, Mikami K, et al. Association of serum phytoestrogen concentration and dietary habits in a sample set of the JACC Study. J Epidemiol. 2005;15(Suppl 2):S196–202. doi: 10.2188/jea.15.S196. doi: 10.2188/jea.15.S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes S, Peterson TG, Coward L. Rationale for the use of genistein-containing soy matrices in chemoprevention trials for breast and prostate cancer. J Cell Biochem Suppl. 1995;22:181–7. doi: 10.1002/jcb.240590823. doi: 10.1002/jcb.240590823. [DOI] [PubMed] [Google Scholar]
- 14.Peeters PH, Slimani N, van der Schouw YT, Grace PB, Navarro C, Tjonneland A, et al. Variations in plasma phytoestrogen concentrations in European adults. J Nutr. 2007;137:1294–300. doi: 10.1093/jn/137.5.1294. [DOI] [PubMed] [Google Scholar]
- 15.Kim W, Ryan CJ. Quo vadis: Advanced prostate cancer-clinical care and clinical research in the era of multiple androgen receptor-directed therapies. Cancer. 2015;121:361–71. doi: 10.1002/cncr.28929. doi: 10.1002/cncr.28929. [DOI] [PubMed] [Google Scholar]
- 16.Powell IJ, Meyskens FL., Jr African American men and hereditary/familial prostate cancer: Intermediate-risk populations for chemoprevention trials. Urology. 2001;57(4 Suppl 1):178–81. doi: 10.1016/s0090-4295(00)00968-7. doi: 10.1016/S0090-4295(00)00968-7. [DOI] [PubMed] [Google Scholar]
- 17.Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: Potential effects on male offspring. Br J Cancer. 1988;57:216–8. doi: 10.1038/bjc.1988.46. doi: 10.1038/bjc.1988.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winters SJ, Brufsky A, Weissfeld J, Trump DL, Dyky MA, Hadeed V. Testosterone, sex hormone-binding globulin, and body composition in young adult African American and Caucasian men. Metabolism. 2001;50:1242–7. doi: 10.1053/meta.2001.26714. doi: 10.1053/meta.2001.26714. [DOI] [PubMed] [Google Scholar]
- 19.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92:2519–25. doi: 10.1210/jc.2007-0028. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 20.Orwoll ES, Nielson CM, Labrie F, Barrett-Connor E, Cauley JA, Cummings SR, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010;95:E151–60. doi: 10.1210/jc.2009-2435. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–26. doi: 10.1093/jnci/88.16.1118. doi: 10.1093/jnci/88161118. [DOI] [PubMed] [Google Scholar]
- 22.Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab. 2011;6:437–51. doi: 10.1586/eem.11.20. doi: 10.1586/eem.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuhouser ML. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad A, Biersack B, Li Y, Bao B, Kong D, Ali S, et al. Perspectives on the role of isoflavones in prostate cancer. AAPS J. 2013;15:991–1000. doi: 10.1208/s12248-013-9507-1. doi: 10.1208/s12248-013-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setchell KD. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68(6 Suppl):1333S–46S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 26.Yan GR, Xiao CL, He GW, Yin XF, Chen NP, Cao Y, et al. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics. 2010;10:976–86. doi: 10.1002/pmic.200900662. doi: 10.1002/pmic.200900662. [DOI] [PubMed] [Google Scholar]
- 27.Wuttke W, Jarry H, Seidlová-Wuttke D. Isoflavones – Safe food additives or dangerous drugs. Ageing Res Rev. 2007;6:150–88. doi: 10.1016/j.arr.2007.05.001. doi: 10.1016/j.arr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura K, Tanaka T, Kawashima H, Nakatani T. Involvement of the estrogen receptor beta in genistein-induced expression of p21(waf1/cip1) in PC-3 prostate cancer cells. Anticancer Res. 2008;28:709–14. [PubMed] [Google Scholar]
- 29.Stettner M, Kaulfuss S, Burfeind P, Schweyer S, Strauss A, Ringert RH, et al. The relevance of estrogen receptor-beta expression to the antiproliferative effects observed with histone deacetylase inhibitors and phytoestrogens in prostate cancer treatment. Mol Cancer Ther. 2007;6:2626–33. doi: 10.1158/1535-7163.MCT-07-0197. doi: 10.1158/1535-7163.mct-07-0197. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Eltoum IE, Carpenter M, Lamartiniere CA. Genistein mechanisms and timing of prostate cancer chemoprevention in lobund-wistar rats. Asian Pac J Cancer Prev. 2009;10:143–50. [PubMed] [Google Scholar]
- 31.Krazeisen A, Breitling R, Möller G, Adamski J. Phytoestrogens inhibit human 17beta-hydroxysteroid dehydrogenase type 5. Mol Cell Endocrinol. 2001;171:151–62. doi: 10.1016/s0303-7207(00)00422-6. doi: 10.1016/S0303-7207(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 32.Wuttke W, Jarry H, Seidlova-Wuttke D. Plant-derived alternative treatments for the aging male: Facts and myths. Aging Male. 2010;13:75–81. doi: 10.3109/13685530903440416. doi: 10.3109/13685530903440416. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014;140:116–32. doi: 10.1016/j.jsbmb.2013.12.010. doi: 10.1016/j.jsbmb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int. 2006;89:1121–34. [PubMed] [Google Scholar]
- 35.van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: A systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113:E119–30. doi: 10.1111/bju.12435. doi: 10.1111/bju.12435. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton-Reeves JM, Banerjee S, Banerjee SK, Holzbeierlein JM, Thrasher JB, Kambhampati S, et al. Short-term soy isoflavone intervention in patients with localized prostate cancer: A randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8:e68331. doi: 10.1371/journal.pone.0068331. doi: 10.1371/journal.pone.0068331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams KF, Chen C, Newton KM, Potter JD, Lampe JW. Soy isoflavones do not modulate prostate-specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2004;13:644–8. [PubMed] [Google Scholar]
- 38.Walser-Domjan E, Richard A, Eichholzer M, Platz EA, Linseisen J, Rohrmann S. Association of urinary phytoestrogen concentrations with serum concentrations of prostate-specific antigen in the National Health and Nutrition Examination Survey. Nutr Cancer. 2013;65:813–9. doi: 10.1080/01635581.2013.801999. doi: 10.1080/016355812013801999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye L, Chan MY, Leung LK. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol Cell Endocrinol. 2009;302:73–80. doi: 10.1016/j.mce.2009.01.003. doi: 10.1016/j.mce.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Caceres S, Silvan G, Martinez-Fernandez L, Illera MJ, Millan P, Monsalve B, et al. The effects of isoflavones on androgens and glucocorticoids during puberty on male Wistar rats. Reprod Domest Anim. 2014;49:611–7. doi: 10.1111/rda.12335. doi: 10.1111/rda.12335. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton-Reeves JM, Rebello SA, Thomas W, Slaton JW, Kurzer MS. Soy protein isolate increases urinary estrogens and the ratio of 2:16alpha-hydroxyestrone in men at high risk of prostate cancer. J Nutr. 2007;137:2258–63. doi: 10.1093/jn/137.10.2258. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton-Reeves JM, Rebello SA, Thomas W, Slaton JW, Kurzer MS. Isoflavone-rich soy protein isolate suppresses androgen receptor expression without altering estrogen receptor-beta expression or serum hormonal profiles in men at high risk of prostate cancer. J Nutr. 2007;137:1769–75. doi: 10.1093/jn/137.7.1769. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Fujimoto K, Chihara Y, Torimoto K, Yoneda T, Tanaka N, et al. Isoflavone supplements stimulated the production of serum equol and decreased the serum dihydrotestosterone levels in healthy male volunteers. Prostate Cancer Prostatic Dis. 2009;12:247–52. doi: 10.1038/pcan.2009.10. doi: 10.1038/pcan.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton-Reeves JM, Vazquez G, Duval SJ, Phipps WR, Kurzer MS, Messina MJ. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: Results of a meta-analysis. Fertil Steril. 2010;94:997–1007. doi: 10.1016/j.fertnstert.2009.04.038. doi: 10.1016/j.fertnstert.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 45.Ozasa K, Nakao M, Watanabe Y, Hayashi K, Miki T, Mikami K, et al. Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer Sci. 2004;95:65–71. doi: 10.1111/j.1349-7006.2004.tb03172.x. doi: 10.1111/j.1349-7006.2004.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, et al. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2009;100:1817–23. doi: 10.1038/sj.bjc.6605073. doi: 10.1038/sj.bjc.6605073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heald CL, Ritchie MR, Bolton-Smith C, Morton MS, Alexander FE. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br J Nutr. 2007;98:388–96. doi: 10.1017/S0007114507700703. doi: 10.1017/s0007114507700703. [DOI] [PubMed] [Google Scholar]
- 48.Sugiyama Y, Masumori N, Fukuta F, Yoneta A, Hida T, Yamashita T, et al. Influence of isoflavone intake and equol-producing intestinal flora on prostate cancer risk. Asian Pac J Cancer Prev. 2013;14:1–4. doi: 10.7314/apjcp.2013.14.1.1. doi: 10.7314/APJCP.2013.14.11. [DOI] [PubMed] [Google Scholar]
- 49.Travis RC, Allen NE, Appleby PN, Price A, Kaaks R, Chang-Claude J, et al. Prediagnostic concentrations of plasma genistein and prostate cancer risk in 1,605 men with prostate cancer and 1,697 matched control participants in EPIC. Cancer Causes Control. 2012;23:1163–71. doi: 10.1007/s10552-012-9985-y. doi: 10.1007/s10552-012-9985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganry O. Phytoestrogens and prostate cancer risk. Prev Med. 2005;41:1–6. doi: 10.1016/j.ypmed.2004.10.022. doi: 10.1016/j.ypmed.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 51.Akaza H. Prostate cancer chemoprevention by soy isoflavones: Role of intestinal bacteria as the “second human genome”. Cancer Sci. 2012;103:969–75. doi: 10.1111/j.1349-7006.2012.02257.x. doi: 10.1111/j.1349-7006.2012.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hedelin M, Bälter KA, Chang ET, Bellocco R, Klint A, Johansson JE, et al. Dietary intake of phytoestrogens, estrogen receptor-beta polymorphisms and the risk of prostate cancer. Prostate. 2006;66:1512–20. doi: 10.1002/pros.20487. doi: 10.1002/pros.20487. [DOI] [PubMed] [Google Scholar]
- 53.Low YL, Taylor JI, Grace PB, Dowsett M, Folkerd E, Doody D, et al. Polymorphisms in the CYP19 gene may affect the positive correlations between serum and urine phytoestrogen metabolites and plasma androgen concentrations in men. J Nutr. 2005;135:2680–6. doi: 10.1093/jn/135.11.2680. [DOI] [PubMed] [Google Scholar]
- 54.Sonoda T, Suzuki H, Mori M, Tsukamoto T, Yokomizo A, Naito S, et al. Polymorphisms in estrogen related genes may modify the protective effect of isoflavones against prostate cancer risk in Japanese men. Eur J Cancer Prev. 2010;19:131–7. doi: 10.1097/CEJ.0b013e328333fbe2. doi: 10.1097/CEJ.0b013e328333fbe2. [DOI] [PubMed] [Google Scholar]
- 55.Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S-equol, an estrogen receptor ß agonist. Nutr Rev. 2011;69:432–48. doi: 10.1111/j.1753-4887.2011.00400.x. doi: 10.1111/j.1753-4887.2011.00400.x. [DOI] [PubMed] [Google Scholar]
- 56.Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction. 2012;143:247–60. doi: 10.1530/REP-11-0369. doi: 10.1530/rep-11-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar R, Verma V, Jain A, Jain RK, Maikhuri JP, Gupta G. Synergistic chemoprotective mechanisms of dietary phytoestrogens in a select combination against prostate cancer. J Nutr Biochem. 2011;22:723–31. doi: 10.1016/j.jnutbio.2010.06.003. doi: 10.1016/j.jnutbio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Dong X, Xu W, Sikes RA, Wu C. Combination of low dose of genistein and daidzein has synergistic preventive effects on isogenic human prostate cancer cells when compared with individual soy isoflavone. Food Chem. 2013;141:1923–33. doi: 10.1016/j.foodchem.2013.04.109. doi: 10.1016/j.foodchem.2013.04.109. [DOI] [PubMed] [Google Scholar]
- 59.Kumar NB, Cantor A, Allen K, Riccardi D, Besterman-Dahan K, Seigne J, et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59:141–7. doi: 10.1002/pros.10362. doi: 10.1002/pros.10362. [DOI] [PubMed] [Google Scholar]
- 60.Hedlund TE, Maroni PD, Ferucci PG, Dayton R, Barnes S, Jones K, et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J Nutr. 2005;135:1400–6. doi: 10.1093/jn/135.6.1400. [DOI] [PubMed] [Google Scholar]
- 61.Swami S, Krishnan AV, Peehl DM, Feldman D. Genistein potentiates the growth inhibitory effects of 1,25-dihydroxyvitamin D3 in DU145 human prostate cancer cells: Role of the direct inhibition of CYP24 enzyme activity. Mol Cell Endocrinol. 2005;241:49–61. doi: 10.1016/j.mce.2005.05.001. doi: 10.1016/j.mce.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Guy L, Védrine N, Urpi-Sarda M, Gil-Izquierdo A, Al-Maharik N, Boiteux JP, et al. Orally administered isoflavones are present as glucuronides in the human prostate. Nutr Cancer. 2008;60:461–8. doi: 10.1080/01635580801911761. doi: 10.1080/01635580801911761. [DOI] [PubMed] [Google Scholar]
- 63.Miltyk W, Craciunescu CN, Fischer L, Jeffcoat RA, Koch MA, Lopaczynski W, et al. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am J Clin Nutr. 2003;77:875–82. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- 64.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 65.Rannikko A, Petas A, Rannikko S, Adlercreutz H. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. Prostate. 2006;66:82–7. doi: 10.1002/pros.20315. doi: 10.1002/pros.20315. [DOI] [PubMed] [Google Scholar]
- 66.Gardner CD, Oelrich B, Liu JP, Feldman D, Franke AA, Brooks JD. Prostatic soy isoflavone concentrations exceed serum levels after dietary supplementation. Prostate. 2009;69:719–26. doi: 10.1002/pros.20922. doi: 10.1002/pros.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu A, Bray TM, Helferich WG, Doerge DR, Ho E. Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp Biol Med (Maywood) 2010;235:90–7. doi: 10.1258/ebm.2009.009128. doi: 10.1258/ebm.2009.009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1–7. doi: 10.1080/01635580701413934. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 69.Chen JJ, Chang HC. By modulating androgen receptor coactivators, daidzein may act as a phytoandrogen. Prostate. 2007;67:457–62. doi: 10.1002/pros.20470. doi: 10.1002/pros.20470. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Raffoul JJ, Che M, Doerge DR, Joiner MC, Kucuk O, et al. Prostate cancer treatment is enhanced by genistein in vitro and in vivo in a syngeneic orthotopic tumor model. Radiat Res. 2006;166(1 Pt 1):73–80. doi: 10.1667/RR3590.1. doi: 10.1667/rr3590.1. [DOI] [PubMed] [Google Scholar]
- 71.Chau MN, El Touny LH, Jagadeesh S, Banerjee PP. Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3. Carcinogenesis. 2007;28:2282–90. doi: 10.1093/carcin/bgm148. doi: 10.1093/carcin/bgm148. [DOI] [PubMed] [Google Scholar]
- 72.Ajdzanovic V, Sosic-Jurjevic B, Filipovic B, Trifunovic S, Manojlovic-Stojanoski M, Sekulic M, et al. Genistein-induced histomorphometric and hormone secreting changes in the adrenal cortex in middle-aged rats. Exp Biol Med (Maywood) 2009;234:148–56. doi: 10.3181/0807-RM-231. doi: 10.3181/0807-rm-231. [DOI] [PubMed] [Google Scholar]
- 73.Davis JN, Kucuk O, Sarkar FH. Expression of prostate-specific antigen is transcriptionally regulated by genistein in prostate cancer cells. Mol Carcinog. 2002;34:91–101. doi: 10.1002/mc.10053. doi: 10.1002/mc.10053. [DOI] [PubMed] [Google Scholar]
- 74.Mahmoud AM, Zhu T, Parray A, Siddique HR, Yang W, Saleem M, et al. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS One. 2013;8:e78479. doi: 10.1371/journal.pone.0078479. doi: 10.1371/journal.pone.0078479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pihlajamaa P, Zhang FP, Saarinen L, Mikkonen L, Hautaniemi S, Jänne OA. The phytoestrogen genistein is a tissue-specific androgen receptor modulator. Endocrinology. 2011;152:4395–405. doi: 10.1210/en.2011-0221. doi: 10.1210/en.2011-0221. [DOI] [PubMed] [Google Scholar]
- 76.Bar-El DS, Reifen R. Soy as an endocrine disruptor: Cause for caution. J Pediatr Endocrinol Metab. 2010;23:855–61. doi: 10.1515/jpem.2010.138. doi: 10.1515/jpem.2010.138. [DOI] [PubMed] [Google Scholar]


