Abstract
Background. Shiga toxin (Stx) is the primary virulence factor of Stx-producing Escherichia coli (STEC). STEC can produce Stx1a and/or Stx2a, which are antigenically distinct. However, Stx2a-producing STEC are associated with more severe disease than strains producing both Stx1a and Stx2a.
Methods and Results. To address the hypothesis that the reason for the association of Stx2a with more severe disease is because Stx2a crosses the intestinal barrier with greater efficiency that Stx1a, we covalently labeled Stx1a and Stx2a with Alexa Fluor 750 and determined the ex vivo fluorescent intensity of murine systemic organs after oral intoxication. Surprisingly, both Stxs exhibited similar dissemination patterns and accumulated in the kidneys. We next cointoxicated mice to determine whether Stx1a could impede Stx2a. Cointoxication resulted in increased survival and an extended mean time to death, compared with intoxication with Stx2a only. The survival benefit was dose dependent, with the greatest effect observed when 5 times more Stx1a than Stx2a was delivered, and was amplified when Stx1a was delivered 3 hours prior to Stx2a. Cointoxication with an Stx1a active site toxoid also reduced Stx2a toxicity.
Conclusions. These studies suggest that Stx1a reduces Stx2a-mediated toxicity, a finding that may explain why STEC that produce only Stx2a are associated with more severe disease than strains producing Stx1a and Stx2a.
Keywords: Shiga toxin, Stx1a, Stx2a, Escherichia coli, STEC, oral intoxication
(See the editorial commentary by Steiner on pages 1214–5.)
Shiga toxin (Stx)–producing Escherichia coli (STEC) is a gram-negative enteric pathogen responsible for serious foodborne disease. A single serotype, O157:H7, is the most frequently reported cause of illness due to STEC in the United States [1]. The Centers for Disease Control and Prevention estimates that O157:H7 strains account for approximately 63 000 of the 175 000 estimated STEC cases each year. Cattle and other ruminants are the natural reservoir for STEC [2–4]. Infection with STEC most frequently occurs after ingestion of contaminated food, but other sources include contaminated swimming or well water or contact with cattle or infected individuals [5, 6]. The primary disease manifestation associated with STEC infection is hemorrhagic colitis. Approximately 10%–20% of individuals will progress to hemolytic uremic syndrome (HUS), a serious sequela characterized by thrombocytopenia, hemolytic anemia, and renal failure [7]. Children aged <5 years have the highest incidence of HUS, and STEC-mediated HUS is the leading cause of pediatric acute renal failure [8, 9].
STEC can encode either Stx1a and/or Stx2a, which are highly similar but antigenically distinct AB5 toxins with identical modes of action [10]. Upon binding the functional receptor, Gb3 [11], Stx is endocytosed and undergoes retrograde transport to the cytoplasm, where it acts as an N-glycosidase that inactivates ribosomes, which, in turn, stops protein synthesis and causes cell death [12]. Treatment of STEC-infected individuals is limited to supportive therapy, as antibiotics can increase the likelihood of developing HUS [7].
Epidemiological studies indicate that an individual is more likely to develop HUS if infected with an O157:H7 strain expressing Stx2a only than with strains expressing Stx1a only or both Stx1a and Stx2a [13–17]. It is not clear why the pathogenicity of a strain that produces only Stx2a is greater than that of a strain that expresses both Stx1a and Stx2a. However, we recently showed that the oral 50% lethal dose (LD50) of Stx2a in mice is 2.8 µg, whereas Stx1a-intoxicated mice failed to exhibit morbidity or mortality even at a dose of 157 µg/mouse [18]. We hypothesized, therefore, that Stx1a might have a reduced capacity to cross the intestinal barrier as compared to Stx2a. Further, we speculated that Stx1a may even interfere with the toxicity of Stx2a when both toxins are present, which, if true, might explain the reduced pathogenicity of a strain that produces both toxins. Since Stx intoxication models recapitulate at least some of the kidney pathology associated with STEC infection [18, 19], we used a murine oral intoxication model in this study to evaluate the effect of Stx1a and Stx2a cointoxication as compared to Stx2a intoxication alone. We found, to our surprise, that similar levels of Stx1a and Stx2a reach the kidney after oral intoxication. We then demonstrated that Stx1a reduced the morbidity and mortality associated with Stx2a when gavaged at the same time. These results may explain the epidemiological observation that STEC infection with Stx2a-positive strains are more severe than Stx1a/Stx2a-positive strains.
MATERIAL AND METHODS
Purification of Stx1a, SW09, and Stx2a
The toxins or toxoid were purified from E. coli DH5α lysates that contained Stx1a (expressed from pLPSH3 [20]), SW09 (an Stx1 active-site toxoid encoded on pSW09 [21]), or Stx2a (from pJES120 [22]). The proteins were purified by affinity chromatography with monoclonal antibody (Ab) raised against the B subunit of Stx1a (13C4 [23]) or Stx2a (BC5 BB12 [24]) as appropriate, over AminoLink Coupling Resin columns as described previously [18].
Stx Labeling
Stx1a and Stx2a were labeled with Alexa Fluor 750 (AF750) NHS ester (Life Technologies) according to the manufacturer's instructions. The unincorporated free dye was separated from fluorescently labeled Stx, Stx-AF750, by size-exclusion chromatography as described by Millipore (available at: http://www.millipore.com/techpublications/tech1/6djryj). The degree of labeling was determined according to the manufacture's instructions. The Vero cell cytotoxicity of Stx-AF750 was equivalent to that of unlabeled Stx (data not shown).
Mice
All mouse studies were approved by the Institutional Animal Care and Use Committee of the Uniformed Services University of the Health Sciences. These studies were conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals [25]. We used 5–6-week-old female BALB/c mice from Charles River Laboratories (Wilmington, Massachusetts). Food and water were removed 18 or 2 hours, respectively, prior to all intragastric intoxication experiments.
Ex Vivo Fluorescent Imaging
Six days prior to intoxication, mice were housed with TEK-Fresh bedding (Harlan) and provided an alfalfa-free, purified diet (TD.94048, Harlan) to eliminate chlorophyll and minimize gastrointestinal autofluorescence. Experimental animals were intoxicated intragastrically with 150 µg of Stx1a-AF750 or Stx2a-AF750, while control animals received phosphate-buffered saline (PBS) spiked with AF750. Experimental and control mice were necropsied after 6 or 24 hours. Four additional control mice that did not receive AF750 were necropsied to determine intrinsic autofluorescence. The following organs were imaged ex vivo with the Kodak In Vivo MS FX Pro (Bruker): stomach, small intestine, cecum, large intestine, kidneys, liver, spleen, heart, lungs, thymus, and brain. The excitation filter was set at 740 nm, and the emission filter was set at 790 nm, with 4 × 4 binning. Images were pseudo-colored to illustrate the intensity spectrum. Bruker Molecular Imaging Software (v.7.2.0.21148) automatically defined each organ as a region of interest (ROI), for which the mean fluorescence intensity (MFI) was determined. The MFI is calculated as [(ROI sum fluorescence) − (ROI background fluorescence)]/[ROI area], in photons/second/millimeter squared. The MFI allows for direct comparison of differentially sized ROIs. Autofluorescence control (no AF750) MFI values were subtracted from each experimental value to determine the corrected MFI. The Stx1a-AF750–corrected MFI values were multiplied by 1.04 to compensate for the difference in degree of labeling (1.82), compared with Stx2-AF750 (1.9).
Cointoxication Experiments
Stxs were diluted in PBS. Mice were intoxicated intragastrically with equal volumes of either 5.8 µg of Stx2a (2 times the Stx2a oral LD50 [18]) or 5.8 µg of Stx2a combined with Stx1a at one of the following doses: 1.16 µg (0.2 times the Stx2a dose), 5.8 µg (the Stx2a dose), 29 µg (5 times the Stx2a dose), or 58 µg (10 times the Stx2a dose). In other studies, 29 µg of Stx1a or an equal volume of PBS was delivered intragastrically 3 hours prior to intragastric intoxication with 5.8 µg of Stx2a. For some studies, mice were intoxicated with 5.8 µg of Stx2a and 29 µg of SW09 at 0 or −3 hours. Finally, mice were gavaged with 29 µg of Stx1a that had been incubated with 100 µg of the human/mouse-chimerized 13C4, cαStx1 [26], or isotype control human immunoglobulin G 1κ (huIgG1κ; Sigma) for 1 hour at 22°C, followed by intragastric intoxication with 5.8 µg of Stx2a 3 hours later. Incubation with cαStx1 resulted in a 29-fold neutralization of Stx1a in vitro, while the huIgG1 did not neutralize (data not shown). Morbidity and mortality were monitored for 18 days. In accordance with the American Veterinary Medical Association guidelines on euthanasia, any mouse that exhibited signs of extreme morbidity was humanely euthanized. In this study, no mice were euthanized because of extreme morbidity.
Serum Biochemistry and Histopathologic Analyses
We collected serum and necropsied kidneys from mice as previously published [18]. A portion of the serum was used to determine the level of neutrophil gelatinase-associated lipocalin (NGAL) with the Quantikine enzyme-linked immunosorbent assay (RnDsystems). The rest of the serum sample was sent to VRL (Rockville, Maryland) for performance of a panel of renal chemistry analyses. Kidney sections were prepared as described previously and stained with hematoxylin-eosin (H-E) or left unstained [18]. H-E–stained slides were read by a veterinary pathologist blinded to study group identifiers.
Immunodot Blots
Relative expression levels of Stx1a and Stx2a from STEC strains 933 [27] and 2812 [28] were determined as previously described [29]. Briefly, overnight cultures grown in Luria Bertani broth were sonicated (10 seconds on and 20 seconds off, for 4 minutes) and centrifuged (20 000g), and the supernatant was filtered (0.22 µm). Lysates were serially diluted 1:2 in PBS, and 300 µL of each dilution was applied to a 0.45-μm nitrocellulose membrane contained within a 96-well Minifold I Dot-Blot apparatus (Whatman). Primary Abs, polyclonal rabbit α-Stx2a [30], and α-Stx1a [31], were precleared against an Stx-negative O157:H7 strain and diluted 1:154 or 1:200, respectively, to normalize for differences in Ab reactivity. The secondary Ab was goat anti-rabbit horseradish peroxidase diluted 1:200. Stx expression was determined from the integrated pixel density of the largest positive dilution with Adobe Photoshop CS5 Extended (version 12.0 × 32).
Immunofluorescence
Unstained kidney slides were deparaffinized and processed as described previously [18]. The primary Abs used were cαStx1 and polyclonal rabbit anti-Stx2a. Secondary Abs were goat anti-human Alexa Fluor 488 or goat anti-rabbit Alexa Fluor 594. All Abs were diluted 1:500 in Ab diluent. Slides were counterstained with 4’,6-diamidino-2-phenylindole and observed on an Olympus BX60 microscope with a BX-FLA fluorescence attachment.
RESULTS
Similar Levels of Stx1a-AF750 and Stx2a-AF750 Disseminate to the Kidneys
To test the hypothesis that Stx1a is less toxic than Stx2a by the oral route because that toxin transits less efficiently than Stx2a from the intestine to the kidney, we fluorescently labeled both toxins and gavaged them individually into mice. Both Stx1a-AF750 and Stx2a-AF750 were observed in systemic organs 6 hours after intoxication (Figure 1A and 1B) and remained at 24 hours (Supplementary Figure 1A and 1B), although the overall intensity was reduced at the later time point. The greatest fluorescent intensity was located in the kidneys for both Stx1a-AF750 (Figure 1A) and Stx2a-AF750 (Figure 1B). Fluorescence was also detected in the liver and occasionally the spleen; however, the intensity was minimal and not statistically different (P > .093) from that observed in AF750-spiked PBS–treated controls (Figure 1A and 1B). The fluorescent signal from the gastrointestinal tract was saturated for both Stx-AF750 and AF750-spiked PBS–treated controls (data not shown).
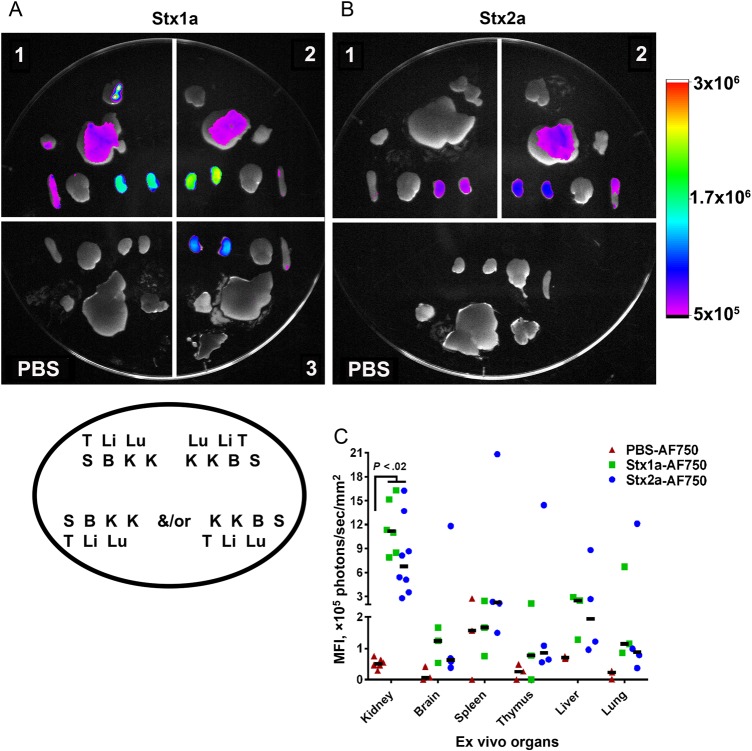
Figure 1.
Fluorescence of ex vivo organs from mice intoxicated with Shiga toxin 1a (Stx1a)–AF750 (A) and Stx2a-AF750 (B) 6 hours after intoxication. Stx1a and Stx2a exhibited a similar dissemination pattern 6 hours after intoxication, with the greatest fluorescent intensity in the kidneys. A and B, Each number represents an individual experimental mouse; Stx1a-AF750: n = 3; Stx2a-AF750: n = 4 (2 biological replicates; the second replicate is depicted). Organ placement for all samples follow the legend depicted in the oval under panel A. B, brain; K, kidney; Li, liver; Lu, lung; S, spleen; T, thymus. The intensity scale to the right is equivalent for both images. C, Quantification of fluorescent intensity of ex vivo organs. The mean fluorescence intensity (MFI) was statistically greater in the kidneys of Stx1a-intoxicated and Stx2a-intoxicated mice as compared to phosphate-buffered saline (PBS)–treated controls (P < .02). There was no statistical difference between Stx1a-AF750 and Stx2a-AF750 in any organ (P > .08). Statistical significance was determined by the Kruskal–Wallis test with the Dunn correction for multiple comparisons. Each symbol is an individual organ; left and right kidneys are included separately. The black bar represents the median value.
To quantify organ fluorescence, we determined the MFI from organs at 6 hours (Figure 1C) and 24 hours after intoxication (Supplementary Figure 1C). The MFI at 6 hours was significantly greater in the kidneys of Stx1a-AF750–intoxicated and Stx2a-AF750–intoxicated animals as compared to PBS-treated controls (P < .02; Figure 1C). There was no difference in the amount of fluorescence from kidneys of mice intoxicated with Stx1a-AF750 or Stx2a-AF750 (P > .8). For all other organs, the MFI for animals gavaged with Stx1a-AF750, Stx2a-AF750, or AF750 PBS was similar at the 6 hours time point. The same pattern of Stx-AF750 dissemination was observed at 24 hours after intoxication (Supplementary Figure 1C).
Stx1a and SW09 Reduce Stx2a Toxicity After Intragastric Cointoxication
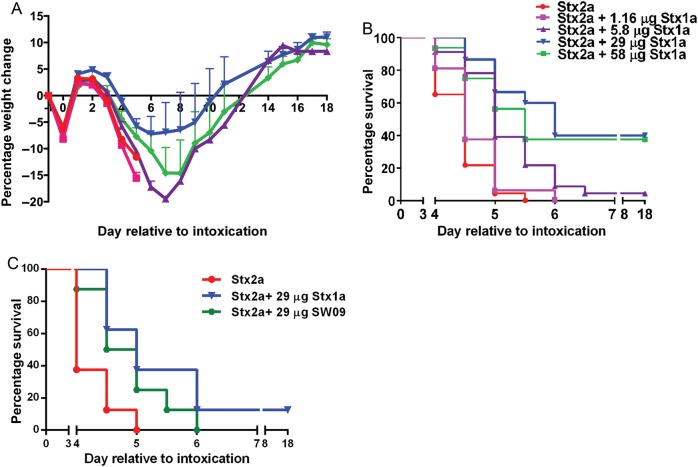
Since equivalent amounts of Stx1a-AF750 and Stx2a-AF750 transited to the kidneys, we next assessed whether Stx1a impedes the toxicity of Stx2a. We orally intoxicated mice with 5.8 µg of Stx2a in combination with Stx1a at multiple concentrations or with Stx2a only. All mice were gavaged with an equal volume of toxin or toxins. Mice intoxicated with only Stx2a lost weight faster (Figure 2A) and had a significantly shorter mean time to death (MTD) as compared to the cointoxication cohorts (P = .0001; Figure 2B). There was a trend toward decreased weight loss as the Stx1a concentration increased, up to 29 µg, especially on days 5–10 (Figure 2A). Furthermore, the groups that received 5.8, 29, or 58 µg of Stx1a in combination with Stx2a exhibited a significant extension in MTD, compared with the group that received Stx2a (P < .0001; Figure 2B). The extension of the MTD was Stx1a-dose dependent, as determined by the log-rank test for trend (P < .0001; Figure 2B). The greatest effect of cointoxication (ie, reduced weight loss and extension of MTD) was seen in the group that received 29 µg of Stx1a (5 times the Stx2a dose). Cointoxication with Stx2a and the Stx1 toxoid, SW09, also significantly extended the MTD as compared to Stx2a alone (P = .032; Figure 2C). Additionally, SW09 was equivalent to Stx1a in the capacity to extend the survival of mice intoxicated with a lethal dose of Stx2a (Figure 2C).
Figure 2.
Shiga toxin 1a (Stx1a) and SW09 delayed Stx2a toxicity after intragastric cointoxication as compared to Stx2a alone. A, Mean percentage weight change in the experimental groups over the course of the experiment. The sample size was 16–23 mice for each group over 3 biological replicates. Error bars indicate standard error of the mean. B, Mouse survival percentage over time for each group. Stx1a significantly increased the mean time to death, as determined by the log-rank test for trend (P = .0001). Cointoxication with Stx2a and 5.8, 29, or 58 µg of Stx1a significantly extended the mean time to death as compared to intragastric intoxication with Stx2a alone (P < .0001). C, Mean time to death after cointoxication with SW09 and Stx2a was significantly delayed as compared to that after intoxication with Stx2a alone (P = .032). Survival was equivalent after cointoxication with Stx2a and either SW09 or Stx1a (P = .18).
Renal Serum Biochemistry Values Trend Toward Normal After Cointoxication, Compared With Intoxication With Stx2a Alone
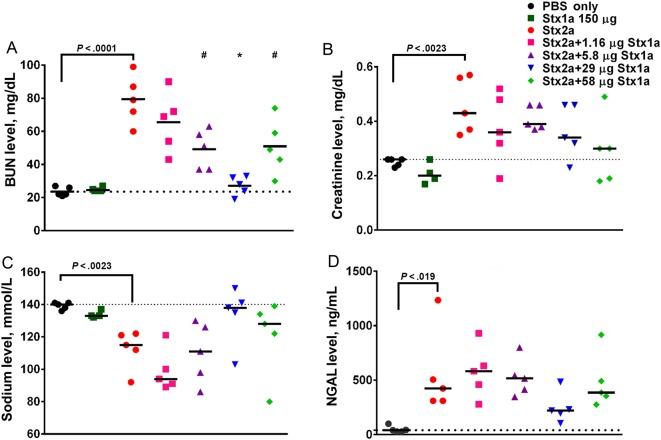
We first determined the effect of oral intoxication with 150 µg of Stx1a alone in serum biochemistry analyses since that dose does not cause morbidity or mortality [18]. Stx1a did not affect the BUN, creatinine, or sodium biochemistry values at that dose (Figure 3A–C). To assess kidney function in cointoxicated animals, we collected serum from the mice 72 hours after intoxication, when Stx2a-mediated renal damage would be expected but before the point at which the damage would be lethal, to try to maximize the difference(s) among the groups. As we showed previously [18], intragastric intoxication with Stx2a alone significantly increased the BUN and creatinine levels and reduced the sodium levels, compared with values for PBS-treated controls (P < .023; Figure 3A–C). However, cointoxication with Stx2a and increasing concentrations of Stx1a significantly lowered the BUN levels (P < .016; Figure 3A) while the creatinine (Figure 3B) and sodium (Figure 3C) levels trended toward control values. A similar trend toward control values in cointoxicated mice as compared to those that received Stx2a alone was observed for chloride and potassium (Supplementary Figure 2A and 2B). The renal injury biomarker NGAL was elevated after intoxication with Stx2a as compared to PBS (P = .019). However, there was no difference in NGAL levels between the Stx2a and cointoxicated groups. Nevertheless, cointoxication with 29 µg of Stx1a prevented a significant increase in NGAL, compared with findings for PBS-treated controls (P = .53; Figure 3D). Additionally, cointoxication with 29 µg of Stx1a resulted in the lowest combined pathology score, which represents the least renal damage, of the mice that received Stx2a (Supplementary Figure 3).
Figure 3.
Renal panel serum biochemistry values trended toward phosphate-buffered saline (PBS) control values after intragastric cointoxication with Shiga toxin 1a (Stx1a) as compared to intoxication with Stx2a alone. A, Intragastric intoxication with Stx2a significantly increased the blood urea nitrogen (BUN) values as compared to those in the PBS-treated control (P < .0001, by the t test). Cointoxication with 5.8 or 58 µg of Stx1a resulted in significantly lower BUN values (P < .016). Cointoxication with 29 µg of Stx1a significantly lowered the BUN values, compared with Stx2a alone (P < .0001), and resulted in BUN values equivalent to those for control mice (P = .973). B, Administration of Stx2a significantly increased the creatinine level above that for PBS-treated control mice (P = .0023, by the t test). Cointoxication with Stx1a resulted in a decrease of creatinine values toward control levels. C, The sodium levels significantly decreased after Stx2a intoxication, compared with those in PBS-treated controls (P = .0013, by the t test). Cointoxication with Stx1a resulted in a trend toward normal sodium values, with the greatest effect seen in the group that received 29 µg. D, Stx2a neutrophil gelatinase associated-lipocalin (NGAL) levels were significantly elevated, compared with those in PBS-treated controls, as measured by the t test (P = .019). There was no significant difference between the Stx2a group and any of the cointoxicated groups (0.274); however, when compared to the PBS-treated controls, NGAL values in all groups were significantly elevated (P < .03), except after cointoxication with Stx2a and 29 µg Stx1a, which resulted in NGAL values equivalent to those of control mice (P = .53). Serum was collected 3 days after intoxication. Each symbol represents an individual animal; the black bar represents the median value of the group. The dotted line represents the median value of the PBS-treated control group. The statistical difference between the PBS and Stx2a recipients was determined by a 2-tailed t test. The difference between the Stx2a and the cointoxicated groups was determined by 1-way analysis of variance. *P < .0001 and **P < .016.
Stx1a and Stx2a Are Found in Distinct Locations in Sections of Kidney From Cointoxicated Mice
We first stained sections of kidney from mice intragastrically intoxicated with 150 µg of Stx1a alone (Supplementary Figure 4A). Stx1a appeared to stain distal tubule epithelial cells, the same location that we previously identified for Stx2a [18]. Next, we stained a kidney section for both Stx1a and Stx2a from a cointoxicated mouse. Both Stxs were bound to tubule epithelial cells, but Stx1a and Stx2a were located at discrete locations in the section (Supplementary Figure 4B).
Cointoxication With Delivery of Stx1a 3 Hours Before Stx2a Intoxication Further Reduces Stx2a-Mediated Toxicity
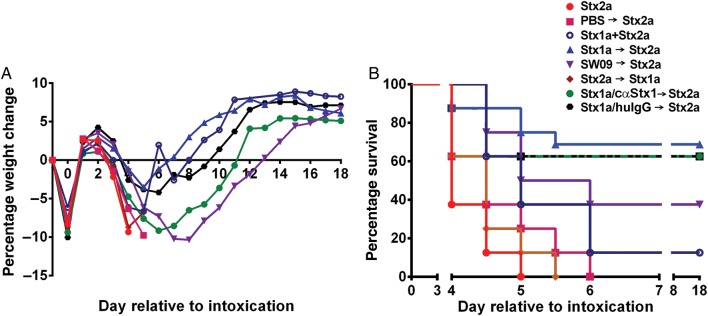
We next asked whether the effect of cointoxication with Stx1a could be amplified if the toxin was given 3 hours prior to Stx2a intragastric intoxication (Figure 4A and 4B). Mice intoxicated with Stx1a 3 hours prior to Stx2a receipt showed decreased weight loss (Figure 4A) and exhibited a significant extension in MTD (P = .016; Figure 4B) as compared to mice given the toxins at the same time. Additionally, mice intoxicated with SW09 3 hours before Stx2a receipt had an equivalent extension in MTD as those that received Stx1a (P = .16). One mouse was responsible for the extreme weight loss denoted by the mean value for the SW09 group; otherwise, that group had a mean weight loss equivalent to that of the group that received Stx1a. There was no difference in weight loss (Figure 4A) or survival (Figure 4B) among mice that received either Stx2a only, PBS prior to Stx2a, or Stx2a prior to Stx1a (P > .07). Finally, partial neutralization of Stx1a by cαStx1 resulted in a trend toward greater weight loss as compared to the group that received huIgG1 as control (Figure 4A), although survival in those groups was equivalent (Figure 4B).
Figure 4.
Addition of Shiga toxin 1a (Stx1a) 3 hours prior to Stx2a intoxication amplified the cointoxication morbidity and survival benefits. A, Mean percentage weight change over time. B, Mouse survival over the course of the experiment. Survival was equivalent after Stx2a intoxication, whether mice received phosphate-buffered saline (PBS) 3 hours prior to or at the time of intoxication (P = .07). Delivery of Stx2a 3 hours prior to Stx1a also resulted in survival equivalent to that of Stx2a delivery alone (P = .19). Stx1a intoxication 3 hours prior to Stx2a intoxication significantly improved survival as compared to concurrent cointoxication (P = .016). When delivered 3 hours prior to Stx2a, Stx1a and SW09 equivalently extended survival (P = .16). Stx1a preincubation with either cαStx1 or human immunoglobulin G (huIgG) had no effect on survival as compared to Stx1a, delivered 3 hours prior to Stx2a (P = .69 and P = .76, respectively). The results represent 1–2 biological replicates with 8–16 mice per group. The plus sign indicates cointoxication at same time; the arrow indicates 3 hours between Stx intoxication.
Stx1a Is Expressed at Higher Levels Than Stx2a in Wild-Type STEC
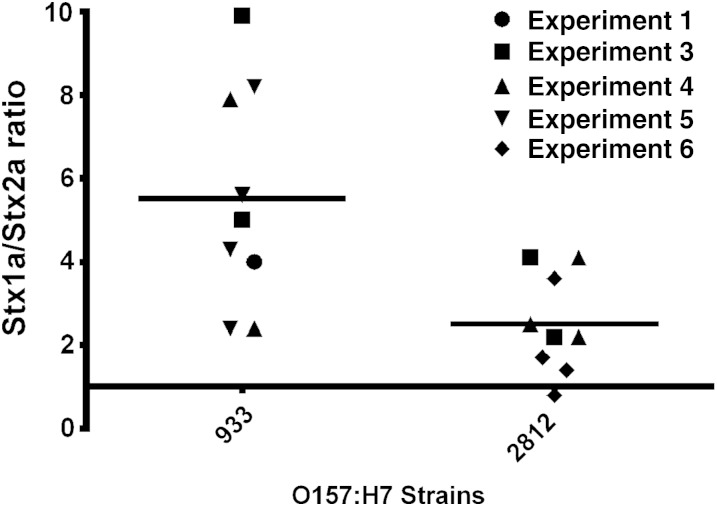
We next determined whether the ratios of Stx1a and Stx2a used to cointoxicate mice were physiologically relevant for wild-type STEC strains. We tested expression levels from whole culture sonic lysates to account for cell-associated and secreted Stx. Stx1a expression was approximately 2.7-fold to 5.4-fold greater than Stx2a expression in the strains (Figure 5); however, strain 933 expressed greater overall levels of Stx than strain 2812 (data not shown).
Figure 5.
Shiga toxin 1a (Stx1a) is expressed at higher levels than Stx2a in wild-type Stx-producing Escherichia coli strains. The mean Stx1a to Stx2a ratio (±SD) for strain 933 was 5.4 ± 0.7, while the mean ratio for strain 2812 was 2.7 ± 0.4. Results are representative of 9 independent cultures from 3 or 4 biological replicates grown for 18–20 hours.
DISCUSSION
The major findings of this study are that equivalent concentrations of Stx1a and Stx2a transit to the kidney after intragastric intoxication and that Stx1a reduces Stx2a-mediated toxicity after intragastric cointoxication. In addition, we found that more Stx1a than Stx2a is produced by STEC, so the cointoxication ratios used in this study are physiologically relevant. Combined, these results help to explain the epidemiological observation that outbreaks associated with Stx2a-only STEC strains are more severe than outbreaks with strain positive for both Stx1a and Stx2a.
The cointoxication model simulated the reduced severity of symptoms associated with SteC strain positive for Stx1a and Stx2a as compared to those positive for Stx2a only. We used multiple ratios of Stx1a to determine the dose with the greatest effect. There was a dose response, such that increased concentrations of Stx1a were associated with increased survival. Additionally, extension of survival after cointoxication with the Stx1a toxoid and Stx2a was equivalent to that observed after administration of Stx1a and Stx2a. This latter result supports our belief that the Stx1a B subunit is responsible for interference of Stx2a-mediated toxicity. However, we are not sure why morbidity appeared to be greater when10 times more Stx1a than Stx2a was delivered, compared with 5 times more Stx1a. It is possible that the high combined concentration of total Stx resulted in the toxic effect that, nevertheless, was still lower than that of Stx2a alone.
We did not anticipate that equal concentrations of Stx1a and Stx2a would disseminate to the kidneys, because we did not observe morbidity or mortality after intragastric intoxication with up to 157 µg of Stx1a, a dose 50 times the Stx2a intragastric LD50 [18]. We are confident that the observed fluorescence is representative of the Stx concentration, since the MFI from control animals gavaged with AF750-spiked PBS was minimal, a finding that suggests that unincorporated free dye is unable to transit out of the intestine. Contrary to a previous report [32], we did not observe elevated levels of Stx1a in the lungs or brain; however, the difference between our results and those of the earlier publication may be due to the method of intoxication (ie, intragastric vs intravenous). Our results suggest that the lack of Stx1a-mediated oral toxicity is not due to dispersed receptor binding and sequestration from the kidneys.
Since cointoxication extended MTD as compared to intoxication by Stx2a alone, we hypothesized that kidney function, as defined by renal serum biochemistry findings, would also be improved in cointoxicated mice. We found that cointoxication with Stx2a and Stx1a prevented some of the change in biochemistry values, compared with mice intoxicated with Stx2a only, with a trend toward PBS-treated control levels. There was a dose response, such that the serum biochemistry values of the group intoxicated with 5 times more Stx1a than Stx2a were the closest to control levels. The histology scores from those same animals were low overall, due to the early time point of necropsy for serum collection, but the 29-µg (5 times) Stx1a cointoxication group had the lowest combined pathology score. The results from the survival studies combined with kidney function support our hypothesis that Stx1a interferes with Stx2a toxicity.
Although we do not know the exact mechanism responsible for the increased survival after cointoxication, it is likely that the delay in or prevention of Stx2a-mediated toxicity occurs at the target site of the kidney. Support for this prediction is that ex vivo imaging detected both Stxs at equal concentrations in the kidneys but not together in other organs. We demonstrated that the Stx1a B subunit is responsible for the increased survival after cointoxication, since the Stx1a toxoid protected mice from Stx2a. We hypothesize, therefore, that Stx1a reduces Stx2a toxicity by interfering with receptor binding or by altering internalization/trafficking in the target endothelial cells of the kidney. It is known that the localization and composition of Gb3 is critical for Stx binding [11, 33–38], that Stx1a can induce a change in the Gb3 membrane composition to promote lipid raft formation [39], and that Stx1a is more strongly associated with lipid rafts than Stx2a [40]. We believe that, since high levels of Stx1a are required for mouse lethality, the cointoxicated mice survive the Stx1a insult and do not succumb to Stx2a, since it is prevented from binding and uptake. To explain those cases for which there is delay in death after cointoxication, we propose that once sufficient Stx1a is endocytosed into the cell and the toxin receptors are recycled, Stx2a would be able to bind the now available Gb3, be internalized, and cause toxicity. Another possible mechanism by which Stx1a could impede Stx2a would be with a delay in transit out of the gastrointestinal tract. Although it has been shown that excess Stx2a does not affect Stx1a translocation across intestinal epithelial cells [41], the effect of excess Stx1a on translocation of Stx2a has not been reported. We could not address the question of whether Stx1a inhibits Stx2a translocation from the gut with Stx1a-AF750 and Stx2a-AF750 because that study would require extremely large quantities of the toxins to be able to detect the toxins in the kidney.
When we delivered Stx1a 3 hours prior to Stx2a, we were able to further reduce Stx2a-mediated toxicity as compared to when the toxins were given at the same time. The 3-hour lead time may allow Stx1a to transit to the site of action or saturate translocation pathways. There was no survival benefit when Stx2a was delivered prior to Stx1a, which indicates that Stx1a cannot rescue a mouse from Stx2a, a finding that further supports the hypothesis that Stx1a may interfere with receptor binding or selection.
The Stx expression profiles of wild type STEC strains 933 and 2812 support our cointoxication studies. Both strains expressed greater concentrations of Stx1a than Stx2a, although the ratio of Stx1a to Stx2a was greater for strain 933 than for strain 2812. Of note is the fact that the outbreak associated with 933 resulted in no HUS cases, whereas the 2812-linked illnesses caused 45 cases of HUS and 3 deaths [27, 42]. Our results suggest that the percentage of patients infected with STEC who progress to HUS could be affected by the ratio of Stx1a and Stx2a produced by the infecting strain.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Mark Smith, VMD, for his pathology review; and Dr Cara Olsen, for facilitation of statistical analyses.
Disclaimer. The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense, the Uniformed Services University of the Health Sciences, or the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH (grant R37 AI020148 to A. D. O.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ et al. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis 2011; 17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslam M, Nattress F, Greer G, Yost C, Gill C, McMullen L. Origin of contamination and genetic diversity of Escherichia coli in beef cattle. Appl Environ Microbiol 2003; 69:2794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GL, Hollingsworth J, Morris JG Jr. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol Rev 1996; 18:29–51. [DOI] [PubMed] [Google Scholar]
- 4.Hancock DD, Besser TE, Rice DH, Tarr PI. Ecology of Escherichia coli O157:H7 in cattle and impact of management practices. In: Kaper JB, O'Brien AD, eds. Escherichia coli O157:H7 and other Shiga toxin-producing E coli strains. Washington, DC: American Society for Microbiology, 1998:85–91. [Google Scholar]
- 5.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg Infect Dis 2005; 11:603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton BC, Jones TF, Vugia DJ et al. Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996–2005. J Infect Dis 2011; 204:263–7. [DOI] [PubMed] [Google Scholar]
- 7.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005; 365:1073–86. [DOI] [PubMed] [Google Scholar]
- 8.Gerber A, Karch H, Allerberger F, Verweyen HM, Zimmerhackl LB. Clinical course and the role of shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997–2000, in Germany and Austria: a prospective study. J Infect Dis 2002; 186:493–500. [DOI] [PubMed] [Google Scholar]
- 9.Spinale JM, Ruebner RL, Copelovitch L, Kaplan BS. Long-term outcomes of Shiga toxin hemolytic uremic syndrome. Pediatr Nephrol 2013. [DOI] [PubMed] [Google Scholar]
- 10.Fraser ME, Fujinaga M, Cherney MM et al. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem 2004; 279:27511–7. [DOI] [PubMed] [Google Scholar]
- 11.Lingwood CA. Role of verotoxin receptors in pathogenesis. Trends Microbiol 1996; 4:147–53. [DOI] [PubMed] [Google Scholar]
- 12.Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem 1988; 171:45–50. [DOI] [PubMed] [Google Scholar]
- 13.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol 1999; 37:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleanthous H, Smith HR, Scotland SM et al. Haemolytic uraemic syndromes in the British Isles, 1985–8: association with verocytotoxin producing Escherichia coli. Part 2: Microbiological aspects. Arch Dis Child 1990; 65:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orth D, Grif K, Khan AB, Naim A, Dierich MP, Wurzner R. The Shiga toxin genotype rather than the amount of Shiga toxin or the cytotoxicity of Shiga toxin in vitro correlates with the appearance of the hemolytic uremic syndrome. Diagn Microbiol Infect Dis 2007; 59:235–42. [DOI] [PubMed] [Google Scholar]
- 16.Neupane M, Abu-Ali GS, Mitra A, Lacher DW, Manning SD, Riordan JT. Shiga toxin 2 overexpression in Escherichia coli O157:H7 strains associated with severe human disease. Microb Pathog 2011; 51:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scotland SM, Willshaw GA, Smith HR, Rowe B. Properties of strains of Escherichia coli belonging to serogroup O157 with special reference to production of Vero cytotoxins VT1 and VT2. Epidemiol Infect 1987; 99:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo LM, Melton-Celsa AR, Smith MA, Smith MJ, O'Brien AD. Oral intoxication of mice with Shiga toxin type 2a (Stx2a) and protection by anti-Stx2a monoclonal antibody 11E10. Infect Immun 2014; 82:1213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pai CH, Kelly JK, Meyers GL. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun 1986; 51:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tesh VL, Samuel JE, Perera LP, Sharefkin JB, O'Brien AD. Evaluation of the role of Shiga and Shiga-like toxins in mediating direct damage to human vascular endothelial cells. J Infect Dis 1991; 164:344–52. [DOI] [PubMed] [Google Scholar]
- 21.Wen SX, Teel LD, Judge NA, O'Brien AD. Genetic toxoids of Shiga toxin types 1 and 2 protect mice against homologous but not heterologous toxin challenge. Vaccine 2006; 24:1142–8. [DOI] [PubMed] [Google Scholar]
- 22.Lindgren SW, Melton AR, O'Brien AD. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect Immun 1993; 61:3832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strockbine NA, Marques LR, Holmes RK, O'Brien AD. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun 1985; 50:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downes FP, Barrett TJ, Green JH et al. Affinity purification and characterization of Shiga-like toxin II and production of toxin-specific monoclonal antibodies. Infect Immun 1988; 56:1926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. National Research Council CftUotGftCaUoLA, Institute for Laboratory Animal Research. Guide for the care and use of laboratory animals. 8 ed Washington, DC: National Academies Press, 2011. [Google Scholar]
- 26.Edwards AC, Melton-Celsa AR, Arbuthnott K et al. Vero cell neutralization and mouse protective efficacy of humanized monoclonal antibodies against Escherichia coli toxins Stx1 and Stx2. In: Kaper JB, O'Brien AD, eds. Escherichia coli O157:H7 and other Shiga toxin-producing E coli strains. Washington, DC: ASM Press, 1998:388–92. [Google Scholar]
- 27.Riley LW, Remis RS, Helgerson SD et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med 1983; 308:681–5. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien AD, Melton AR, Schmitt CK, McKee ML, Batts ML, Griffin DE. Profile of Escherichia coli O157:H7 pathogen responsible for hamburger- borne outbreak of hemorrhagic colitis and hemolytic uremic syndrome in Washington. J Clin Microbiol 1993; 31:2799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zangari T, Melton-Celsa AR, Panda A et al. Enhanced virulence of the Escherichia coli O157:H7 spinach-associated outbreak strain in two animal models is associated with higher levels of Stx2 production after induction with ciprofloxacin. Infect Immun 2014; 82:4968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindgren SW, Samuel JE, Schmitt CK, O'Brien AD. The specific activities of Shiga-like toxin type II (SLT-II) and SLT-II-related toxins of enterohemorrhagic Escherichia coli differ when measured by Vero cell cytotoxicity but not by mouse lethality. Infect Immun 1994; 62:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien AD, LaVeck GD, Griffin DE, Thompson MR. Characterization of Shigella dysenteriae 1 (Shiga) toxin purified by anti-Shiga toxin affinity chromatography. Infect Immun 1980; 30:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutjes NW, Binnington BA, Smith CR, Maloney MD, Lingwood CA. Differential tissue targeting and pathogenesis of verotoxins 1 and 2 in the mouse animal model. Kidney Int 2002; 62:832–45. [DOI] [PubMed] [Google Scholar]
- 33.Ali S, Brockman HL, Brown RE. Structural determinants of miscibility in surface films of galactosylceramide and phosphatidylcholine: effect of unsaturation in the galactosylceramide acyl chain. Biochemistry 1991; 30:11198–205. [DOI] [PubMed] [Google Scholar]
- 34.Arab S, Lingwood CA. Intracellular targeting of the endoplasmic reticulum/nuclear envelope by retrograde transport may determine cell hypersensitivity to verotoxin via globotriaosyl ceramide fatty acid isoform traffic. J Cell Physiol 1998; 177:646–60. [DOI] [PubMed] [Google Scholar]
- 35.Kiarash A, Boyd B, Lingwood CA. Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues. J Biol Chem 1994; 269:11138–46. [PubMed] [Google Scholar]
- 36.Pellizzari A, Pang H, Lingwood CA. Binding of Verocytotoxin 1 to its receptor is influenced by differences in receptor fatty acid content. Biochemistry 1992; 31:1363–70. [DOI] [PubMed] [Google Scholar]
- 37.Bauwens A, Betz J, Meisen I, Kemper B, Karch H, Muthing J. Facing glycosphingolipid-Shiga toxin interaction: dire straits for endothelial cells of the human vasculature. Cell Mol Life Sci 2013; 70:425–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obrig TG, Louise CB, Lingwood CA, Boyd B, Barley-Maloney L, Daniel TO. Endothelial heterogeneity in Shiga toxin receptors and responses. J Biol Chem 1993; 268:15484–8. [PubMed] [Google Scholar]
- 39.Falguieres T, Romer W, Amessou M et al. Functionally different pools of Shiga toxin receptor, globotriaosyl ceramide, in HeLa cells. FEBS J 2006; 273:5205–18. [DOI] [PubMed] [Google Scholar]
- 40.Tam P, Mahfoud R, Nutikka A et al. Differential intracellular transport and binding of verotoxin 1 and verotoxin 2 to globotriaosylceramide-containing lipid assemblies. J Cell Physiol 2008; 216:750–63. [DOI] [PubMed] [Google Scholar]
- 41.Hurley BP, Jacewicz M, Thorpe CM et al. Shiga toxins 1 and 2 translocate differently across polarized intestinal epithelial cells. Infect Immun 1999; 67:6670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell BP, Goldoft M, Griffin PM et al. A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experience. JAMA 1994; 272:1349–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.