Abstract
Chronic infection with the bacterial Helicobacter pylori is a major cause of gastric and duodenal ulcer disease, gastric mucosal atrophy, and cancer. H. pylori–induced expression of the intestinal epithelial–specific transcription factor caudal-related homeobox 2 (Cdx2) contributes to intestinal metaplasia, a precursor event to gastric cancer. Given a role for the bacterial pattern recognition molecule nucleotide-binding oligomerization domain 1 (NOD1) in the innate immune response to bacterial infection, we investigated mechanisms used by NOD1 to regulate H. pylori infection and its propensity towards the development of intestinal metaplasia. We found that Cdx2 was induced by H. pylori infection in both normal and neoplastic gastric epithelial cells in a manner that was inversely related to NOD1 signaling. Mechanistic investigations revealed that Cdx2 induction relied upon activation of NF-κB but was suppressed by NOD1-mediated activation of TRAF3, a negative regulator of NF-κB. In vivo, prolonged infection of NOD1-deficient mice with H. pylori led to increased Cdx2 expression and intestinal metaplasia. Furthermore, gastric epithelial cells from these mice exhibited increased nuclear expression of the NF-κB p65 subunit and decreased expression of TRAF3. Overall, our findings illuminated a role for NOD1 signaling in attenuating H. pylori–induced Cdx2 expression in gastric epithelial cells, suggesting a rationale to augment NOD1 signaling in H. pylori–infected patients to limit their risks of accumulating precancerous gastric lesions.
Introduction
Chronic gastric infection with Helicobacter pylori (H. pylori) can cause intestinal metaplasia, a pathologic change that is frequently a precursor of gastric cancer (1). Intestinal metaplasia is, at least in part, caused by H. pylori–induced expression of caudal-related homeobox 2 (Cdx2), a protein involved in the normal differentiation of the intestinal epithelium, which in this context causes aberrant intestinalization of the gastric epithelium (2–4). Although Cdx2 acts as a tumor suppressor in the small and large intestine, its aberrant expression in the gastric mucosa is considered to be pro-oncogenic. This is supported by recent studies showing that Cdx2 provides “lineage-specific” survival for already deregulated cancer cells via its capacity to activate the Wnt/catenin signaling pathway (5).
Nucleotide-binding oligomerization domain 1 (NOD1) is an intracellular component of the innate immune system, which is highly expressed in epithelial cells and is activated by a peptide derived from peptidoglycan (PGN), a component of the bacterial wall injected into the H. pylori–infected cell by type IV secretion system (6). It has been demonstrated in studies of H. pylori infection in NOD1-deficient mice that absence of NOD1 results in greatly enhanced bacterial burden (7, 8), and polymorphisms in the NOD1 gene correlate with H. pylori infection–related diseases (9, 10). However, the role of NOD1 in the regulation of H. pylori–induced gastric intestinal metaplasia is unknown.
Materials and Methods
Animals
NOD1-deficient mice were obtained as previously described (8). C57BL/6 (NOD1-intact) mice were purchased from CLEA Japan. Both NOD1-deficient and NOD1-intact mice were reared in the same animal facility in Tohoku University (Sendai, Miyagi, Japan). Mice were handled according to the Regulations for Animal Experiments and Related Activities at Tohoku University.
Cell lines
Human gastric cancer–derived epithelial cell lines AGS and KATOIII were purchased from ATCC, where the cell lines had been authenticated by short tandem repeat profiling. Human cancer–derived epithelial cell lines GCIY and NUGC-4 were obtained from Tohoku University Cell Bank, where the cell lines had been authenticated by short tandem repeat analysis. The normal murine gastric epithelial cell line GSM06 was obtained from Riken Cell Bank, where the cell line had been authenticated by simple sequence length polymorphism analysis. Cells were maintained according to the manufacturers’ protocols, and the cell lines were carefully checked for morphologic consistency by microscope. In addition, cultures of the cell lines were checked for mycoplasma contamination using Cycleave PCR Mycoplasma Detection Kit (TaKaRa).
In vitro H. pylori infection experiments
GCIY, AGS, and GSM06 were infected with 5 × 107 CFU/mL cytotoxin-associated gene pathogenicity island (cagPAI)-positive H. pylori (strain 43504, ATCC), which corresponds to 50 multiplicity of infection (MOI). In the indicated experiment, GCIY cells were infected with 50 MOI cagPAI− H. pylori (Microbiological Research Institute, Tokushima, Japan). Total RNA was collected using TRIzol reagent (Invitrogen) and subjected to RT-PCR using Super Script III First-Strand Synthesis System (Invitrogen). The synthesized cDNA was subjected to semiquantitative PCR for Cdx2, MUC2, and GAPDH as previously described (11). Similarly, total protein was collected from the infected and noninfected cells and was subjected to Western blotting as previously described (8). In the indicated experiments, the cells were treated with NF-κB inhibitor BAY11-7082 (Calbiochem) or were transfected with NOD1-siRNA (Dharmacon) or TRAF3-expressing plasmid (InvivoGen) or incubated with 100 ng/mL iE-DAP (Invivo-Gen) prior to infection.
Luciferase assay
Transcription factor–binding site in the 5′-promoter region of human Cdx2 was analyzed using MatInspector software (Genomatix). Next, the 5′-promoter region of Cdx2 was obtained by PCR and ligated into Luciferase Reporter Vector-pGL3 (Pro-mega) using DNA Ligation Kit (Takara). The latter was transfected into GCIY cells together with pRL-TK plasmid (Promega) using Trans-IT-LT1 Transfection Reagent (Mirus). Transfected cells were infected with H. pylori, and the relative luciferase activity was measured using Dual-Luciferase Reporter Assay System (Pro-mega). In the indicated experiments, pNF-κB-Luc reporter plas-mid (Clontech) was transfected into the cells with TRAF3-expressing plasmid (InvivoGen).
Electrophoretic mobility shift assay
Nuclear extracts were obtained from H. pylori–infected and uninfected GCIY cells and incubated with appropriate oligonucleotides representing the NF-κB–binding site in the Cdx2 promoter. Electrophoretic mobility shift assay (EMSA) was performed using Gel-Shift Kit from Panomics.
Establishment of NOD1 knockdown stable cell line
GSM06 cells were transfected with mouse NOD1-shRNA vector (OriGene), using a 4D-Nucleofector System (Lonza). A stable NOD1-deficient clone was established by selection with puromycin (Thermo Scientific). Vector with noneffective scrambled shRNA (OriGene) was used as control.
qPCR
Total RNA was extracted from gastric epithelial cells and murine gastric mucosa, and the synthesized cDNA was subjected to qPCR (StepOnePlus, Applied Biosystems) for NOD1, Cdx2, MUC2, trefoil factor 2 (TFF2), TFF3, TRAF3, TNFα, and β-actin. The relative amount of gene expression was normalized using β-actin mRNA and shown in arbitrary units. Each sample was performed in triplicate.
In vivo H. pylori infection
Five 8-week-old male NOD1-intact and NOD1-deficient mice were anesthetized and orally infected with 2.5 × 108 CFU cagPAI+ H. pylori (strain 43504, ATCC: previously reported to colonize mice; refs. 12–14) on days 0, 2, and 4 as described previously (8). Five NOD1-intact and five NOD1-deficient mice without oral H. pylori infection were also used as uninfected controls. After confirming the colonization of H. pylori in the stomach by quantitative culture on Columbia HP agar plates (Becton Dickinson) at 5 weeks, the infected mice were maintained under normal housing conditions until sacrifice at 12 months after oral H. pylori administration. At that point, the chronic infection of mice was confirmed by quantitative culture, and their gastric mucosae were processed for hematoxylin–eosin (H&E) staining to detect inflammatory cell infiltration and histologic changes associated with intestinal metaplasia. Two NOD1-deficient and two NOD1-intact mice were sacrificed at 8 months after infection to evaluate the histologic changes in their stomachs at an earlier time point. Inflammation was scored using the criteria developed by Rogers and colleagues (15). Intestinal metaplasia was evaluated by the ratio of metaplastic glands to total glands. In addition, H. pylori colonization was evaluated by quantitative culture as previously described (8). Immunohistochemical examination of gastric tissues was evaluated using antibodies to detect Cdx2 (BioGenex) and NF-κB subunit (p65; Cell Signaling Technology), while total RNA was extracted for qPCR analysis as described above. The in vivo infection experiment was repeated five times.
Statistical analysis
Student t test was used to evaluate the significance of the differences between two groups. In the case where multiple samples were compared, Dunnett test was performed to evaluate the significance of the differences. A value of P < 0.05 was considered as statistically significant.
Results
H. pylori infection of gastric epithelial cell lines induces/ enhances Cdx2 expression
The occurrence of gastric intestinal metaplasia during H. pylori infection has been reported to be dependent on induction of Cdx2 expression in gastric epithelial cells (3). Thus, in initial studies, we evaluated Cdx2 expression and other epithelial cell differentiation markers in human gastric cancer cell lines before and after infection with H. pylori. Among the evaluated cell lines, uninfected GCIY cells expressed gastric-type mucins, mucin 1 (MUC1) and MUC5AC, but not an intestinal-type mucin MUC2; more importantly, they expressed very low level of Cdx2 (Supplementary Fig. S1). This expression pattern indicated that, with respect to intestinal metaplasia, uninfected GCIY cells are similar to epithelial cells of the gastric mucosa not infected by H. pylori (3, 16). In contrast, AGS, NUGC-4, and KATOIII cells expressed Cdx2 and MUC2 prior to infection with H. pylori.
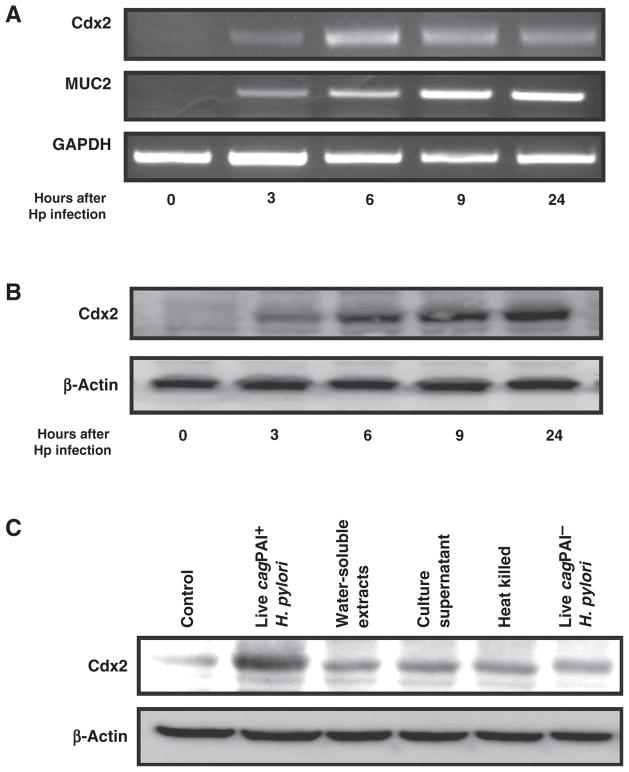
With this information in hand, we next addressed whether H. pylori infection induces increased Cdx2 expression in cell lines with and without baseline Cdx2 expression. Accordingly, the induction of Cdx2 was evaluated both in cultures of GCIY and AGS cells infected with cagPAI+ H. pylori. The expression of Cdx2 mRNA (as well as MUC2 mRNA) was induced in GCIY cells at 3 hours after H. pylori infection (Fig. 1A); in addition, Cdx2 protein was expressed at this time point and then increased progressively over 24 hours (Fig. 1B). In parallel studies, the preexisting level of Cdx2 present in AGS cells increased progressively during 24 hours of infection (Supplementary Fig. S2). In studies of both cell lines, visual assessments of Cdx2 increases were confirmed by densitometry. These results indicated that in vitro H. pylori infection results in the increased expression of Cdx2 in epithelial cell lines, regardless of preexistent Cdx2 expression levels.
Figure 1.
cagPAI+ H. pylori induces Cdx2 in human gastric epithelial cells. A, total RNA extracted from GCIY cells infected with 5 × 107 CFU/mL cagPAI+ H. pylori (Hp) were subjected to RT-PCR. B and C, cell lysates were extracted from H. pylori (Hp)-infected GCIY cells (B) and GCIY cells stimulated with the indicated H. pylori-related products (C), and were subjected to Western blotting.
cagPAI is an important H. pylori virulence factor that, in epidemiologic studies, has been shown to confer a higher risk for the occurrence of infection-associated gastric carcinoma (17, 18). Furthermore, its injection into gastric epithelial cells via a type IV secretion system results in the entry of cytotoxin-associated gene A (CagA) and PGN, the source of NOD1 ligand, and thus, the activation of signaling pathways relevant to Cdx2 expression (19).
We therefore investigated whether Cdx2 induction by H. pylori infection requires bacteria expressing cagPAI. Although cagPAI− H. pylori, heat-killed H. pylori, and culture supernatants of infected cells induced Cdx2 expression to some extent, optimal induction of Cdx2 expression required live cagPAI+ H. pylori (Fig. 1C). These results, thus, show that intracellular entry of H. pylori components are necessary for maximal H. pylori induction of Cdx2 expression in an epithelial cell line.
H. pylori infection–induced Cdx2 expression is dependent on NF-κB
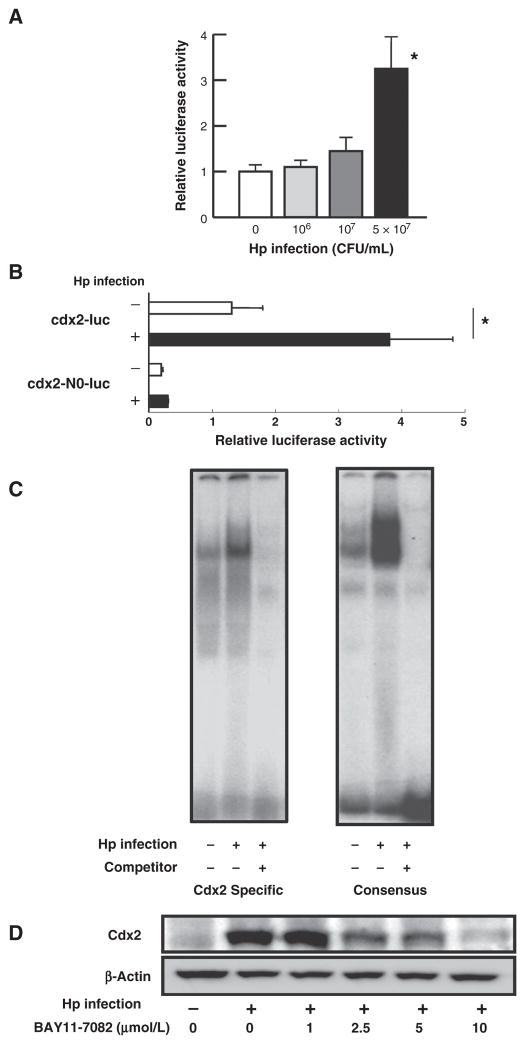
H. pylori infection of epithelial cells has been shown to result in NF-κB activation, and the analysis of the 5′-promoter region of Cdx2 showed that it contains an NF-κB–binding site (20, 21). We therefore investigated if H. pylori induction of Cdx2 expression is dependent on NF-κB signaling. In initial studies, we transfected GCIY cells with a Cdx2 promoter–reporter plasmid (Supplementary Fig. S3), containing a promoter construct with an intact NF-κB–binding site (cdx2-luc) or a construct not containing this binding site (cdx2-N0-luc) and then subjected the cells to H. pylori infection. Cells expressing cdx2-luc, but not cdx2-N0-luc, exhibited enhanced promoter activity upon infection with H. pylori (Fig. 2A and B). These data thus provide initial evidence that NF-κB activation is involved in the induction of Cdx2 expression upon H. pylori infection.
Figure 2.
H. pylori (Hp) induction of Cdx2 is dependent on NF-κB. A and B, GCIY cells transfected with cdx2-luc or cdx2-N0-luc plasmids with control pRL-TK plasmid were infected with the indicated amount (or 5 ×107 CFU/mL in B) of H. pylori; cell lysates were assessed for luciferase activity. C, nuclear extracts of H. pylori–infected GCIY cells were subjected to EMSA. D, GCIY cells were infected with 5 × 107 CFU/mL H. pylori in the absence or presence of BAY11-7082; cell lysates were subjected to Western blotting. Results shown in A and B indicate means ± SD. *, P < 0.05 as compared with uninfected cells.
In further studies, we sought to confirm the role of NF-κB activation in Cdx2 induction. First, we subjected nuclear extracts of cells obtained from H. pylori–infected or uninfected GCIY cell cultures to EMSA using 32P-labeled oligonucleotides that contain either NF-κB consensus–binding sequences or the NF-κB–binding sequence present in the Cdx2 promoter. Enhanced binding of nuclear extracts to NF-κB–binding sequences in the Cdx2 promoter was seen in cells derived from H. pylori–infected cultures, but not in those derived from uninfected cultures (Fig. 2C). Second, we assessed the effect of BAY11-7082, an NF-κB inhibitor on Cdx2 expression, in H. pylori–infected cells. BAY11-7082 inhibited Cdx2 induction in cells from such cultures in a dose-dependent manner (Fig. 2D) that paralleled its ability to inhibit NF-κB expression in H. pylori–infected cells (Supplementary Fig. S4). Together, these studies buttress the idea that Cdx2 expression induced by H. pylori infection does in fact depend, at least in part, on NF-κB activation.
NOD1 signaling has an inhibitory effect on Cdx2 induction by H. pylori
Activation of NOD1 plays an important host defense role in acute gastric infection with H. pylori by inducing the expression of bactericidal mediators (7, 8, 22). It was therefore possible that NOD1 signaling also regulates H. pylori induction of Cdx2. To examine this question, we conducted a series of studies addressing the role of NOD1 signaling on H. pylori induction of Cdx2 mainly in AGS cells, i.e., cells that had previously been shown to support a full range of NOD1 signaling effects (8).
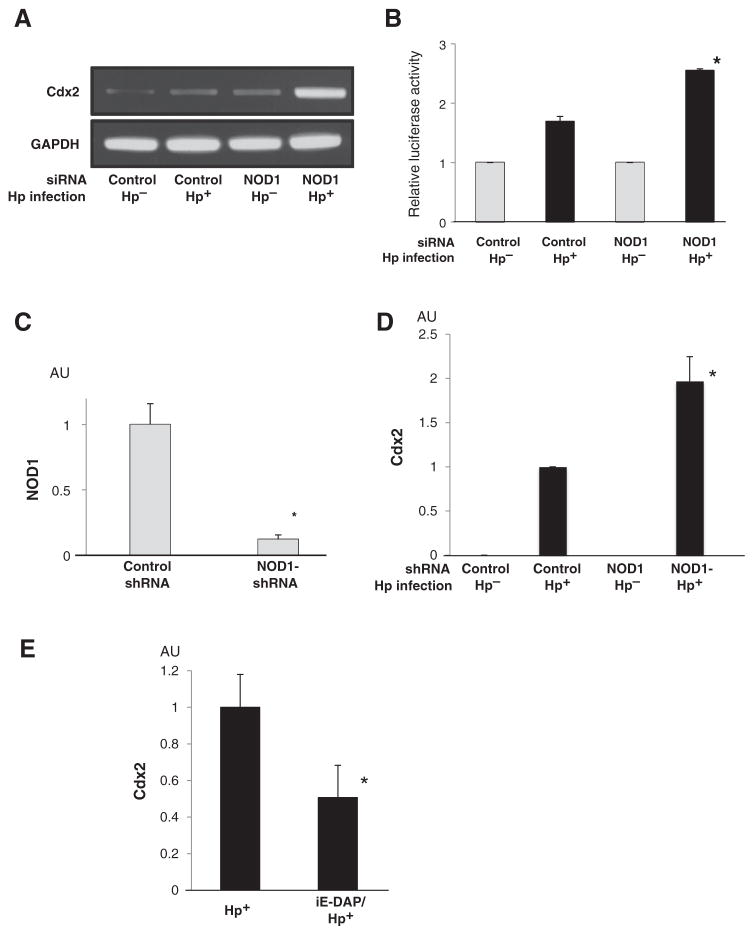
In initial studies, we evaluated Cdx2 expression in H. pylori–infected AGS cells transfected with NOD1-specific siRNA, previously evaluated for its capacity to substantially inhibit NOD1 expression (Supplementary Fig. S5). We found that AGS cells transfected with NOD1-siRNA exhibited greatly enhanced H. pylori–induced Cdx2 expression compared with cells transfected with control siRNA (Fig. 3A). In addition, NOD1-siRNA significantly enhanced H. pylori–induced Cdx2 promoter activity (Fig. 3B). These results suggested that NOD1 has an inhibitory effect on H. pylori–induced Cdx2 expression.
Figure 3.
NOD1 suppresses Cdx2 expression induced by H. pylori (Hp) infection. A, total RNA extracted from AGS cells transfected with the indicated siRNA and infected with H. pylori was subjected to RT-PCR. B, AGS cells were cotransfected with the indicated siRNA, cdx2-luc reporter vector, and pRL-TK plasmid; cell lysates were subjected to luciferase assay. C, total RNA extracted from GSM06 cell stably expressing either control shRNA or NOD1-shRNA was subjected to qPCR. D, GSM06 cells stably expressing NOD1-shRNA were infected with 5 × 107 CFU/mL H. pylori. Total RNA was extracted and subjected to qPCR. E, GCIY cells were infected with H. pylori with or without preincubation with iE-DAP. Total RNA was extracted and subjected to qPCR. Results shown in B–E indicated as means ± SD. *, P < 0.05 as compared with H. pylori-infected cells transfected with control siRNA (B), control shRNA transfected cells (C and D), and non-pretreated cells (E). AU, arbitrary units.
In complementary studies, we determined whether the above findings obtained with AGS cells (a human gastric cancer cell line) were also observed with GSM06 cells, a normal murine gastric epithelial cell line, which expresses neither MUC2 nor Cdx2 prior to H. pylori infection (Supplementary Fig. S6). In preliminary studies, we established that GSM06 cells, stably expressing NOD1-shRNA, exhibit an 88% reduction in NOD1 mRNA expression compared with GSM06 cells expressing control shRNA (Fig. 3C). We then infected the shRNA-transfected cells with H. pylori and assessed their expression of Cdx2. GSM06 cells exhibited rapid upregulation of Cdx2 upon infection with H.pylori, indicating that such upregulation occurs in normal epithelial cells as well as gastric cancer cell lines (Fig. 3D). In addition, GSM06 cells expressing NOD1-shRNA exhibited significantly higher expression of Cdx2 compared with the cell line expressing control shRNA. This result showed that NOD1 inhibition of Cdx2 upregulation during H. pylori infection is also observed in normal gastric epithelial cells and thus reinforced our findings obtained with gastric cancer cell lines.
The above results, showing that the downregulation of NOD1 signaling enhances H. pylori induction of Cdx2, suggested that the stimulation of cells with NOD1 ligand during H. pylori infection might downregulate Cdx2 expression induced by H. pylori infection. In studies designed to address this possibility, we assessed the effect of NOD1 ligand stimulation on Cdx2 expression induced in cultures of H. pylori–infected gastric epithelial cells. Preincubation of GCIY cells with NOD1 ligand (iE-DAP) led to greatly reduced Cdx2 expression in H. pylori–infected cells (Fig. 3E). Similarly, preincubation of AGS cells with iE-DAP exhibited a lesser but, nevertheless, significant reduction in H. pylori–induced Cdx2 expression (Supplementary Fig. S7). In studies of either type of cells, stimulation with iE-DAP alone had no effect on Cdx2 expression (Supplementary Fig. S8). These studies thus reinforce the idea that NOD1 signaling has a negative effect on H. pylori–induced Cdx2 expression.
TRAF3, a downstream signaling molecule induced by NOD1, downregulates Cdx2
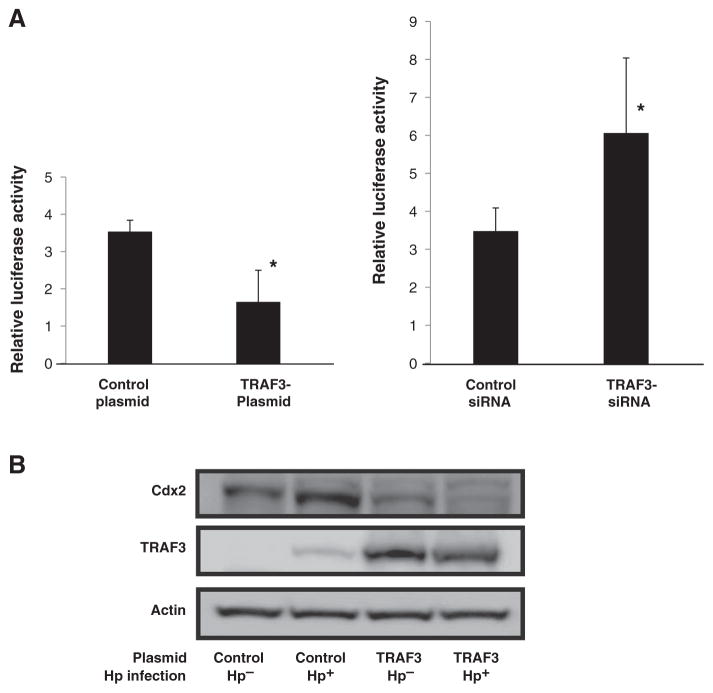
In previous studies, we showed that in epithelial cells, RICK, activated by NOD1 stimulation, binds to TRAF3 and thus sets in motion a signaling pathway that leads to the production of type I IFN (8). In addition, we showed that downregulation of TRAF3 in NOD1 ligand–stimulated cells leads to enhanced NF-κB reporter activity. Thus, as had been noted previously in other experimental systems (23), increased TRAF3 expression suppresses the NF-κB signaling pathway. Since, as shown above, Cdx2 expression is under the control of NF-κB, these previous studies suggested that NOD1 might be regulating Cdx2 via its effect on TRAF3. To examine this possibility, we first determined the effect of TRAF3 regulation on NF-κB reporter activity in cultures of AGS cells infected with H. pylori. Cotransfection of an NF-κB reporter plasmid with a TRAF3 expression plasmid led to a significant reduction in reporter activation, whereas, in contrast, cotransfection of the same reporter plasmid with TRAF3-siRNA led to enhanced reporter activation (Fig. 4A). Thus, TRAF3 expression does indeed downregulate NF-κB activation in H. pylori–infected cells. We next determined the effect of TRAF3 overexpression on the induction of Cdx2 in cultures of AGS cells infected with H. pylori. The level of Cdx2 mRNA was upregulated by H. pylori infection, as noted previously, and such enhancement was accompanied by low-level expression of TRAF3 (Fig. 4B). However, Cdx2 expression was greatly downregulated in cells transfected with a TRAF3-expressing plasmid. Interestingly, such downregulation was particularly evident in H. pylori–infected cells, possibly reflecting the fact that in such cells, the transfected TRAF3 was being more efficiently activated by the increased NOD1 signaling resulting from H. pylori infection. In any case, these results suggested that TRAF3, a downstream signaling molecule of NOD1, could negatively regulate Cdx2 expression induced by H. pylori.
Figure 4.
TRAF3 suppresses NF-κB activation and Cdx2 expression. A, AGS cells transfected with an NF-κB reporter plasmid together with pRL-TK plasmid and cotransfected with either a TRAF3 expression plasmid or TRAF3-siRNA were infected with H. pylori (Hp), and relative luciferase activity was measured. Results, means ± SD. *, P < 0.05 as compared with control plasmid or control siRNA–transfected cells. B, AGS cells transfected with either a control plasmid or a TRAF3 expression plasmid were cultured in either H. pylori–infected or uninfected media. After 24 hours, cell lysates were obtained and subjected to Western blotting.
Effect of NOD1 signaling on the occurrence of intestinal metaplasia and the expression of Cdx2 during in vivo H. pylori infection
In a final series of studies, we determined whether the NOD1-mediated negative regulation of Cdx2 expression, observed in the above in vitro studies, was also manifested in physiologic gastric epithelial cells in vivo. To this end, we orally infected NOD1-intact and NOD1-deficient mice with cagPAI+ H. pylori and then, 8 or 12 months after infection, i.e., after prolonged H. pylori infection, determined the occurrence of intestinal metaplasia and the expression of Cdx2 in the gastric epithelium.
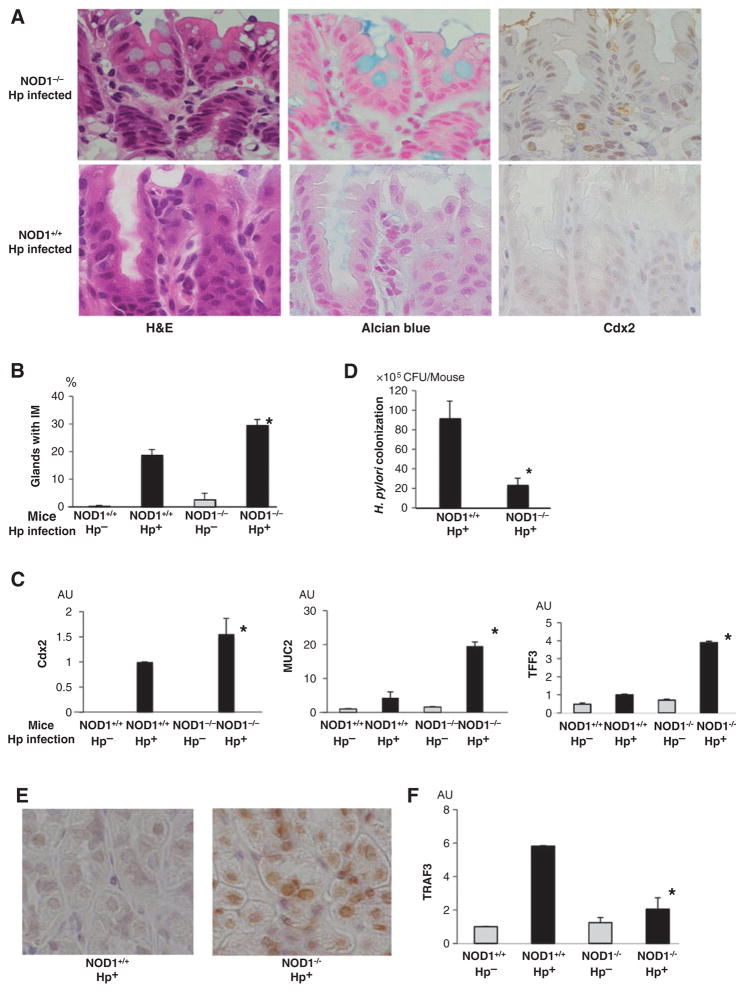
As indicated previously by Goldenring and Nomura, intestinal metaplasia in mice is best identified by both morphologic criteria, i.e., the presence of goblet cells (containing mucus that can be stained by Alcian blue) in the gastric mucosa and the presence of various markers previously shown to be associated with this morphologic change (24). Accordingly, we first determined goblet cell frequencies in the gastric tissues obtained from mice infected with H. pylori for 12 months. We found that goblet cells were more frequently seen in tissue from NOD1-deficient mice than in that of NOD1-intact mice, as assessed by H&E-stained tissue and by Alcian blue–stained tissue (Fig. 5A and B). Similar histologic changes were observed in mice infected with H. pylori for 8 months (data not shown).
Figure 5.
NOD1 deficiency enhances the formation of gastric intestinal metaplasia during in vivo H. pylori (Hp) infection. A, H&E staining, Alcian blue staining, and immunohistochemistry for Cdx2 of gastric mucosa removed from H. pylori–infected NOD1-deficient and NOD1-intact mice. B, percentage of glands exhibiting intestinal metaplasia (IM) in the stomachs of NOD1-intact and NOD1-deficient mice. C, total RNA extracted from the stomachs of these mice was subjected to qPCR. D, loads of H. pylori in the stomachs of NOD1-intact and NOD1-deficient mice 12 months after initiation of H. pylori infection. E, gastric expression of NF-κB p65 in the stomach of NOD1-intact and NOD1-deficient mice. F, total RNA extracted from the stomachs of chronically infected mice was subjected to qPCR. Results shown in B, C, D, and F indicate means ± SD.*, P < 0.05 as compared with H. pylori–infected NOD1-intact mice. AU, arbitrary units.
Also pursuing the Goldenring/Nomura criteria for the identification of intestinal metaplasia, we next assessed Cdx2 expression in tissues derived from mice after 12 months of H. pylori infection. Both immunohistologic analysis and qPCR studies disclosed that NOD1-deficient tissue exhibited higher levels of Cdx2 than NOD1-intact tissue (Fig. 5A and C). In addition, mRNA levels of MUC2 and TFF3 [other epithelial cell markers of intestinal metaplasia (25)] were significantly higher in gastric tissues from NOD1-deficient mice as compared with NOD1-intact mice. In contrast to these findings in infected mice, Cdx2 expression evaluated by immunohistologic analysis was barely seen in tissues from either NOD1-intact or NOD1-deficient mice without H. pylori infection (data not shown).
The above morphologic and marker studies showed that prolonged H. pylori infection is associated with a greater level of intestinal metaplasia in NOD1-deficient mice than in NOD1-intact mice. Two additional observations relating to this conclusion suggest that these differences were not simply due to the severity of the H. pylori infection in these mice. First, the lamina propria of NOD1-deficient mice and NOD1-intact mice with prolonged H. pylori infection displayed equal levels of inflammation, suggesting that increased intestinal metaplasia expression and higher Cdx2 levels in NOD1-deficient mice were not due to the possibility that these mice have a more severe chronic gastric inflammation (Supplementary Fig. S9). This was supported by the fact that H. pylori–infected NOD1-intact and NOD1-deficient mice expressed equally increased levels of TNFα compared with the uninfected mice (Supplementary Fig. S10). Second, quantification of bacteria in gastric tissues disclosed that after prolonged H. pylori infection, NOD1-deficient mice displayed a substantially lower H. pylori bacterial load than NOD1-intact mice with prolonged infection (Fig. 5D). This observation is consistent with previous studies that have shown that gastric tissue that displays extensive intestinal metaplasia is less able to support H. pylori colonization (26). Taken together, these observations support the notion that the more severe intestinal metaplasia and increased Cdx2 in NOD1-deficient mice were not simply due to more intense chronic infection and inflammation.
Signaling pathway activation in NOD1-intact and NOD1-deficient tissue from mice with prolonged H. pylori infection
The above in vitro studies showed that epithelial cells with reduced NOD1 levels exhibit increased NF-κB activation, and this accounts, at least in part, for the increased expression of Cdx2. To determine if a similar phenomenon occurs in vivo, we immunostained the gastric tissue obtained from mice after prolonged H. pylori infection for NF-κB. Immunostaining of NF-κB p65 disclosed strikingly increased staining in epithelial cell nuclei of NOD1-deficient tissue as compared with NOD1-intact tissue (Fig. 5E), indicating that during H. pylori infection, NOD1 inhibits NF-κB expression in vivo as well as in vitro.
In parallel studies, we determined tissue expression of TRAF3, the NOD1 signaling component shown above, to inhibit NF-κB activation and to downregulate Cdx2 in overexpression studies. TRAF3 mRNA levels were more than 3-fold higher in the tissue of NOD1-intact mice as compared with that of NOD1-deficient (Fig. 5F).
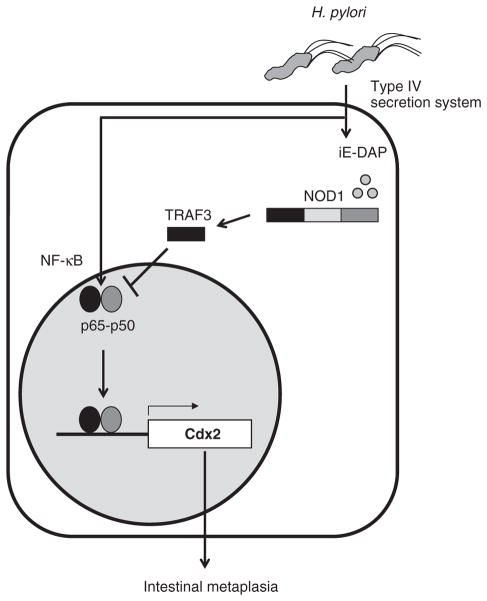
These findings are compatible with the results of in vitro studies of epithelial cells discussed above in that they suggest that NOD1 signaling results in downregulation of Cdx2 via TRAF3 inhibition of NF-κB (Fig. 6).
Figure 6.
Schematic view of the suppressive role of NOD1 on H. pylori infection–induced Cdx2 induction.
As such, they support the notion that the mechanism of NOD1 downregulation of Cdx2 derived from the study of epithelial cell lines also obtains in physiologic epithelial cells.
Discussion
Previous studies addressing the role of NOD1 in H. pylori infection of the gastric mucosa clearly showed that this innate immune receptor is an important part of the mucosal host defense program (7, 8). These studies, however, did not consider the possibility that NOD1 activation may also play a role during established infection that addresses H. pylori induction of Cdx2 and IM, a feature of chronic H. pylori infection that is considered a precursor to gastric cancer (1). In this study, we fill this gap with studies showing that NOD1 activation inhibits infection-induced Cdx2 expression both in vitro and in vivo. In addition, we show in extensive in vivo studies that the absence of NOD1 leads to enhanced intestinal metaplasia in mice with prolonged H. pylori infection and thus that NOD1 inhibits this precancerous change.
In our initial studies, we found that H. pylori infection of both cancer cell line cells and nonneoplastic cell line led to prompt and robust expression of Cdx2. In addition, we showed that such induction was greatly enhanced by H. pylori expression of the cagPAI virulence factor possibly because CagA induces activation of NF-κB (27). This in vitro Cdx2 induction system allowed us to probe the effect of NOD1 signaling on H. pylori Cdx2 induction and, subsequently, to conduct studies that defined the mechanism of such effects. Our basic observation was that downregulation of NOD1 signaling led to increased H. pylori–induced Cdx2 expression, and upregulation had the opposite effect. These in vitro studies thus clearly established that NOD1 signaling has a regulatory function with respect to H. pylori induction of Cdx2.
In further studies focused on the nature of the NOD1 signaling mechanism responsible for downregulating Cdx2 induction, we showed initially that increased Cdx2 expression in gastric cancer cells lines by H. pylori infection depends on NF-κB activation. This finding is consistent with previous reports showing that H. pylori infection causes the activation of NF-κB (28) and that the activation of this transcription factor is involved in the induction of Cdx2 expression (20, 21). We then showed that NF-κB activation is negatively regulated by NOD1 signaling probably because such signaling activates TRAF3 (8, 29). This possibility was in fact supported by in vitro studies in which we showed that transfection of an epithelial cell line with a TRAF3 expression vector down-regulated H. pylori–induced NF-κB activity, whereas TRAF3-siRNA upregulated such activity; in addition, overexpression of TRAF3 greatly inhibited Cdx2 expression especially in H. pylori–infected cells in which NOD1 signaling (and presumably TRAF3 activation) was enhanced. Finally, the idea that NOD1 signaling inhibits H. pylori–induced Cdx2 expression by negative regulation of NF-κB was supported by in vivo studies in which we showed that prolonged infection with H. pylori in NOD1-deficient mice as compared with that in NOD1-intact mice was accompanied by greatly increased nuclear expression of NF-κB p65 associated with greatly decreased expression of TRAF3.
Our observation that NOD1 signaling regulates H. pylori–induced Cdx2 expression in epithelial cells infected by H. pylori in vitro was fully corroborated by in vivo studies in which we showed that NOD1-deficient mice manifest greatly increased intestinal metaplasia both 8 and 12 months after infection. Moreover, the gastric mucosa of NOD1-deficient mice expressed increased levels of Cdx2 and other markers of intestinal metaplasia. Finally, as already mentioned, the gastric mucosa of NOD1-deficient mice displayed molecular changes in NF-κB and TRAF3, indicative of increased Cdx2 expression. These studies thus supported the notion that NOD1 signaling regulates Cdx2 in physiologic epithelial cells as well as in epithelial cell lines.
One important difference between H. pylori–induced Cdx2 expression in vitro and in vivo is that in vitro, such induction is immediate, whereas, in vivo, it is delayed and evident only after months of infection. This delayed appearance of Cdx2 (and accompanying intestinal metaplasia) may be due to the presence of cytokines in the in vivo environment that have an inhibitory effect on infection-induced Cdx2 expression. For instance, IFNγ, an inflammatory cytokine induced by H. pylori infection in vivo has been shown to cause dephosphorylation of STAT3 (30), another factor that is activated by CagA (31) that can induce Cdx2 expression (32). Whatever the explanation, the difference in the in vitro versus in vivo time course of H. pylori–induced Cdx2 expression by no means invalidates the NOD1-regulatory effect; on the contrary, it indicates that the latter is operating at a level not affected by secondary effects of the infection.
Recent studies have suggested that Helicobacter infection of the gastric mucosa can induce another type of precancerous change known as spasmolytic polypeptide–expressing metaplasia (SPEM; refs. 18, 33), which is characterized by parietal cell atrophy and the expression of TFF2 (24). As in the case of a recent study of mice infected with Helicobacter felis (18), we did find some evidence that SPEM was also induced in our chronic H. pylori infection model in that TFF2 levels in the infected mice were increased compared with the uninfected mice. However, this TFF2 increase was not as high as that of TFF3 and did not differ between NOD1-deficient and NOD1-intact mice (data not shown). Thus, it seems likely that TFF2-associated SPEM in H. pylori infection is not regulated by NOD1; nevertheless, further studies are warranted.
In previous studies, chronic infection or excessive responses to commensal organisms has been linked to the development of neoplasia in several gastrointestinal or even nongastrointestinal organs (34–36). The mechanism proposed to explain these associations involved activation of Toll-like receptor (TLR) signaling and its subsequent effects on cell survival and activation. It should be noted, however, that in a recent report, Banerjee and colleagues showed that although there was no difference in inflammation scores, mice lacking MyD88, an adaptor molecule involved in TLR signaling, exhibited early and rapid advancement to gastric dysplasia in response to H. felis infection compared with their littermate controls (37). Thus, the relation of innate signaling to neoplasia is not necessarily proneoplastic. This is supported, to some extent, by the studies reported here, which show that a deficiency in innate immune signaling, in this case NOD1 deficiency, can in fact, lead to the appearance of factors such as Cdx2 and the promotion of precancerous lesions via abrogation of the regulation of NF-κB signaling.
The relation of gastric NOD1 responses to H. pylori infection and induction of gastric cancer is complex because on one hand, such responses can protect against the initiation of infection and, on the other hand, can intensify inflammation once infection has been established. The protective effect has been demonstrated in studies showing that type I interferon responses induced by NOD1 stimulation reduce the level of H. pylori infection (8) and that β-defensin production induced by NOD1 exerts a negative influence on H. pylori survival (38). The reciprocal proinflammatory effect has been shown in studies showing that NOD1 signaling enhances epithelial chemokine responses and that an H. pylori organism bearing a mutation in its NOD1-stimulating peptidoglycan induces decreased inflammation and malignant transformation in vivo (39). These negative and positive effects on H. pylori–induced inflammation, however, are probably independent from the effects of NOD1 on Cdx2 expression reported here, as the latter influences malignant transformation in gastric tissue harboring a chronic and stable infection. That such an antimalignant NOD1 effect does, in fact, operate in human populations with chronic H. pylori infection and thus alters the latter’s outcome is suggested by a recent study showing that a certain NOD1 polymorphism in a large Chinese population confers a decreased risk of intestinal metaplasia; this finding thus opens the door to the possibilities that subtle molecular differences in NOD1 structure leading to increased NOD1 responses may downregulate Cdx2 expression (40); in addition, it has been shown that in humans, NOD1 expression is decreased in cancerous gastric tissue, but not noncancerous tissue (39).
In conclusion, we show here that H. pylori infection leads to increased expression of Cdx2 and that insufficiency of the innate immunity–related molecule NOD1 enhanced such expression and was associated with increased appearance of precancerous intestinal metaplasia in the gastric mucosa. Thus, our findings suggest that augmentation of NOD1 signaling in patients with H. pylori infection may inhibit not only gastric colonization of this organism but the capacity of this infection, once established, to induce tumor development.
Supplementary Material
Acknowledgments
The authors thank Ms. Kiiko Ogashiwa for her help in histology.
Grant Support
This work was supported by JSPS KAKENHI 25460925.
Footnotes
Disclosure of Potential Confiicts of Interest
No potential confiicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Authors’ Contributions
Conception and design: N. Asano, W. Strober, T. Shimosegawa
Development of methodology: N. Asano, A. Imatani
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): N. Asano, A. Imatani, T. Watanabe, J. Fushiya, Y. Kondo, N. Ara, K. Uno
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): N. Asano, A. Imatani, T. Watanabe, T. Koike, W. Strober
Writing, review, and/or revision of the manuscript: N. Asano, T. Watanabe, W. Strober
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Y. Kondo, X. Jin, K. Iijima, T. Koike
Study supervision: W. Strober, T. Shimosegawa
References
- 1.Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut. 1991;32:1110–3. doi: 10.1136/gut.32.10.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, et al. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–96. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 3.Barros R, Camilo V, Pereira B, Freund JN, David L, Almeida R. Pathophysiology of intestinal metaplasia of the stomach: emphasis on CDX2 regulation. Biochem Soc Trans. 2010;38:358–63. doi: 10.1042/BST0380358. [DOI] [PubMed] [Google Scholar]
- 4.Mutoh H, Hakamata Y, Sato K, Eda A, Yanaka I, Honda S, et al. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470–9. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 5.Salari K, Spulak ME, Cuff J, Forster AD, Giacomini CP, Huang S, et al. CDX2 is an amplified lineage-survival oncogene in colorectal cancer. Proc Natl Acad Sci U S A. 2012;109:E3196–205. doi: 10.1073/pnas.1206004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44:239–48. doi: 10.1007/s00535-009-0014-1. [DOI] [PubMed] [Google Scholar]
- 7.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–74. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, et al. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest. 2010;120:1645–62. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikawa T, Asano N, Imatani A, Ohyauchi M, Fushiya J, Kondo Y, et al. Gene polymorphisms of NOD1 and interleukin-8 influence the susceptibility to erosive esophagitis in Helicobacter pylori infected Japanese population. Hum Immunol. 2012;73:1184–9. doi: 10.1016/j.humimm.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstiel P, Hellmig S, Hampe J, Ott S, Till A, Fischbach W, et al. Influence of polymorphisms in the NOD1/CARD4 and NOD2/CARD15 genes on the clinical outcome of Helicobacter pylori infection. Cell Microbiol. 2006;8:1188–98. doi: 10.1111/j.1462-5822.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohyauchi M, Imatani A, Yonechi M, Asano N, Miura A, Iijima K, et al. The polymorphism interleukin 8 -251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54:330–5. doi: 10.1136/gut.2003.033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y, et al. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–85. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahm KB, Lee KM, Kim YB, Hong WS, Lee WH, Han SU, et al. Conditional loss of TGF-beta signalling leads to increased susceptibility to gastrointestinal carcinogenesis in mice. Aliment Pharmacol Ther. 2002;16(Suppl 2):115–27. doi: 10.1046/j.1365-2036.16.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 14.Olczak AA, Seyler RW, Jr, Olson JW, Maier RJ. Association of Helicobacter pylori antioxidant activities with host colonization proficiency. Infect Immun. 2003;71:580–3. doi: 10.1128/IAI.71.1.580-583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–15. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 16.Silva E, Teixeira A, David L, Carneiro F, Reis CA, Sobrinho-Simoes J, et al. Mucins as key molecules for the classification of intestinal metaplasia of the stomach. Virchows Arch. 2002;440:311–7. doi: 10.1007/s004280100531. [DOI] [PubMed] [Google Scholar]
- 17.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 18.Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, Fox JG, et al. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–94. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–7. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 20.Huo X, Zhang HY, Zhang XI, Lynch JP, Strauch ED, Wang JY, et al. Acid and bile salt-induced CDX2 expression differs in esophageal squamous cells from patients with and without Barrett’s esophagus. Gastroenterology. 2010;139:194–203. doi: 10.1053/j.gastro.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Domon-Dell C, Wang Q, Chung DH, Di Cristofano A, Pandolfi PP, et al. PTEN and TNF-alpha regulation of the intestinal-specific Cdx-2 homeobox gene through a PI3K, PKB/Akt, and NF-kappaB-dependent pathway. Gastroenterology. 2002;123:1163–78. doi: 10.1053/gast.2002.36043. [DOI] [PubMed] [Google Scholar]
- 22.Hirata Y, Ohmae T, Shibata W, Maeda S, Ogura K, Yoshida H, et al. MyD88 and TNF receptor-associated factor 6 are critical signal transducers in Helicobacter pylori-infected human epithelial cells. J Immunol. 2006;176:3796–803. doi: 10.4049/jimmunol.176.6.3796. [DOI] [PubMed] [Google Scholar]
- 23.He JQ, Oganesyan G, Saha SK, Zarnegar B, Cheng G. TRAF3 and its biological function. Adv Exp Med Biol. 2007;597:48–59. doi: 10.1007/978-0-387-70630-6_4. [DOI] [PubMed] [Google Scholar]
- 24.Goldenring JR, Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 25.Winn B, Tavares R, Fanion J, Noble L, Gao J, Sabo E, et al. Differentiating the undifferentiated: immunohistochemical profile of medullary carcinoma of the colon with an emphasis on intestinal differentiation. Hum Pathol. 2009;40:398–404. doi: 10.1016/j.humpath.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yabuki N, Sasano H, Tobita M, Imatani A, Hoshi T, Kato K, et al. Analysis of cell damage and proliferation in Helicobacter pylori-infected human gastric mucosa from patients with gastric adenocarcinoma. Am J Pathol. 1997;151:821–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb A, Yang XD, Tsang YH, Li JD, Higashi H, Hatakeyama M, et al. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242–9. doi: 10.1038/embor.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099–109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 29.Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci U S A. 2008;105:3503–8. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang P, Hwa V, Rosenfeld RG. Interferon-gamma-induced dephosphorylation of STAT3 and apoptosis are dependent on the mTOR pathway. Exp Cell Res. 2006;312:1229–39. doi: 10.1016/j.yexcr.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Bronte-Tinkew DM, Terebiznik M, Franco A, Ang M, Ahn D, Mimuro H, et al. Helicobacter pylori cytotoxin-associated gene A activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res. 2009;69:632–9. doi: 10.1158/0008-5472.CAN-08-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobler L, Pera M, Garrido M, Iglesias M, de Bolos C. CDX2 can be regulated through the signalling pathways activated by IL-6 in gastric cells. Biochim Biophys Acta. 2014;1839:785–92. doi: 10.1016/j.bbagrm.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–46. [PubMed] [Google Scholar]
- 34.Datta A, Sinha-Datta U, Dhillon NK, Buch S, Nicot C. The HTLV-I p30 interferes with TLR4 signaling and modulates the release of pro- and anti-inflammatory cytokines from human macrophages. J Biol Chem. 2006;281:23414–24. doi: 10.1074/jbc.M600684200. [DOI] [PubMed] [Google Scholar]
- 35.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–68. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 36.Aravalli RN. Role of innate immunity in the development of hepatocellular carcinoma. World J Gastroenterol. 2013;19:7500–14. doi: 10.3748/wjg.v19.i43.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee A, Thamphiwatana S, Carmona EM, Rickman B, Doran KS, Obonyo M. Deficiency of the myeloid differentiation primary response molecule MyD88 leads to an early and rapid development of helicobacter-induced gastric malignancy. Infect Immun. 2014;82:356–63. doi: 10.1128/IAI.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grubman A, Kaparakis M, Viala J, Allison C, Badea L, Karrar A, et al. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell Microbiol. 2010;12:626–39. doi: 10.1111/j.1462-5822.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 39.Suarez G, Romero-Gallo J, Piazuelo MB, Wang G, Maier RJ, Forsberg LS, et al. Modification of Helicobacter pylori peptidoglycan enhances NOD1 activation and promotes cancer of the stomach. Cancer Res. 2015;75:1749–59. doi: 10.1158/0008-5472.CAN-14-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li ZX, Wang YM, Tang FB, Zhang L, Zhang Y, Ma JL, et al. NOD1 and NOD2 genetic variants in association with risk of gastric cancer and its precursors in a Chinese population. PLoS One. 2015;10:e0124949. doi: 10.1371/journal.pone.0124949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.