Abstract
Background:
Ultrasound imaging is an ideal tool for stellate ganglion block (SGB) due to clarity, portability, lack of radiation, and low cost. Ultrasound guided anterior approach requires the application of pressure to the anterior neck and is associated with more risk of injury to inferior thyroid artery, vertebral artery, and esophagus. The lateral approach does not interfere with nerve or vascular structures. Blockade at the C6 vertebral level results in more successful sympathetic blockade of the head and neck with less sympathetic blockade of the upper extremity compared to sympathetic blockade at C7 vertebral level, which produces successful sympathetic blockade of upper extremity. This is helpful in patients of complex regional pain syndrome of the upper limb. Hence, we conducted a study using the lateral approach at C7 level.
Materials and Methods:
Ultrasound guided SGBs using lateral in-plane technique at C7 level were given in 20 patients suffering from chronic pain patients of upper extremity, head, and neck using 4 ml of 0.25% bupivacaine and 1 ml of 40 mg triamcinolone. The patients were assessed for a numeric pain intensity score (NPIS), the rise in axillary temperature, the range of motion of joints of upper extremity, and resolution of edema at various time intervals up to 3 months.
Results:
NPIS showed a statistically significant decrease from baseline at 30 min, which was sustained till 3rd month. The rise in axillary temperature after the block was statistically significant, which was sustained till 2nd week. The edema score decreased significantly at all-time intervals (P ≤ 0.001). The restriction of motion in all joints of upper limb decreased from 13 to 3 patients.
Conclusion:
There is a significant variation in the anatomy of stellate ganglion at the level of C6 and C7. Ultrasound guided lateral approach increases the efficacy of SGB by deposition of drug subfascially with real-time imaging.
Keywords: Complex regional pain syndrome, lateral approach, stellate ganglion block, ultrasound guidance
Introduction
Stellate ganglion block (SGB) is a common intervention in the diagnosis and management of sympathetically mediated pain and vascular insufficiency of the upper extremities, head, and neck. This block has also been used for the treatment of phantom pain, postherpetic neuralgia, cancer pain, cardiac arrhythmias, orofacial pain, and vascular headache.[1]
The stellate ganglion lies medial to the scalene muscles, lateral to longus colli muscle, esophagus, trachea, and recurrent laryngeal nerve, anterior to C7 transverse process and prevertebral fascia, superior to the subclavian artery, and posterior to vertebral vessels.[2,3] The close proximity of stellate ganglion to various critical structures can result in a number of life-threatening complications.[4] Various modalities to localize stellate ganglion have varied to the use of fluoroscopy, computerized tomography, magnetic resonance imaging, and radionucleotide tracers. However, these techniques may not be practical in clinical practice as they are time-consuming, costly, involve radiation exposure, and may cause damage to the important structures situated near the stellate ganglion.[3,5,6,7,8] The use of ultrasound improves safety of procedure by direct visualization of the related anatomical structures minimizing the risk of injury to neighboring structures. It helps in the deposition of drug subfascially with real-time imaging.[8] The two approaches by ultrasound include anterior paratracheal and lateral. In anterior approach, the transducer is placed at the level of C6 or C7 to visualize important structures and then gently pressed between the carotid artery and the trachea to retract the carotid artery laterally and to position the transducer close to longus colli, but is associated with more risk of injury to inferior thyroid artery, vertebral artery, and esophagus.[9,10]
Gofeld et al. attempted to find a pathway for needle placement, away from the vital neck structures. In the first study, identification of anatomy and simulation of injection were performed by bilateral ultrasonographic examination in 10 volunteers, at C6. After analyzing images, the anterior technique seemed suboptimal as the displacement of carotid artery and thyroid by force could produce vascular and visceral damage. Hence, a new lateral intramuscular pathways was simulated. This required experimental validation. On the basis of three-dimensional sonography and image reconstruction in 10 healthy volunteers, a lateral trajectory for needle placement was simulated. Its accuracy was checked in cadavers with methylene blue injections using 5 ml of bupivacaine and iohexol mixture in seven patients. The spread of contrast was verified fluoroscopically. They concluded that lateral approach did not interfere with nerve or vascular structure. Furthermore, the imaging analysis supported their earlier conclusion that cervical sympathetic trunk lies on the surface of longus colli muscle. The longus colli muscle is thicker than can be inferred from literature. The routine blind injection may result in intramuscular deposition of injectate. Anatomical dissection in cadavers confirmed subfascial spread of dye and staining of the sympathetic trunk between C4 and T1 levels.[9]
Hence, we planned to use a lateral approach for SGB. Studies have shown that blockade at the C6 vertebral level results in more successful sympathetic blockade to the head, and neck with less sympathetic blockade to the upper extremity compared to sympathetic blockade at C7 vertebral level, which produces successful sympathetic blockade of upper extremity.[1,7,11] This is because of the presence of Kuntz's nerves. Methylene blue spread with classical blind approach at C6 has been thoracoscopically visualized at stellate ganglion in only 45% while with same technique at C7 the dye has been seen at the ganglion in 63% of cases.[12] This promoted us to use C7 level as it would be better in cases of complex regional pain syndrome (CRPS) of upper limbs.
Materials and Methods
After approval by the Institutional Review Board, 20 patients suffering from chronic pain in upper extremity, head, and neck presenting to our pain clinic were included in the study after informed consent. Patients suffering from chronic pain in upper extremity, head, and neck due to CRPS Types I and II, phantom limb pain, brachial plexus neuritis, with numeric pain intensity score (NPIS) ≥4 who failed to respond to 4 weeks of conservative treatment with opioids; antidepressants or neuromodulator drugs were included in the study. They were excluded from the study, if they were taking medications affecting the sympathetic functions such as beta blockers had a history of upper limb cellulitis, recent myocardial infarction, glaucoma, pathological bradycardia, and deranged coagulation profile.
The patients were thoroughly assessed and investigated including coagulation profile. The severity of pain was evaluated by NPIS (0-10) where 0 stands for no pain and 10 for worst possible pain.[13] The affected limb was examined for signs of sympathetic overactivity such as hyperalgesia, allodynia, changes in skin color, texture, trophic changes in hair, nails, and sweating abnormality. Axillary temperature of the affected side was measured. Edema was scored as 0 - no edema, 1 - mild edema, 2 - severe edema with skin tight and shiny.[14] The restriction of a range of motion was assessed as 0 - no restriction of movement, 1 - restriction, but some movement present, 2 - severe restriction with no movements.[14] Pain disability index (0-70) was calculated by asking for patient's participation in seven categories of life including family responsibility, recreation, social activity, occupation, life-supporting activities, self-care, and sexual activity. Each category was scored from 0 to 10, and the index was calculated by adding them.[13]
All the blocks were performed in the operation theater with SonoSite M-Turbo using 6-13 MHz probe by the same anesthesiologist for technical accuracy. Intravenous midazolam in a dose of 0.05 mg/kg was given to all patients. The patient was placed in the supine position with neck turned to the opposite side. After asepsis of neck and transducer, it was placed on the neck to enable the cross-sectional (transverse) visualization of anatomical structures at the level C6 and C7 vertebrae. The carotid artery, internal jugular vein, vertebral artery, thyroid gland, trachea, longus colli covered with prevertebral fascia, and transverse process of C7 were visualized [Figure 1]. The color Doppler mode was used before needling to avoid penetration of vertebral artery and inferior thyroid vessels [Figure 2]. Immediately posterior to the transducer 2 ml of 2% lignocaine was infiltrated. Under real-time, a 23 gauge spinal needle was inserted using in-plane technique to reach the longus colli [Figure 3]. Once needle entered the prevertebral fascia of longus colli 1 ml of saline was injected after confirming negative aspiration for blood and cerebrospinal fluid. If the spread was appropriate, as evident by clear separation of tissue planes 4 ml 0.25% bupivacaine and 1 ml of 40 mg triamcinolone was injected. However, if the spread was observed above the fascia or within the muscle, the needle was gently repositioned. Time for the location of stellate ganglion, time to administer the block with ultrasound (time from introduction of the spinal needle to its position in the longus colli muscle) and the distance traversed by the needle and were noted.
Figure 1.
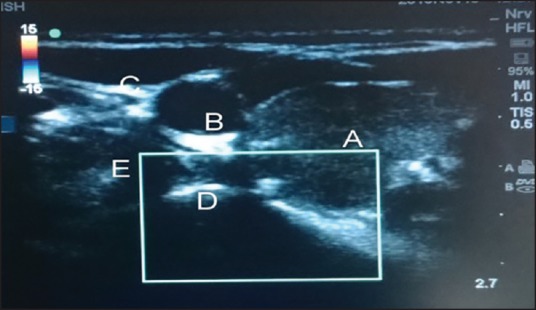
Transverse view at C7 level: (a) Thyroid gland (b) carotid artery (c) internal jugular vein (d) transverse process of C7 (e) longus colli muscle
Figure 2.
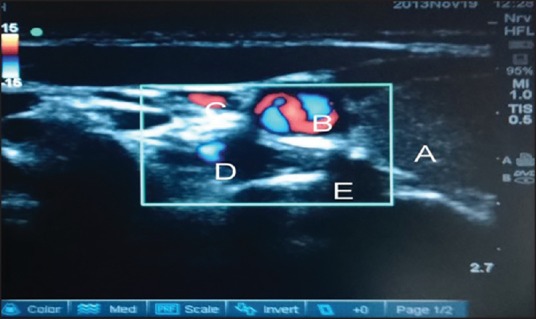
Color Doppler mode used before needling to identify major vessels. (a) Thyroid gland (b) carotid artery (c) internal jugular vein (d) vertebral artery (e) transverse process of C7
Figure 3.
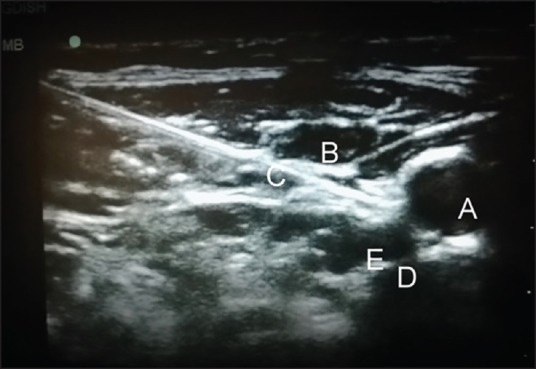
Needle path in real-time imaging (a) carotid artery (b) internal jugular vein (c) needle piercing the fascia covering longus colli (d) transverse process of C7 (e) longus colli
The time of onset of Horner's syndrome and the presence of any complications such as dysphagia, hoarseness, foreign body sensation in the throat, hematoma, weakness in limbs, and respiration insufficiency were noted. The patient's vital parameters were monitored. The patients were evaluated for rise in axillary temperature and NPIS at 30 min and then at 1st, 2nd, 4th, 8th, and 24th h. In addition, the patients were followed up for evaluation of edema, signs of sympathetic overactivity, restriction of range of motion, pain disability index, presence of paresthesias, and delayed side effects weekly up to 1 month and monthly for 3 months. Quality of block was graded as excellent (NPIS = 0), good (NPIS = 1-2), satisfactory (NPIS <3), or unsatisfactory (NPIS >3). The duration of complete pain relief (NPIS 0) and the duration of partial pain relief (defined as NPIS 3 points lower than pre-block value) were noted. Patient satisfaction score was noted at the end of the study from 0 to 10 (0 - completely dissatisfied and 10 - fully satisfied).
Statistical analysis
Statistical analysis was performed using the SPSS statistical package (version 17.0). Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as absolute numbers and percentage. Continuous variables including NPIS, restriction of range of motion, edema scores, and rise in axillary temperature values over time were analyzed using repeated measures of analysis of variance followed by Bonferroni's post hoc testing to compare the differences within different time intervals. A P < 0.05 was considered statistically significant.
Results and Observation
The demographic data including age, sex, and hand dominance is shown in Table 1. Thirteen patients had CRPS, three had phantom pain, two had thoracic postherpetic neuralgia, and two patients had brachialgia. The duration between first symptom and the first SGB administered ranged from 9 months to >2 years. Ten patients (50%) had duration of 0-6 months, seven patients (35%) had 7 months to 2 years, and three patients (15%) had duration of more than 2 years. The technical parameters during administration of the block are shown in Table 2.
Table 1.
Demographic data
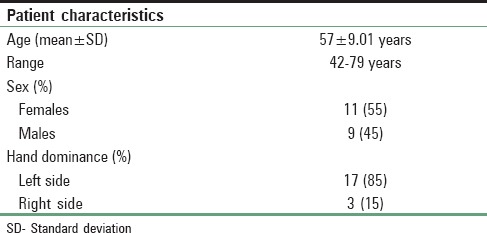
Table 2.
Technical parameters

NPIS showed a statistically significant decrease from baseline at 30 min, which was sustained till 1st week, 1st month, 2nd month, and 3rd month, respectively (P < 0.001). There was an increase in NPIS from 2nd month to 3rd month (P = 0.258) [Tables 3 and 4, Figure 4]. Complete pain relief was observed in two patients for 2 months and one patient for 1 month. These patients continued to have partial pain relief thereafter. Seventeen patients had partial pain relief throughout the study period. This could be explained as all the patients in our study were suffering from chronic pain of different etiology. The rise in axillary temperature after the block was statistically significant at 30 min, which was sustained till 2nd week. The temperature of affected extremity decreased at 3rd week, 1st month, 2nd month, and 3rd month, respectively though it was above the baseline always (P < 0.001) [Tables 3 and 4, Figure 5]. There was a significant decrease in temperature between 1st month and 3rd month (P = 0.001). Nine patients had edema prior to block. The edema score decreased significantly at all-time intervals (P ≤ 0.001) [Table 4 and Figure 6]. The number of patients having restriction of motion in all joints of upper limb decreased from 13 to 3 patients at 1st month and one patient in 2nd month, respectively. No patient had restriction at 3rd month [Table 4 and Figure 7]. Eleven patients (55%) had signs and symptoms of sympathetic overactivity, paresthesias, abnormal skin texture, hair loss, and nail dystrophy in the affected extremity. Allodynia and hyperalgesia are resolved by 1 week along with improvement in skin texture. Paresthesias resolved by 3 weeks. Partial hair growth was seen by 2nd month though no changes were observed in nails. The pain disability index showed improvement at 1st week and was sustained thereafter (P < 0.001) [Table 4].
Table 3.
Outcome measurement
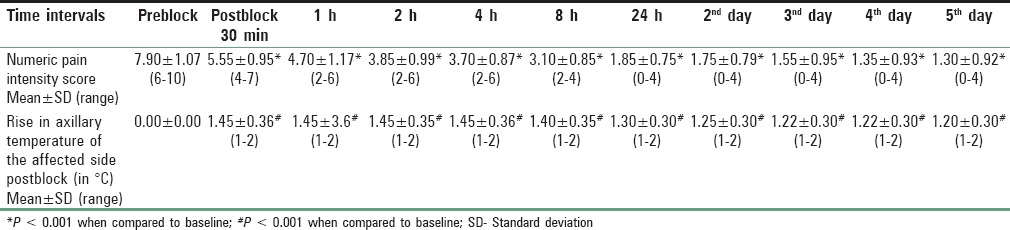
Table 4.
Outcome measurements
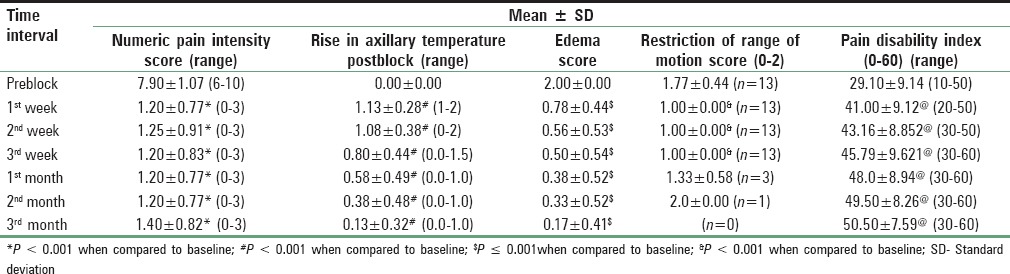
Figure 4.
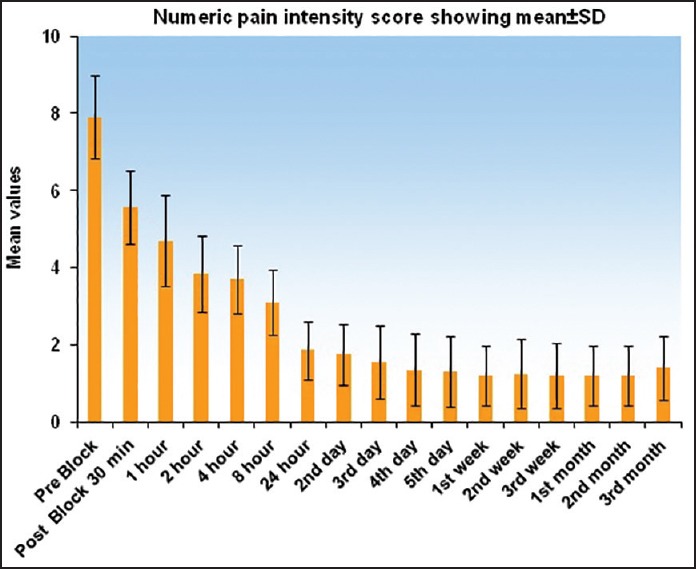
Graph showing numeric pain intensity score (0-10) at various time intervals. Graph shows decreasing trend in the score and a slight rise at 3rd month
Figure 5.
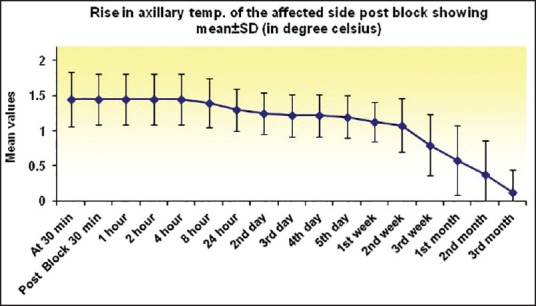
Graph showing a change in axillary temperature of affected limb at various time intervals. Sustained rise was observed up to 2nd week, and it decreased thereafter, but did not reach the baseline until 3rd month
Figure 6.
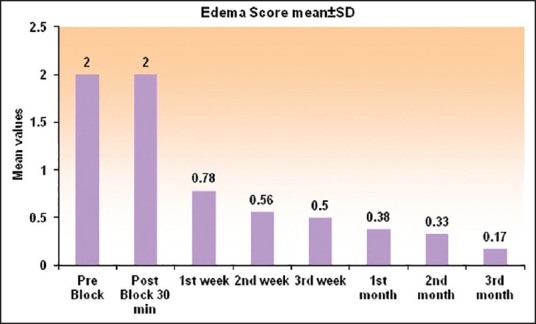
Histogram showing the edema score (0-2) at various time intervals. Score showed a sustained decrease up to 3 months
Figure 7.
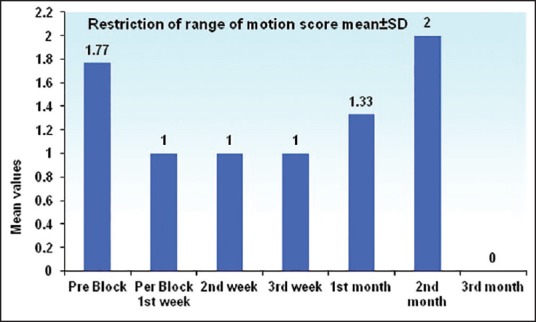
Histogram for restriction of range of motion for affected joints of upper limb at different time intervals n= 13 up to 3 weeks, n= 3 at 1st month, n= 1 at 2nd month, and n= 0 at 3rd month
Hoarseness of voice was seen in four patients. There was no delayed complication. The quality of block was reported excellent by three patients (15%) and good by 17 patients (85%). Patient satisfaction score was 9.40 ± 1.09 (range 7-10).
Discussion
CRPS is a clinical diagnosis based on the criteria, which includes hyperalgesia, sudomotor, and trophic changes. Management of CRPS involves multidisciplinary coordinated approach and aims at functional restoration. Physical therapy plays a critical role in achieving the goal and includes exercises within patient tolerance.[15] Medications and regional blocks improve patient's pain to help them in doing exercises and improving the range of motion. Sympathetic nerve blocks have been shown to be effective in improving patients tolerance.[16] Nerve injury is often accompanied by dysfunction of the peripheral sympathetic system, which causes microcirculatory disturbances.
Narouze et al. emphasized the importance of ultrasound guidance during SGB performed under fluoroscopy. Once 22 gauge needle had passed through the skin and subcutaneous tissue with fluoroscopic guidance aiming at C6 transverse process, ultrasound was used to verify needle placement. The needle was aiming at thyroid tissue anteriorly and esophagus posteriorly, which was not visible in fluoroscopy. At this point, the needle was withdrawn and reinserted, and then advanced with real-time imaging into prevertebral fascia. The use of sonography improved patient safety by avoiding soft tissue structures in needle path.[8] Kapral et al. used SGB in 12 patients by classic blind approach (8 ml of 0.25% bupivacaine) who also received the block under ultrasound guidance (5 ml of 0.25% bupivacaine) the next day. All the patients in ultrasound guided group developed more rapid onset of the block within 10 min compared with 10 in the blind group. Three patients in the blind group developed hematoma.[17]
Bhatia et al. compared out of plane anterior approach with an in-plane lateral approach for SGB. In 100 patients, USG was used to scan the regional anatomy of neck at bilateral sixth and seventh cervical vertebrae. It was found out that esophagus was lateral to the airway in 50% (48% on left and 2% on right) and 74% (72% on left and 2% on right) at C6 and C7, respectively. These patients were at risk of esophageal injury with a nonultrasound guided anterior approach. The mean distance between the lateral esophageal border and medial wall of carotid was found 9 mm. At C6 and C7 levels, 29% and 43% of subjects had blood vessels (especially thyroid vessels) susceptible to puncture within the simulated anterior needle path. The vertebral artery was entering the foramen transversum at or above C6 in 7% of subjects. They concluded that ultrasound guided lateral approach at C6 level conferred a greater margin of safety for performing SGB. A preprocedure ultrasound scan was suggested to choose the appropriate approach and needle path.[10]
The decrease in NPIS and improvement in pain disability index in our study is comparable to the study by Ackerman and Zhang. The scoring of pain disability index was done in a manner opposite to Ackerman and Zhang due to the higher grade being considered better by patients in our set up. The pain disability index improved as the patient was able to better participate in family responsibility, recreation, and social activity. Sexual activity could not be assessed in our set up as the patients were uncomfortable in disclosure. In addition, they performed laser Doppler flux difference of affected extremity.[13] The decrease in edema score, decreased restriction of motion in joints of extremity and patient satisfaction score in our study is comparable to the study by Toshniwal et al. However, the duration of pain relief was longer in their study, which could be explained due to continuous infusions for 5 days.[14]
Horner's syndrome was present in all our patients due to the injections being subfascial Shibata et al. performed subfascial injections in 26 patients and suprafascial in seven patients due to the smaller length of needles. They also noted a significant change in temperature of the ipsilateral arm compared to contralateral arm (2.14 ± 1.39°C) with subfascial injections, but not with suprafascial injection (0.67 ± 0.25°C). Hoarseness occurred in four of seven patients in the suprafascial group.[18] In our study, four patients complained of hoarseness, this could be explained by spillage of the drug to the area of recurrent laryngeal nerve. One patient in our study complained of paresthesias during the performance of block, which subsided with redirection of needle. Shankar and Simhan reported paresthesias in one patient during ultrasound guided SGB due to poor visualization of the needle tip. The paresthesia resolved after the needle was reintroduced at a different angle and was attributed to neuronal contact.[19] Cardiac arrest has been reported even with ultrasound guided SGB. The authors had given 10 ml of 0.25% bupivacaine in 2 ml increments, and the syringe was aspirated for blood each time. They attributed the cause to be injection into a vertebral artery.[20] Subsequently Narouze emphasized the importance of serpentine inferior thyroid artery while performing SGB. The artery originates from thyrocervical trunk, passes through thyroid gland then it curves medially behind the carotid artery passing from lateral to medial end at either C7 or C6 -C7 level taking serpentine course and here it can be injured by using the anterior paratracheal approach at this level. He attributed inferior thyroid vessels to be the major cause of retropharyngeal hematoma because of its vulnerable and variable anatomy. He emphasized the importance of prescan of neck in short axis view before performing the block to identify relevant anatomy, using color Doppler flow to detect any vessels and plan a safe path for needle placement.[21]
Limitation of our study included that continuous infusions of local anesthetic were not used, which would have avoided fluctuations in pain better and could have increased the duration of pain relief. There was no placebo group. Laser Doppler flow of the affected hand was not performed to assess vascular flow. Restriction of a range of motion was assessed by a subjective score rather than goniometry, which would have been more objective. We recommend future research on ultrasound guided SGB by conducting adequately powered studies involving the larger population.
Conclusion
Ultrasound guided SGB using the lateral in-plane approach at C7 level is safe, effective for management of chronic pain of upper extremity, head, and neck. The addition of steroid to the local anesthetic mixture sustained the effect up to 3 months. It provides real-time needle advancement to help the clinician to visualize the needle entry into subfascial plane helping the caudal spread of drug. It also allows direct monitoring of the spread of the local anesthetic and hence complication such as recurrent laryngeal nerve palsies, the intrathecal, epidural, or intravascular spread may be minimized, as well.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Elias M. Cervical sympathetic and stellate ganglion bocks. Pain Physician. 2000;3:294–304. [PubMed] [Google Scholar]
- 2.Raj PP. Stellate ganglion block. In: Waldman Steven D., editor. Interventional Pain Management. Philadelphia: Saunders; 1996. [Google Scholar]
- 3.Hogan QH, Erickson SJ. MR imaging of the stellate ganglion: Normal appearance. AJR Am J Roentgenol. 1992;158:655–9. doi: 10.2214/ajr.158.3.1739014. [DOI] [PubMed] [Google Scholar]
- 4.Higa K, Hirata K, Hirota K, Nitahara K, Shono S. Retropharyngeal hematoma after stellate ganglion block: Analysis of 27 patients reported in the literature. Anesthesiology. 2006;105:1238–45. doi: 10.1097/00000542-200612000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Hogan QH, Erickson SJ, Abram SE. Computerized tomography (CT) guided stellate ganglion blockade. Anesthesiology. 1992;83:424–6. doi: 10.1097/00000542-199209000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Slappendel R, Thijssen HO, Crul BJ, Merx JL. The stellate ganglion in magnetic resonance imaging: A quantification of the anatomic variability. Anesthesiology. 1995;83:424–6. doi: 10.1097/00000542-199508000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Jadon A. Revalidation of a modified and safe approach of stellate ganglion block. Indian J Anesth. 2011;55:52–6. doi: 10.4103/0019-5049.76601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narouze S, Vydyanathan A, Patel N. Ultrasound-guided stellate ganglion block successfully prevented esophageal puncture. Pain Physician. 2007;10:747–52. [PubMed] [Google Scholar]
- 9.Gofeld M, Bhatia A, Abbas S, Ganapathy S, Johnson M. Development and validation of a new technique for ultrasound-guided stellate ganglion block. Reg Anesth Pain Med. 2009;34:475–9. doi: 10.1097/AAP.0b013e3181b494de. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia A, Flamer D, Peng PW. Evaluation of sonoanatomy relevant to performing stellate ganglion blocks using anterior and lateral simulated approaches: An observational study. Can J Anesth. 2012;59:1040–7. doi: 10.1007/s12630-012-9779-4. [DOI] [PubMed] [Google Scholar]
- 11.Harano K. Difference of spread of injectate solutions after stellate ganglion block to sixth and seventh vertebral levels. Anesthesiolgy. 1998;15:871–7. [Google Scholar]
- 12.Feigl GC, Rosmarin W, Stelzl A, Weninger B, Likar R. Comparison of different injectate volumes for stellate ganglion block: An anatomic and radiologic study. Reg Anesth Pain Med. 2007;32:203–8. doi: 10.1016/j.rapm.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Ackerman WE, Zhang JM. Efficacy of stellate ganglion blockade for the management of type 1 complex regional pain syndrome. South Med J. 2006;99:1084–8. doi: 10.1097/01.smj.0000233257.76957.b2. [DOI] [PubMed] [Google Scholar]
- 14.Toshniwal G, Sunder R, Thomas R, Dureja GP. Management of complex regional pain syndrome type I in upper extremity-evaluation of continuous stellate ganglion block and continuous infraclavicular brachial plexus block: A pilot study. Pain Med. 2012;13:96–106. doi: 10.1111/j.1526-4637.2011.01285.x. [DOI] [PubMed] [Google Scholar]
- 15.Kurvers HA, Jacobs MJ, Beuk RJ, Van den Wildenberg FA, Kitslaar PJ, Slaaf DW, et al. Reflex sympathetic dystrophy: Evolution of microcirculatory disturbances in time. Pain. 1995;60:333–40. doi: 10.1016/0304-3959(94)00133-y. [DOI] [PubMed] [Google Scholar]
- 16.Cepeda MS, Lau J, Carr DB. Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: A narrative and systematic review. Clin J Pain. 2002;18:216–33. doi: 10.1097/00002508-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Kapral S, Krafft P, Gosch M, Fleischmann D, Weinstabl C. Ultrasound imaging for stellate ganglion block: Direct visualization of puncture site and local anesthetic spread. A pilot study. Reg Anesth. 1995;20:323–8. [PubMed] [Google Scholar]
- 18.Shibata Y, Fujiwara Y, Komatsu T. A new approach of ultrasound-guided stellate ganglion block. Anesth Analg. 2007;105:550–1. doi: 10.1213/01.ane.0000265691.02963.a4. [DOI] [PubMed] [Google Scholar]
- 19.Shankar H, Simhan S. Transient neuronal injury followed by intravascular injection during an ultrasound guided stellate ganglion block. Anesth Pain Med. 2013;2:134–7. doi: 10.5812/aapm.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rastogi S, Tripathi S. Cardiac arrest following stellate ganglion block performed under ultrasound guidance. Anesthesia. 2010;65:1042. doi: 10.1111/j.1365-2044.2010.06487.x. [DOI] [PubMed] [Google Scholar]
- 21.Narouze S. Beware of the “serpentine” inferior thyroid artery while performing stellate ganglion block. Anesth Analg. 2009;109:289–90. doi: 10.1213/ane.0b013e3181a20197. [DOI] [PubMed] [Google Scholar]


