Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a pleiotropic cytokine that plays a critical role in regulating myeloid cell host defense. In this study, we demonstrated that GM-CSF signaling plays an essential role in antifungal defense against Aspergillus fumigatus. Mice that lack the GM-CSF receptor β chain (GM-CSFRβ) developed invasive hyphal growth and exhibited impaired survival after pulmonary challenge with A. fumigatus conidia. GM-CSFRβ signaling regulated the recruitment of inflammatory monocytes to infected lungs, but not the recruitment of effector neutrophils. Cell-intrinsic GM-CSFRβ signaling mediated neutrophil and inflammatory monocyte antifungal activity, because lung GM-CSFRβ−/− leukocytes exhibited impaired conidial killing compared with GM-CSFRβ+/+ counterparts in mixed bone marrow chimeric mice. GM-CSFRβ−/− neutrophils exhibited reduced (hydrogenated) nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in vivo. Conversely, administration of recombinant GM-CSF enhanced neutrophil NADPH oxidase function, conidiacidal activity, and lung fungal clearance in A. fumigatus–challenged mice. Thus, our study illustrates the functional role of GM-CSFRβ signaling on lung myeloid cell responses against inhaled A. fumigatus conidia and demonstrates a benefit for systemic GM-CSF administration.
Keywords: GM-CSF, Aspergillus, neutrophils, monocytes, ROS
Aspergillus fumigatus is a pathogenic mold that forms ubiquitous airborne conidia (vegetative spores) [1]. Invasive aspergillosis represents a common cause of infection-related death in patients with leukemia and allogeneic hematopoietic cell transplant recipients. In murine and human studies, quantitative and qualitative defects in neutrophils and monocytes are associated with conidial germination into tissue-invasive hyphae and represent major clinical risk factors for the development of invasive aspergillosis [1–5]. Beyond patients with hematologic cancers, humans with defects in granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling are susceptible to invasive mold infections [6].
GM-CSF was first identified as a pleiotropic cytokine with primary functions in regulating myeloid cell survival, proliferation, and differentiation [7, 8]. The GM-CSF receptor (GM-CSFR/Csf2r) is a heterodimer composed of a specific ligand-binding subunit (GM-CSFRα/Csf2ra) and a common signal-transduction subunit (GM-CSFRβ/Csf2rb) [9]. GM-CSF–deficient (GM-CSF−/−) mice are susceptible to a wide range of pathogens that include Mycobacterium tuberculosis [10], Pseudomonas aeruginosa [11], Pneumocystis carinii [12], and Plasmodium chabaudi [13]). Although GM-CSF enhances neutrophil phagocytic and microbicidal properties [14, 15], the mechanisms by which GM-CSF signaling promote defense against pathogenic yeasts and molds remains incompletely defined. In a systemic Candida albicans challenge model, natural kill (NK) cell–derived GM-CSF enhances neutrophil antifungal activity [16]. For the intracellular fungal pathogen Histoplasma capsulatum, macrophage GM-CSF signaling sequesters zinc ion from phagosomes to the Golgi apparatus and increases macrophage reactive oxygen species (ROS) production [17]. These findings provide a mechanistic explanation for the observation that GM-CSF depletion is lethal for H. capsulatum–infected mice, whereas GM-CSF administration promotes fungal clearance [18].
GM-CSF is critical for alveolar macrophage differentiation and mendelian defects in GM-CSF signaling, including mutations in the GM-CSF receptor β chain [19], or anti-GM-CSF autoantibodies lead to dysregulated surfactant homeostasis and to pulmonary alveolar proteinosis (PAP) [19, 20]. Not only are patients with PAP susceptible to invasive aspergillosis [6], but among patient with PAP who have opportunistic infections, those with fungal infections have the lowest survival rates [6]. Although GM-CSF has been used as a Food and Drug Administration–approved drug for PAP [21], little is known about the mechanism by which defective GM-CSF signaling impairs pulmonary innate immune responses against A. fumigatus.
In this study, we demonstrated that GM-CSFRβ signaling is critical for survival and control of hyphal formation in A. fumigatus–challenged mice. GM-CSFRβ signaling regulates conidial uptake and killing by neutrophils and inflammatory monocytes in a cell-intrinsic manner, in part by regulating the oxidative burst in vivo. Our data further illustrate that GM-CSF supplementation enhances antifungal activity of lung neutrophils and inflammatory monocytes and accelerate fungal clearance in otherwise immunocompetent mice.
MATERIALS AND METHODS
Mice
GM-CSFRβ−/− (stock No. 005940), p91phox−/− (stock No. 002365), C57BL/6 (CD45.2+), and C57BL/6.SJL (CD45.1+) mice were purchased from Jackson Laboratories. All mouse strains were bred and housed in the Memorial Sloan Kettering Cancer Center Research Animal Resource Center under specific pathogen-free conditions. All animal experiments were conducted with sex- and age-matched mice and performed with Institutional Animal Care and Use Committee approval.
Mixed bone marrow (BM) chimeric mice were generated by reconstituting lethally irradiated (900 cGy) recipient mice (F1 progeny from C57BL/6 and C57B/6.SJL cross; CD45.1+CD45.2+) with 2–5 × 106 CD45.2+GM-CSFRβ−/− and CD45.1+C57BL/6.SJL GM-CSFRβ+/+ BM cells and rested for 6–8 weeks before experimental use.
Analysis of Infected Mice
Mice were inoculated with 3–8 × 107 Af293 or CEA10 conidia via the intratracheal route as in [22, 23]. We used fluorescent Aspergillus reporter (FLARE) conidia to measure conidial uptake by and viability in leukocytes as described elsewhere [22, 23]. Bronchoalveolar lavage (BAL) and lung cell suspensions were prepared as described elsewhere [24] and stained with anti-Ly6C (clone AL-21), anti-Ly6G (1A8), anti-CD11b (M1/70), anti-CD11c (HL3), anti-CD45.1 (A20), anti-CD45.2 (104), anti-Ly6B.2 (7/4), anti–major histocompatibility complex (MHC) class II (M5/114.15.2) and with a viability dye (eBioscience; catalog No. 65-0865-14) and plated for colony-forming units (CFUs). Neutrophils were identified as CD45+CD11b+Ly6CloLy6G+Ly6B.2+, inflammatory monocytes as CD45+CD11b+Ly6ChiLy6G−CD11c−MHC-II−, and monocyte-derived dendritic cells (Mo-DCs) as CD45+CD11c+MHC-II+Ly6CloLy6G− cells. Flow cytometric data were collected on a BD LSR II and analyzed with FlowJo software, version 9.8.2.
BAL lactate dehydrogenase levels were measured using the CytoTox96 nonradioactive cytotoxicity assay kit (Promega; catalog No. G1780). Lungs were homogenized in 2 mL of phosphate-buffered saline (PBS) containing 0.025% Tween 20 for enzyme-linked immunosorbent assays. For histology, perfused lungs were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin-eosin or Gomori ammoniacal silver. Images were captured using a Zeiss Mirax Midi slide scanner with a ×20x/0.8-numerical aperture objective and analyzed using Panoramic Viewer software (version 1.15.3).
For GM-CSF supplementation experiments, wild-type (WT) mice were injected with 500 ng of recombinant mouse GM-CSF (Gemini; catalog No. 300–308P) in PBS intraperitoneally at 24 hours before infection (day -1) and at the time of infection (day 0). The endotoxin level was <1 endotoxin units/μg, as measured by limulus amebocyte lysate analysis.
Neutrophil ROS and FLARE Assays
BAL cells from infected mixed BM chimeric mice were cultured with 5 μmol/L CM-H2DCFDA (General Oxidative Stress Indicator; Life Technologies) at 5 μmol/L in Hank's balanced salt solution for 45 minutes at 37oC, stained with a viability dye to exclude dead cells, and analyzed using flow cytometry. BM neutrophils were enriched from WT or p91phox−/− mice using density gradient centrifugation. Neutrophils were cocultured with AF293 FLARE conidia at a 1:1 ratio for 16 hours at 37°C and analyzed with flow cytometry.
Statistical Analysis
All results are expressed as mean (standard error of the mean [SEM]) values derived from 3 independent experiments, unless stated otherwise. The Mann–Whitney U test was used for unpaired 2-group comparisons, and the Wilcoxon signed rank test for paired 2-group comparisons. Nonparametric 2-way analysis of variance was used for multiple-group comparison. Survival data were analyzed using log-rank test unless stated otherwise. All statistical analyses were performed with GraphPad Prism software (version 6.0c).
RESULTS
GM-CSF Receptor Signaling and Murine Survival After A. fumigatus Challenge
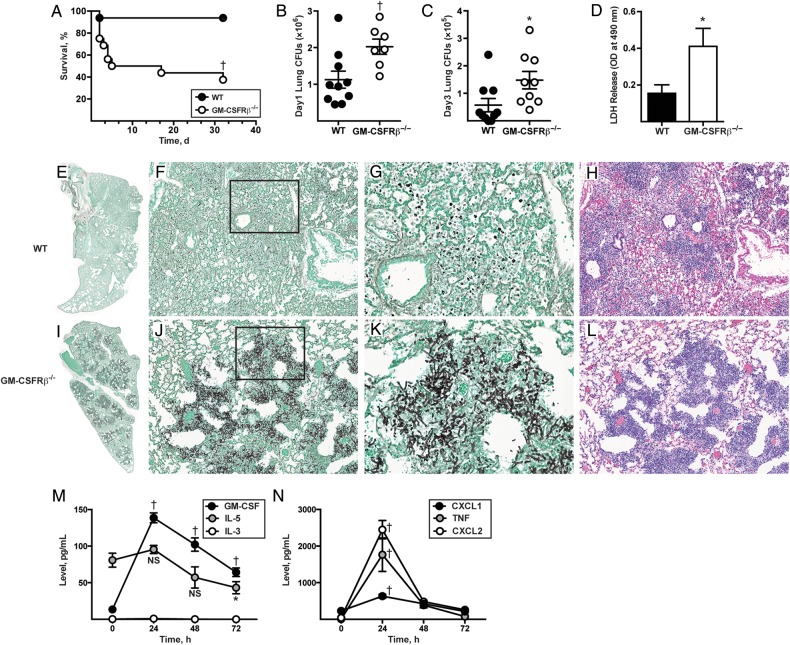
To define the role of GM-CSFR receptor β chain (GM-CSFRβ) signaling during respiratory fungal challenge, GM-CSFRβ−/− and C57BL/6 control mice were challenged with 8 × 107 A. fumigatus CEA10 conidia and monitored for survival. Fifty percent of GM-CSFRβ−/− mice died by day +5, and the survival rates for GM-CSFRβ−/− and WT mice on day 32 were 37.5% and 93.7%, respectively (Figure 1A). The increased mortality rate correlated with a higher lung fungal burden at 24 and 72 hours after infection in GM-CSFRβ−/− mice, as measured by CFUs (inoculum, 3 × 107 conidia; Figure 1B and 1C) and greater lung damage at 48 hours after infection, as judged by BAL fluid lactate dehydrogenase release (inoculum, 8 × 107 conidia; Figure 1D).
Figure 1.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor β chain (GM-CSFRβ) signaling is critical for survival, fungal clearance, and lung integrity during Aspergillus fumigatus challenge. A–D, Wild-type (WT) and GM-CSFRβ−/− mice were challenged with 8 × 107 CEA10 conidia and monitored for survival (Kaplan–Meier survival plot of WT [black circles; n = 16] and GM-CSFRβ−/− [white circles; n = 16] mice; data pooled from 2 independent experiments) (A); challenged with 3 × 107 CEA10 conidia tested for lung fungal burden 24 (B) hours and 72 (C) hours after infection (representative data from 3 experiments [B]) and 1 experiment [C]); and challenged with 8 × 107 CEA10 conidia and examined for bronchoalveolar lavage fluid lactate dehydrogenase (LDH) levels 48 hours after infection (bar graphs show mean [standard error of the mean {SEM}] from an experiment with 6–10 mice per genotype) (D). E–L, Representative micrographs of hematoxylin-eosin– and Gomori ammoniacal silver–stained lung sections from WT and GM-CSFRβ−/− mice 48 hours after infection. Images were captured at ×2 (E, I), ×20 (F, H, J, L), and ×60 (G, K) magnification, with G and K corresponding to insets in F and J, respectively. M, N, Graph shows mean (SEM) lung cytokine and chemokine levels at the indicated time points in C57BL/6 WT mice infected with 3 × 107 CEA10 conidia, with representative data from 1 of 2 independent experiments (5, 8, 9 and 8 mice at 0, +24, +48, and +72 hours, respectively). *P < .05; †P < .01. Abbreviations: CFUs, colony-forming units; IL-3, interleukin 3; IL-5, interleukin 5; NS, not significant; TNF, tumor necrosis factor.
Although C57BL/6 mice formed widespread inflammatory infiltrates in lung sections harvested 48 hours after infection, we did not observe evidence of hyphal tissue invasion (Figure 1E–H). In contrast, GM-CSFRβ−/− mice developed invasive aspergillosis; lung sections showed widespread hyphal tissue invasion and destruction of bronchoalveolar architecture (Figure 1I–L). GM-CSFRβ−/− lungs contained extensive, multifocal lesions with necrotic cells and masses of fungal hyphae (Figure 1I–L). In sum, these results indicate that GM-CSFRβ chain signaling is essential for effective fungal clearance and murine survival.
Because GM-CSFRβ can participate in interleukin 3 (IL-3) and interleukin 5 (IL-5) signaling as well, we measured the induction of GM-CSF, IL-3, and IL-5 during respiratory fungal challenge (Figure 1M). We observed a 10-fold increase in lung GM-CSF level and no significant change in IL-5, and we did not detect IL-3 in the lung at any time point tested. The kinetics of GM-CSF induction mirror those of the inflammatory cytokines tumor necrosis factor, CXCL1, and CXCL2, which have well-described roles in host defense (Figure 1N) [25–27].
GM-CSFRβ Chain Signaling and Lung Leukocyte Recruitment
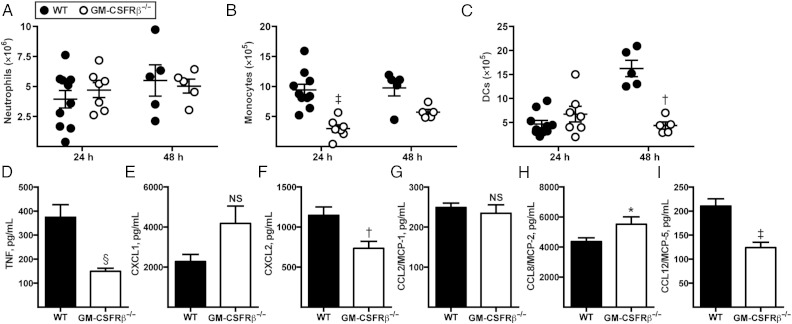
To dissect the mechanism by which GM-CSFRβ signaling mediates host protection against A. fumigatus, we monitored innate immune cell recruitment during respiratory challenge. Lung neutrophil numbers were similar in C57BL/6 and GM-CSFRβ−/− mice at both 24 and 48 hours after infection (Figure 2A). However, early inflammatory monocyte recruitment (24 hours after infection) was decreased in GM-CSFRβ−/− mice, and this trend continued 48 hours after infection (Figure 2B). At 24 hours after infection, lung Mo-DC numbers were similar between WT and GM-CSFRβ−/− mice, but at 48 hours after infection, Mo-DCs in GM-CSFRβ−/− mice failed to expand (Figure 2C). Given the developmental relationship between inflammatory monocytes and descendant Mo-DCs [24] during respiratory A. fumigatus challenge, these data suggest that the failure to observe Mo-DC expansion was related to a reduced lung inflammatory monocyte influx. Furthermore, we observed significant reductions in tumor necrosis factor, CXCL2, and monocyte chemotactic protein-5 (60%, 37%, and 42%, respectively) in the lungs of GM-CSFRβ−/− mice compared with WT mice (Figure 2D–I). We did not observe reductions in CXCL1, CCL2, or CCL8 lung levels in GM-CSFRβ−/− mice. Thus, genetic deletion of GM-CSFR signaling had a variable impact on the production of inflammatory and chemotactic mediators in the lung.
Figure 2.
Granulocyte-macrophage colony-stimulating factor receptor β chain (GM-CSFRβ) signaling and neutrophil and monocyte recruitment during respiratory fungal infection. A–C, Wild-type (WT) mice were infected with 3 × 107 CEA10 conidia, and recruitment of neutrophils (A), inflammatory monocytes (B), and monocyte-derived dendritic cells (DCs) (C) in lungs was measured with flow cytometry at the indicated time points; representative data from 3 independent experiments are shown (WT: n = 10, GM-CSFRβ−/−: n = 7). D–I, Graphs show mean (standard error of the mean) lung cytokine and chemokine levels 24 hours after infection in C57BL/6 WT or GM-CSFRβ−/− mice infected with 3 × 107 CEA10 conidia; representative data from 2 independent experiments are shown (WT: n = 10; GM-CSFRβ−/−: n = 13). *P < .05; †P < .01; ‡P < .001; §P < .0001. Abbreviations: MCP-5, monocyte chemotactic protein 5; NS, not significant; TNF, tumor necrosis factor.
Cell-Intrinsic Role of GM-CSFR-β Signaling in Conidial Uptake and Killing
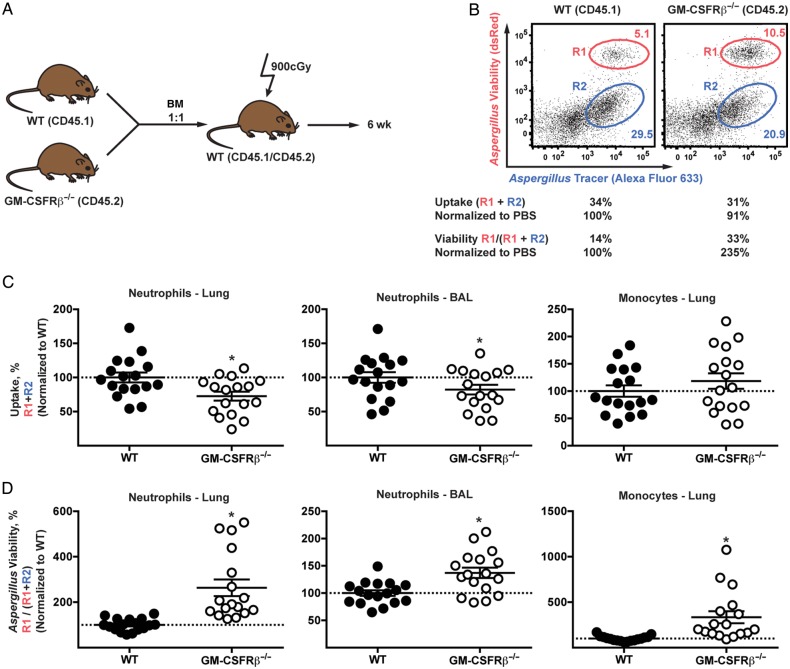
To determine whether leukocyte-intrinsic GM-CSFRβ signaling regulates neutrophil antifungal responses, we generated mixed BM chimeric mice by injecting CD45.2+GM-CSFRβ−/− and CD45.1+ WT BM cells into lethally-irradiated CD45.1+CD45.2+ recipients (Figure 3A). The advantage of using mixed BM chimeric mice was that gene-deficient and gene-sufficient leukocytes were compared functionally side by side within the same lung inflammatory context in the absence of dysregulated lung surfactant homeostasis [28, 29].
Figure 3.
Cell intrinsic role of granulocyte-macrophage colony-stimulating factor receptor β chain (GM-CSFRβ) signaling in antifungal activity in neutrophils and monocytes. A–D, Mixed bone marrow (BM) chimeric mice of wild-type (WT) and GM-CSFRβ−/− were generated and infected with 3 × 107 AF293 fluorescent Aspergillus reporter (FLARE). Bronchoalveolar lavage (BAL) fluid and lungs were harvested at 48 hours after infection and fungal uptake and viability were analyzed with flow cytometry. A, Schematic showing how to generate mixed BM chimeric mice. B, Representative plots showing fungal uptake and killing in neutrophils from WT and GM-CSFRβ−/− compartment showing live (R1) and dead (R2) conidia. C, D, Fungal uptake (C) and viability (D) in neutrophils and monocytes were measured, and monocytes were identified as CD45+CD11b+Ly6ChiLy6G−Ly6B.2+. Data from 3 independent experiments were pooled and normalized, and relative levels were indicated (n = 17). *P < .001. Abbreviation: PBS, phosphate-buffered saline.
Using FLARE conidia in mixed BM chimeric mice, we compared neutrophil and inflammatory monocyte-mediated conidial uptake and killing (Figure 3B–D). FLARE conidia encode a fungal viability indicator (ie, DsRed or TdTomato) and contain a tracer fluorophore (ie, Alexa Fluor 633) [22, 23]. FLARE conidia emit 2 fluorescence signals (DsRed/TdTomato and Alexa Fluor 633) when the fungal cell is alive, and emit a single fluorescence signal (Alexa Fluor 633 only) when the fungal cell is killed. This approach allows us to distinguish fungus-engaged leukocytes from bystander leukocytes and determine the frequency of fungus-engaged leukocytes that contain either live or killed fungal cells in the lung.
In a representative example depicted in Figure 3B, lung neutrophils were analyzed on the basis of conidial uptake and viability within the leukocyte 48 hours after infection. R1 and R2 gate represent neutrophils containing live and dead conidia, respectively. The frequency of fungus-engaged neutrophils (conidial uptake frequency, gate R1 + R2) was slightly reduced for the GM-CSFRβ−/− cells compared with WT counterparts. More strikingly, the frequency of fungus-engaged cells that contain live conidia was increased among GM-CSFRβ−/− compared with WT neutrophils (neutrophil conidial viability, R1/[R1 + R2]), indicating a defect in conidial killing. Normalized data pooled from 3 experiments showed that conidial uptake by lung and BAL fluid GM-CSFRβ−/− neutrophils was decreased, on average, by 27.4% (SEM, 6.4%) and 17.8% (7.1%), respectively, compared with WT counterparts isolated from the same lung (Figure 3C; gate R1 + R2). We did not observe a statistically significant change in inflammatory monocyte conidial uptake in the lung among cells of both genotypes (Figure 3C).
The frequency of neutrophils that contain live conidia was 2.6-fold and 1.3-fold higher for lung and BAL fluid GM- CSFRβ−/− neutrophils compared with WT counterparts (Figure 3D). Similarly, the frequency of lung inflammatory monocytes that contain live conidia was 3.3-fold higher for GM-CSFRβ−/− than for WT cells (Figure 3D). The fungal killing defect in GM-CSFRβ−/− neutrophils and inflammatory monocytes was not due to impaired cellular viability, as measured by annexin V and viability dye staining (see Supplementary Figure 1). Thus, these data indicate that neutrophils and inflammatory monocytes require cell-intrinsic GM-CSFRβ signaling to achieve full conidiacidal activity.
GM-CSFRβ Signaling and Neutrophil ROS Production During Respiratory Fungal Challenge
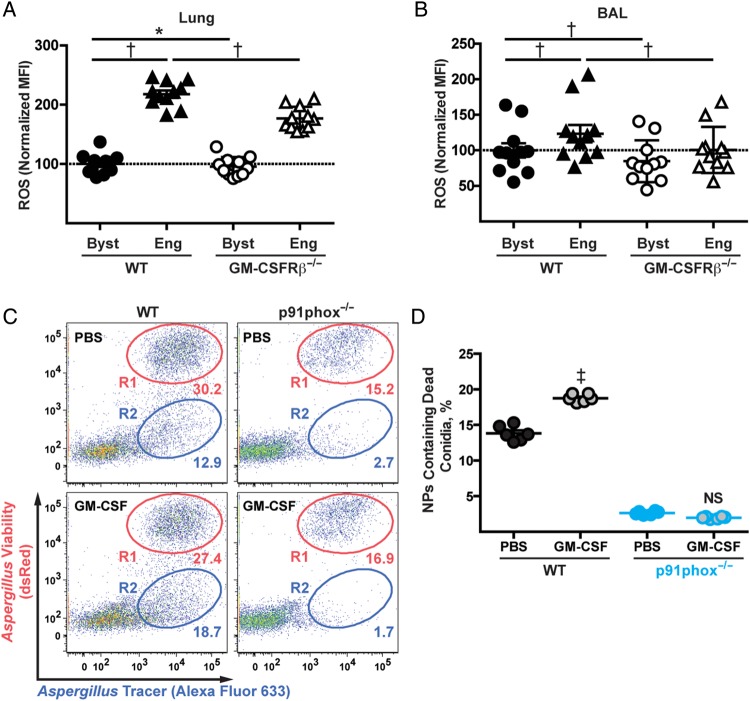
To examine conidiacidal effector mechanisms controlled by GM-CSFRβ signaling, we measured in vivo neutrophil ROS production in congenically marked GM-CSFRβ−/− and GM-CSFRβ+/+ neutrophils in the lungs of mixed BM chimeric mice using the experimental scheme described in Figure 3A. We challenged mixed BM chimeric mice with Alexa Fluor 633–labeled AF293 conidia to distinguish bystander and fungus-engaged neutrophils.
Pooled data from multiple experiments were normalized to a neutrophil ROS median fluorescence intensity of 100 in lung and BAL GM-CSFRβ+/+ bystander cells (Figure 4A and 4B, black circles). The normalized ROS median fluorescence intensity for GM-CSFRβ−/− bystander neutrophils was very similar to that of WT counterparts (Figure 4A and 4B, white circles). However, neutrophil ROS induction that was triggered by conidial uptake was significantly attenuated in GM-CSFRβ−/− neutrophils compared with WT neutrophils isolated from the same lungs (Figure 4A) and airways (Figure 4B). These data demonstrate that neutrophil-intrinsic GM-CSFRβ signaling partially regulates ROS induction during respiratory A. fumigatus challenge.
Figure 4.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor β chain (GM-CSFRβ) signaling and neutrophil reactive oxygen species (ROS) production. Mixed bone marrow chimeric mice of wild-type (WT) and GM-CSFRβ−/− were infected with 3 × 107 AF293 fluorescent Aspergillus reporter (FLARE) and lungs and bronchoalveolar lavage (BAL) were harvested at 24 hours after infection. A, B, ROS production in neutrophils from lungs (A) and BAL fluid (B) were analyzed with flow cytometry. Median fluorescence intensity (MFI) for ROS levels was measured and normalized to the ROS levels in WT bystander neutrophils; data from 2 independent experiments were pooled and relative values are presented (n = 11). C, Neutrophils were enriched from the bone marrow of WT or p91phox−/− mice and cocultured with FLARE, with or without recombinant GM-CSF. Representative plots show fungal uptake and killing in neutrophils from WT and p91phox−/− mice, with live (R1) and dead (R2) conidia. D, Frequency of neutrophils (NPs) containing dead conidia (R2) was measured. C, D, Data from 1 experiment (n = 6). *P < .01; †P < .001; ‡P < .0001. Abbreviations: Byst, bystander neutrophils; Eng, fungus-engaged neutrophils; NS, not significant; PBS, phosphate-buffered saline.
To determine whether GM-CSF regulates neutrophil (hydrogenated) nicotinamide adenine dinucleotide phosphate (NADPH) oxidase–dependent antifungal activity, we cocultured FLARE conidia and neutrophils isolated from WT or NADPH oxidase–deficient mice (p91phox−/−), with or without exogenous GM-CSF. On average, the addition of GM-CSF increased the frequency of WT neutrophils that contain dead conidia from 13.8% to 18.7%, whereas GM-CSF had no effect on fungal killing by p91phox−/− neutrophils in vitro (Figure 4C and 4D). These data indicate that GM-CSF can enhance neutrophil NADPH oxidase–dependent conidial killing in vitro.
GM-CSF Administration and Neutrophil and Inflammatory Monocyte Antifungal Activity
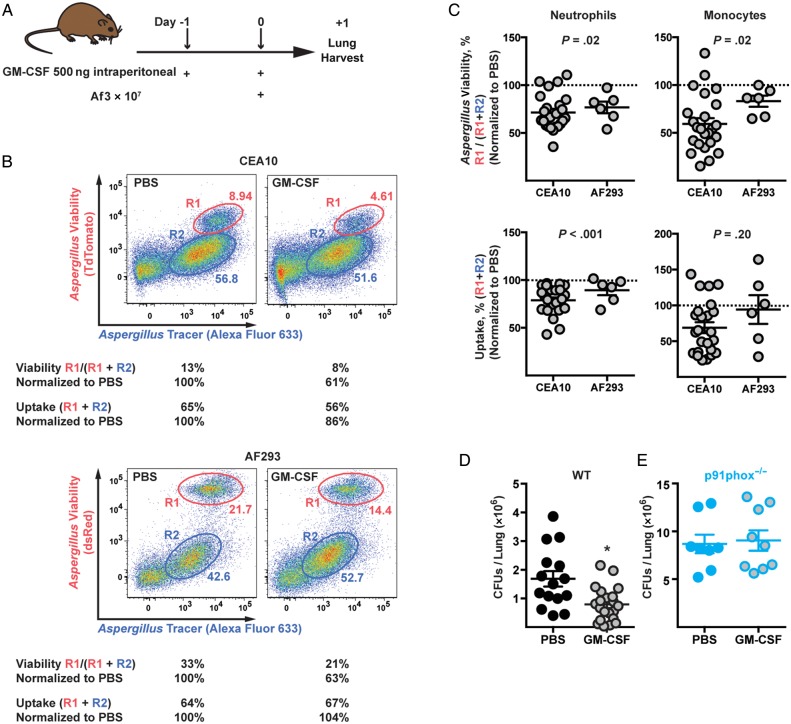
To determine whether exogenous GM-CSF enhanced antifungal responses by innate immune cells in vivo, we injected GM-CSF (dose, 500 ng) or PBS into mice via the intraperitoneal route and challenged the animals with 2 strains of FLARE conidia via the intratracheal route (Figure 5A). We used 2 strains of FLARE conidia to ensure that the effects of GM-CSF were general and not limited to a single clinical isolate.
Figure 5.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) administration can enhance fungal killing and clearance in the lung. A, Schematic of experimental design. B, Representative plot shows lung neutrophils analyzed on the basis of conidial uptake and viability. The R1 gates represent fungus-engaged neutrophils with live conidia, and the R2 gates, fungus-engaged neutrophils with dead conidia. C, Dot plots show normalized conidial viability in (top row) and conidial uptake by (bottom row) lung neutrophils (left panels) and inflammatory monocytes (right panels) isolated from C57BL/6 mice challenged with CEA10 fluorescent Aspergillus reporter (FLARE) or Af293 FLARE conidia. Data were normalized to phosphate-buffered saline (PBS) controls (dotted lines) from 3 independent experiments (CEA10; n = 20 for PBS, n = 24 for GM-CSF) and 1 experiment (AF293; n = 7 for PBS, n = 6 for GM-CSF), and relative values are presented. D, Fungal burden in the lungs from PBS (n = 15) or GM-CSF (n = 21) injected wild-type (WT) mice challenged with CEA10 FLARE conidia. E, Fungal burden in the lungs from PBS- (n = 8) or GM-CSF– (n = 9) injected p91phox−/− mice challenged with CEA10 FLARE conidia. *P < .01. Abbreviations: Af, Aspergillus fumigatus; CFUs, colony-forming units.
GM-CSF administration enhanced neutrophil-mediated conidial killing, as judged by the reduced frequency of fungus-engaged neutrophils that contain live conidia among fungus-engaged neutrophils (see Figure 5B for representative example), irrespective of the fungal strain used. For data presented in Figure 5B and in the dot plots in Figure 5C, neutrophil conidial viability was normalized to results obtained with PBS-treated mice.
On average, GM-CSF injection reduced the frequency of neutrophils and inflammatory monocytes that contain live CEA10 conidia by 28.6% (SEM, 3.8%) and 40.6% (6.0%), respectively, and that contain Af293 conidia, by 23.3% (6.0%) and 16.9% (5.8%), respectively (Figure 5C). These data indicate that GM-CSF enhances conidial killing by neutrophils and inflammatory monocytes. We observed a marginal reduction in the frequency of fungus-engaged neutrophils by 21.3% (SEM, 2.9%) and 10.7% (5.1%) for CEA10 and AF293 FLARE conidia in GM-CSF–treated mice, respectively, but we observed no significant difference in inflammatory monocyte-mediated conidial uptake (Figure 5C).
To determine whether the enhanced conidial killing observed in GM-CSF–treated mice influenced fungal clearance, lung CFUs were measured in both groups and found to 53% lower in GM-CSF–treated mice than in PBS-treated control mice (Figure 5D). Thus, enhanced neutrophil and inflammatory monocyte conidial killing in GM-CSF–treated mice is associated with more rapid conidial clearance in the lung. To determine whether the accelerated fungal clearance seen in GM-CSF–treated C57BL/6 mice depends on NADPH oxidase (Figure 5D), we conducted a similar set of experiments in p91phox−/− mice. In this setting, we did not observe a significant difference in CFUs between GM-CSF– and PBS-treated groups (Figure 5E), consistent with the idea that the protective effect of GM-CSF depends, at least in significant part, on intact NADPH oxidase activity in the lung.
DISCUSSION
In this study, we demonstrated a cell-intrinsic role of GM-CSFRβ signaling in neutrophil and inflammatory monocyte activity against A. fumigatus conidia. Mice genetically deficient in GM-CSFRβ signaling were susceptible to respiratory A. fumigatus challenge and hyphal tissue invasion. The development of invasive aspergillosis in GM-CSFRβ−/− mice correlated with impaired inflammatory monocyte trafficking and a defect in neutrophil and inflammatory monocyte conidiacidal activity, in part via a reduced oxidative burst. The conidiacidal defect in GM-CSFRβ−/− neutrophils and inflammatory monocytes was observed in mixed chimeric hosts, consistent with a cell-intrinsic defect that is not dependent on host tissue context, exemplified by excess surfactant production in globally-deficient GM-CSFRβ−/− hosts. Furthermore, administration of exogenous GM-CSF enhanced neutrophil and inflammatory monocyte antifungal activity in otherwise immunocompetent mice and accelerated lung fungal clearance. In this study, we focused on the antifungal activity of neutrophils and inflammatory monocytes, because fungal clearance depends on these 2 cell subsets in otherwise immunocompetent mice [3, 4].
Recombinant GM-CSF (ie, sargramostin, molgramostin) is a Food and Drug Administration–approved drug and has been used to shorten neutropenia in patients that are undergoing myeloablative chemotherapy [30] and to treat patients with PAP [31]. Recent preclinical studies support a role for GM-CSF in patients with severe combined neutropenia due to mendelian defects in Jagunal homolog 1 [32] and in individuals with central nervous system candidiasis due to mendelian defects in CARD9 [33]. A small series of case reports describe the impact of GM-CSF on disseminated aspergillosis in patients with a monocyte defect in antifungal activity and with advanced AIDS [34, 35]. In cyclophosphamide- and corticosteroid-treated mice challenged with A. fumigatus via the respiratory route, intranasal recombinant GM-CSF was associated with a reduced lung fungal burden [36], though the underlying mechanism of protection was not examined. In vitro studies supported the notion that GM-CSF reverses suppressive effects of corticosteroids by augmenting NF-κB activation, inflammatory cytokine production, and oxidative function in peripheral blood mononuclear cells and macrophages challenged with A. fumigatus [37, 38], but how these observations related to lung fungal clearance remained unclear.
Our present findings indicate that GM-CSF signaling calibrates the fungicidal activity of effector neutrophils and inflammatory monocytes in the lung in a cell-intrinsic manner, in part by regulating the oxidative burst. Our data do not formally rule out the possibility that IL-3 or IL-5 may regulate GM-CSFRβ chain–dependent signaling events, and future experiments could address this possibility. However, neither IL-3 nor IL-5 were induced in the respiratory A. fumigatus challenge model.
Although the results of this study imply a direct link between GM-CSF signaling, NADPH oxidase activity, and conidial killing in vitro and in the lung, the studies were not designed to test whether GM-CSF is the dominant regulator of NADPH oxidase activity during respiratory fungal challenge. We believe that additional, GM-CSF–independent, mechanisms regulate neutrophil and inflammatory monocyte NADPH oxidase activity during respiratory fungal challenge [39]. Furthermore, our data do not formally exclude the possibility that GM-CSF may activate NADPH oxidase–independent mechanisms in the lung, although we did not detect a benefit in administering GM-CSF to p91phox−/− mice that were challenged with A. fumigatus. A limitation of our study is that we did not distinguish whether GM-CSF signaling in neutrophils or inflammatory monocytes is more critical for fungal clearance. Although it is likely that both innate effector cells contribute to GM-CSF–dependent fungal clearance, the recent development of GM-CSFRβfl/fl mice coupled with neutrophil-restricted (ie, Mrp8-Cre mice) and inflammatory monocyte–restricted (ie, CCR2-Cre-ERT2 mice) conditional ablation strategies [40] will allow this question to be addressed in future studies.
A recent study profiled lung neutrophils isolated from sterile inflammatory lesions and described a gene signature that is characterized by elevated CD54 and dectin-2 expression and by interleukin 1β promoter activation after exposure to GM-CSF. In vitro, recombinant GM-CSF induced these neutrophil phenotypic changes most robustly compared with 65 other cytokines tested [41]. Taylor and colleagues demonstrated similar phenotypic changes in a subset of murine neutrophils isolated from animals that received a subcutaneous injection of heat-killed swollen A. fumigatus conidia. In this model, neutrophils expressed the transcription factor RAR-related orphan receptor gamma t and initiated an autocrine interleukin 17 signaling pathway in a dectin-2–, interleukin 6–, and interleukin 23–dependent manner [42]. These primed neutrophils displayed augmented ROS production and antifungal activity after a secondary challenge with live conidia in a corneal model of fungal disease. Neutrophil priming in this model did not require T, B, NK, NK T, and innate lymphoid cells (ILCs). It remains unclear whether additional cytokines, including GM-CSF, participate in or augment the priming process described in this model.
Various lung cell types can produce GM-CSF and GM-CSF messenger RNA and/or protein have been detected in hematopoietic and nonhematopoietic cells that include myeloid, endothelial, fibroblastic, bronchial, tracheal, and in type II alveolar epithelial cells [43, 44]. In this study, we were not able to define whether GM-CSFRβ signaling depends on GM-CSF from focal or diffuse cellular sources. Although NK cells contribute to fungal clearance and survival in a neutropenic model of invasive aspergillosis via an interferon γ–dependent mechanism [45, 46], NK cells (and other interleukin 2 receptor γ chain–and recombinase-activating gene–dependent leukocytes) are dispensable for conidial clearance in otherwise immunocompetent mice [3]. In contrast, a dendritic cell–NK cell-neutrophil axis was essential for fungal clearance, and NK cell–derived GM-CSF played a major role in activating neutrophil antifungal responses in a systemic candidiasis model [16]. In the gastrointestinal tract, macrophage interactions with commensal bacteria regulate type 3 ILC–dependent GM-CSF production and the induction of oral tolerance [47]. Thus, the induction signals and cellular source(s) of GM-CSFR ligands in the lung during respiratory fungal challenge remains to be defined, though they are likely to involve different cellular networks than at other anatomic sites.
There remains a critical knowledge gap in our understanding of whether immunomodulatory agents, such as GM-CSF, can improve clinical outcomes beyond those achieved with contemporary antifungal therapy [48]. A recent prospectively randomized controlled study suggested that prophylactic GM-CSF, compared with G-CSF, reduced both long-term mortality rates due to invasive fungal disease, primarily because of a reduction in candidiasis, and transplantation-related and cumulative mortality rates [49]. The results in the current study suggest that defective GM-CSF signaling heightens host risk for invasive aspergillosis and that GM-CSF supplementation may be particularly effective in enhancing the fungicidal properties of lung neutrophils and monocytes during acute infection as a therapeutic adjunct. Given the rising incidence of antifungal drug resistance among clinical and environmental Aspergillus isolates [50], further studies to optimize adjunctive treatment strategies are timely and warranted.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Ingrid Leiner for technical assistance.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish or preparation of manuscript.
Financial support. This work was supported in part by the National Institutes of Health (NIH) (grants R01 AI093808 and R21 AI105617 to T. M. H.), the NIH/National Cancer Institute Cancer Center (support grant P30 CA008748 to Memorial Sloan Kettering Cancer Center), the Burroughs Wellcome Fund (Investigators in the Pathogenesis of Infectious Diseases awards to T. M. H. and R. A. C.), and the Lucille Castori Center for Microbes, Inflammation and Cancer (postdoctoral fellowship to A. J.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Segal BH. Aspergillosis. N Engl J Med 2009; 360:1870–84. [DOI] [PubMed] [Google Scholar]
- 2.Bonnett CR, Cornish EJ, Harmsen AG, Burritt JB. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus conidia. Infect Immun 2006; 74:6528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinosa V, Jhingran A, Dutta O et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog 2014; 10:e1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 2009; 200:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordonnier C, Ribaud P, Herbrecht R et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis 2006; 42:955–63. [DOI] [PubMed] [Google Scholar]
- 6.Punatar AD, Kusne S, Blair JE, Seville MT, Vikram HR. Opportunistic infections in patients with pulmonary alveolar proteinosis. J Infect 2012; 65:173–9. [DOI] [PubMed] [Google Scholar]
- 7.Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem 1977; 252:1998–2003. [PubMed] [Google Scholar]
- 8.Metcalf D. Hematopoietic cytokines. Blood 2008; 111:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocyte-macrophage colony-stimulating factor. Crit Rev Immunol 2005; 25:405–28. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Juarrero M, Hattle JM, Izzo A et al. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol 2005; 77:914–22. [DOI] [PubMed] [Google Scholar]
- 11.Ballinger MN, Paine R III, Serezani CH et al. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 2006; 34:766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paine R III, Preston AM, Wilcoxen S et al. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J Immunol 2000; 164:2602–9. [DOI] [PubMed] [Google Scholar]
- 13.Riopel J, Tam M, Mohan K, Marino MW, Stevenson MM. Granulocyte-macrophage colony-stimulating factor-deficient mice have impaired resistance to blood-stage malaria. Infect Immun 2001; 69:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisbart RH, Kwan L, Golde DW, Gasson JC. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood 1987; 69:18–21. [PubMed] [Google Scholar]
- 15.Lopez AF, Nicola NA, Burgess AW et al. Activation of granulocyte cytotoxic function by purified mouse colony-stimulating factors. J Immunol 1983; 131:2983–8. [PubMed] [Google Scholar]
- 16.Whitney PG, Bar E, Osorio F et al. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog 2014; 10:e1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 2013; 39:697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deepe GS Jr, Gibbons R, Woodward E. Neutralization of endogenous granulocyte-macrophage colony-stimulating factor subverts the protective immune response to Histoplasma capsulatum. J Immunol 1999; 163:4985–93. [PubMed] [Google Scholar]
- 19.Tanaka T, Motoi N, Tsuchihashi Y et al. Adult-onset hereditary pulmonary alveolar proteinosis caused by a single-base deletion in CSF2RB. J Med Genet 2011; 48:205–9. [DOI] [PubMed] [Google Scholar]
- 20.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001; 15:557–67. [DOI] [PubMed] [Google Scholar]
- 21.Tazawa R, Inoue Y, Arai T et al. Duration of benefit in patients with autoimmune pulmonary alveolar proteinosis after inhaled granulocyte-macrophage colony-stimulating factor therapy. Chest 2014; 145:729–37. [DOI] [PubMed] [Google Scholar]
- 22.Shepardson KM, Jhingran A, Caffrey A et al. Myeloid derived hypoxia inducible factor 1-alpha is required for protection against pulmonary Aspergillus fumigatus infection. PLoS Pathog 2014; 10:e1004378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jhingran A, Mar KB, Kumasaka DK et al. Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell Rep 2012; 2:1762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohl TM, Rivera A, Lipuma L et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 2009; 6:470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehrad B, Strieter RM, Standiford TJ. Role of TNF-α in pulmonary host defense in murine invasive aspergillosis. J Immunol 1999; 162:1633–40. [PubMed] [Google Scholar]
- 26.Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, Standiford TJ. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol 1999; 163:6086–94. [PubMed] [Google Scholar]
- 27.Jhingran A, Kasahara S, Shepardson KM et al. Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog 2015; 11:e1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanley E, Lieschke GJ, Grail D et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A 1994; 91:5592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dranoff G, Crawford AD, Sadelain M et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994; 264:713–6. [DOI] [PubMed] [Google Scholar]
- 30.Antman KS, Griffin JD, Elias A et al. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on chemotherapy-induced myelosuppression. N Engl J Med 1988; 319:593–8. [DOI] [PubMed] [Google Scholar]
- 31.Tazawa R, Hamano E, Arai T et al. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2005; 171:1142–9. [DOI] [PubMed] [Google Scholar]
- 32.Wirnsberger G, Zwolanek F, Stadlmann J et al. Jagunal homolog 1 is a critical regulator of neutrophil function in fungal host defense. Nat Genet 2014; 46:1028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavino C, Cotter A, Lichtenstein D et al. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis 2014; 59:81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandera A, Trabattoni D, Ferrario G et al. Interferon-γ and granulocyte-macrophage colony stimulating factor therapy in three patients with pulmonary aspergillosis. Infection 2008; 36:368–73. [DOI] [PubMed] [Google Scholar]
- 35.Abu Jawdeh L, Haidar R, Bitar F et al. Aspergillus vertebral osteomyelitis in a child with a primary monocyte killing defect: response to GM-CSF therapy. J Infect 2000; 41:97–100. [DOI] [PubMed] [Google Scholar]
- 36.Quezada G, Koshkina NV, Zweidler-McKay P, Zhou Z, Kontoyiannis DP, Kleinerman ES. Intranasal granulocyte-macrophage colony-stimulating factor reduces the Aspergillus burden in an immunosuppressed murine model of pulmonary aspergillosis. Antimicrob Agents Chemother 2008; 52:716–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brummer E, Kamberi M, Stevens DA. Regulation by granulocyte-macrophage colony-stimulating factor and/or steroids given in vivo of proinflammatory cytokine and chemokine production by bronchoalveolar macrophages in response to Aspergillus conidia. J Infect Dis 2003; 187:705–9. [DOI] [PubMed] [Google Scholar]
- 38.Roilides E, Holmes A, Blake C, Venzon D, Pizzo PA, Walsh TJ. Antifungal activity of elutriated human monocytes against Aspergillus fumigatus hyphae: enhancement by granulocyte-macrophage colony-stimulating factor and interferon-γ. J Infect Dis 1994; 170:894–9. [DOI] [PubMed] [Google Scholar]
- 39.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol 2008; 30:279–89. [DOI] [PubMed] [Google Scholar]
- 40.Croxford AL, Lanzinger M, Hartmann FJ et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 2015; 43:502–14. [DOI] [PubMed] [Google Scholar]
- 41.Yao Y, Matsushima H, Ohtola JA, Geng S, Lu R, Takashima A. Neutrophil priming occurs in a sequential manner and can be visualized in living animals by monitoring IL-1β promoter activation. J Immunol 2015; 194:1211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor PR, Roy S, Leal SM Jr et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat Immunol 2014; 15:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Churchill L, Friedman B, Schleimer RP, Proud D. Production of granulocyte-macrophage colony-stimulating factor by cultured human tracheal epithelial cells. Immunology 1992; 75:189–95.1537596 [Google Scholar]
- 44.Blau H, Riklis S, Kravtsov V, Kalina M. Secretion of cytokines by rat alveolar epithelial cells: possible regulatory role for SP-A. Am J Physiol 1994; 266:L148–55. [DOI] [PubMed] [Google Scholar]
- 45.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest 2003; 112:1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SJ, Hughes MA, Burdick M, Strieter RM, Mehrad B. Early NK cell-derived IFN-γ is essential to host defense in neutropenic invasive aspergillosis. J Immunol 2009; 182:4306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mortha A, Chudnovskiy A, Hashimoto D et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014; 343:1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marr KA, Schlamm HT, Herbrecht R et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 2015; 162:81–9. [DOI] [PubMed] [Google Scholar]
- 49.Wan L, Zhang Y, Lai Y et al. Effect of granulocyte-macrophage colony-stimulating factor on prevention and treatment of invasive fungal disease in recipients of allogeneic stem-cell transplantation: a prospective multicenter randomized phase IV trial. J Clin Oncol 2015; 33:3999–4006. [DOI] [PubMed] [Google Scholar]
- 50.van der Linden JW, Camps SM, Kampinga GA et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 2013; 57:513–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.