Abstract
Background:
Liver donors are subjected to specific postresection hemodynamic changes. The aim was to monitor these changes and to evaluate the effect of magnesium sulfate infusion (MgSO4) on these changes together with total anesthetic agents consumption.
Patients and Methods:
A total of 50 donors scheduled for right hepatotomy were divided into two equal groups. Controls (C) received saline and magnesium group (Mg) received MgSO4 10% (30 mg/kg over 20 min) administered immediately after induction of anesthesia, followed by infusion (10 mg/kg/h) till the end of surgery. Hemodynamics, transesophageal Doppler (TED) data and anesthetic depth guided by Entropy were recorded.
Results:
Postresection both groups demonstrated an increase in heart rate (HR) and cardiac output (COP) in association with lowering of systemic vascular resistance (SVR). The increase in HR with Mg was lower when compared with C, P = 0.00. Increase in COP was lower with Mg compared to (C) (6.1 ± 1.3 vs. 7.5 ± 1.6 L/min, P = 0.00) and with less reduction in SVR compared to C (1145 ± 251 vs. 849.2 ± 215 dynes.s/cm5, P < 0.01), respectively. Sevoflurane consumption was lower with Mg compared to C (157.1 ± 35.1 vs. 187.6 ± 25.6 ml, respectively, P = 0.001). Reduced fentanyl and rocuronium consumption in Mg group are compared to C (P = 0.00). Extubation time, postoperative patient-controlled fentanyl were lower in Mg than C (P = 0.001).
Conclusion:
TED was able to detect significant hemodynamic changes associated with major hepatotomy. Prophylactic magnesium helped to reduce these changes with lower anesthetic and analgesics consumption and an improvement in postoperative pain relief.
Keywords: Hemodynamics, liver resection, magnesium
Introduction
Volunteers donating their right liver lobe for the purpose of adult live donor liver transplantation (LDLT) are subjected to specific postresection hemodynamic changes. In a recent study by El Sharkawy et al., the heart rate (HR), stroke volume (SV), and cardiac index (CI) measured by transesophageal Doppler (TED), were found to immediately increase after right hepatotomy in association with a significant reduction in the systemic vascular resistance (SVR).[1] Similar changes in (cardiac output [COP]) were also previously described in 2002 by Niemann et al., among patients with healthy livers subjected to major hepatic resection for the same purpose of live donation for liver transplantation.[2] These hemodynamic changes could be of a multifactorial origin and would require close monitoring to ensure the volunteers perioperative safety during the process of their donation. The use of TED monitoring can help to diagnose and manage these changes with a minimally invasive approach. The aim was to monitor these changes with the TED probes and to evaluate the possible effect of prophylactic magnesium sulfate infusion (MgSO4) on these hemodynamics changes and on the total anesthetic agents and analgesics consumption.
Patients and Methods
Approval was obtained from the Local Ethics and Research Committee (IRB 0073/2013). The study was also registered at the Pan African Clinical Trial Registry of South Africa (PACTRA201404000809236), website (www.pactr.org). These prospective randomized control trial (RCT) (double-blinded) involved 50 adults live liver donors from both sex undergoing right hepatotomy. Inclusion criteria were: Age 18-40 years, American Society of Anesthesiologists classification I, and normal liver function tests, renal function tests, and serum electrolyte. Exclusion criteria included any donor is not fulfilling the inclusion criteria, unwilling to participate and with a history of cardiac arrhythmia.
The 50 donors scheduled for right hepatotomy were divided randomly by closed, opaque envelopes into two equal groups. The Pharmacy Department supplied the infusions to the Anesthesia Department prior to the planned surgery. Both the anesthesia provider and the assessors were blind to the content of the infusion. Sealed opaque envelopes were only opened by the pharmacist to allocate the patient to his group. Controls (C) received saline boluses and infusion as placebo. The magnesium group (Mg) received MgSO4 (Memphis Company for Pharmaceutical and Chemical Industries, ElAmirya, Cairo, Egypt.) intravenous infusion starting 15 min after induction of general anesthesia, MgSO4 (10%), a bolus of 30 mg/kg over an hour before induction and 10 mg/kg/h by continuous infusion with a syringe pump over the entire operation period until the end of skin wound closure. Hemodynamic changes monitored with continuous pressure transduced invasive arterial blood pressure (mean arterial blood pressure [MAP]), central venous pressure (CVP), and TED (CardioQ Deltex Medical, Chichester, UK). TED measured parameters include are: SV in ml, corrected flow time (FTc) in m sec, CI in L/m2, SVR in dyns.s/cm5, and COP in L/min. After preoxygenation by 80% O2 for 3 min, general anesthesia was induced with propofol 2 μg/kg IV, fentanyl 1 μg/kg IV, and rocuronium 0.6 mg/kg IV followed by oral endotracheal intubation after loss of train of four. Maintenance of general anesthesia with a mixture of sevoflurane and 50% oxygen in air, fentanyl, and keeping entropy (anesthesia depth monitor) between 40 and 60, mechanical ventilation was performed for all patients with a closed system (Datex Omeda, GE, USA) adjusted to keep SaO2 >95% and end-tidal CO2 between 35 and 40 mmHg. Rocronium was administrated according to a nerve stimulator and increment dose of fentanyl to provide balanced general anesthesia. Boluses of colloids were administered, guided by an algorithm depending on the Doppler parameters estimations of SV and FTc. This algorithm was similar to that used by Sinclair et al.[3] A 200-ml aliquot of 6% hydroxyethyl starch in saline (6% HES 130/0.4 Voluven®; Fresenius-Kabi, Bad Homburg, Germany) were given in response to the FTc values. A continuous infusion of Ringers acetate was set at 6 ml/kg/h. Intraoperative fluid balance: Blood products requirements and both crystalloid and colloid consumption in ml. Patients were extubated, either in the operating room or postoperatively, when they fulfilled standard clinical criteria (adequate protective reflexes, adequate oxygenation, and stable hemodynamic). All patients were studied at the following times: 15 min after induction of anesthesia (T1); laparotomy: Immediately after the abdominal fascia opening (T2); during hepatotomy phase (T3); at the end of surgery (T4). Postoperative pain score and anesthetic requirements were reported. Total inhalational anesthetic requirement (ml) calculated by S/5e Anesthesia Monitor by GE Health Care, Finland (formerly Datex-Ohmeda, Helsinki, Finland) and muscle relaxant (mg) consumed.
Sample size and power of the study
In the present study, a was set to 0.05, and maximum accepted = 20% with a minimum power of the study of 80%. Primary outcome of this RCT was SVR (dyns.s/cm5) between the two groups. Calculated sample size = 25. Calculation of sample size was done using (IBM SPSS Sample power) software and was also confirmed using Lenth Java Applets for Power and Sample Size (Computer software).[4]
Statistical procedure
Data were collected and entered into the computer using Statistical Package for Social Science program for statistical analysis. Data were entered as numerical or categorical, as appropriate. Kolmogorov-Smirnov test was carried out and revealed no significance in the distribution of variables, so all variables included in the study are normally distributed and parametric statistics were carried out. Exploration of the data: This yielded complete descriptive statistics including the minimum and maximum, range, mean, standard deviation, median, and inter-quartile range for each variable. Comparisons were carried out between the two studied groups using the independent t-test. Box and Whiskers graph were done. The chi-square test and Fisher exact test were used to measure the association between qualitative variables. Correction of the P value for multiple testing was set P = 0.01 to detect significance (Bonforroni correction of multiple comparisons). Hence, in the present study, an alpha level was set to 1% with a significance level of 99%, and a beta error accepted up to 20% with a power of the study of 80%.
Results
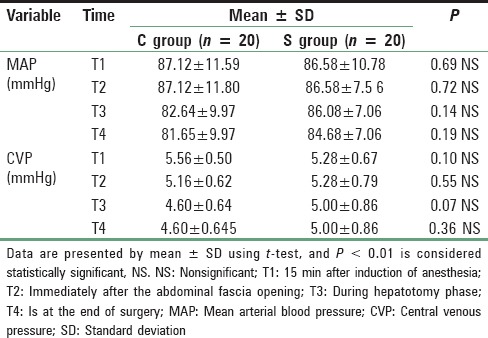
Patients in both groups were well matched for age, weight, height, and sex; P > 0.05. Mean age in magnesium group was 37.80 ± 6.44 years (range 19-40), and in the control group mean was 36.68 ± 7.67 years (range 19-40). Mean height in magnesium group was 170.76 ± 4.65 cm range (160-180) while in control group mean height was 171.44 ± 3.00 cm range (165-185), 74.88 ± 5.90 kg was the mean weight in magnesium group range (65-90), and in control group mean was 73.32 ± 4.007 kg range (65-80), male to female ratio 16/6 in magnesium group and 18/7 in control group. These differences recorded no statistically significant differences [Table 1]. Lower HR with Mg compared to C all over the time P < 0.01 [Figure 1]. Increase in COP of TED was significantly lower postresection with Mg compared to Controls (C) (6.1 ± 1.3 vs. 7.5 ± 1.6 L/min, P < 0.01) and with lesser reduction in SVR compared to C (1145 ± 251 vs. 849.2 ± 215 dynes.s/cm5, P < 0.01), respectively [Table 2 and Figure 2]. No significant difference in TED; FTc and CVP were reported between both groups at any stage. FTc and CVP during resection were in Mg group 355.84 ms and 5 ± 0.86 mmHg versus 367.35 ms and 4.60 (0.64) mmHg in C, respectively, P > 0.05 [Tables 3 and 4]. Sevoflurane consumption was lower with Mg 157.1 ± 35.1 versus 187.6 ± 25.6 ml in C, respectively. Reduced fentanyl and rocuronium consumption were observed in Mg group 400.3 ± 63.7 μg and 202.0 ± 26.9 mg compared to 560.0 ± 49.90 mg, 271.7 ± 29.4 mg in C, respectively, P < 0.01. Operative time and blood loss were 7.52 ± 0.7 h and 500 ± 0.7 ml in Mg compared to 7.16 ± 0.74 h and 498.74 in C group [Table 3].
Table 1.
Demographic data in the two study groups

Figure 1.
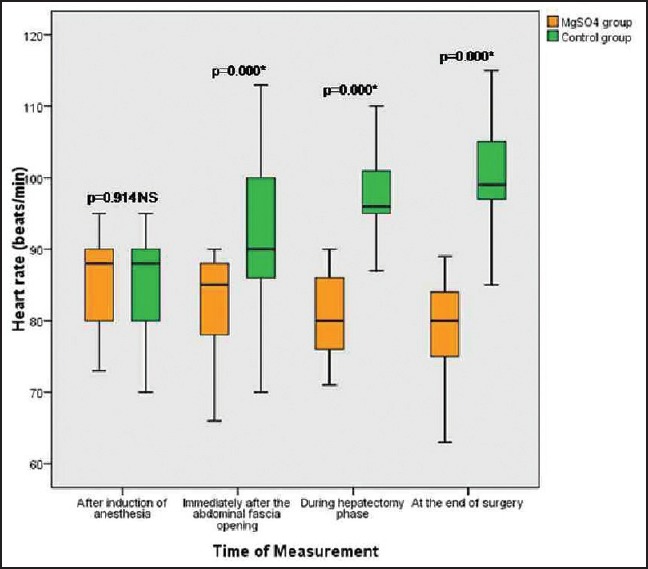
Box and whiskers graph of heart rate (beat/min) in the two studied groups, *significant P < 0.01, NS: Nonsignificant
Table 2.
COP (L/min) and corrected flow time (ms) in both groups
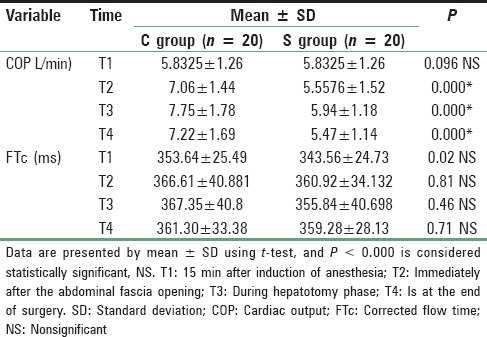
Figure 2.
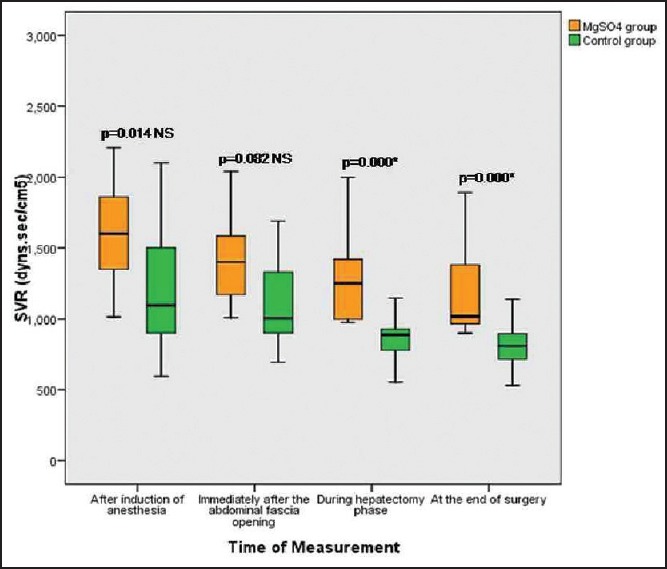
Box and whiskers graph of systemic vascular resistance (dyns.s/cm5) in the two studied groups, *significant P < 0.01, NS: Nonsignificant
Table 3.
Operative data and anesthetic requirement
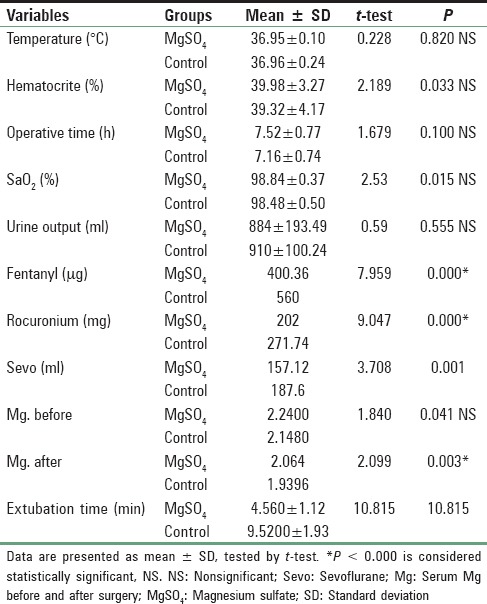
Table 4.
MAP (mmHg) and CVP (mmHg) in both groups
Mean graft weight were 874.5 ± 137 g in Mg versus 870 ± 130 g in C, respectively, P > 0.05. Mean extubation time was 4.56 ± 1.1 min for Mg versus 9.52 ± 1.93 min for C respectively, P < 0.01 [Table 3]. Postoperative patient-controlled fentanyl requirements and visual analog scale were lower with Mg versus C, 70 ± 14.1 μg/h versus 114 ± 9.09 μg/h; and 1.2 ± 0.8 versus 3.8 ± 0.9, respectively, P < 0.01.
Discussion
The safety of the donors has the highest priority when LDLT is performed.[5] Donors can be subjected to significant hemodynamic changes as reported in results of this current study, which could be attributed to the liver resection procedure itself and/or to the magnitude of the resection. Most of the donations are not routinely performed under continuous COP monitoring.[2] The minimally invasive transesophageal probe used in this current study was proposed as an alternative method to the traditional invasive pulmonary artery catheters used for COP monitoring. Hemodynamic stability can be achieved by maintaining an adequate COP and avoiding excessive bleeding. A significant increase in the HR during and after the hepatotomy phase for both groups was observed, but with least effect on the MAP. Splanchnic mediators as endotoxin, released during liver surgery, may explain these significant hemodynamic changes associated with the liver resection procedure.[2] Boermeester et al. showed that endotoxin-neutralizing proteins significantly altered hemodynamic in rats undergoing partial hepatotomy.[6] Similar hemodynamic changes were reported by El Sharkawy et al.[1] and Niemann et al.[2] Marinangeli et al. demonstrated that the HR did increase after resection and that the MAP was not significantly changed similar to our results.[7] In this current study, MgSO4 intravenous infusion was able to reduce this increase in HR. This finding could be explained by the stabilizing effect and the anti-arrthymoginic effect of magnesium. Manaa and Alhabib found similar results and concluded that MgSO4 reduced the HR and MAP.[8]
The surgical technique used during the procedure of resection, in this current study also played an important role to reduce blood loss, despite a CVP ranging between 6 and 7 mmHg. The blood loss was kept to the minimal with no blood transfusion required in any case.
The CVP readings in our study, were not in correlation with the (FTc) data of the Doppler; this could be contributed to the technical difference and anatomic variation between both sites of measurements.
Stroke volume and COP derived from TED measurements tended to increase after right hepatotomy in association with a significant reduction in SVR for both groups; these changes were significantly less in magnesium treated group. Similar changes were previously described by Niemann et al.[2] and El Sharkawy et al.[1] Marinangeli et al. also reported that the CO and CI increased after liver resection.[7] Several experimental studies had also shown that significant changes in hepatosplanchnic and systemic hemodynamics do happen after major hepatotomy, this could be due to rapid regenerative response and activity of the remnant liver parenchyma, with increased demand for oxygen consumption in relation to the extent of regeneration and consequently an increase in hepatosplanchnic blood flow, or to the significant increase in prostacyclin after hepatotomy.[9,10,11] In contrast to our current study findings, Boermeester et al. reported depressed hemodynamic parameters in rats (CO decreased 40%) after partial hepatotomy, and this might be due to species differences.[6] Nakaigawa et al. had studied the effects of bolus doses of MgSO4 on the hemodynamic state, and indicated that the depressant effect of MgSO4 on cardiac function was offset by lowering of peripheral vascular resistance, so that cardiac pump function remained effective.[11] Friedman et al. concluded that COP was lowered after usage of magnesium chloride.[12] Vicković, S concluded that MgSO4 as an adjuvant to anesthesia reduces hemodynamic changes during anesthesia.[13]
The finding in the current study, that the reduction in SVR postresection was less with the magnesium infusion may be related to the potential beneficial effects of the magnesium infusion on systemic inflammation and endothelial function.[14]
This current study also demonstrated that the general anesthetic requirements (inhalational agents, muscle relaxant, and intravenous fentanyl) monitored with Entropy, were significantly lower in the magnesium group, this might be due to the fact that magnesium is known to be a calcium channel blocker and N-Methyl-D-Aspartate (NMDA) receptor antagonist with anti-nociceptive effects. Manaa and Alhabib found similar results in their study and conclude that MgSO4 was a safe and cost-effective supplement with a general anesthetic regimen including propofol, fentanyl, and rocuronium as it reduces the total anesthetic requirements.[8] Akhtar et al. showed that that magnesium could contribute in reducing the intraoperative anesthetic requirement.[15] The extubation time was shorter in magnesium treated group than with the controls; this might be due to the less amount of muscle relaxants used with the magnesium and to the less anesthetic agents consumed. Similar results were shown by Ferasatkish et al.[16]
In contrast Ray et al. demonstrated that the intraoperative use of MgSO4 caused delayed recovery, which may be related to dosage protocol used in their study.[17]
The serum magnesium levels tend to be reduced intraoperatively in both the studied groups also reported by Yassen et al.[18] and Külpmann et al.,[19] but this reduction was less in the magnesium treated group. The use of MgSO4 in our study, reduces the postoperative analgesic requirement with a better postoperative pain score, this might be due to the competitive antagonistic effect of MgSO4 on the NMDA receptors and to the blockade of calcium channels. Pastore et al. demonstrated that the intravenous infusion of MgSO4 during spinal anesthesia improves the quality of analgesia and reduces the postoperative consumption of analgesics.[20]
Conclusion
Significant hemodynamic changes in the form of increased COP and reduced SVR were detected with the use of TED monitoring after right hepatotomy for live liver donation. These changes would not be diagnosed if TED monitoring were not in use, these changes could help in the management of any diagnosed hypotension episodes during the procedure of the liver resection. Prophylactic intraoperative magnesium infusion improved the hemodynamic changes associating the process of live donor liver right hepatotomy. Magnesium infusion managed to reduce the significant increase in HR and COP postliver resection. Magnesium infusion reduced the intraoperative inhalational agent consumption and the postoperative intravenous opioids requirements. No side effects were observed with the magnesium infusion.
This study can recommend a close hemodynamic observation and monitoring for the volunteers undergoing live liver donation for the purpose of liver transplantation to increase safety measures and ensure adequate diagnosis and management of any hemodynamic changes.
The use of TED monitoring should be encouraged as it provides valuable hemodynamic information about the volunteers particularly postresection, this information about COP and SVR is provided in a minimally invasive method.
Further studies in depth are required to investigate and monitor the types of cytokines released and its rate, as well as to study the magnesium effect on these cytokines released during the process of major liver resection.
The justification of the use of central venous catheters during this type of surgery should be further studied in view of the allegations that the pressure measured through these catheters might be unreliable due to the use of surgical retractors and manipulations of the liver during the process of resection. The use of TED probes should be looked into as an alternative and less invasive method.
The use of the FTc parameter (ms) of the TED should be investigated as an alternative for the CVP mmHg readings for guided fluid management, and protocols should be designed to use it intraoperatively. Magnesium infusion can be considered as suggested part of the intraoperative management protocol for live liver donors, but further studies on a larger scale is still required to improve the evidence-based ranking before implementing it as a routine management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.El Sharkawy OA, Refaat EK, Ibraheem AE, Mahdy WR, Fayed NA, Mourad WS, et al. Transoesophageal Doppler compared to central venous pressure for perioperative hemodynamic monitoring and fluid guidance in liver resection. Saudi J Anaesth. 2013;7:378–86. doi: 10.4103/1658-354X.121044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niemann CU, Roberts JP, Ascher NL, Yost CS. Intraoperative hemodynamics and liver function in adult-to-adult living liver donors. Liver Transpl. 2002;8:1126–32. doi: 10.1053/jlts.2002.36493. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: Randomised controlled trial. BMJ. 1997;315:909–12. doi: 10.1136/bmj.315.7113.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field A, editor. Discovering Statistics Using SPSS. 2nd ed. London, California, New Delhi: SAGE Publications Ltd; 2006. Sample Size Calculation; pp. 143–217. [Google Scholar]
- 5.Keeffe EB. Assessment of the alcoholic patient for liver transplantation: Comorbidity, outcome, and recidivism. Liver Transpl Surg. 1996;2(5 Suppl 1):12–20. [PubMed] [Google Scholar]
- 6.Boermeester MA, Houdijk AP, Straatsburg IH, van Noorden CJ, van Leeuwen PA. Organ blood flow after partial hepatectomy in rats: Modification by endotoxin-neutralizing bactericidal/permeability-increasing protein (rBPI23) J Hepatol. 1999;31:905–12. doi: 10.1016/s0168-8278(99)80293-1. [DOI] [PubMed] [Google Scholar]
- 7.Marinangeli F, Ciccozzi A, Angeletti C, Guetti C, Aloisio T, Paladini A, et al. Hemodynamic changes during hepatic vascular exclusion: Use of intraoperative transesophageal echocardiography a case series. Anesthesiology 2011. 2011:1–6. doi:10.5402/2011/278545. [Google Scholar]
- 8.Manaa EM, Alhabib AF. Effect of magnesium sulfate on the total anesthetic and analgesic requirements in neurosurgery. J Neurol Neurophysiol. 2012;S11:2–5. [Google Scholar]
- 9.Lai OF, Chow PK, Tan S, Song IC, Soo KC, Aw SE, et al. Changes in prostaglandin and nitric oxide levels in the hyperdynamic circulation following liver resection. J Gastroenterol Hepatol. 2000;15:895–901. doi: 10.1046/j.1440-1746.2000.02295.x. [DOI] [PubMed] [Google Scholar]
- 10.Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95:634–42. doi: 10.1093/bja/aei223. [DOI] [PubMed] [Google Scholar]
- 11.Nakaigawa Y, Akazawa S, Shimizu R, Ishii R, Ikeno S, Inoue S, et al. Effects of magnesium sulphate on the cardiovascular system, coronary circulation and myocardial metabolism in anaesthetized dogs. Br J Anaesth. 1997;79:363–8. doi: 10.1093/bja/79.3.363. [DOI] [PubMed] [Google Scholar]
- 12.Friedman HS, Nguyen TN, Mokraoui AM, Barbour RL, Murakawa T, Altura BM. Effects of magnesium chloride on cardiovascular hemodynamics in the neurally intact dog. J Pharmacol Exp Ther. 1987;243:126–30. [PubMed] [Google Scholar]
- 13.Vicković S, Pap D, Pjević M, Uvelin A. Magnesium sulphate as an adjuvant to anesthesia in patients with arterial hypertension. Eur J Anaesthesiol. 2013;30:25–8. doi: 10.20471/acc.2016.55.03.20. [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr. 2007;85:1068–74. doi: 10.1093/ajcn/85.4.1068. [DOI] [PubMed] [Google Scholar]
- 15.Akhtar MI, Ullah H, Hamid M. Magnesium, a drug of diverse use. J Pak Med Assoc. 2011;61:1220–5. [PubMed] [Google Scholar]
- 16.Ferasatkish R, Dabbagh A, Alavi M, Mollasadeghi G, Hydarpur E, Moghadam AA, et al. Effect of magnesium sulfate on extubation time and acute pain in coronary artery bypass surgery. Acta Anaesthesiol Scand. 2008;52:1348–52. doi: 10.1111/j.1399-6576.2008.01783.x. [DOI] [PubMed] [Google Scholar]
- 17.Ray M, Bhattacharjee DP, Hajra B, Pal R, Chatterjee N. Effect of clonidine and magnesium sulphate on anaesthetic consumption, haemodynamics and postoperative recovery: A comparative study. Indian J Anaesth. 2010;54:137–41. doi: 10.4103/0019-5049.63659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yassen K, Tamimi W, Jiffry B, Sadek A, Abdulkareem AA. Changes of serum magnesium and phosphate concentrations during and after hepatic resections for cirrhotic patients. Alex J Anaesth Intensive Care. 2014;8:85–9. [Google Scholar]
- 19.Külpmann WR, Rademacher E, Bornscheuer A. Ionized magnesium concentration during liver transplantation, resection of the liver and cardiac surgery. Scand J Clin Lab Invest Suppl. 1996;224:235–43. doi: 10.3109/00365519609088643. [DOI] [PubMed] [Google Scholar]
- 20.Pastore A, Lanna M, Lombardo N, Policastro C, Iacovazzo C. Intravenous infusion of magnesium sulphate during subarachnoid anesthesia in hip surgery and its effect on postoperative analgesia: Our experience. Transl Med UniSa. 2013;5:18–21. [PMC free article] [PubMed] [Google Scholar]


