Abstract
Background. Toll-like receptor 4 (TLR4) is a critical receptor involved in the sensing of gram-negative bacterial infection. However, the roles of TLR4 in sepsis are cell-type specific. Dendritic cells (DCs) are known to play a central role in microbial detection, alerting the immune system to the presence of infection and coordinating adaptive immune response. The goal of this study was to investigate the impact of DC-specific TLR4 signaling on host defense against intra-abdominal polymicrobial sepsis.
Methods. C57BL/6, global Tlr4 knockout, cell-specific knockout control, and CD11c-specific Tlr4-/- mice underwent cecal ligation and puncture (CLP).
Results. Specific deletion of TLR4 on DCs in mice improved survival and enhanced bacterial clearance. Deletion of TLR4 on DCs was associated with lower levels of circulating interleukin 10 (IL-10), higher polymorphonuclear leukocyte (PMN) accumulation in the peritoneal cavity, and higher expression of chemokine (C-X-C motif) receptor 2 (CXCR2) on PMNs after CLP. In vitro studies of DC and neutrophil cocultures confirmed that TLR4-dependent secretion of IL-10 from DCs regulated neutrophil CXCR2 expression.
Conclusions. Our data shed light on a previously unrecognized role for TLR4 signaling on DCs in driving IL-10 secretion during sepsis and, through this pathway, regulates PMN recruitment via suppression of CXCR2 expression.
Keywords: TLR4, dendritic cell, CLP, sepsis, PMN recruitment, IL-10, CXCR2
Polymicrobial intra-abdominal infections resulting from perforation and/or necrosis of the gastrointestinal tract are an important etiology of sepsis. Failure to clear the infections leads to septic shock resulting from sustained and excessive activation of a systemic inflammatory response [1]. Toll-like receptor 4 (TLR4) plays a central role in the response to intra-abdominal sepsis through the sensing of lipopolysaccharide (LPS) from gram-negative bacteria abundant in enteric microbes. Although TLR4 seemed like an attractive therapeutic target, a trial testing a TLR4/MD2 antagonist in patients with severe sepsis or septic shock failed to improve 28-day mortality [2]. The reasons for the lack of benefit can be ascribed to redundancies between innate immune sensing pathways and to a failure to appreciate the diverse and cell-specific functions of TLR4 in the host response during bacterial sepsis.
TLR4 is expressed on different subsets of immune and nonimmune cells [3–6], and the function of TLR4 varies by cell type. For example, we have shown that TLR4 is essential to LPS uptake by hepatocytes [7–9]. In clear distinction, TLR4 activation in macrophages leads to enhanced phagocytosis and inflammatory mediator production [7, 10]. To our knowledge, the contribution of TLR4 on other relevant cell types to the host response to sepsis has not been investigated.
Dendritic cells (DCs) play an essential role in detecting microbial invasion and in guiding host antimicrobial responses [11]. CD11c+ DCs become activated in the spleen and then lymph nodes by 8 hours following cecal ligation and puncture (CLP) [12], a model that recapitulates the features of intra-abdominal sepsis due to gastrointestinal perforation. Furthermore, the percentages of splenic and peritoneal DCs precipitously drop within 24 hours after CLP [13, 14]. Deletion of CD11c+ DCs results in greater mortality after CLP, confirming the key role of these professional antigen-presenting cells to the host survival response in sepsis [15]. The specific function of DC subsets or pattern-recognition receptors on DCs in the initial host response to intra-abdominal sepsis has not been defined. We show here that selective deletion of TLR4 from CD11c+ DCs results in enhanced bacterial clearance following CLP-induced sepsis. This appears to be a consequence of the downregulation of CXCR2 on polymorphonuclear leukocytes (PMNs) by DC-derived interleukin 10 (IL-10), leading to impaired PMN chemotaxis. This finding demonstrates the central role for DCs in regulating PMN responses in intra-abdominal sepsis and the specific role of TLR4 in promoting IL-10 production in CD11c+ DCs.
MATERIALS AND METHODS
Reagents
Ultrapure LPS (Escherichia coli 0111:B4) was obtained from List Biological Laboratories. Low-molecular-weight polyinosinic-polycytidylic acid was purchased from InvivoGen. Neutralizing IL-10 antibody (Ab; clone IESS-2A5) was from eBioscience, while mouse immunoglobulin G1 isotype control Ab and recombinant IL-10 were obtained from BD Bioscience.
Animals
Animal protocols were approved by the Animal Care and Use Committee of the University of Pittsburgh, and the experiments were performed in strict adherence to the National Institutes of Health Guidelines for the Use of Laboratory Animals. Male C57BL/6 (wild type [WT]) and CD11c-Cre mice were specific-pathogen-free animals, weighed approximately 25 g, and were from Jackson Laboratories. Male Tlr4loxP/loxP (Flox) mice, cell-specific (DC-Tlr4-/-) mice, and global Tlr4 knockout (Tlr4-/-) mice were bred and cohoused at our facility and used at the age of 8–12 weeks. All mice developed were on a C57BL/6 genetic background. Flox and cellular-specific Tlr4-/- mice were generated as previously described [7, 16].
CLP
Sepsis was induced by CLP as previously described [7]. In brief, laparotomy was performed under isoflurane anesthesia (Piramal Critical Care). The cecum (0.5 cm) was ligated and punctured twice with a 22-gauge needle. In some experiments, mice also received antibiotics (Primaxin 25 mg/kg; Merck) subcutaneously every 12 hours, starting 2 hours after CLP. At the study end point, mice were anesthetized with isoflurane and euthanized by opening the chest cavity and withdrawing blood by cardiac puncture.
Isolation of Splenic DCs and PMNs
Mouse spleens were collected under sterile conditions and cut into pieces. The spleen pieces were transferred into collagenase (1 mg/mL in Roswell Park Memorial Institute [RPMI] 1640 medium) and incubated at 37°C for 1 hour. The collagenase solution containing splenocytes were filtered through a 70-µm cell strainer. Erythrocytes were lysed with lysis solution. Cells were spun down at 800g for 5 minutes and washed twice in phosphate-buffered saline (PBS). Cells were resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1000 UI/mL penicillin-streptomycin. The splenocytes were further separated by using MACS magnetic bead isolations (Miltenyi Biotec). Cells were mixed with anti-CD11c or Ly6G magnetic beads to positively select for DCs and PMNs, respectively.
In Vitro Generation of DCs
Bone marrow specimens from the femurs and tibias of WT or Tlr4-/- mice were collected under sterile conditions and incubated with collagenase (1 mg/mL in RPMI 1640 medium) at 37°C for 1 hour. The collagenase solution containing released cells was filtered through a 70-µm cell strainer. Cells were spun down at 800g for 5 minutes. Erythrocytes were lysed with lysis solution. Erythroid cells, T and B lymphocytes, natural killer (NK) cells, and granulocytes were removed by incubation with anti-TER-119, CD3, B220, NK-1.1, and GR1, followed by rabbit complement (Cederlane). Bone marrow cells were cultured in RPMI 1640 medium with 10% FBS, glutamine, nonessential amino acids, sodium pyruvate, HEPES, 2-mercaptoethanol, pen strep, granulocyte-macrophage colony-stimulating factor (20 ng/mL; R&D Systems), and interleukin 4 (10 ng/mL; Peprotech). Medium and cytokines were renewed every 2 days. Immature DCs were purified after 6 days of culture by positive isolation with MACS beads labeled with anti-CD11c (Milteny Biotec).
Adoptive Transfer of DCs
Bone marrow–derived DCs from WT or Tlr4-/- mice were generated in vitro as described above, and 5 × 105 DCs were resuspended in PBS and injected intraperitoneally into DC-Tlr4-/- mice immediately after CLP.
Bacterial Culture
Peritoneal lavage fluid (PLF) and blood specimens for bacterial culture were collected as mice were euthanized at time points after CLP. PLF and blood specimens were subjected to serial 10-fold dilutions and cultured at 37°C overnight in 5% sheep blood agar (Teknova). Colony-forming units (CFUs) were quantified by manual counting.
Assessment of Cytokine Levels
Plasma, PLF, and cell culture supernatant samples were analyzed using interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), IL-10, interleukin 12 (IL-12), CXCLl, CXCL2, CXCL5, and CCL2 enzyme-linked immunosorbent assay kits from R&D Systems.
Flow Cytometry
Cells were blocked for Fc receptors with anti-mouse CD16/32 (BD Bioscience) for 5 minutes and then were stained with fluorochrome-conjugated Ab for 30 minutes at 4°C in the dark. Data were acquired with a BD FACS LSR Fortessa flow cytometer (BD Bioscience) and analyzed with FlowJo analytical software (TreeStar). Each experiment was repeated 3 times.
Comparative Polymerase Chain Reaction (PCR) Analysis
Total RNA was extracted with the RNeasy mini extraction kit (Qiagen) according to the manufacturer's instructions. Two-step, real-time reverse-transcription PCR (RT-PCR) was performed as previously described [17] with forward and reverse primer pairs prevalidated and specific for Cxcr2 (Qiagen). All samples were assayed in duplicate and normalized to the actin messenger RNA (mRNA) abundance.
Statistical Analysis
All data were analyzed using GraphPad Prism software (GraphPad Software). Data were analyzed using a 2-tailed t test. For measurements of bacterial CFU, groups were compared using the nonparametric Mann–Whitney U test. Survival data were analyzed using the log-rank test. A P value of <.05 was considered statistically significant for all experiments. All values are presented as mean values ± SD, except for bacterial counts, for which median values are designated.
RESULTS
DC-Specific TLR4 Regulates Bacterial Clearance and Thus Regulates Systemic Inflammation and Survival After CLP
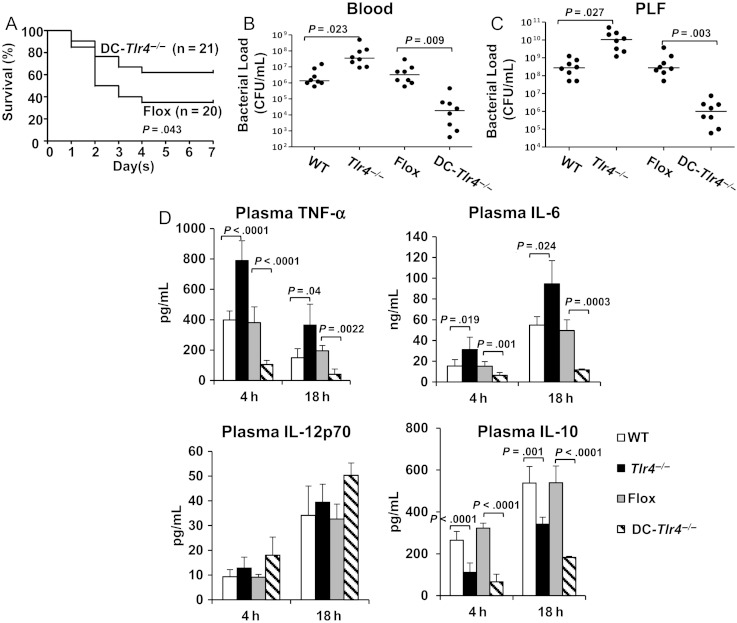
We have previously shown that both global deletion of Tlr4, as well as deletion of TLR4 specifically on myeloid cells, results in impaired bacterial clearance and reduced survival following CLP [7]. To establish the role of TLR4 on CD11c+ DCs in polymicrobial sepsis, CLP without antibiotics was performed on DC-Tlr4-/- mice and their controls (Flox). Surprisingly, deletion of Tlr4 expression specifically on DCs significantly improved survival after CLP (P = .04 vs Flox mice; Figure 1A). These results indicate that TLR4 signaling on CD11c+ DCs is crucial for host survival after CLP.
Figure 1.
Dendritic cell (DC)–specific Toll-like receptor 4 (TLR4) regulates bacterial clearance and thus regulates systemic inflammation and survival after cecal ligation and puncture (CLP). Wild-type (WT), global Tlr4-/-, Flox (cell-specific Tlr4-/- control), and DC-Tlr4-/- mice were subjected to CLP without antibiotic treatment. A, Seven-day survival after CLP. Data are from 20 mice per group from 2 separate experiments. Statistical difference was tested using the log-rank test. B and C, Blood and peritoneal lavage fluid (PLF) were collected at 18 hours after CLP. Bacterial counts in blood (B) and peritoneal lavage fluid (C). Data are from 8 mice per group from 2 separate experiments. Statistical difference was tested using a nonparametric Mann–Whitney U statistics. D, Plasma cytokine level. Blood was collected 4 and 18 hours after CLP. Plasma tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interleukin 12p70 (IL-12p70), and interleukin 10 (IL-10) concentrations were measured by enzyme-linked immunosorbent assay. Data are means ± SD from 8 mice per group from 4 separate experiments. Statistical difference was tested using a 2-tailed paired t test. Abreviation: CFU, colony-forming units.
To test whether differences in bacterial clearance account for the improved survival in DC-Tlr4-/- mice, the number of CFUs was measured in the blood and PLF 18 hours after CLP. For all results, WT mice served as controls for the global Tlr4-/- mice, and mice from the Flox parent strain served as controls for DC-Tlr4-/- mice. Consistent with our previous results [7], bacterial counts in global Tlr4-/- mice were significantly higher than those in WT mice in the blood and PLF (Figure 1B and 1C). However, bacterial levels in DC-Tlr4-/- mice were 2 logs lower than those found in Flox controls (Figure 1B and 1C), suggesting that TLR4 expression on CD11c+ DCs leads to decreased bacterial clearance after CLP. To ensure that the insertion of the cre recombinase had no effect, we subjected mice expressing the CD11c-driven cre recombinase to CLP and found no impact on blood or peritoneal bacterial levels, percentages of peritoneal DCs or PMNs, or circulating IL-6 levels after CLP when compared to WT mice (Supplementary Figure 1A–D).
Circulating TNF-α and IL-6 levels were significantly higher in global Tlr4-/- mice than in WT mice, whereas TNF-α and IL-6 levels were lower in DC-Tlr4-/- mice than in the Flox controls (Figure 1D). Therefore, cytokine markers of the systemic inflammatory response correlated with bacterial load. Plasma IL-10 levels were significantly lower in global Tlr4-/- and DC-Tlr4-/- mice as compared to their respective controls (Figure 1D). However, no significant difference was detected in the plasma IL-12p70 concentration between any of the strains following CLP (Figure 1D). Thus, IL-10 appeared to be regulated independently of bacterial load and in a DC-specific TLR4–dependent manner.
DC-Specific TLR4 Regulates PMN Recruitment to Peritoneum After CLP
To determine whether TLR4 status regulates the relative PMN and DC recruitment in the peritoneal cavity, PLF was collected 4 and 18 hours after CLP, and the abundance of PMNs (CD11b+Ly6G+ cells) and DCs (CD11c+ cells) was assessed by flow cytometry. Interestingly, the absolute numbers and percentages of PMNs in PLF were significantly higher in global Tlr4-/- and DC-Tlr4-/- mice than those in their respective controls (Figure 2A), indicating that DC-specific TLR4 is an important regulator of PMN recruitment after CLP. The percentage of CD11c+ cells relative to total cells in the peritoneal cavity was decreased 18 hours after CLP, compared with control (Supplementary Figure 2A and 2B). This reduction in relative abundance was similar across all 4 strains. However, the total number of peritoneal CD11c+ cells increased following CLP, and this increase was greater in the global Tlr4-/- and DC-Tlr4-/- strains (Supplementary Figure 2C), suggesting that TLR4 may be involved in either the recruitment or turnover of CD11c+ cells in the peritoneal cavity following CLP.
Figure 2.
Dendritic cell (DC)–specific Toll-like receptor 4 (TLR4) regulates polymorphonuclear leukocyte (PMN) recruitment to peritoneum after cecal ligation and puncture (CLP). Wild-type (WT), global Tlr4-/-, Flox, and DC-Tlr4-/- mice were subjected to CLP without antibiotic treatment. Peritoneal lavage fluid (PLF) was collected 4 and18 hours after CLP. A, Percentage and number of PMNs were measured by flow cytometry. Number indicates percentage of CD11b and Ly6G double-positive cells. The total number of PMNs in the peritoneal cavity is specified as the mean ± standard deviation (SD) for 6 mice per group. Statistical difference was tested using a 2-tailed paired t test. B, Chemokine level in PLF. PLF was collected 4 and 18 hours after CLP. CXCL1, CXCL2, CXCL5, and CCL2 concentrations in PLF were measured by enzyme-linked immunosorbent assay. Data are means ± SD from 8 mice per group from 2 separate experiments. Statistical difference was tested using a 2-tailed paired t test. C, CXCR2 expression on peritoneal PMNs. PLF was collected from out-of-box control mice (Cont) and 4 and 18 hours after CLP. Mean fluorescence intensity (MFI) for CXCR2 expression was measured by flow cytometry. Data are means ± SD for 3 mice in the control group and 6 mice per CLP group. Statistical difference was tested using a 2-tailed unpaired t test.
PMN recruitment to a site of infection is known to be driven by chemokines [18]. Unexpectedly, concentrations of CXCL1, CXCL2, CXCL5, and CCL2 in plasma (Supplementary Figure 3A–D) and PLF (Figure 2B) were significantly lower in DC-Tlr4-/- mice than in their controls 18 hours after CLP. These chemokine levels were significantly higher in global Tlr4-/- mice than in WT controls (Figure 2B). Therefore, levels of chemokines appear to correlate with bacterial levels but not with the number of PMNs in the peritoneal cavity.
PMN surface CXCR2 (CXC chemokine receptor 2) expression is known to be suppressed in severe sepsis [19]. The engagement of CXCR2 by chemokines recruits PMNs to infection sites. To test whether DC-specific TLR4 regulates CXCR2 expression on PMNs, peritoneal PMNs were analyzed by flow cytometry for CXCR2 expression. The mean fluorescence intensity (MFI) of CXCR2 was lower in all strains of mice 4 and 18 hours after CLP, compared with naive controls (Figure 2C). Interestingly, the MFI of CXCR2 in Tlr4-/- and DC-Tlr4-/- mice was significantly higher than in their respective controls at both 4 and 18 hours after CLP (Figure 2C). These findings suggest that signaling by DC-specific TLR4 suppresses PMN recruitment to the peritoneum through a mechanism that determines CXCR2 expression on PMNs after CLP.
Adoptive Transfer of WT DCs Impairs Bacterial Clearance and PMN Recruitment in DC-Tlr4-/- Mice After CLP
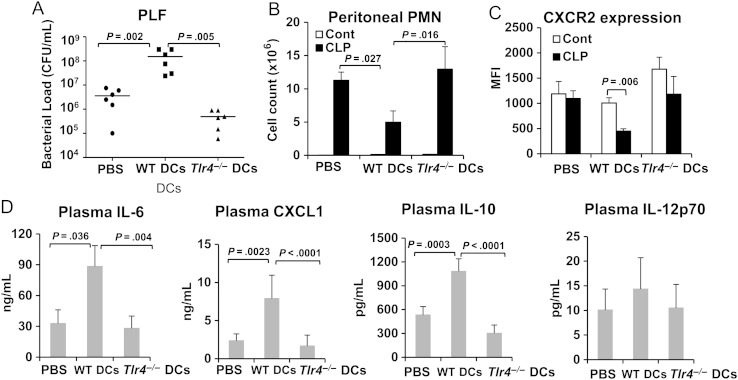
Although CD11c is expressed highly by conventional DCs, it is also present at lower levels on the surface of plasmacytoid DCs and macrophage subsets [20]. Therefore, we performed adoptive transfer experiments to establish whether conventional DCs are one of the CD11c+ cells that regulate bacterial clearance and PMN influx in a TLR4-dependent manner during CLP-induced sepsis. Immediately after CLP, DC-Tlr4-/- mice were injected intraperitoneally with WT or Tlr4-/- bone marrow–derived DCs generated in vitro (5 × 105 cells/mouse). DC-Tlr4-/- mice injected with PBS without cells were used as the control for mice that underwent adoptive transfer with DCs. Mice were euthanized 18 hours after CLP. As expected, the bacterial load in PLF was significantly higher in DC-Tlr4-/- mice that received WT DCs than in mice that received Tlr4-/- DCs and PBS (Figure 3A). PMN counts in PLF were inversely correlated to bacterial load (Figure 3B). Furthermore, peritoneal PMN CXCR2 expression in the mice that received WT DCs after CLP was approximately 50% lower when compared to PMNs from mice that received WT DCs without CLP (Figure 3C). In contrast, only a modest decrease in PMN CXCR2 expression was detected in the PBS-treated and Tlr4-/- DC–transfer groups after CLP (Figure 3C). Circulating IL-6 and CXCL1 levels correlated to bacterial load (Figure 3D). Plasma IL-10 concentrations increased dramatically in mice that received WT DCs as compared to mice given PBS or Tlr4-/- DCs after CLP (Figure 3D). Plasma IL-12p70 levels were similar in all 3 groups (Figure 3D). These data confirm that DCs are one of the CD11c+ cell types that regulate bacterial clearance and PMN responses through a TLR4-dependent mechanism in CLP-induced sepsis.
Figure 3.
Adoptive transfer of wild-type dendritic cells (DCs) impairs bacterial clearance and polymorphonuclear leukocyte (PMN) recruitment in DC-Tlr4-/- mice after cecal ligation and puncture (CLP). DC-Tlr4-/- mice were subjected to CLP without antibiotic administration. Immediately after CLP, DC-Tlr4-/- mice were intraperitoneally injected with wild-type (WT) DCs or Tlr4-/- DCs (0.5 million cells/mouse). Mice injected with WT DCs, Tlr4-/- DCs, or phosphate-buffered saline (PBS) were subjected to CLP. Peritoneal lavage fluid (PLF) and plasma were collected 18 hours after CLP. Control mice (Cont) are mice only subjected to injection. A, Bacterial counts in PLF. Data are from 6 mice per group from 2 separate experiments. Statistical difference was tested using a nonparametric Mann–Whitney U test; *P < .05 vs Tlr4-/- DCs and PBS. B and C, PMNs in PLF were analyzed by flow cytometry for total number of PMNs (B) and mean fluorescence intensity (MFI) for CXCR2 expression (C). D, Circulating cytokine and chemokine levels. Plasma interleukin 6 (IL-6), CXCL1, interleukin 10 (IL-10), and interleukin 12p70 (IL-12p70) concentrations were measured by enzyme-linked immunosorbent assay. Data are means ± SD from 3 mice per control group and 6 mice per CLP group. Statistical difference was tested using the 2-tailed unpaired t test. Abreviation: CFU, colony-forming units.
CLP With Antibiotics Reveals a Role for DC-Specific TLR4 in IL-10 Production in Polymicrobial Sepsis
The above results in the CLP model without antibiotics indicate that DC-specific TLR4 is important for bacterial clearance and PMN trafficking. To evaluate the impact of DC-specific TLR4 on the systemic inflammatory response independent of variations in bacterial clearance, CLP was performed with antibiotic treatment. Antibiotic treatment reduced levels of bacteria (to undetectable levels in blood and by 2–4 logs in the peritoneum) and equalized the bacterial counts between all 4 mouse strains. As expected, more PMNs (CD11b+Ly6G+ cells) were recruited to the peritoneum in global Tlr4-/- and DC-Tlr4-/- mice after CLP as compared to the respective control mice (Figure 4A). PMN CXCR2 expression decreased in the WT and Flox strains 18 hours after CLP, whereas no significant reduction of CXCR2 was detected in the global Tlr4-/- or DC-Tlr4-/- mice (Figure 4B). These results further confirm that DC-specific TLR4 regulates PMN recruitment via downregulation of CXCR2 on PMNs. The systemic inflammatory response, measured by IL-6, CXCL1, CXCL2, and CCL2 levels, was suppressed in the global Tlr4-/- mice but not the DC-Tlr4-/- mice (Figure 4C). These results suggest that DC-specific TLR4 influences the systemic inflammatory response indirectly by impacting the efficiency of bacterial clearance after CLP. Interestingly, plasma IL-10 levels (Figure 4C) were significantly lower in global Tlr4-/- and DC-Tlr4-/- mice, while plasma IL-12p70 concentrations (Figure 4C) were lower in the Tlr4-/- mice but not the DC-Tlr4-/- mice, as compared to their controls. These results indicate that DC-specific TLR4 is important for systemic release of IL-10 during sepsis.
Figure 4.
Cecal ligation and puncture (CLP) with antibiotics reveals a role for dendritic cell (DC)–specific Toll-like receptor 4 (TLR4) in interleukin 10 (IL-10) release in polymicrobial sepsis. Mice were subjected to CLP with the administration of the antibiotic Primaxin (25 mg/kg, subcutaneously starting 2 hours after CLP and then every 12 hours). Peritoneal lavage fluid (PLF) and plasma was collected 18 hours after CLP. Control mice (Cont) are out-of-box mice. A and B, Polymorphonuclear leukocyte (PMN) count (A) and mean fluorescence intensity for CXCR2 expression on peritoneal PMNs (B) were measured by flow cytometry. C, Circulating cytokine and chemokine level. Plasma interleukin 6 (IL-6), CXCL1, CXCL2, CXCL2, interleukin 10 (IL-10), and interleukin 12p70 (IL-12p70) concentrations were measured by enzyme-linked immunosorbent assay. Data are means ± SD for 3 mice per control group and 6 mice per CLP group. Statistical difference was tested using a 2-tailed unpaired t test.
TLR4 Signaling on DCs Regulates CXCR2 Expression on PMNs via IL-10 After LPS Stimulation In Vitro
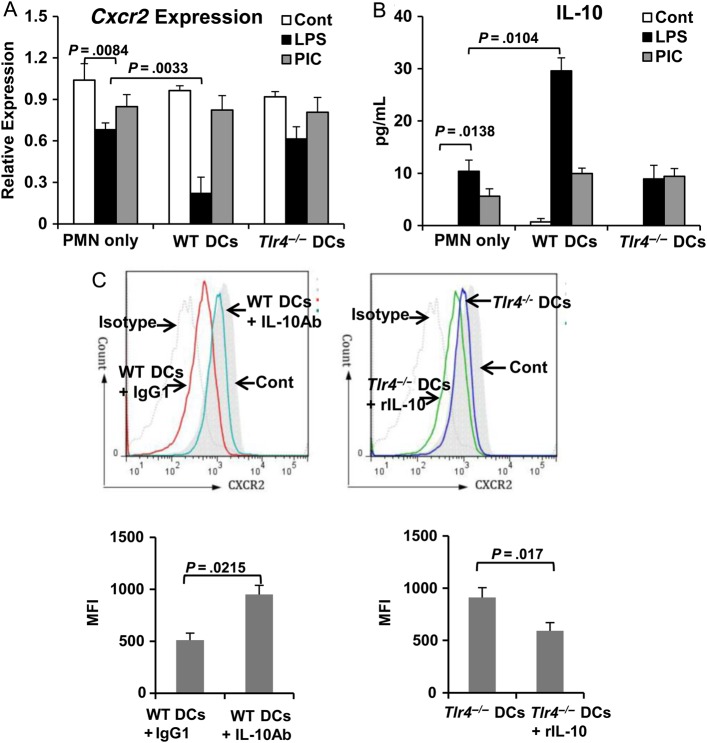
To confirm the role of DC-specific TLR4 in the regulation of PMN CXCR2 expression, WT splenic PMNs (Ly6G+) were cocultured with splenic WT DCs or Tlr4-/- DCs. The expression of Cxcr2 was quantitated using real-time PCR. Cxcr2 expression on PMNs decreased 4 hours after LPS stimulation (100 ng/mL), compared with findings for unstimulated control PMNs (Figure 5A). The Cxcr2 expression decreased further after LPS stimulation in the coculture with WT DCs but not with Tlr4-/- DCs (Figure 5A). Importantly, IL-10 levels in medium was inversely correlated with the Cxcr2 expression level (Figure 5B). Furthermore, Cxcr2 expression in coculture with WT DCs or Tlr4-/- DCs remained at levels similar to those for unstimulated controls after stimulation with polyinosinic-polycytidylic acid (a TLR3 ligand; 10 µg/mL) for 4 hours (Figure 5A). These results indicate that stimulation of TLR4 on DCs selectively downregulates PMN Cxcr2 expression, which may be mediated via IL-10.
Figure 5.
Dendritic cell (DC)–specific Toll-like receptor 4 (TLR4) regulates CXCR2 expression in polymorphonuclear leukocytes (PMNs) via interleukin 10 (IL-10) after lipopolysaccharide (LPS) stimulation in vitro. DCs (CD11c+) and PMNs (Ly6G+) were isolated from spleen and stimulated with LPS (100 ng/mL) or polyinosinic-polycytidylic acid (PIC; 10 µg/mL) for 4 hours. A, The relative expression of Cxcr2 was quantitated by real-time reverse-transcription polymerase chain reaction analysis. B, IL-10 concentration in medium was measured by enzyme-linked immunosorbent assay. C, PMNs cocultured with wild-type (WT) DCs or Tlr4-/- DCs in the presence of LPS (100 ng/mL) were treated with IL-10 antibody (1 mg/mL) or recombinant IL-10 (20 ng/mL), respectively. PMNs cocultured with WT DCs treated with immunoglobulin G1 (IgG1) served as a control for IL-10 antibody treatment. Cxcr2 expression was measured by flow cytometry. Data are mean values ± SD from 3 experiments. Statistical difference was tested using by the 2-tailed paired t test. Abbreviation: MFI, mean fluorescence intensity.
IL-10 is known to inhibit PMN recruitment [21]. To further test the role of IL-10 in the regulation of PMN CXCR2 expression, PMNs cocultured with WT DCs plus LPS were treated with IL-10 Ab (1 mg/mL). IL-10 Ab prevented the loss of surface CXCR2 expression on PMNs in response to LPS (Figure 5C). Conversely, addition of recombinant IL-10 (20 ng/mL) reduced surface CXCR2 expression on PMNs cocultured with Tlr4-/- DCs plus LPS (Figure 5C). These data demonstrate that DCs downregulate PMN CXCR2 expression by secreting IL-10 through a TLR4-dependent mechanism.
DISCUSSION
Activation of TLR on DCs contributes to DC maturation and regulates DC antigen presentation and T-cell differentiation [22]. For infections that involve gram-negative bacteria, TLR4 is central to this integrated response through the sensing of LPS [23]. Indeed, DC responses are altered in global Tlr4 knockout or mutant mice in CLP-induced sepsis [13, 14]. Here, using a cell-selective Tlr4 deletion strategy, we demonstrate a major role for TLR4 signaling on DCs in polymicrobial sepsis and that many of the effects are mediated through DC-derived IL-10 release. DC-derived IL-10 secretion downregulates PMN CXCR2 expression, PMN accumulation in the peritoneal cavity, and bacterial clearance. These observations, combined with our previous results [7], provide insights into the diverse and cell-selective roles of TLR4 in polymicrobial sepsis.
In clear contrast to our previous studies showing that deletion of Tlr4 from myeloid cells leads to less effective bacteria clearance in a CLP model without antibiotics, deletion of TLR4 from CD11c+ DCs leads to a marked improvement in bacterial clearance and host survival. CD11c is expressed highly by conventional DCs, a subset of myeloid DCs, and to a lesser extent by inflammatory macrophages and by macrophage subsets associated with epithelia [20]. The fact that we were able to recapitulate the TLR4-specific responses with adoptive transfer of Tlr4+/+ conventional DCs into DC-Tlr4-/- mice confirms that conventional DCs are one of the CD11c+ cell types involved in our observed CD11c-restricted, TLR4-dependent responses. The central role of conventional DCs in gram-negative bacterial and polymicrobial intra-abdominal responses is not entirely unexpected. In CD11c-DTR mice, deletion of CD11c+ cells resulted in reduced host survival after CLP [24]. Early activation of DCs has been documented in the spleen and lymph nodes in CLP-induced sepsis [12]. The capacity of splenic DCs to secrete IL-12 is suppressed after CLP, whereas IL-10 release is enhanced [25]. Others have shown that TLR4 stimulation drives IL-10 production by DCs in vitro [26] and in vivo during sepsis [12, 27]. We found that DC-specific TLR drives IL-10 production in CLP-induced sepsis, with or without antibiotics. This distinction is important because, in the CLP model, systemic inflammation correlates with bacteria load in the absence of antibiotics, while bacterial counts are similar when antibiotics are used early. Even with antibiotics, only IL-10 exhibited significant dependence on DC-specific TLR4. We have previously shown that plasma concentrations of systemic inflammatory cytokines, such as IL-6, are lower in myeloid-specific Tlr4-/- mice in a CLP model with antibiotic administration [7]. While TLR4 expression on myeloid cells contributes to the systemic release of different inflammatory mediators, TLR4 signaling on DCs appears to be a key step for IL-10 secretion in CLP-induced sepsis.
Our results show that a major consequence of DC-derived IL-10 is to reduce CXCR2 expression on PMNs through a mechanism that suppresses CXCR2 mRNA levels. This is associated with suppression of PMN accumulation in the peritoneal cavity. DCs are known to control PMN homeostasis, and deletion of DCs results in higher numbers of PMNs in peripheral organs, such as the lung [28] and spleen [29]. Whether this effect on baseline PMN homeostasis is due to IL-10 is unknown, but IL-10 deletion has been shown to enhance PMN recruitment into the peritoneal cavity following E. coli inoculation [30]. Scott et al [31] reported that treatment with anti–IL-10 Ab enhanced PMN recruitment in the liver after CLP. The mechanism of IL-10 regulation of PMN recruitment is unclear. We provide evidence that the target of DC-derived IL-10 is the amount CXCR2 on the surface of PMNs.
CXCR2 is essential for PMN chemotaxis in response to CXC chemokines [32]. PMNs from patients with sepsis have reduced surface expression of CXCR2 [19, 24]. PMN CXCR2 expression is also reduced in experimental sepsis [33], and blockade of CXCR2 in mice leads to reduction in PMN recruitment to the lung after CLP in mice [34]. Others have shown that PMN CXCR2 expression is regulated by inducible nitric oxide synthase during sepsis [33]. Our data link TLR4 stimulation on DCs to PMN CXCR2 expression via IL-10. IL-10 has been shown to protect organs from secondary damage from excessive inflammation during septic insults [35]. Therefore, the balance between the beneficial protective effects of IL-10 and the detrimental consequences of sustained or excessive IL-10 production on bacterial clearance may be skewed in CLP-induced sepsis in the absence of antibiotics, where a large microbial burden overwhelms the clearance capacity of the host.
In summary, we provide compelling evidence that TLR4 on DCs regulates IL-10 production, which in turn regulates PMN recruitment into the peritoneal cavity during intra-abdominal sepsis. Our findings suggest that modulation of DC-specific TLR4 signaling maybe a new therapeutic strategy for DC immunotherapy in inflammatory disease. Furthermore, these findings, together with our previous findings, indicate the importance of understanding the diverse and cell-specific roles of critical innate immune receptors in the integrated host response to serious infections.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work is supported by the National Institutes of Health (grant R01-GM-050441 to T. R. B.), the National Natural Science Foundation of China (grants 81170296 and 81471841 to T. M.), and the Surgical Infection Society Junior Faculty Fellowship program (award to M. D.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature 2002; 420:885–91. [DOI] [PubMed] [Google Scholar]
- 2.Opal SM, Laterre PF, Francois B et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 2013; 309:1154–62. [DOI] [PubMed] [Google Scholar]
- 3.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 2011; 8:292–300. [DOI] [PubMed] [Google Scholar]
- 4.Bauer S, Muller T, Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol 2009; 653:15–34. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Sun R. Toll-like receptors in acute liver injury and regeneration. Int Immunopharmacol 2011; 11:1433–41. [DOI] [PubMed] [Google Scholar]
- 6.Vodovotz Y, Liu S, McCloskey C, Shapiro R, Green A, Billiar TR. The hepatocyte as a microbial product-responsive cell. J Endotoxin Res 2001; 7:365–73. [PubMed] [Google Scholar]
- 7.Deng M, Scott MJ, Loughran P et al. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J Immunol 2013; 190:5152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott MJ, Billiar TR. Beta2-integrin-induced p38 MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of lipopolysaccharide by hepatocytes. J Biol Chem 2008; 283:29433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott MJ, Liu S, Shapiro RA, Vodovotz Y, Billiar TR. Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism. Hepatology 2009; 49:1695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand RJ, Kohler JW, Cavallo JA, Li J, Dubowski T, Hackam DJ. Toll-like receptor 4 plays a role in macrophage phagocytosis during peritoneal sepsis. J Pediatr Surg 2007; 42:927–32. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Venet F, Chung CS, Lomas-Neira J, Ayala A. Changes in dendritic cell function in the immune response to sepsis. Cell- & tissue-based therapy. Expert Opin Biol Ther 2007; 7:929–38. [DOI] [PubMed] [Google Scholar]
- 12.Flohe SB, Agrawal H, Schmitz D, Gertz M, Flohe S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol 2006; 79:473–81. [DOI] [PubMed] [Google Scholar]
- 13.Ding Y, Chung CS, Newton S et al. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock 2004; 22:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pene F, Courtine E, Ouaaz F et al. Toll-like receptors 2 and 4 contribute to sepsis-induced depletion of spleen dendritic cells. Infect Immun 2009; 77:5651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scumpia PO, McAuliffe PF, O'Malley KA et al. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol 2005; 175:3282–6. [DOI] [PubMed] [Google Scholar]
- 16.Sodhi CP, Neal MD, Siggers R et al. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012; 143:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng M, Loughran PA, Zhang L, Scott MJ, Billiar TR. Shedding of the tumor necrosis factor (TNF) receptor from the surface of hepatocytes during sepsis limits inflammation through cGMP signaling. Sci Signal 2015; 8:ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis 2012; 25:321–7. [DOI] [PubMed] [Google Scholar]
- 19.Cummings CJ, Martin TR, Frevert CW et al. Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J Immunol 1999; 162:2341–6. [PubMed] [Google Scholar]
- 20.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol 2008; 181:5829–35. [DOI] [PubMed] [Google Scholar]
- 21.Corsetti PP, de Almeida LA, Carvalho NB et al. Lack of endogenous IL-10 enhances production of proinflammatory cytokines and leads to Brucella abortus clearance in mice. PloS One 2013; 8:e74729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004; 5:987–95. [DOI] [PubMed] [Google Scholar]
- 23.Maeshima N, Fernandez RC. Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Fron Cell Infect Microbiol 2013; 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams JM, Hauser CJ, Livingston DH, Lavery RF, Fekete Z, Deitch EA. Early trauma polymorphonuclear neutrophil responses to chemokines are associated with development of sepsis, pneumonia, and organ failure. J Trauma 2001; 51:452–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 25.Aachoui Y, Leaf IA, Hagar JA et al. Caspase-11 protects against bacteria that escape the vacuole. Science 2013; 339:975–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigt H, Muhlradt PF, Emmendorffer A, Krug N, Braun A. Synthetic mycoplasma-derived lipopeptide MALP-2 induces maturation and function of dendritic cells. Immunobiology 2003; 207:223–33. [DOI] [PubMed] [Google Scholar]
- 27.Higgins SC, Lavelle EC, McCann C et al. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J Immunol 2003; 171:3119–27. [DOI] [PubMed] [Google Scholar]
- 28.Schindler D, Gutierrez MG, Beineke A et al. Dendritic cells are central coordinators of the host immune response to Staphylococcus aureus bloodstream infection. Am J Pathol 2012; 181:1327–37. [DOI] [PubMed] [Google Scholar]
- 29.Autenrieth SE, Warnke P, Wabnitz GH et al. Depletion of dendritic cells enhances innate anti-bacterial host defense through modulation of phagocyte homeostasis. PLoS Pathog 2012; 8:e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sewnath ME, Olszyna DP, Birjmohun R, ten Kate FJ, Gouma DJ, van Der Poll T. IL-10-deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J Immunol 2001; 166:6323–31. [DOI] [PubMed] [Google Scholar]
- 31.Scott MJ, Hoth JJ, Turina M, Woods DR, Cheadle WG. Interleukin-10 suppresses natural killer cell but not natural killer T cell activation during bacterial infection. Cytokine 2006; 33:79–86. [DOI] [PubMed] [Google Scholar]
- 32.Boppana NB, Devarajan A, Gopal K et al. Blockade of CXCR2 signalling: a potential therapeutic target for preventing neutrophil-mediated inflammatory diseases. Exp Biol Med 2014; 239:509–18. [DOI] [PubMed] [Google Scholar]
- 33.Rios-Santos F, Alves-Filho JC, Souto FO et al. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. Am J Respir Crit Care Med 2007; 175:490–7. [DOI] [PubMed] [Google Scholar]
- 34.Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol 2004; 76:58–64. [DOI] [PubMed] [Google Scholar]
- 35.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med 2002; 30:S58–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.