Abstract
Purpose
Common advice for lower urinary tract symptoms (LUTS) of frequency, urgency and related bother includes elimination of potentially irritating beverages (coffee, tea, alcohol, and carbonated and/or artificially sweetened beverages). The purpose of this study was to determine compliance with standardized instruction to eliminate these potentially irritating beverages, whether LUTS improved after instruction, and if symptoms worsened with partial reintroduction.
Design
The three-phase fixed sequence design was: 1) baseline, 2) eliminate potentially irritating beverages listed above, and 3) reintroduce at 50% of baseline volume, with a washout period between each 3-day phase. We asked participants to maintain total intake volume by swapping in equal amounts of non-potentially irritating beverages (primarily water).
Subjects and Setting
The study sample comprised 30 community-dwelling women recruited through newspaper advertisement.
Methods
Quantification measures included 3-day voiding diaries and detailed beverage intake, and LUTS questionnaires completed during each phase.
Results
During Phase 2, we found significant reduction in potentially irritating beverages but complete elimination was rare. Despite the protocol demands, total beverage intake was not stable; mean (± standard deviation) daily total intake volume dropped by 6.2±14.9oz (p=0.03) during Phase 2. In Phase 3, the volume of total beverage intake returned to baseline, but intake of potentially irritating beverages also returned to near baseline rather than 50% as requested by protocol. Despite this incomplete adherence to study protocols, women reported reduction in symptoms of urge, inability to delay voiding, and bother during both phases (p≤0.01). The number of voids per day decreased on average by 1.3 and 0.9 voids during phases 2 and 3 respectively (p=0.002 and p=0.035).
Conclusions
Education to reduce potentially irritating beverages resulted in improvement in LUTS. However, eliminating potentially irritating beverages was difficult to achieve and maintain. Study findings do not allow us to determine if LUTS improvement was attributable to intake of fewer potentially irritating beverages, reduced intake of all beverages, the effect of self-monitoring, or some combination of these factors.
Keywords: overactive bladder, bladder irritants, incontinence, lower urinary tract symptoms, artificial sweeteners, caffeine, women
Introduction
Lower urinary tract symptoms (LUTS) including urinary urgency, voiding frequency and related bother, with or without incontinence, were reported in 30% of women in a recent community-based study.1 Frequency (defined as 8 or more voids per day2) and urgency are associated with anxiety, reduced health related quality of life, and depression.3
There is general agreement among health professionals and the public that certain beverages act as bladder irritants. Community-dwelling women accessing web-based information even from respected organizations such as the National Association for Continence4 and the Mayo Clinic5 will readily find advice to reduce coffee, tea, alcohol, and carbonated and artificially sweetened beverages. We will refer to these in total as “potentially irritating beverages” (PIBs).
Despite the widespread notion that PIBs intake is associated with LUTS, evidence to either support or refute the efficacy of this behavioral intervention is scant. We tested the effects of instructing women to eliminate PIBs for the purpose of reducing LUTS. We also tested whether LUTS resume with reintroduction of PIBs. Specifically, in a pre- and post-test design, we tested the following hypotheses: 1) LUTS are significantly reduced as measured by 3-day diaries (primary outcome) and questionnaires (secondary outcome) after women view a DVD instructing them to eliminate PIBs from their diets and 2) LUTS (measured via voiding diary and questionnaires) increase with reintroduction of PIBs at half the woman’s baseline volume.
Methods
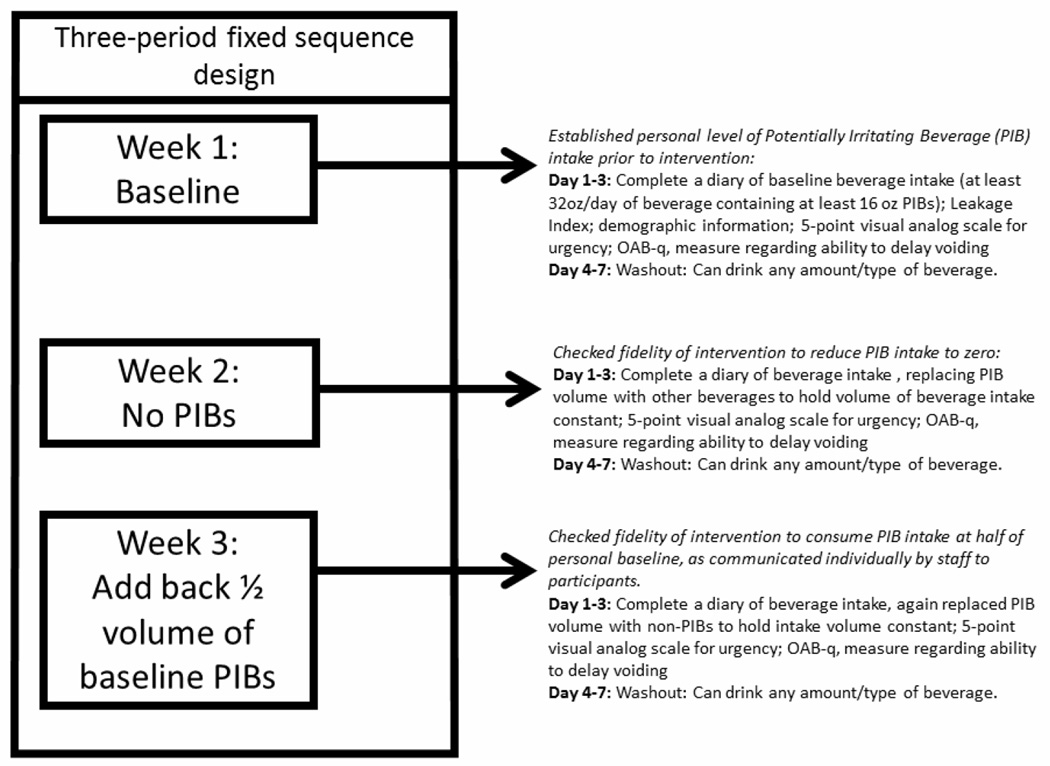
We employed a 3-phase fixed sequence design (Figure 1). An instructional DVD, detailed below, introduced participants to expectations for each phase including when reminder information would be provided. In Phase 1, participants were asked to complete a 3-day beverage intake and bladder habits diary along with symptom questionnaires, reflecting usual (baseline) beverage intake patterns. During Phase 2, participants was asked to document LUTS while completely eliminating of PIBs. During Phase 3, participants were asked to document LUTS while reducing their baseline intake of PIBs by 50%. To keep total intake volume consistent during the three phases, participants were to swap in non-PIBs (mostly water). A 4-day wash-out period was introduced between each of the 3-day study phases so that participants could have a break from the tedious effort of daily diary recording. Participants were told that this was a rest period from the study and that they could drink whatever they wanted during the 4 days between baseline Phase 1 recording and Phase 2 recording, and between Phase 2 recording and Phase 3 recording.
Figure 1.
Study Design
Study Sample
We recruited community-dwelling women through a newspaper advertisement. We chose community-dwelling women rather than patients presenting for care because our target population was women accessing information available to the public, regardless of whether they had sought counselling from a health care provider. Initial screening occurred by telephone; women were invited to participate in the study if they reported consuming a daily intake of ≥32 ounces total beverages and ≥16 ounces of PIBs. We chose the screening threshold of ≥32 ounces total beverage intake to avoid concern for dehydration should total volume not be maintained over the study. We chose the screening cut point of ≥16 ounces/day of PIBs as a compromise between needing to show intervention-related reduction in PIBs intake and being inclusive in sampling women who consume any PIBs.
Additional inclusion criteria included answering “yes” to 2 of the following 3 questions: “Do you experience frequent urination, defined as greater than 8 times per day or 2 times at night?”, “Do you experience frequent (greater than every 2 waking hours) strong feelings of urgency to empty your bladder?”, and “Do you experience frequent (routinely >2 times) night-time urination?” We based the wording of these questions on the 2002 International Continence Society (ICS) definitions of urinary frequency and urgency.6
We excluded women who were currently pregnant, <12 months postpartum, breastfeeding, had a history of vaginal or bladder-related surgery, or taking diuretic medications. We also excluded women with the following medical conditions: diabetes mellitus, multiple sclerosis, muscular dystrophy, cerebral palsy, dementia, Alzheimer’s disease, stroke, or spinal cord injury. Women with current symptoms of dysuria or a history of frequent urinary tract infections (defined as >4/year) were excluded. We excluded men as our intended population of community dwelling adults with LUTS was likely to include men in the latter half of their fifth decade and above, when the common condition of prostate gland enlargement might confound the outcome variables of frequency. Study procedures were reviewed and approved by IRBMED, University of Michigan: HUM00050865. All participants signed an informed consent document.
Study Instruments
We adapted a traditional bladder diary format to provide more granular detail for beverage consumption (Appendix 1). Briefly, women recorded the following information daily for 3 consecutive days: time of beverage intake, type of beverage intake (in detail) and volume consumed, time of voids, voided volume, number of incontinence episodes, time to bed and time awake.
During each phase of the study, participants also completed 3 brief questionnaires on LUTS. This first was a visual analog scale that assessed urinary urgency for each day a voiding diary was completed. This urgency scale was adapted from Bower and colleagues. They used the question “Whenever I need to go to the toilet I can…” and offered 5 response options presented equidistantly on a 10 unit undemarcated line. The response options were: 1)make the wee go away, 2) easily hold on, 3) wait a little while, 4) hardly wait, and 5) feel wee already coming out.”.7 We replaced the term “wee” with the terms “urine” or “urge” and treated the options as an ordered 5-point choice rather than as an equidistant labeling on a 10-unit scale, for cultural relevancy and improved face validity on response to pilot testing in our setting. The question was placed on the 3-day diary at the end of each day’s recording, for each phase of the study.
The OAB-q is a reliable and valid measure used to differentiate between normal and clinically diagnosed continent and incontinent participants with LUTS8. It was administered to assess symptom bother and health-related quality of life associated with a variety of different LUTS. The original instrument includes 33 items, but we used only the first eight questions that pertained to bother, and which are designed to be scored separately. We modified the 8 questions by changing the introductory phrase “During the past 4 weeks, how bothered were you by…” to “During the past 3 days, how bothered were you by…” to characterize the bother of LUTS under the 3-day conditions of each phase
Women were also asked at the end of the 3-day diary logging, during each phase, to evaluate their ability to delay voiding that day. This modified grading bladder fullness scale is an ordinal scale with different time-lengths of ability to delay relative to desire to void. The scale was modified from one suggested in a study performed by De Wachter and Wyndaele.9 We modified by replacing the word “voiding” with the word “urinating,” to render the responses more relevant to participants, and we made slight adjustments to wording and time increments for clarity and logic (Appendix 2).
Study Procedures
Descriptive data, including age, height, weight, race, parity, education, and yearly income were collected. Baseline diary and symptom questionnaires were also obtained. These baseline measures, as well as repeated measures of diary and symptom questionnaires for each phase of the study, were distributed and returned via US mail. Each woman received a plastic container (sometimes referred to as a “Texas hat”) for measuring voided urine.
These community-dwelling women were provided with an instructional DVD about study procedures and expectations. The DVD contained a voice-over slide presentation, which played like a video. It was 34 slides long and required about 30 minutes to view. Two of the 34 slides were devoted to defining “potentially irritating beverages,” which in this study we described as coffee (even decaf), tea (even decaf), alcohol, carbonated, and artificially sweetened beverages. The remainder of the slides included a welcome slide, background and study purpose, extensive “tips and tricks” for accurate recording on the diary and the location and importance of the questionnaires. The DVD standardized the instruction to remove the potential for bias that comes from extensive nurse contact at face-to-face visits, and to offer convenience for the participants. An added advantage was that women were able to refer back to their DVD at any time throughout the study if they desired a review of instructions. Participants were given the option of viewing the presentation in our office if preferred (for instance, if they did not have computer access or a DVD player). One participant requested this option.
After completion of the Phase 1, a study investigator examined each voiding diary, calculated the amount of PIBs and non-PIBs consumed, and provided individualized mailed instruction to each participant on the desired PIBs and overall fluid consumption during the next two study phases. Participants were asked to complete diaries on the same 3 days of the week for each phase so that typical habits reflective of specific days would be consistent throughout the study period. We also asked women to record an output volume in the diary for every void, even if they did not void into the container provided. We further instructed them to note that it was an estimate in a column labeled for this, rather than leaving that as missing data (appendix A). Women were reimbursed for each completed phase of the study for a possible total of $70.
Data Analysis
Diary data were reduced such that there was a mean 3-day score for each diary variable at each time period. For example, for the urgency visual analogue scale was assessed each day of the 3-day diary7 but the average of the 3 days was used as the score across that phase. Differences between study phases were evaluated using paired 2-sample t-tests were used for variables with a normal distribution and Wilcoxon Signed-Rank tests for variables that were not normally distributed. We used the Bland and Altman measures of agreement graphical methods to portray variance from protocol to maintain total beverage intake or to reduce intake at the appropriate study intervals.10 Statistical Analysis Software version 9.3 (SAS Institute, Inc., Cary, NC) was used for most of the statistical analysis, and SPSS (IBM, Armonk, New York) was used for other selected data management and analysis procedures. A p-value <0.05 was considered statistically significant.
Results
Eighty-six women were screened and 35 were enrolled into the study. Five were excluded from the final analyses for various screening or protocol violations discovered during the later data analysis procedures. Analysis was based on data from 30 women. Their mean age was 57.5±10.2 years (mean ± SD) and 90.0% identified themselves as Caucasian (Table 1). Baseline beverage diary data showed that all met enrollment criteria for PIB consumption (>16oz/day). No woman in this study reported juice as her only potentially irritating beverage.
Table 1.
Participant Characteristics
| Variable | Mean (SD) |
| Age (years) (n=30) | 57.53 (10.24) |
| Weight (pounds) (n=29) | 159.22 (38.21) |
| Height (inches) (n=30) | 64.6 (2.4) |
| BMI (n=29) | 26.8 (6.01) |
| Variable | N (%) |
| Yearly Income (dollars) | |
| <$20,000 | 3 (10.34%) |
| $20,000–40,999 | 9 (31.03%) |
| $41,000–60,000 | 7 (24.14%) |
| >$60,000 | 10 (34.48%) |
| Race | |
| Black/African American | 1 (3.33%) |
| White | 27 (90.0%) |
| Other/did not disclose | 2 (6.66%) |
| Education level | |
| 12th grade | 3 (10%) |
| Some college | 6 (20%) |
| College (4 yrs or tech writing) | 9 (30%) |
| Graduate school | 12 (40%) |
| Total number of children | |
| 0 | 8 (27.59%) |
| 1 | 4 (13.79%) |
| 2 | 8 (27.59%) |
| 3 | 4 (13.79%) |
| 4 | 3 (10.34%) |
| 5 | 0 (0%) |
| 6 | 1 (3.45%) |
| 7 | 1 (3.45%) |
| Number of normal vaginal deliveries | |
| 0 | 11 (37.93%) |
| 1 | 4 (13.79%) |
| 2 | 6 (20.69%) |
| 3 | 4 (13.79%) |
| 4 | 3 (10.34%) |
| 5 | 0 (0%) |
| 6 | 0 (0%) |
| 7 | 1 (3.45%) |
At baseline, average total daily beverage intake was 74.7±26.1 ounces, with PIBs accounting for 63.3±39.3 ounces (85% of total) (Table 2). Despite instructions otherwise, the total fluid volume intake over all diary days of the study was not stable. During Phase 2, when women were instructed to avoid consuming any PIBs, diaries showed wide variability in intake volume. Specifically, the total beverage intake was reduced by a mean of 6.2 ounces, which was significantly lower than Phase 1 (p=0.03) and Phase 3 (p=0.02). During Phase 3, total beverage intake was essentially equal to Phase 1, but again variability was noted between individual diaries. The Bland and Altman scatter plot and accompanying measures of agreement analysis (Figure 2) showed that any individual woman in the study might vary her total beverage intake by 20 ounces or more from phase to phase (nearly 30 ounces from Phase 1 to Phase 2), despite being requested to maintain a steady intake volume.
Table 2.
Results of symptoms and bladder habits at baseline (Phase 1), on attempt at complete elimination of potentially irritating beverages (Phase 2), and on attempt to reintroduce potentially irritating beverages at ½ of baseline (Phase 3).
| Outcomes | Phase 1: Baseline |
Phase 2: All PIBs eliminated |
Phase 3: Add back ½ PIBs |
Phase 1 vs Phase 2 |
Phase 1 vs Phase 3 |
Compare Phase 2 vs Phase 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) |
n | Mean (SD) |
n | Mean (SD) |
Mean (SD) |
p-value | Mean (SD) |
p- value |
Mean (SD) |
p- value |
|
| Average total daily intake per 24 hours over 3 days (oz) | 30 | 74.7 (26.1) |
30 | 68.6 (28.7) |
30 | 74.8 (30.3) |
−6.16 (14.88) |
0.031 | +0.08 (10.03) |
0.965 | +6.24 (13.67) |
0.018 |
| Average "Potentially Irritating Beverage" intake per 24 hours over 3 days (oz) | 30 | 63.3 (39.3) |
30 | 38.0 (37.0) |
30 | 52.8 (27.6) |
−25.3 (53.4) |
0.015 | −10.5 (37.1) |
0.132 | +14.8 | 0.04 |
| Average number of voids per 24 hours over 3 days | 30 | 10.5 (2.64) |
30 | 9.2 (2.71) |
30 | 9.6 (2.86) |
−1.27 (2.05) |
0.002 | −0.9 (2.29) |
0.040 | +0.37 (1.54) |
0.202 |
| Average total daily voided volume per 24 hours over 3 days (oz) | 30 | 77.72 (22.66) |
30 | 70.99 (27.98) |
30 | 72.07 (24.69) |
−6.74 (20.73) |
0.086 | −5.66 (14.0) |
0.035 | +1.08 (14.28) |
0.681 |
| Average self-reported urge symptoms* reflecting on past 24 hours on each day of 3 days (ranging from 1–5 points with higher scores indicating worse urge) | 29 | 3.21 (0.63) |
30 | 2.80 (0.57) |
30 | 2.91 (0.71) |
−0.43 (0.55) |
0.000 | −0.339 (0.67) |
0.011 | +0.11 (0.64) |
0.377 |
| Self-report ability to delay reflecting on past 3 days (ranging from 0–4 points, with higher scores indicating worse ability to delay) | 29 | 2.91 (0.67) |
30 | 2.25 (0.91) |
30 | 2.37 (0.78) |
−0.66 (0.64) |
<0.000 | −0.53 (0.64) |
0.000 | +0.12 (0.73) |
0.387 |
| Self-report of bother reflecting on past 3 days (ranging from 8 –48 points, with higher scores indicating more bother) | 29 | 29.77 (15.49) |
30 | 18.95 (13.4) |
30 | 22.05 (14.78) |
−10.46 (14.82) |
0.001 | −7.65 (12.14) |
0.002 | +3.09 (8.03) |
0.044 |
Figure 2.
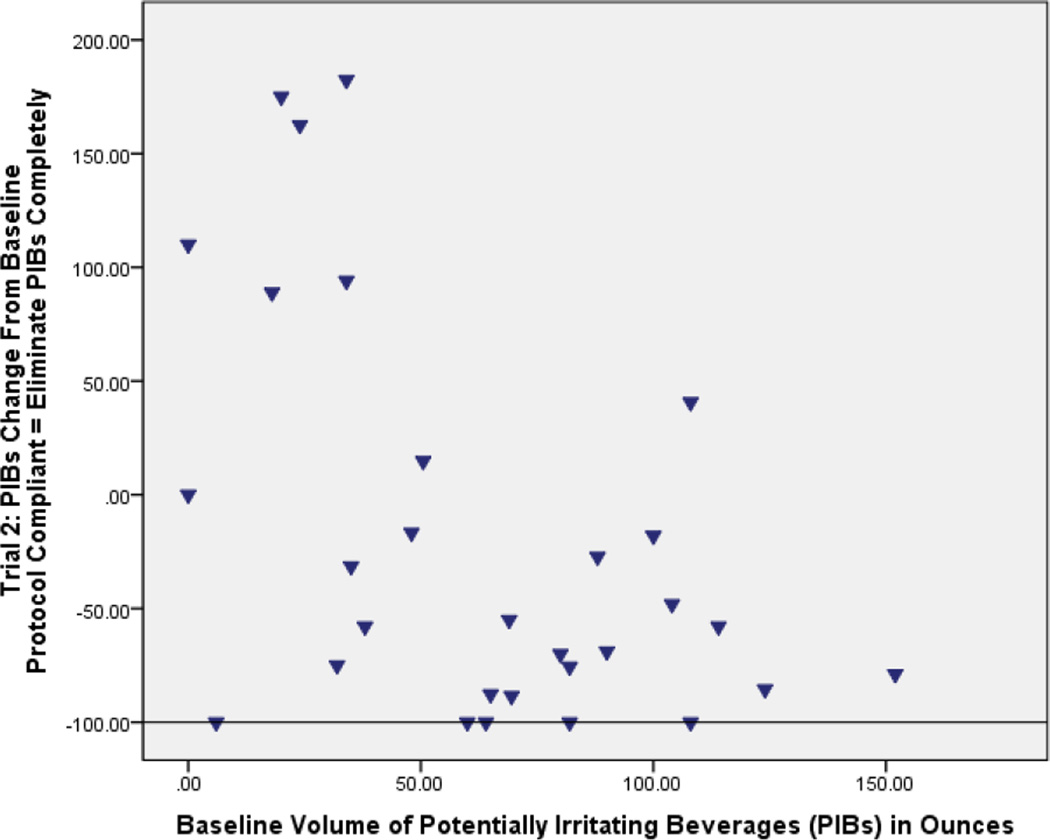
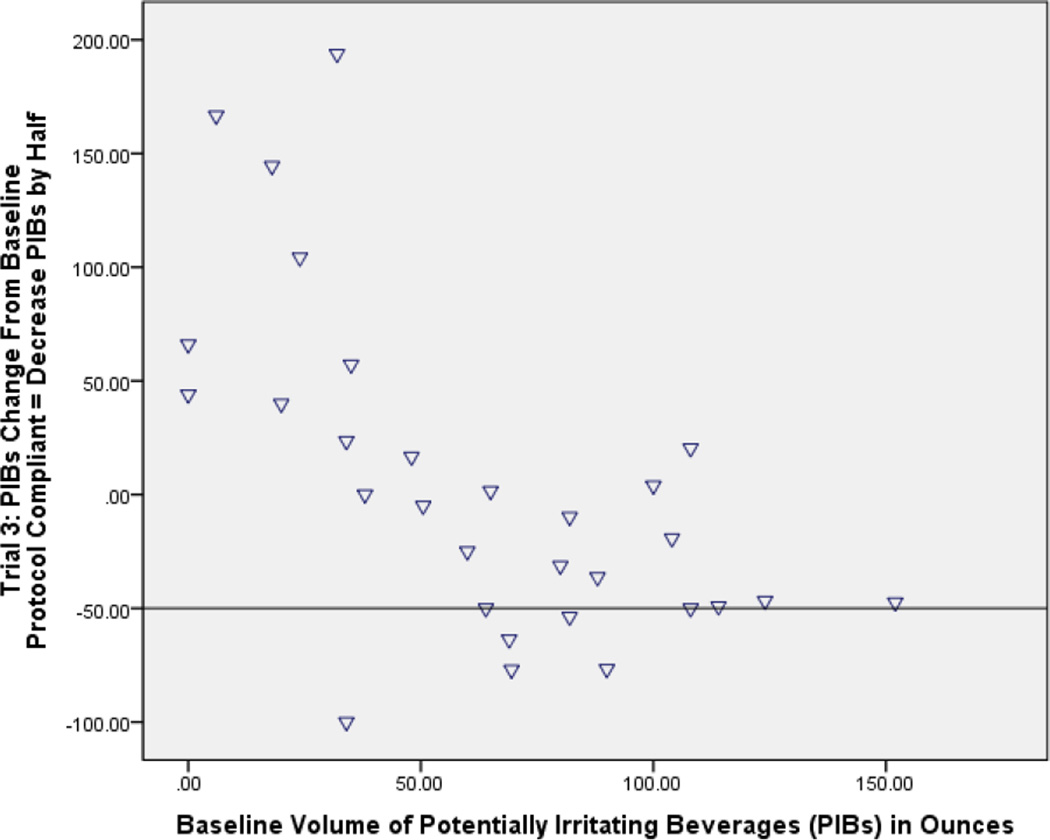
a and b: Bland and Altman plots showing percent change of potentially irritating beverages compared to baseline. The horizontal line portrays the protocol expectation for each phase). At Phase 2 when all potentially irritating beverages should have been eliminated (−100% on the graph), instead several women showed very little change and some ranged up to nearly 200% above baseline (Fig A). At Phase 3, when potentially irritating beverages by protocol should have been down by half (−50% on the graph) from baseline, again a substantial number of women showed little behavioral change or even up to 200% above baseline in one case (Fig B).
Although women were instructed to entirely eliminate PIBs during Phase 2, PIB intake was still on average 38.0 ounces per day, which was a reduction of 25.3 ounces per day from baseline (Table 2). Eight participants reported increasing their intake, one participant did not change her intake volume, five complied with 100% elimination of PIBs, and the remaining 17 women decreased PIB intake to varying degrees (Figure 2a). There was no relationship between reducing PIBs during Phase 2 and original PIB intake volume, as shown in a plot of individual data in Figure 2a.
Participants were individually instructed on how to add back 50% of their personal baseline PIBs intake during Phase 3. Nevertheless, they experienced difficulty achieving this goal, and 11 women (37%) did not reduce the amount of PIBs from baseline at all. Overall, women reported a mean 15% reduction in PIB consumption rather than the requested 50% (Table 2, Figure 2b).
Despite incomplete adherence to the protocol, the greatest reduction in LUTS was achieved during Phase 2, when PIB consumption was lowest (Table 2). When compared to their baseline (Phase 1) self-reported urinary urgency (p=0.0003), ability to delay voiding (p<0.0001) and bother scores (p=0.0007) improved significantly during Phase 2. In addition, the mean daily voided volume was reduced by 6.7±20.8 ounces; this change was consistent with a decrease in total beverage intake. Nevertheless, this difference was not statistically significant compared to baseline, (p=0.09). The number of voids per day in Phase 2 was significantly reduced compared to Phase 1 (10.5 voids vs. 9.2 voids, p=0.002), but it was not different from Phase 3.
When participants were asked to add back some PIBs during Phase 3, there was still a significant reduction in LUTS compared to baseline (Table 2). During this phase, total beverage intake volume was similar to baseline (p=0.1), while the improvements in bladder function demonstrated at Phase 2 were maintained. The number of voids/day continued to be significantly lower than baseline (10.5 voids compared to 9.6 voids; p=0.04). Total voided volume was also significantly reduced by 5.6 ounces (p=0.04) relative to baseline. Symptom scores for urgency, ability to delay voiding and degree of bother were all significantly improved compared to baseline (Table 2).
Comparing Phases 2 and 3, women consumed more total beverages and more PIBs in Phase 3 than Phase 2. Voided volume and number of voids per day were similar in the two phases (Table 2). Bother scores did show a significant worsening from Phase 2 to Phase 3 (p=0.04), although both phases were improved compared to baseline.
Discussion
This feasibility study evaluated the effects on LUTS of two different intake volumes of PIBs, none or half of baseline consumption, while holding total beverage intake constant by swapping in primarily plain water. While we noted LUTS improvement with PIB reduction, truly eliminating PIBs proved difficult for a majority of study participants. As a result, we cannot yet conclude if the improvement we noted was from fewer PIBs, reduced total intake of beverages, the effect of self-monitoring bladder habits, or some combination of these.
Complete elimination of PIBs was not achieved by many women in this study. We therefore recommend that clinicians carefully consider the difficulty of achieving and adhering to PIB elimination as a treatment strategy. We did not specifically analyze reasons for lack of adherence to PIB and overall fluid intake, so we can only speculate about the numerous possible reasons women were unable to eliminate PIBs. Many PIBs are habit-forming11, 12, and many social events are tied to PIB intake. As a result, persons may find it difficult to avoid consuming a cup of coffee at a meeting or an alcoholic beverage as when participating in an after-work social gathering. Based on these considerations, we believe that partial elimination of PIBs may be a more feasible goal. Women still improved in our study when average PIBs intake was reduced to almost half of their initial intake, making this still a reasonable treatment option. The cultural, societal, familial, biological and other influence on PIB intake are an important future area of future investigation.
We observed wide variability in daily fluid intake across the study phases, despite instructions to try to maintain the same cumulative volume of beverage intake. Multiple reasons may account for this variability, including physical activity levels, weather conditions, and their effects on thirst.
While women’s LUTS improved based on group averages at both study phases, variability in the concentration of certain ingredients or in total amount of irritating substances in these beverages renders it impossible to draw conclusions about the relationship of LUTS to each specific substances or the cumulative amount consumed. All we can determine from this 3-phase study design is that following instruction to reduce total amount of PIBs while swapping in non-PIB alternatives, while recording intake, output and LUTS women achieved reductions in LUTS. [0]Our findings were confounded by inconsistent protocol compliance across the women and wide variability in concentration or total amount of potentially irritating substances in these commonly consumed beverages.
While the effect of various PIBs on LUTS is clinically relevant, we cannot draw conclusions based on specific ingredients of each PIB. For example, we did not assess the type and strength of coffees consumed. Similarly, findings from this study cannot differentiate which of the multiple ingredients in a diet cola that may act as a bladder irritant, considering it is carbonated, caffeinated, artificially sweetened, and acidic. We hope to study this interesting question in future work.
There are pros and cons to the various study designs and protocols available. A laboratory study that rigorously controls participants’ intake would offer more precise determination of ingredients and control for overall intake volume. However, since LUTS are driven by the social environment, evaluation of these environmentally triggered symptoms (e.g. urgency on arrival home) poses a validity issue. A community-based study that limits ingredients by providing pre-packaged standardized amounts of an ingredient of interest is a feasible and likely worthwhile design, but does not reflect the reality of our complex beverage-choice environment and is unlikely to provide real-world clinical treatment guidance. A randomized controlled trial asking participants in the intervention group to eliminate all PIBs is a logical next step and has the advantage of a control group who is equally exposed to the simple learnings that can occur from self-monitoring, such as awareness of how much one is actually drinking. However, since we do not yet fully understand the reasons for lack of compliance to PIB reduction, it seems logical to initiate investigation with real-life, qualitative work to learn more about this phenomenon. Clearly, the data from our study show that reliance on simple instruction to “eliminate coffee, tea, alcohol, and carbonated beverages” is not adequate motivation for women to do so. This area is also worthy of further investigation.
A relationship between PIBs such as the ones measured in our study and LUTS has been demonstrated in prior studies, but many of these studies have focused only on caffeine. In one study, mean dietary caffeine intake of women with detrusor overactivity on cystometry was significantly higher than that of women without detrusor overactivity.13 Total beverage intake and bladder capacity were not reported. Other studies link a decrease in caffeine consumption to reduction in LUTS. A recent pilot study of 11 women demonstrated a reduction in urgency and frequency, quality of life and bladder symptoms in general while drinking decaffeinated drinks compared to when they drank caffeinated beverages14. In a study by Bryant and colleagues, 26 adult participants who routinely consumed at least 100 mg of caffeine per day were randomly assigned to an intervention or control group.15 All participants received education about bladder training, but participants in the control group continued caffeine intake, while the intervention group received additional education on caffeine reduction. The intervention group achieved a significant reduction in daily episodes of leakage. It is unclear if other beverages were substituted for caffeine, so symptom reduction could have resulted from a decrease in total volume intake.
The biochemical mechanism of how caffeine contributes to LUTS is unclear, but does not appear to be the same in all women. This was supported by a study in which a standardized drink of caffeine was associated with a rise in bladder pressure only in a group of women with detrusor overactivity compared to asymptomatic women.16 It can be hypothesized that caffeine is perhaps metabolized differently in symptomatic women, or that some other factor predisposes symptomatic women to bladder symptoms when exposed to caffeine. Nevertheless, our knowledge of these mechanisms are not entirely understood, as another study showed. In that cohort, decreasing overall beverage intake significantly improved LUTS, but decreasing caffeine intake did not result in significant improvement when replacing caffeinated beverages with non-caffeinated drinks.17
The question of different PIBs affecting LUTS differently is a fascinating one that additionally merits further work. If different PIBs have different effects on symptoms, this would be welcome and germane information to patients. This is suggested in a recent study that showed that women who increased consumption of coffee or soda, especially diet soda, experienced an increase in LUTS.18
We also included as PIBs those containing alcohol and artificial sweeteners. Data concerning sugar substitutes in humans are limited, but laboratory studies have shown that ascorbic acid and citric acid augment bladder muscle contraction via a hypothesized enhanced calcium ion influx.19 Three artificial sweeteners, acesulfame K, aspartame, and sodium saccharin have been evaluated using isolated animal detrusor specimens and found to stimulate the contractile response of bladder muscle and significantly enhance contractions.
The strengths of our study, which is one of the only of its kind studying this important clinical recommendation, include enrolling community-dwelling women who may be amenable to modification of PIBs versus treatment with drugs or other interventions with a higher likelihood of side effects. We used diaries to minimize recall bias, and we had good compliance with diary recording throughout the 3 phases of the study. We believe this was partially due to the provided detailed instruction and “tips and tricks” on easing the burden of the diary recording in the standardized educational slideshow. Diary recording is a burden for patients, and hence we abided by recommendations of only three days at a time, followed by four days of non-recording.20 While this likely did ease the burden on study participants, it raises other interesting potential implications as the “wash-out period” most likely included increased PIB intake compared to the study phases, likely skewing results of the first diary day of each study period. This supported using an average value of the three study periods for our data interpretations. The trade-off of preventing recording fatigue was important to preserve subject completion of the study.
Limitations
Our three phase study design limited our ability to make causal inferences. Whether a DVD-based slide show delivery of information is the best method of study instruction is unknown, as are the many social factors discussed above regarding societal encouragement of PIB consumption. While our diary permitted the collection of a large amount of discrete data, the intensive self-monitoring that occurs with use of intake-output diaries acted as intervention. Translating this into clinical practice is always complex, and we tried to mitigate the artificial environment of a study versus real-world clinical care by keeping the study period short, providing tips on PIBs substitution and allowing subjects the washout period. All of these factors, however, are also limitations. We do not know from this study whether the length of the intervention influenced the outcomes, nor in what direction. We also do not know how the washout period influenced the outcomes. It is one thing to commit to a 3-day period of limiting PIBs, but quite another to do so for the duration.
We recommend that future studies better document specific PIBs and sweetener intake to improve counseling about specific PIBs. Compliance on counselling women about giving up sweeteners versus caffeine, for example, may increase if women are allowed to keep one or the other. Additionally, PIBs are not only found in beverages. We did not measure the presence of sweeteners in food, such as yogurt. We did not collect data on reasons why so many of our participants were unable to eliminate PIBs, and recommend future investigation into the barriers of changing this intake pattern in future studies.
Additionally, given the primarily Caucasian and well-educated demographics of our sample, our current results cannot be generalized to the broader public. Future studies will ideally include a wide range of demographic backgrounds.
Conclusions
Findings from this study supports instructing women to reduce PIBs to improve LUTS. Nevertheless, the reasons why reduction in PIB intake alleviates LUTS remain unclear. LUTS improvement observed in this study may result from changing the type of beverages consumed, changing overall volume of beverages consumed, or some combination of these factors. We believe that findings from this feasibility study set the stage for additional research. In addition, we believe that the data collection tool used in this study offers a level of precision previously not available to clinicians or researchers interested in the relationship between PIB consumption and LUTS.
Acknowledgments
Funding sources:
This project was funded by The Michigan Center for Health Intervention, University of Michigan School of Nursing, National Institutes of Health, National Institute of Nursing Research (P30 NR009000-01). Additional investigator support was provided through the Office for Research on Women's Health (ORWH) Specialized Center of Research (SCOR) on Sex and Gender Factors Affecting Women's Health and National Institute on Child and Human Development (NICHD) (Grant #P50 HD044406)).
Appendix 1
Three-day diary (last page shown)
| Time Arise: | _______________ | Trial: | 1 | 2 | 3 |
| Time to Bed: | _______________ | Today I should: | Maintain usual habits | Drink no "irritating" beverages | Drink____oz. of “irritating” beverages |
| Beverage Intake | Urine Output | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beverage Type |
Caffeinated | Artificially Sweetened |
Beverage Amount (ounces) |
Volume of Urine Output | Episodes of Leakage |
||||||
| Yes ✓ |
No ✓ |
Yes ✓ |
No ✓ |
Urine Amount (oz) |
Measured ✓ |
Close estimate ✓ |
Wild Guess ✓ |
✓✓✓ ✓ |
|||
| Morning (6am-noon) | |||||||||||
| Afternoon (noon-6pm) | |||||||||||
| Evening (6pm-midnight) | |||||||||||
| Night (midnight-6am) | |||||||||||
Appendix 2
Over the past 3 days, when you urinated, what was your usual perception of your bladder fullness? (Adapted with permission from De Wachter & Wyndaele)8
| 0 | No bladder sensation |
| 1 | Urinating could easily be delayed for more than 30–60 minutes |
| 2 | Urinating could only be delayed for 30 minutes |
| 3 | Urinating could only be delayed for 5 minutes |
| 4 | Immediate urinating was mandatory and/or fear of leakage |
Footnotes
Conflict of Interest: None of the authors have a conflict of interest.
References
- 1.Coyne KS, Sexton CC, Bell JA, et al. The prevalence of (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB-POLL. Neurourol Urodyn. 2013;32(3):230–237. doi: 10.1002/nau.22295. [DOI] [PubMed] [Google Scholar]
- 2.Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Neurourol Urodyn. 2011;30(1):2–12. doi: 10.1002/nau.21036. [DOI] [PubMed] [Google Scholar]
- 3.Milsom I, Kaplan SA, Coyne KS, et al. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology. 2012;80(1):90–96. doi: 10.1016/j.urology.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 4.National Association for Continence. Diet and Daily Habits: Can this Affect Your Bladder or Bowel Control? [Accessed on 1/17/14]; http://www.nafc.org/bladder-bowel-health/frequently-asked-questions/diet-and-daily-habits/ [Google Scholar]
- 5.Mayo Clinic. Overactive Bladder: Lifestyle and home remedies. [Accessed on 1/17/14]; http://www.mayoclinic.org/diseases-conditions/overactive-bladder/basics/lifestyle-home-remedies/con-20027632. [Google Scholar]
- 6.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187(1):116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 7.Bower WF, Moore KH, Adams RD. A pilot study of the home application of transcutaneous neuromodulation in children with urgency or urge incontinence. J Urol. 2001;166:2420–2422. [PubMed] [Google Scholar]
- 8.Coyne K, Revickil D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563–574. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 9.De Wachter S, Wyndaele JJ. Frequency-volume charts: A tool to evaluate bladder sensation. Neurourol Urodyn. 2003;22:638–642. doi: 10.1002/nau.10160. [DOI] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet, I. :307–310. [PubMed] [Google Scholar]
- 11.Alati R, Betts KS, Williams GM, Najman JM, Hall WD. Generational Increase in Young Women’s Drinking: A Prospective Analysis of Mother-Daughter Dyads. JAMA Psychiatry. 2014;71(8):952–957. doi: 10.1001/jamapsychiatry.2014.513. Published online June 25, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths RR, Woodson PP. Caffeine physical dependence: a review of human and laboratory animal studies. Psychopharmacology. 1988;94:437–451. doi: 10.1007/BF00212836. [DOI] [PubMed] [Google Scholar]
- 13.Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: A case-control study. Obstet Gynecol. 2000;96(1):85–89. doi: 10.1016/s0029-7844(00)00808-5. [DOI] [PubMed] [Google Scholar]
- 14.Wells MJ, Jamieson K, Markham TCW, Green SM, Fader MJ. The effect of caffeinated versus decaffeinated drinks on overactive bladder: A double-blind, randomized, crossover study. J Wound Ostomy Continence Nurs. 2014;41(4):371–378. doi: 10.1097/WON.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 15.Bryant CM, Dowell CJ, Fairbrother G. Caffeine reduction education to improve urinary symptoms. Br J Nurs. 2002;11(8):560–565. doi: 10.12968/bjon.2002.11.8.10165. (2002) [DOI] [PubMed] [Google Scholar]
- 16.Creighton SM, Stanton SL. Caffeine: Does it affect your bladder? British Journal of Urology. 1990;66:613–614. doi: 10.1111/j.1464-410x.1990.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 17.Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol. 2005;174:187–189. doi: 10.1097/01.ju.0000162020.10447.31. [DOI] [PubMed] [Google Scholar]
- 18.Maserejian NN, Wager CG, Giovannucci EL, Curto TM, McVary KT, McKinlay JB. Intake of caffeinated, carbonated, or citrus beverage types and development of lower urinary tract symptoms in men and women. Am J Epidemiol. 2013;177(12):1399–1410. doi: 10.1093/aje/kws411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasgupta J, Elliott RA, Tincello DG. Modification of rat detrusor muscle contraction by ascorbic acid and citric acid involving enhanced neurotransmitter release and Ca2+ influx. Neurourol Urodyn. 2009;28:542–548. doi: 10.1002/nau.20701. [DOI] [PubMed] [Google Scholar]
- 20.Brown JS, McNaughton KS, Wyman JF, Burgio KL, Harkaway R, Bergner D, et al. Measurement characteristics of a voiding diary for use by men and women with overactive bladder. Urology. 2003 Apr;61(4):802–809. doi: 10.1016/s0090-4295(02)02505-0. DOI: http://dx.doi.org/10.1016/S0090-4295(02)02505-0. [DOI] [PubMed] [Google Scholar]