Abstract
Background. Human immunodeficiency virus (HIV)–infected individuals may have poorer serological responses to syphilis treatment and may be more likely to experience neurosyphilis. Treponema pallidum is cleared from sites of infection by opsonization, ingestion, and killing by macrophages.
Methods. Serum samples from 235 individuals with syphilis were tested for T. pallidum–specific opsonic activity. Blood T. pallidum concentrations were determined by real-time polymerase chain reaction amplification of the tp0574 gene, and T. pallidum was detected in cerebrospinal fluid (CSF) by reverse-transcriptase polymerase chain reaction of 16S ribosomal RNA.
Results. Opsonic activity was higher with higher serum rapid plasma reagin titers (P < .001), and in those treated for uncomplicated syphilis before serum collection (P < .001). Opsonic activity was lower in HIV-infected than in HIV-uninfected individuals even after the above factors were taken into account (P = .006). In participants in whom blood T. pallidum was detectable, those with the highest opsonic activity had lower blood T. pallidum concentrations. In multivariable analyses, there was not a significant relationship between opsonic activity and detection of T. pallidum in CSF or CSF-VDRL reactivity.
Conclusions. Serum T. pallidum–specific opsonic activity is significantly lower in HIV-infected individuals. Impaired T. pallidum–specific immune responses could contribute to differences in the course of disease or treatment response.
Keywords: syphilis, immune response, opsonic antibody, neuroinvasion, neurosyphilis
In the United States, the number of cases of early syphilis has steadily increased since 2000; similar increases have been seen in Western Europe and elsewhere. In 2013, the most recent year for which detailed US data are available, the number of cases was the highest since 1995. Rates were greatest among men, 75% of whom were men who have sex with men, and approximately half were infected with human immunodeficiency virus (HIV) [1]. Since the advent of HIV, reports have suggested that the course of syphilis may differ between HIV-infected and HIV-uninfected individuals. These have described poorer serological response to standard syphilis therapy [2–4] and increased risk of neurosyphilis [5, 6]. Both of these outcomes are less likely in individuals receiving effective antiretroviral therapy [7–9], suggesting a link to host immune function. Because there have been no large, population-based studies, consensus on the magnitude of increased risk, if any, among HIV-infected individuals with syphilis has not been reached. The Centers for Disease Control and Prevention guidelines recommend more frequent serological follow-up after treatment of early and late syphilis in HIV-infected than in HV-uninfected individuals [10].
We reasoned that differences in HIV-infected patients' treatment response or likelihood of neurosyphilis could be due to defects in the specific host immune response to infection. Treponema pallidum subsp. pallidum (hereafter T. pallidum) is cleared from local sites of infection by opsonization, followed by ingestion and killing by activated macrophages. Opsonophagocytosis of T. pallidum by peritoneal macrophages in the presence of serum samples from infected animals (immune serum samples) has been demonstrated in the rabbit model of infection, where it is mediated by pathogen-specific immunoglobulin G and is independent of complement [11–14]. Although individual T. pallidum proteins have been identified as targets of opsonic antibody in the rabbit model [15–17], the full spectrum of opsonins and identification of those that are important for control of infection remain unknown.
Opsonophagocytosis of T. pallidum by human peripheral blood monocytes in the presence of immune human serum samples has been demonstrated on a more limited basis than in the rabbit model [18, 19], and the relationship with HIV has not been investigated. Compared with HIV-uninfected individuals, HIV-infected individuals show decreased opsonic activity against other pathogens, including Streptococcus pneumoniae [20, 21] and parasitized red blood cells in pregnancy-associated malaria [22, 23]. The goal of the current study was to determine whether HIV-infected patients with syphilis have less T. pallidum–specific opsonic antibody activity in serum samples than HIV-uninfected patients with syphilis and whether serum opsonic antibody activity is related to development of neurosyphilis.
METHODS
Study Participants
Participants were enrolled in a study of cerebrospinal fluid (CSF) abnormalities in syphilis conducted in Seattle, Washington, from July 2001 through December 2013 [24]. Study eligibility criteria included clinical or serological evidence of syphilis and assessment by the referring provider that the patient was at risk for neurosyphilis. Reasons for referral to the study included (1) neurological findings, particularly vision or hearing loss; (2) serum rapid plasma reagin (RPR) titer ≥1:32; and (3) in HIV-infected individuals, peripheral blood CD4+ T-cell count ≤350/µL. The latter criteria for risk of neurosyphilis are based on published data [7, 24, 25]. Participants underwent a structured history and neurological examination that included assessment of vision and hearing, lumbar puncture, and venipuncture. The study protocol was reviewed and approved by the University of Washington Institutional Review Board, and human experimentation guidelines were followed in the conduct of this research. Written informed consent was obtained from all participants.
Because our goal was to assess the relationship between serum opsonic antibody activity, HIV infection, and neurosyphilis, participants included in this analysis were a convenience sample chosen to overrepresent HIV-uninfected individuals and those who were treated for neurosyphilis based on clinical or CSF abnormalities relative to the overall study population. Participants had to have reactive serum RPR tests and reactive serum treponemal tests (primarily T. pallidum particle agglutination test or a commercial enzyme immunoassay), and could not have had a previous diagnosis of neurosyphilis.
Laboratory Methods
General
Plasma HIV RNA, CSF-VDRL tests, and enumeration of peripheral blood CD4+ T cells and CSF white blood cells were performed in a Clinical Laboratory Improvement Amendments–approved hospital clinical laboratory. Serum RPR tests were performed in a single research laboratory using published methods [26]. Identification of T. pallidum 16S ribosomal RNA in CSF was performed using reverse-transcriptase polymerase chain reaction (RT-PCR), as described elsewhere [24].
Measurement of Serum Opsonic Activity
Monocytes were obtained from the University of Nebraska Medical Center Elutriation Core. Briefly, 2 × 105 cells per well were cultured in sterile 24-well culture plates on sterile 12 mm cover slips with 0.4 mL per well of Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 1% L-glutamine, 1 ng/mL macrophage colony-stimulating factor, 100 µg/mL gentamicin, and 10 µg/mL ciprofloxacin at 37°C in 5% carbon dioxide to generate monocyte-derived macrophages (MDMs). Half-medium changes were performed every 2–3 days for 9 days.
The Nichols strain of T. pallidum was propagated in and harvested from New Zealand White rabbits as described elsewhere [27]. Animal care was provided in full accordance with established guidelines, and experimental procedures were conducted under protocols approved by the University of Washington Institutional Animal Care and Use Committee.
On day 9 of monocyte cell culture, 100 µL of treponemes in Dulbecco's modified Eagle medium (5 × 107/mL), with supplements but without macrophage colony-stimulating factor, was mixed with 100 µL of (1) heat-inactivated serum from individual study participants, (2) normal human AB serum (Atlanta Biologicals) that was confirmed to be fluorescent treponemal antibody absorption test nonreactive (negative control), or (3) a pool of serum from 58 patients with syphilis (positive control; RPR titer 1:128) and added to wells. The multiplicity of infection was 25:1, with a final concentration of 17% human serum per well, and each serum sample was tested in quadruplicate. Plates were incubated at 37°C in 5% carbon dioxide for 4 hours. After incubation, the medium was removed, and the cover slips were washed 3 times with warm medium, air dried for 30 minutes, fixed with 4% formaldehyde with 0.1% Triton X-100, and stored at 4°C. For staining, the formaldehyde mixture was removed and cover slips were washed twice with phosphate-buffered saline (PBS) to remove residual formaldehyde before immunofluorescence detection of T. pallidum.
Immunofluorescence detection of T. pallidum was performed using as the primary antibody a pool of immune rabbit serum, diluted 1:400 in PBS with 2% Tween-80, and as the secondary antibody fluorescein isothiocyanate–conjugated goat anti-rabbit immunoglobulin G (Sigma-Aldrich), diluted 1:400 in PBS with 2% Tween-80. Cover slips were incubated with 200 µL of the primary antibody for 1 hour at room temperature, washed 3 times with PBS with 0.05% Tween-20, incubated with 200 µL of secondary antibody for 1 hour at room temperature, and then washed as described above. Cover slips were counterstained with 300 µL of Evans blue for 15 seconds, washed thoroughly with water, dried at room temperature for 1 hour, mounted onto slides with mounting medium (90% glycerol and 10% Tris [1 mol/L]), and scored using a fluorescence microscope. The number of macrophages containing internalized T. pallidum (visible as fluorescent vacuoles in the cytoplasm of the macrophages) was determined in a blinded fashion for 100 macrophages per cover slip and expressed as a proportion.
Measurement of Blood T. pallidum Concentration
To determine the concentration of T. pallidum in blood, 0.5 mL whole blood in ethylenediaminetetraacetic acid (EDTA) tubes was mixed with 0.5 mL of 2× lysis buffer (20 mmol/L Tris-Hydrochloride, 0.2 mol/L EDTA, and 1% sodium dodecyl sulfate), stored at −80°C, thawed, and extracted with the QIAamp DNA Blood Midi Kit (Qiagen), according to the manufacturer's instructions. DNA was precipitated with 2.5 volumes of 100% ethanol, 0.1 volumes of 3 mol/L sodium acetate, and 1 µL of glycogen overnight at −20°C. DNA pellets were washed twice with 75% ethanol and resuspended in 60 µL of molecular-grade water.
A portion of the tp0574 (Tp47) gene was amplified from 5 µL of DNA in a 20 µL reaction containing 1× TaqMan Fast Advanced Master Mix, 0.3 µmol/L sense (CAA GTA CGA GGG GAA CAT CG) and antisense (TGA TCG CTG ACA AGC TTA GG) primers, and 0.1 µmol/L TaqMan probe (CGG AGA CTC TGA TGG ATG CTG CAG TT, labeled on the 5′ end with 6-FAM and on the 3′ end with TAMRA) in a MicroAmp Optical 384-Well Reaction Plate (Applied Biosystems) on the Viia 7 Real-Time PCR System (Applied Biosystems). Cycling conditions were 50°C for 2 minutes and 95°C for 30 seconds, followed by 45 cycles of 95°C for 5 seconds and 60°C for 20 seconds, with data acquisition during the 60°C step. Results were analyzed using the Viia 7 software program, and the number of copies of the tp0574 gene per microliter was used to calculate the number of T. pallidum bacterial copies per milliliter of blood. All samples were tested in triplicate. The limit of detection of the assay was approximately 5 copies/mL.
Statistical Methods
We found that normal donor MDMs incubated with the positive control serum pool differed in their ability to ingest opsonized T. pallidum, with a range of 20%–47% of macrophages containing internalized T. pallidum. To account for this variability, the proportion of macrophages containing internalized T. pallidum for each replicate for individual patients was normalized to the average of the positive control serum pool in each assay. This ratio is termed the opsonic index. Descriptive statistics are reported as numbers with percentages or medians with interquartile ranges. Associations between categorical variables were assessed with χ2 or Fisher exact tests, associations between continuous and categorical variables with Mann–Whitney U tests, and associations between continuous variables with Spearman rank correlation coefficients. Linear and logistic regression was used for multivariable analyses. In these models, opsonic index and T. pallidum copies per milliliter were log-transformed to approximate a normal distribution. All tests were 2 tailed, and differences were considered statistically significant at P < .05.
RESULTS
Participant Characteristics
The characteristics of the 235 individuals included in the analysis are shown in Table 1. Seventy-two percent of participants had early syphilis (defined as ≤1 year duration), and 28% had late latent syphilis or syphilis of unknown duration. A total of 123 individuals were treated for uncomplicated syphilis (ie, not neurosyphilis) with ≥1 doses of 2.4 million units of intramuscular benzathine penicillin G (n = 110) or with 100 mg oral doxycycline twice a day (n = 13; median duration of treatment, 9 days [interquartile range, 6–21]) before study entry. Of the 160 HIV-infected individuals for whom HIV treatment status was known, 90 (56.3%) were taking antiretroviral medications. The median CD4+ T-cell count within 90 days of study entry in the HIV-infected participants was 409/µL (251–587, n = 151). Compared with HIV-uninfected participants, HIV-infected participants were more likely to be men, were older, were more likely to have early syphilis and to have higher serum RPR titers, and were less likely to have been treated for uncomplicated syphilis before study entry. As planned, the proportions with laboratory or clinical evidence of neurosyphilis and the proportion subsequently treated for neurosyphilis did not differ between the groups.
Table 1.
Characteristics of Study Participants
| Characteristic | Participants, No. (%)a |
|||
|---|---|---|---|---|
| All Participants (n = 235) | HIV-Uninfected (n = 64) | HIV-Infected (n = 171) | P Valueb | |
| Male sex | 225 (95.7) | 56 (87.5) | 169 (98.8) | .001 |
| Age, median (IQR), y | 38 (32–45) | 36 (25–44) | 39 (33–45) | .01 |
| Early stage syphilisc | 170 (72.3) | 39 (60.9) | 131 (76.6) | .02 |
| Symptomatic syphilisc | 115 (48.9) | 27 (42.2) | 88 (51.5) | NS |
| 1/serum RPR titer, median (IQR) | 64 (32–256) | 64 (32–128) | 128 (32–256) | .003 |
| Treated for uncomplicated syphilis before study entryd | 123 (52.3) | 46 (71.9) | 77 (45.0) | <.001 |
| Previous episode of syphilis | 57 (24.3) | 11 (17.2) | 46 (26.9) | NS |
| Reactive CSF-VDRL | 67 (28.5) | 18 (28.1) | 49 (28.7) | NS |
| Vision or hearing losse | 50 (22.1) | 11 (17.5) | 39 (23.9) | NS |
| Treated for neurosyphilis | 115 (48.9) | 30 (46.9) | 85 (49.7) | NS |
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IQR, interquartile range; NS, not significant; RPR, rapid plasma reagin.
a Data represent No. (%) of participants unless otherwise specified.
b P values represent comparison of HIV-uninfected and HIV-infected participants. Differences between groups were determined with χ2, Fisher exact, or Mann–Whitney U tests.
c Early stage syphilis includes primary, secondary, and early latent stages; late stage syphilis includes late latent stage and syphilis of unknown duration; symptomatic stage syphilis includes primary and secondary stages.
d Uncomplicated syphilis was defined as syphilis other than neurosyphilis.
e Vision or hearing loss could not be determined for 1 HIV-uninfected participant and 8 HIV-infected participants owing to preexisting abnormalities.
Opsonic Index, HIV, and Blood T. pallidum Concentration
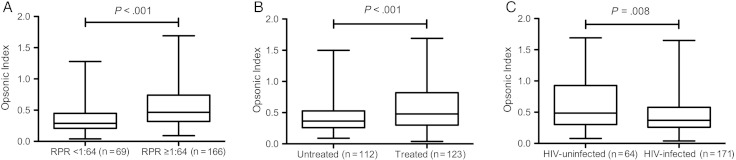
The opsonic index was significantly higher in those with serum RPR titers ≥1:64 than in those with titers <1:64, higher in those treated for uncomplicated syphilis before serum collection than in those who were untreated, and higher in HIV-uninfected than in HIV-infected participants (Figure 1). Opsonic index did not differ by syphilis stage, sex, or age. In multivariable linear regression analysis, opsonic index remained significantly higher in HIV-uninfected than in HIV-infected individuals (P = .006) after accounting for serum RPR titer (P < .001) and treatment for uncomplicated syphilis (P = .003); there was no interaction between HIV status and treatment for uncomplicated syphilis before serum collection. Among the HIV-infected participants, there was no significant relationship between opsonic index and plasma HIV RNA copy number, peripheral blood CD4+ T-cell concentrations, or use of antiretroviral medications.
Figure 1.
Box-and-whisker plots of opsonic index of serum samples in participants with low versus high serum rapid plasma reagin (RPR) titers (A); participants who were not treated for uncomplicated syphilis before collection of serum samples versus those who were treated for uncomplicated syphilis before collection of serum samples (B); and human immunodeficiency virus (HIV)–uninfected versus HIV-infected individuals (C). Lower border of box represents the 25th percentile and upper border the 75th percentile, with the median between them; whiskers represent minimum and maximum values. Differences between groups were determined using Mann–Whitney U tests.
As expected, compared with individuals who were treated for uncomplicated syphilis before collection of serum, T. pallidum DNA was more commonly detected in blood from participants who were not treated for uncomplicated syphilis before collection of serum (5 [4.1%] of 122 vs 45 [41.3%] of 109; P < .001). Among those who were untreated, T. pallidum DNA was more commonly detected in blood from those with RPR titers ≥1:64 than from those with lower titers (39 [50.0%] of 78 vs 6 [19.4%] of 31; P = .003) and from those with early syphilis than from those with late syphilis or syphilis of unknown duration (39 [54.9%] of 71 vs 6 [15.8%] of 38; P < .001), but detection of T. pallidum DNA in blood did not differ significantly by HIV status.
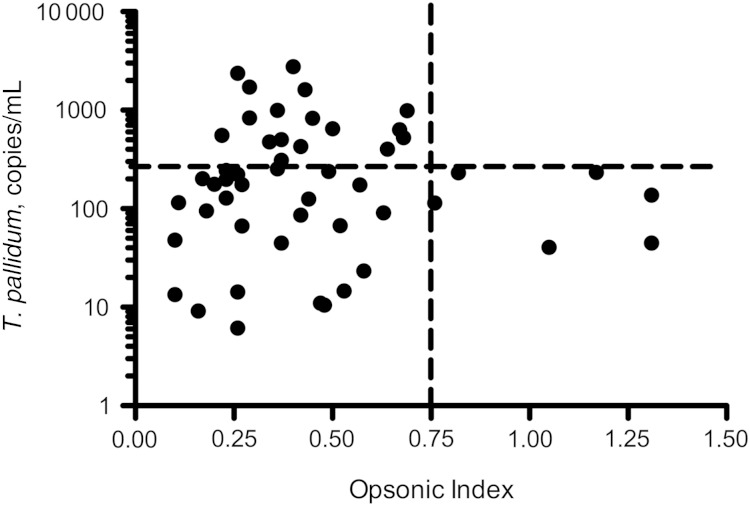
Overall, T. pallidum was detected in blood from 50 (21.7%) of 231 participants; 4 were HIV-uninfected and 46 were HIV-infected. The relationship between opsonic index and blood T. pallidum concentration was examined in those in whom blood T. pallidum was detectable. In this group, those with the highest opsonic indexes (≥0.75) had lower blood T. pallidum concentrations. Specifically, among the 6 samples with opsonic indexes ≥0.75, none contained more than 234 copies/mL organisms, compared with 20 (45.5%) of 44 with opsonic indexes <0.75 (P = .07) (Figure 2).
Figure 2.
Scatterplot of blood Treponema pallidum concentration versus opsonic index of serum samples in participants with detectable T. pallidum DNA in blood. Horizontal line is at a T. pallidum concentration of 234 copies/mL blood, and vertical line at an opsonic index of 0.75.
Opsonic Index and Neurosyphilis
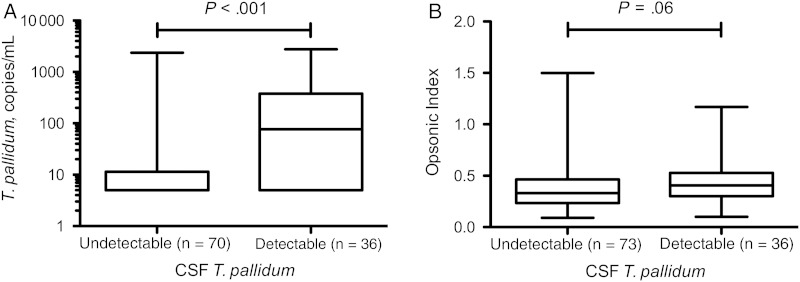
To examine the relationship between serum opsonic index and neurological outcome, we used detection of T. pallidum in CSF as the definition of neuroinvasion, and we used CSF-VDRL reactivity as the definition of neurosyphilis. Compared with patients treated for uncomplicated syphilis before study entry, T. pallidum was more commonly detected in CSF by RT-PCR (neuroinvasion) in untreated patients (3 [2.7%] of 110 vs 36 [33.0%] of 109; P < .001). Among untreated patients, neuroinvasion was more commonly seen in individuals with serum RPR titers ≥1:64 compared with those with lower titers (35 [44.9%] of 78 vs 1 [3.2%] of 31; P < .001), but did not differ significantly by syphilis stage or HIV status. Among untreated patients, blood T. pallidum concentration was significantly higher in those with detectable CSF T. pallidum than in those in whom T. pallidum was not detected in CSF. (Figure 3A). Also among untreated patients, opsonic index was significantly higher in those with detectable CSF T. pallidum than in those in whom T. pallidum was not detected in CSF (Figure 3B), although this difference did not reach statistical significance (P = .06). This relationship was further weakened when serum RPR titer and blood T. pallidum concentration were taken into account in multivariable logistic regression (data not shown).
Figure 3.
Box-and-whisker plots of blood Treponema pallidum concentration (A) and opsonic index of serum samples (B) in untreated participants who had undetectable and detectable cerebrospinal fluid (CSF) T. pallidum by reverse-transcriptase polymerase chain reaction. Lower border of box represents the 25th percentile and upper border the 75th percentile, with the median between them; whiskers represent minimum and maximum values. Differences between groups were determined using Mann–Whitney U test.
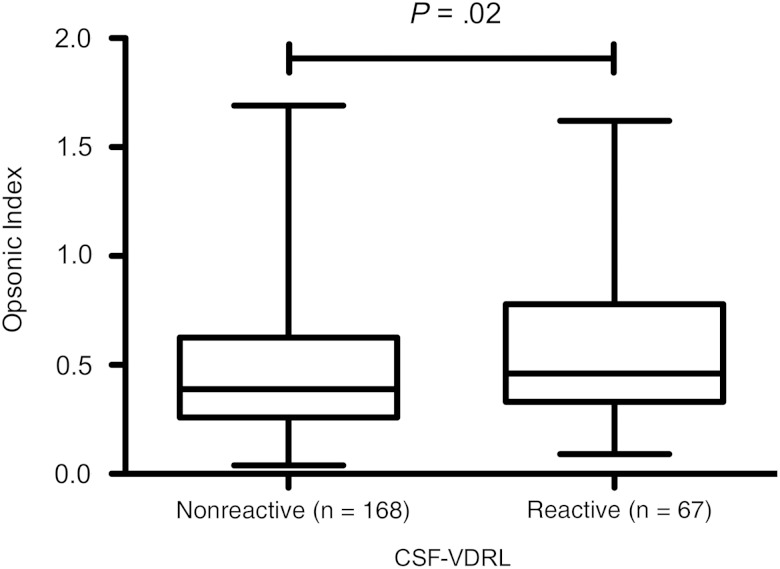
Among all participants, reactive CSF-VDRL was more common in those with serum RPR titers ≥1:64 than in those with lower titers (60 [36.1%] of 166 vs 7 [10.1%] of 69; P < .001) and more common in those with late stage than in those with early stage syphilis (29 [44.6%] of 65 vs 38 [22.4%] of 170; P = .001). Compared with patients who had a nonreactive CSF-VDRL, opsonic indexes were significantly higher among those with reactive CSF-VDRL (Figure 4), but this relationship was also lost when serum RPR titer and syphilis stage were taken into account in multivariable logistic regression (data not shown).
Figure 4.
Box-and-whisker plot of opsonic index of serum samples in participants with nonreactive and reactive cerebrospinal fluid (CSF) VDRL tests. Lower border of box represents the 25th percentile and upper border the 75th percentile, with the median between them; whiskers represent minimum and maximum values. Differences between groups were determined using Mann–Whitney U test.
DISCUSSION
We measured T. pallidum–specific opsonic activity of serum samples from 171 HIV-infected and 64 HIV-uninfected patients with syphilis. In multivariable analysis, opsonic activity was significantly greater in HIV-uninfected individuals, even after controlling for treatment of uncomplicated syphilis before serum collection and higher serum RPR titers, both of which remained significant in the model. About half of our study subjects were treated for uncomplicated syphilis before study entry. Most commonly, this occurred because the lumbar puncture could not be performed on the day that the patient presented with newly diagnosed syphilis. Treatment for uncomplicated syphilis pending lumbar puncture was given to prevent infection of sexual contacts.
Higher serum opsonic activity in individuals who were treated for uncomplicated syphilis before study entry could be due to antibiotic-related rapid destruction of organisms that expose the host to a greater concentration of targets of opsonic antibody, leading to development of greater opsonic activity. The relationship between higher serum RPR titer and higher opsonic activity is less easy to explain in our study. Whereas serum from rabbits immunized with VDRL antigen is opsonic [28], serum VDRL titers in infected animals correlate with serum opsonic activity early but not later in infection [12]. In our study, opsonic activity was not related to syphilis stage, but, among untreated individuals, serum RPR titers were higher in those with detectable blood T. pallidum concentrations. This relationship could suggest that higher opsonic activity might be driven by higher organism burden, but, as discussed below, our results suggest that opsonic activity may be lower, not higher, in individuals with higher blood T. pallidum concentrations.
Case reports and small series have suggested a greater likelihood of syphilis treatment failure [2–4] and development of neurosyphilis [5, 6] in HIV-infected than in HIV-uninfected patients. One possible explanation for these observations is a defect in immune-mediated mechanisms of clearance of T. pallidum from sites of infection, a process that requires opsonic antibody [11–14]. We hypothesized that, compared to HIV-uninfected individuals with syphilis, serum from HIV-infected individuals with syphilis would have lower opsonic activity and that this would be accompanied by a higher organism burden in blood. Our results show robustly that serum opsonic activity is significantly lower in HIV-infected individuals, even taking into account other factors that influence opsonic activity. Although we were able to show an inverse relationship between opsonic activity and organism burden in blood, this finding was less robust, but perhaps not unexpected. Ingestion and killing of T. pallidum by macrophages occurs at local sites of infection, such as skin or oral or genital mucosa; it probably does not take place in blood. In primary syphilis, T. pallidum disseminates from the local site of infection via the blood and can seed virtually any organ. In secondary syphilis, these disseminated organisms, which express variant TprK antigens [29], escape immune control, leading to additional waves of bacteremia. Thus, detection of blood T. pallidum, while ultimately related to immune control or clearance, is probably not a direct correlate of this process, thus weakening the relationship between serum opsonic activity and blood organism burden.
Neurosyphilis is more common in individuals with higher serum RPR titers [7, 24, 25] and in those with late stage versus early stage syphilis [9]. In addition, several factors that reflect the host immune response increase the likelihood of neurosyphilis, including single-nucleotide polymorphisms in Toll-like receptor genes [9] and, among HIV-infected individuals, lower peripheral blood CD4+ T-cell concentrations [7, 24], detectable plasma HIV RNA [30], and lack of antiretroviral medication use [7, 9]. As such, we anticipated that neurosyphilis would be more common in individuals with lower serum opsonic activity. In contrast, we found that individuals with neuroinvasion (defined by RT-PCR detection of T. pallidum in CSF) or neurosyphilis (defined by a reactive CSF-VDRL) had higher, rather than lower, serum opsonic stageic activity. However, these relationships were lost in multivariable models, leading us to conclude that there was not, at least directly, a relationship between serum opsonic activity and neuroinvasion or neurosyphilis in our study.
Limitations inherent in this work should be acknowledged. Although we measured differences in serum “opsonic activity” in HIV-infected versus HIV-uninfected individuals, we cannot determine whether these differences reflect lower concentration of antibody, diminished antibody binding, responses to different antigen repertoires, or some other factor that results in reduced ingestion of opsonized organisms by MDMs. We are able to estimate only roughly the duration of infection in our study participants based on their syphilis stage. We have no information on dose or strain of infecting organisms, and we are not able to study the course of untreated disease. This latter limitation is particularly noteworthy when we consider the relationship between opsonic activity and neuroinvasion or neurosyphilis. We assessed opsonic activity at a single time point, which may or may not have been remote in time from neuroinvasion or development of neurosyphilis. The level of opsonic activity at those earlier times could have been substantially different than at the time of our assessment. Nonetheless, we found that serum T. pallidum–specific opsonic activity is significantly lower in HIV-infected than in HIV-uninfected individuals with syphilis. This result was robust even taking into account prior treatment for uncomplicated syphilis and serum RPR titer. Impaired T. pallidum–specific immune responses in HIV-infected individuals could contribute to differences in the course of disease or treatment response, both of which may be more common in HIV-infected than in HIV-uninfected patients with syphilis. Future longitudinal studies could address some of these issues, in particular the relationship between serum opsonic activity and serological response to syphilis treatment.
Notes
Financial support. This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (grant NS34235).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2013. Atlanta, GA: US Department of Health and Human Services, 2014. [Google Scholar]
- 2.Blank LJ, Rompalo AM, Erbelding EJ, Zenilman JM, Ghanem KG. Treatment of syphilis in HIV-infected subjects: a systematic review of the literature. Sex Transm Infect 2011; 87:9–16. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Lopez JJ, Guerrero ML, Lujan R, Tostado SF, de Gorgolas M, Requena L. Factors determining serologic response to treatment in patients with syphilis. Clin Infect Dis 2009; 49:1505–11. [DOI] [PubMed] [Google Scholar]
- 4.Horberg MA, Ranatunga DK, Quesenberry CP, Klein DB, Silverberg MJ. Syphilis epidemiology and clinical outcomes in HIV-infected and HIV-uninfected patients in Kaiser Permanente Northern California. Sex Transm Dis 2010; 37:53–8. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MM, Aynalem G, Olea LM, He P, Smith LV, Kerndt PR. A consequence of the syphilis epidemic among men who have sex with men (MSM): neurosyphilis in Los Angeles, 2001–2004. Sex Transm Dis 2008; 35:430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Symptomatic early neurosyphilis among HIV-positive men who have sex with men—four cities, United States, January 2002-June 2004. MMWR Morb Mortal Wkly Rep 2007; 56:625–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS 2008; 22:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Antiretroviral therapy is associated with reduced serologic failure rates for syphilis among HIV-infected patients. Clin Infect Dis 2008; 47:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marra CM, Sahi SK, Tantalo LC et al. Toll-like receptor polymorphisms are associated with increased neurosyphilis risk. Sex Transm Dis 2014; 41:440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 11.Baker-Zander SA, Lukehart SA. Macrophage-mediated killing of opsonized Treponema pallidum. J Infect Dis 1992; 165:69–74. [DOI] [PubMed] [Google Scholar]
- 12.Baker-Zander SA, Shaffer JM, Lukehart SA. Characterization of the serum requirement for macrophage-mediated killing of Treponema pallidum ssp. pallidum: relationship to the development of opsonizing antibodies. FEMS Immunol Med Microbiol 1993; 6:273–9. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer JM, Baker-Zander SA, Lukehart SA. Opsonization of Treponema pallidum is mediated by immunoglobulin G antibodies induced only by pathogenic treponemes. Infect Immun 1993; 61:781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukehart SA, Miller JN. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol 1978; 121:2014–24. [PubMed] [Google Scholar]
- 15.Houston S, Hof R, Honeyman L, Hassler J, Cameron CE. Activation and proteolytic activity of the Treponema pallidum metalloprotease, pallilysin. PLoS Pathog 2012; 8:e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis 2000; 181:1401–13. [DOI] [PubMed] [Google Scholar]
- 17.Centurion-Lara A, Castro C, Barrett L et al. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response [published erratum appears in J Exp Med 1999 Jun 7;189(11):following 1852]. J Exp Med 1999; 189:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore MW, Cruz AR, LaVake CJ et al. Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces gamma interferon production. Infect Immun 2007; 75:2046–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz AR, Ramirez LG, Zuluaga AV et al. Immune evasion and recognition of the syphilis spirochete in blood and skin of secondary syphilis patients: two immunologically distinct compartments. PLoS Negl Trop Dis 2012; 6:e1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi H, Oishi K, Yoshimine H et al. Decreased serum opsonic activity against Streptococcus pneumoniae in human immunodeficiency virus-infected Ugandan adults. Clin Infect Dis 2003; 37:1534–40. [DOI] [PubMed] [Google Scholar]
- 21.Eagan R, Twigg HL 3rd, French N et al. Lung fluid immunoglobulin from HIV-infected subjects has impaired opsonic function against pneumococci. Clin Infect Dis 2007; 44:1632–8. [DOI] [PubMed] [Google Scholar]
- 22.Jaworowski A, Fernandes LA, Yosaatmadja F et al. Relationship between human immunodeficiency virus type 1 coinfection, anemia, and levels and function of antibodies to variant surface antigens in pregnancy-associated malaria. Clin Vaccine Immunol 2009; 16:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keen J, Serghides L, Ayi K et al. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Med 2007; 4:e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marra CM, Maxwell CL, Smith SL et al. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis 2004; 189:369–76. [DOI] [PubMed] [Google Scholar]
- 25.Libois A, De Wit S, Poll B et al. HIV and syphilis: when to perform a lumbar puncture. Sex Transm Dis 2007; 34:141–4. [DOI] [PubMed] [Google Scholar]
- 26.Larsen SA, Pope V, Johnson RE, Kennedy EJ Jr. A manual of tests for syphilis. 9th ed Washington, DC: American Public Health Association, 1998. [Google Scholar]
- 27.Lukehart SA, Marra CM. Isolation and laboratory maintenance of Treponema pallidum. Curr Protoc Microbiol 2007; Chapter 12:Unit 12A 1. [DOI] [PubMed] [Google Scholar]
- 28.Baker-Zander SA, Shaffer JM, Lukehart SA. VDRL antibodies enhance phagocytosis of Treponema pallidum by macrophages. J Infect Dis 1993; 167:1100–5. [DOI] [PubMed] [Google Scholar]
- 29.Reid TB, Molini BJ, Fernandez MC, Lukehart SA. Antigenic variation of TprK facilitates development of secondary syphilis. Infect Immun 2014; 82:4959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumaresq J, Langevin S, Gagnon S et al. Clinical prediction and diagnosis of neurosyphilis in HIV-infected patients with early syphilis. J Clin Microbiol 2013; 51:4060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]