Abstract
Background
Glioma-related immunosuppression is well documented; however, the mechanisms of suppression are not fully understood. Here we explore a role for glioma extracellular vesicles (EVs) as a means of immune modulation.
Methods
Healthy donor peripheral blood mononuclear cells (PBMCs) were incubated with mitogenic stimuli and various concentrations of glioma-derived EVs. Intracellular signaling and cytokine output were determined by protein microarrays, and phenotypic changes were assessed by flow cytometry. Recall antigen testing, mixed lymphocyte reactions, and migration assays analyzed PBMC functional capacity.
Results
Protein microarray data revealed induction of an immunosuppressive phenotype and cytokine output at high tumor-vesicle concentrations but an activated phenotype at low concentrations. T cell activation antigen expression confirmed differential activation profiles. Functional analyses revealed decreased migratory capacity of PBMCs after incubation with EVs; however, recall antigen and mixed lymphocyte tests indicated that activation capacity is still retained in EV-treated cells.
Conclusion
The differential effects of high and low EV concentrations dictate modulatory effects on PBMCs. These data provide a role for EVs at high concentrations for inducing selective tolerance of an immune response in a tumor setting. This suggests that lymphocytes in patients’ circulation are not irreparably impaired, as previously thought, but can be rescued to augment antitumor responses.
Keywords: exosomes/extracellular vesicles, glioma, immune suppression, immunology, T cells
High-grade gliomas (HGGs) are devastating and universally fatal cancers with few treatment options. Traditional therapies marginally extend survival and exhibit minimal success in preventing disease progression.1,2 Newer targeted therapies kill glioma cells in a more localized fashion; however, their clinical success has also been limited.3–6 Thus, the treatments available are often palliative rather than curative. Current clinical trials investigate the potential induction of antitumor immunity to selectively destroy infiltrating glioma while sparing healthy brain tissue.7–9 A better understanding of the convoluted interface between gliomas and the immune system may help overcome tumor-induced suppression. HGGs develop multiple layers of immune evasion and suppression throughout their natural history. Induction of regulatory T cell (Treg) phenotypes and myeloid-derived suppressor cells and absence of danger signals are a few adaptations utilized by gliomas to generate a tumor-tolerant environment.10–12 Recent literature implicates extracellular vesicles (EVs), including exosomes and microvesicles, for modulating the tumor microenvironment. EVs resemble small cellular surrogates that act locally and systemically to transfer relatively large amounts of material in discrete packages. When released from a tumor, they aid in tumor progression by remolding the extracellular matrix, inducing angiogenesis, and promoting immune tolerance.13–17 EVs in the tumor environment contain a variety of immune-modulating capacities, but the processes of immune cell alteration and tumor tolerance produced by EVs are poorly understood. Tumor-derived EVs are promising candidates for targeting constraint of glioma progression and facilitating effective immune strategies.18,19 Previous work revealed a direct correlation between tumor grade and EV release, indicating that more aggressive tumors release more EVs.20–22 The highly malignant nature of gliomas and the concentrations of EVs found in circulation of patients with other tumor types20,23,24 suggest a prominent role of EVs in promoting tumor progression and evading immune surveillance. Specific phenotypic changes, differential effects of concentration, and reversibility of damage to immune cells by EVs have yet to be elucidated in gliomas. Understanding the consequences of tumor-derived EVs on immune modulation is necessary for successful glioma immunotherapy. Here we demonstrate that different concentrations of glioma-derived EVs have differential effects on the cytokine output, intracellular signaling pathways, activation status, and migratory capabilities of healthy PBMCs/T cells, which are likely relevant considerations in terms of peripheral circulation and the localized tumor microenvironment.
Materials and Methods
Cell Lines
UPN933 is a grade III anaplastic oligodendroglioma cell line obtained from a tumor resected at the Children's Hospital Colorado on a study approved by the Colorado Combined Institutional Review Board (COMIRB # 95-100). E3-2 and E6-5 are grade IV glioblastoma (GBM) lines obtained from patients undergoing surgery for intracranial tumors at the University of Colorado Hospital who had consented to COMIRB protocol #13-3007. Tumor cells were dissociated and grown under stem cell-like conditions in supplemented Neurobasal A (NBA) medium (Invitrogen Technologies) as described (see also Supplementary material, Methods).25
Patient Plasma Collection; Healthy Donor Peripheral Blood Mononuclear Cells
Blood was collected in EDTA tubes from patients undergoing surgery for intracranial tumors under COMIRB protocol #13-3007, as well as from healthy donors. Histological examination confirmed GBM diagnosis in samples used in study. Blood was immediately processed by centrifugation to isolate plasma, which was then stored at −80°C. Specimen collection from both patients and healthy donors followed informed written consent. Healthy donor PBMCs and Jurkat cells were prepared as described (see also Supplementary material, Methods).26
Extracellular Vesicle Purification
Extracellular vesicle (EV) purifications from cell culture media and patient plasma were conducted as described (see also Supplementary material, Methods).27 Proteomic and Western blot characterizations and NanoSight measurements were performed as described27 (see also Supplementary material, Methods).
Functional Assays
Healthy donor PBMCs were cultured at 1 × 106 cells in a 24-well plate with 1 mL of medium. Mitogenic stimulation was conducted with 5 μg/mL phytohemagglutinin (PHA, Sigma), along with varying EV concentrations. Cells were incubated for 24 or 72 hours. We assessed proliferation via [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] assay according to manufacturer's protocol (Promega). Values are average-fold change compared with the control group. Cytokine concentrations in culture media were quantified using ELISAs (R & D Systems), and all groups were run in triplicate.
Cell migration was measured in Boyden chambers using permeable cell culture inserts (8 µm pore size, Corning) in a 24-well plate. PBMCs were plated in the top chamber in 300 μL of media without fetal bovine serum (FBS)+/-PHA. 500 μL media + 10% FBS and varying EV concentrations were plated in the bottom chamber; and cells migrated for 24 hours. We wiped nonmigratory cells from the top chamber, and cells on the bottom were counted using a microscope at 40X magnification. Values were defined as average cell number from 3 random microscope fields (±SEM) for each treatment.
To assess the re-stimulation capacity of EV-treated PBMCs (±PHA), cells were challenged with 2 μg/mL tetanus toxin (Astarte Biologics) in some experiments. In other work, cells were removed from EV-containing media by centrifugation, resuspended in new media (with no tumor EVs), and utilized in a modified mixed lymphocyte reaction using unrelated healthy donors' PBMCs. Cells were cultured in triplicate with 200 μL of media in a 96-well plate for 72 hours. Proliferation and ELISA assays were conducted upon completion of incubation, as described above.
Phospho-Kinase and Cytokine Arrays
Phospho-kinase and Cytokine Panel A Proteome Profiler antibody arrays (R&D Systems) were performed according to manufacturer's protocols. After incubation with PHA and EVs, T cells were negatively selected from the PBMC population using Pan T Cell isolation kits (Miltenyi Biotec) prior to lysis. Cell lysates and culture media were incubated with appropriate arrays along with biotinylated detection antibodies. Streptavidin-HRP and chemiluminescent reagents were used to quantify relative protein concentrations. Chemiluminescence was detected using a FluorChemQ developer and quantified with AlphaView software (Protein Simple). Background was subtracted from each membrane, and average luminosity from duplicate spots was compared between treatment groups. Pathway/functional analysis predictions were based on Ingenuity Pathway Analysis (IPA; Ingenuity Systems/QIAGEN). Significance of predicted functional pathways was determined by the strength of probability that the protein changes between groups would produce such effects, based on literature documentation in the IPA database.
Flow Cytometry
For EV binding/incorporation assays, glioma EVs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD), as described,28 and EVs were incubated with PBMCs at 100 or 500 µg/mL for 1 or 4 hours. Dynasore (Sigma) was used to inhibit EV uptake as described.29 Flow cytometric analysis was conducted as described.28
For immune parameters after incubation with PHA and EVs, PBMCs were resuspended at 1 × 106 cells and incubated with viability dye eFluor 780 followed by Fc Receptor Binding Inhibitor (eBioscience). Cells were then placed in staining buffer (PBS+2% FBS) and stained with appropriate fluorochrome-conjugated antibodies for 45 minutes. Different groups of cells were handled as follows: cells were fixed/permeabilized using FoxP3/Transcription Factor Staining Buffer Set (eBioscience) for 45 minutes prior to antibody incubation in staining buffer for 45 minutes. Prior to intracellular cytokine detection, protein transport inhibitor cocktail (eBioscience) was added to the culture for the last 16 hours of incubation. All experiments were analyzed on a BD LSRII flow cytometer (BD Biosciences) using FlowJo software (TreeStar). APC conjugated anti-CD4 and PE conjugated anti-CD8 antibodies were from Novus Biologicals. Fluorescein isothiocyanate (FITC)-conjugated anti-interferon gamma (IFNγ), FITC conjugated anti-FoxP3, PerCP-eFluor 710 conjugated anti-CD25, PE-Cy7 conjugated anti-CD69, and Alexa Fluor700 conjugated anti-CD3 antibodies were from eBioscience.
Statistics
All data shown are represented as mean ± SEM. The fold changes shown represent the percentage difference between specified groups. Student' t test was used to determine differences between variables, with P < .05 considered significant.
Results
Characterization of UPN933 Extracellular Vesicles
UPN933 anaplastic oligodendroglioma EVs were harvested from spent cell media by centrifugation and filtration. EV sizes and concentrations were determined by nanoparticle tracking analysis via NanoSight, with a typical particle size of ∼130–150 nm (Supplementary material, Fig. S1A). Protein analysis by Western blotting showed that UPN933 EVs possess canonical EV proteins such as tetraspanins CD9 and CD63, heat shock proteins HSP90 and HSP/HSC70 (stress-inducible/constitutive), and commonly identified proteins GAPDH and 14-3-3 family members (Supplementary material, Fig. S1B). In addition, UPN933 and its EVs lack detectable amounts of Fas ligand (FasL), while other brain tumor cell lines expressed it (Supplementary material, Fig. S1C).
From EV proteomic analysis (Redzic et al, manuscript in preparation), we identified relevant pathways (and protein members) for ensuing functional studies using the FunRich Functional Enrichment Analysis Tool30 (Supplementary material, Table S1).
Glioma Extracellular Vesicles Bind and Internalize into Peripheral Blood Mononuclear Cells
We tested the binding and internalization of glioma EVs into PBMCs using DiD-labeled 933 and E3-2 EVs in FACS analyses (Supplementary material, Fig. S2). Uptake increased with time and dose and was blocked by the dynamin inhibitor Dynasore (which prevents clathrin-dependent endocytosis).
Extracellular Vesicular Modulation of Cytokine Release is Concentration Dependent
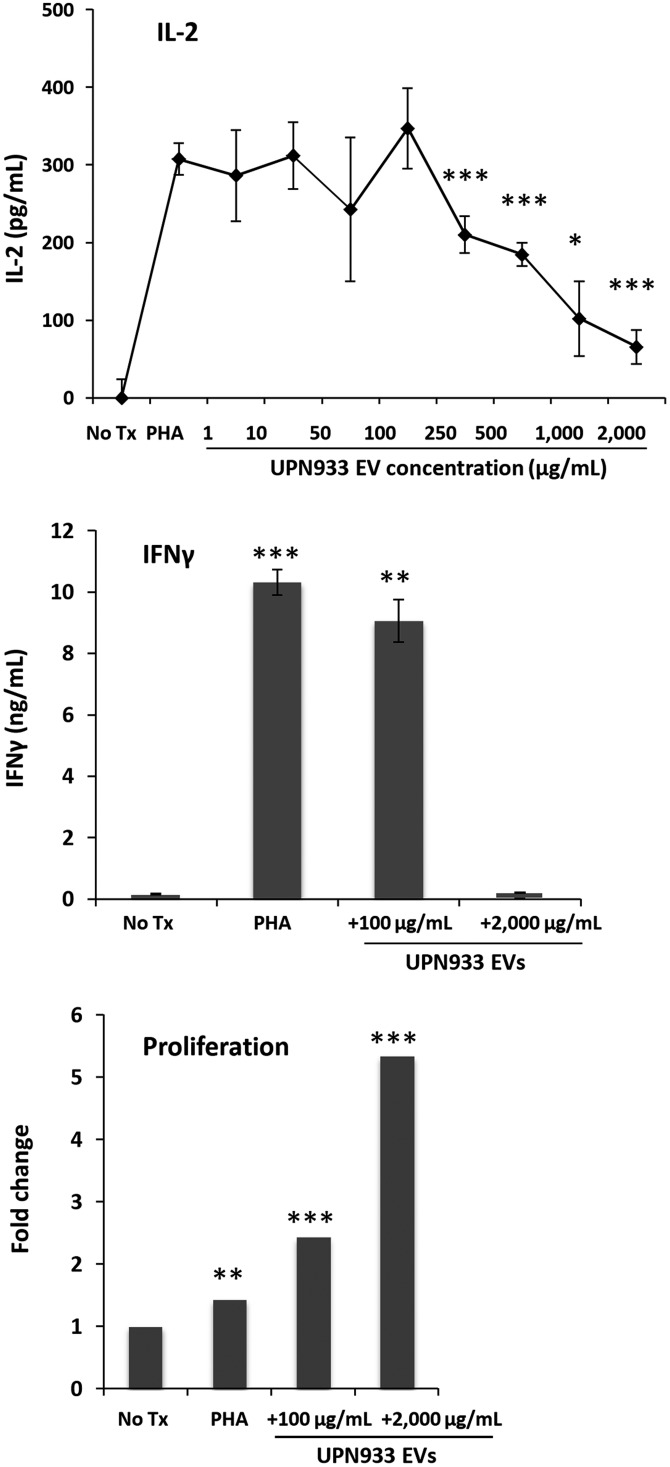
To explore the immunomodulatory effects of tumor-derived EVs at various concentrations, we incubated Jurkat cells with PHA and increasing concentrations of UPN933 EVs for 24 hours. Media IL-2 concentrations increased from PHA-stimulated cells with no significant effect of EV treatment up to 100 μg/mL (Fig. 1A). However, EV concentrations >100 μg/mL resulted in a significant decrease in IL-2 release up to the highest concentration tested. These data display a tipping point at which tumor-derived EVs influence Jurkat cell IL-2 release, which reflects cellular activation capacity. To assess cellular changes on both sides of this tipping point, we designated 100 μg/mL and 2000 μg/mL EVs as low and high concentrations for the remaining studies. To confirm that high EV concentrations were not toxic to immune cells, we repeated the study with low and high EV concentrations (72 h incubation) using healthy donor PBMCs. IFNγ release revealed a similar profile to that of IL-2 in the previous study, with higher concentration of EVs significantly attenuating IFNγ release (Fig. 1B). Low EV concentration induced similar IFNγ output compared with PHA-stimulated cells and did not ablate cytokine release, unlike high EV concentrations. Surprisingly, EV treatment sensitized proliferation in a dose-dependent manner (Fig. 1C). Incubation with high and low EV concentrations along with PHA induced greater proliferation than PBMCs stimulated with PHA alone. These data indicate that tumor-derived EVs are not lethal to naïve PBMCs and that relatively large EV doses mitigate mitogen-driven IFNγ release while promoting proliferation.
Fig. 1.
Extracellular vesicle (EV) concentration differentially affects cytokine output and proliferation of (naïve) PHA-stimulated peripheral blood mononuclear cells (PBMCs). (A) ELISA IL-2 measurements (24 h) from PHA-stimulated Jurkats + various EV concentrations (asterisks = t test vs PHA stimulation). (B) IFNγ ELISA (72 h) from PBMCs + PHA alone or +100 or 2000 µg/mL EVs (t test vs No Tx control). (C) Average PBMC proliferation (72 h) with PHA alone or +100 or 2000 µg/mL EVs. Values displayed as fold change versus No Tx control. No Tx = untreated control cells. *P < .05, **P < .01, ***P < .001.
Intracellular Signaling and Cytokine Output Reveal Selective T Cell Dysfunction at High and Low Tumor EV Concentrations
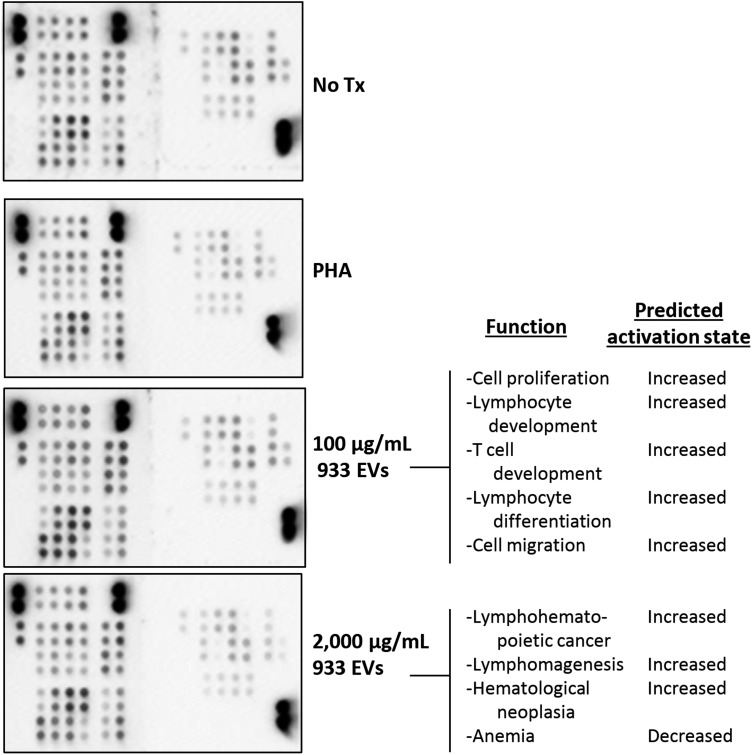
Enhanced proliferation and decreased IFNγ output after exposure to high EV concentrations imply eccentricity of signaling cascades within EV-treated cells. We assessed glioma EV-induced T cell alterations via phospho-kinase and cytokine arrays to detect relative protein concentrations between differentially stimulated groups. PBMCs were incubated for 24 hours with PHA alone or PHA+low or high EV concentrations; T cells were purified and lysed for array exposure and quantification (Fig. 2). Treated PBMC media were used for cytokine analysis (below). Treatment of bulk PBMCs rather than purified T cells alone reflects the in vivo scenario in which lymphocytes besides T cells may impact T cell responses. Differential protein phosphorylation implicated in a variety of signaling cascades revealed EV-induced changes compared with PHA-stimulated cells (Supplementary material, Table S2). IPA algorithms predicted significant downstream effects based on phosphorylation status changes. These include induction of pathways implicated in cellular proliferation, development, and migration of T cells after PBMC incubation with low EV concentrations (Fig. 2). These predictions suggest exacerbation of overall activation and functionality of T cells exposed to low concentrations of tumor-derived EV+PHA compared with PHA alone. Of 45 proteins analyzed, 35 exhibited >10% increase in phosphorylation after exposure to low EV concentrations (Supplementary material, Table S2). Incubation with high EV concentrations, however, does not induce a similar activation profile. The primary functional pathways predicted to increase over PHA-stimulated cells involved proliferation, but no other pathways were predicted to change significantly (Fig. 2).
Fig. 2.
Low extracellular vesicle (EV) concentrations enhance functional T cell signaling; high concentrations enhance proliferation only. Phospho-kinase arrays were incubated with lysates from T cells isolated from EV-treated peripheral blood mononuclear cells. Signal intensities were background-corrected and compared between groups. Ingenuity Pathway Analysis examined relative fold changes and predicted the functional pathways most strongly influenced. (Right), functional pathways predicted to significantly change in T cells incubated with low/high EV concentrations versus PHA-stimulated T cells (P < .05).
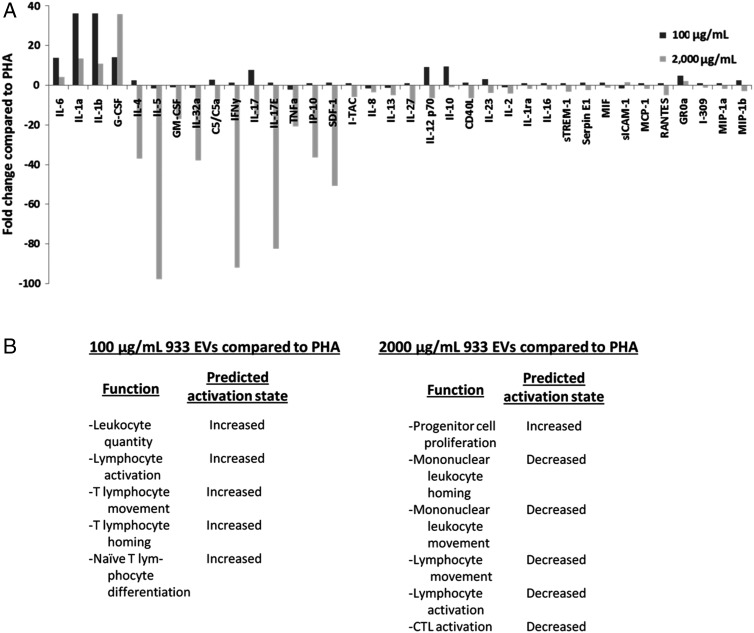
EVs induce differential T cell stimulus and cytokine release in a concentration-dependent fashion, with the associated cytokine profiles having potential downstream consequences (Supplementary material, Table S3, Fig. 3A). Predicted functional changes based on cytokine output indicate enhanced functionality of PBMCs exposed to low EV concentrations for 72 hours (Fig. 3B). The magnitudes and types of cytokines released suggest induced activation, migration, and differentiation of cells in response to low tumor EV concentrations. Exposure to high EV concentrations results in reduced levels of many mitogen-induced signaling molecules. Compared with PHA-stimulated cells, the functional consequences of the cytokine milieu produced by this environment suggest decreases in migratory and activation capabilities (Fig. 3B). Nonetheless, lymphocyte proliferation is still predicted to increase with higher EV concentrations, supporting the unexpected proliferation data (Fig. 1C). The overall predictive IPA analyses, which are based on relative protein changes, reveal a severe functional deficit induced by exposure to high EV concentrations even in the presence of mitogenic stimulation. Low EV concentrations appear to enhance PBMC functions, but to better understand specific phenotypic changes and physiological consequences of altered protein expression, we directly tested activation makers and functional capabilities in remaining studies.
Fig. 3.
PHA-stimulated peripheral blood mononuclear cells exposed to low extracellular vesicle (EV) concentrations release an activated cytokine profile; high EV concentrations impair that activated profile. Culture media from PHA-treated and PHA/EV-treated T-cells were analyzed on protein microarrays for relative cytokine concentrations. Fold changes between groups were analyzed by Ingenuity Pathway Analysis for functional pathways predicted to change (also, Supplementary material, Tables S1 and S2). (A) Relative cytokine/chemokine concentrations detected in media of EV-treated cells compared with cells receiving only PHA stimulation. (B) Predicted significant functional consequences of low (left) or high (right) EV exposure based on all cytokine changes versus PHA (P < .05).
High Tumor-derived EV Concentrations Decrease PBMC/T Cell Activation
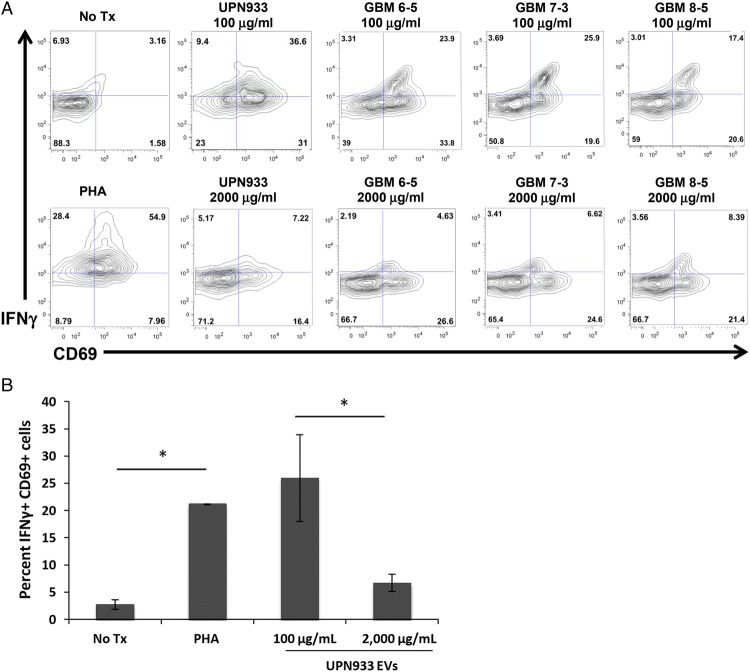
Following incubation of PBMCs with PHA and EVs, we probed cells with anti-CD69 and anti-IFNγ antibodies to assess changes in activation status by fluorescence-activated cell sorting (FACS). We analyzed CD3+/CD4+ cells selected from the lymphocyte gate. Mitogenic stimulation reliably elevated expression of CD69 and intracellular IFNγ after 72 hours of incubation (P = .02) (Fig. 4A and B). Co-incubation with low EV concentrations did not attenuate either parameter but instead trended towards exacerbation of expression, as IPA predicted in the previous study. PBMC incubation with a high concentration of UPN933 EVs constrains the effects of mitogenic stimulation effects on CD69 and IFNγ expression but remained higher than control levels (P = .02). Repeating the incubation with GBM patient plasma-derived EVs demonstrates a very similar trend. High EV concentrations decrease CD69 and IFNγ expression, and low concentrations have little effect compared with PHA stimulation (Fig. 4B). It is important to note that patient plasma EVs contain tumor-derived EVs as well as healthy (normal cell) EVs participating in normal functions, whereas the UPN933 EVs from cell culture are all tumor derived. Thus, variation between samples is expected due to nonselective purification of EVs, but the expression patterns of CD69 and IFNγ nearly mimic those driven by pure tumor EVs (Fig. 4).
Fig. 4.
Tumor-derived extracellular vesicles (EVs) suppress T-cell activation at high, but not low, concentrations. (A) Representative fluorescence-activated cell sorting diagrams of peripheral blood mononuclear cells (PBMCs)/T cells stained for CD69 and intracellular IFNγ. Cells depicted originate from lymphocyte gate and a second CD3+/CD4+ gate. Cell culture conditions, including cell line and EV concentrations and origins, are shown above corresponding figures. (B) Average % IFNγ+/CD69+ cells of all cells assessed. Cells treated with PHA and low EV concentration did not change compared with PHA-stimulated cells, whereas cells incubated with PHA + high EV concentrations attenuated expression of both markers (*P < .05).
Tumor-derived EVs Act as Chemorepellents for Activated PBMCs
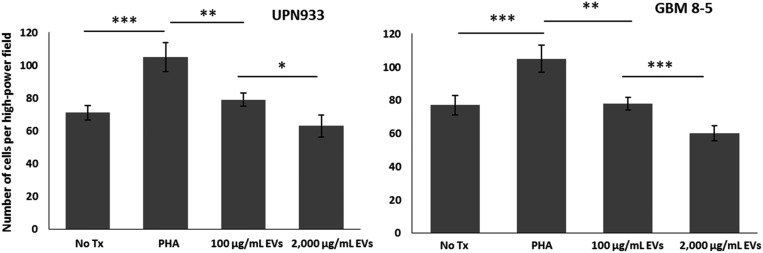
Array studies implied aberrations of migratory pathways within EV-treated PBMCs, and flow cytometry studies revealed differential expression of activation markers. To confirm that these molecular markers were indicative of cellular functionality, we performed migration assays. Briefly, PBMCs (±PHA) were plated in the top of a Boyden chamber, and UPN933 and GBM patient EVs were plated in the bottom well at 100 µg/mL and 2000 µg/mL (with FBS) for 24 hours. PHA stimulation expectedly enhanced migration ∼60 percent (P < .001) (Fig. 5). The presence of low concentration of EVs as putative attractants significantly attenuated PBMC migration (P = .002) but remained greater than control levels of spontaneous migration (P = .03). The presence of high concentrations of glioma EVs as attractants significantly reduced migration compared with low EV concentration (P = .02) and was the same as control levels (P = .71). Overall trends and significance between groups were nearly identical between UPN933 and GBM 8–5 patient EVs (Fig. 5, left and right panels). Although signaling cascades in the array study suggested enhanced functionality of PBMCs in the presence of low concentrations of tumor-derived EVs, migration data reveal an inverse relationship between EV concentration and PBMC migration.
Fig. 5.
Tumor-derived extracellular vesicles (EVs) blunt migration of activated peripheral blood mononuclear cells (PBMCs). Migration chambers contained naïve PBMCs+/-PHA in FBS-free media in top well. Bottom chambers contained media with fetal bovine serum+100 or 2000 µg/mL tumor EVs. No Tx chamber represents PBMCs without PHA stimulation. (Left panel) average migration from 3 random visual fields (40X) on bottom of migration membrane +/-UPN933 EVs present. PHA-treated PBMCs significantly migrated more versus No Tx control (P < .001); 100 µg/mL of EVs as attractants constrained migration of PHA-activated cells (P < .01). 2000 µg/mL EVs as attractant significantly reduced migration versus 100 µg/mL EVs (P < .05). (Right panel) average number of migrated cells ±EVs isolated from glioblastoma (GBM) patient plasma as attractants. The group’s parallel results are seen with cell culture EVs, in which increasing EV concentration significantly decreased PBMC migration. *P < .05, **P < .01, ***P < .001.
PBMCs Remain Suppressed in the Presence of EVs
The studies above suggest an EV-induced deficit in PBMC activation pathways and functionality. We further examined tumor EV effects on other forms of T cell stimulation and asked if the EV-induced impairment was reversible. Supplementary material, Fig S3A reveals that tumor EVs reduce PBMC release of IFNγ when the cells are stimulated with PHA followed by further stimulation with tetanus toxin, while the recall antigen alone combined with PHA almost doubles IFNγ release. However, EVs continue to promote PBMC proliferation at dramatic levels in the presence of tetanus toxin (Supplementary material, Fig S3B).31 Thus, the ratio of cell number to IFNγ release is greatly skewed between the control and EV-treated cells.
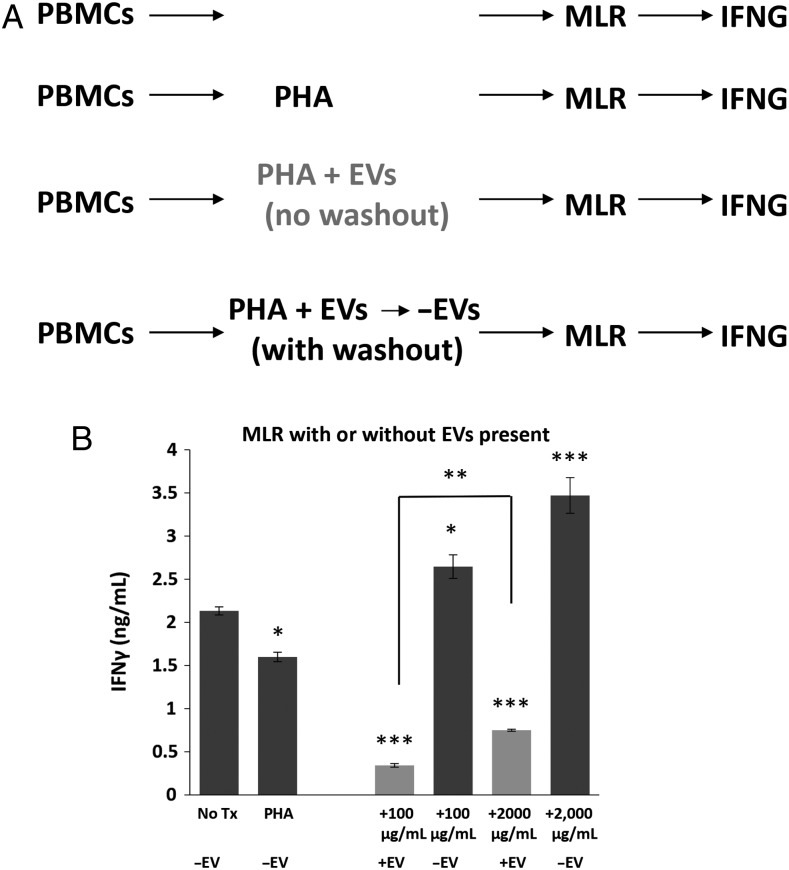
We also incubated PBMCs with PHA±EVs at the 2 concentrations and either washed out the EVs from the cells or allowed the EVs to remain in the culture. We then incubated the PBMCs with unrelated healthy donor PBMCs in the form of a mixed lymphocyte reaction (MLR, Fig 6A). Cells in the MLR release prodigious amounts of IFNγ in the absence of EVs (albeit less when stimulated with PHA, Fig 6B). The continued presence of EVs at both low and high concentrations significantly reduced cytokine release (P < .001), but removal of the EVs prior to MLR allowed for a significant increase in IFNγ output (P = .001). These data indicate the importance of continual EV exposure to PBMC inhibition but also suggest that their removal may permit immune stimulation.
Fig. 6.
Extracellular vesicle (EV)-treated peripheral blood mononuclear cells (PBMCs) retain ability for activation and re-stimulation by mixed lymphocyte reaction (MLR) but only in the absence of EVs. (A) Outline of PBMC treatment: one group of PBMCs was only co-incubated with unrelated HD PBMCs (MLR), and IFNγ release was measured by ELISA; a second PBMC group was PHA-treated and then utilized in MLR, with IFNγ release measured; a third group was PHA-stimulated and treated to low and high EV concentrations followed by MLR (with EVs remaining during the MLR), and IFNγ release measured; a fourth group was treated as above, but EVs were washed out prior to MLR/IFNγ release. (B) IFNγ concentration of media from PBMCs treated as described in (A). Background release of IFNγ without MLR stimulation was 0.158 ng/mL (Fig. 1B). *P < .05, **P < .01, ***P < .001 compared to No Tx control.
Discussion
Our studies assessed the consequences of exposing naïve PBMCs to glioma-derived EVs to better understand EV roles in glioma-induced immune tolerance. There are numerous reports of tumor-derived EV immune suppression.32–34 While this is likely true in gliomas as well,35 there are few reports of glioma EV-mediated immune dysfunction.18 The latter publication involved in vivo (murine) EV treatments and is difficult to compare directly to our work here. Our current studies used relatively high EV concentrations compared with most. We believe that our EV concentrations represent reasonable amounts in blood (at the low end, 100 µg/mL) and possibly in the tumor microenvironment (high end, 2000 µg/mL) based on values in the literature for blood EVs from patients with numerous tumor types (Supplementary material, Table S4) and using a hypothetical tumor model (see Supplemental material and Methods for calculations).
Other than our own work,27 we found only one other publication with similar EV concentrations.36 There, the authors found that healthy donor plasma EVs could suppress activation-driven CD4+ T-cell proliferation by FasL-dependent mechanisms with EV concentrations as high as 500 µg/mL. In our hands, however, UPN933 EVs (and cells) did not appear to express high FasL levels since we could not detect it in Western blots (Supplementary material, Fig. S1C).
As we exposed Jurkat cells to increasing concentrations of glioma EVs, we found a tipping point at which EVs inhibited mitogen-induced IL-2 release. We saw similar suppression of IFNγ output from PHA-activated PBMCs incubated with high EV concentrations, suggesting that the local tumor EV concentration impacts PBMC activation status. Cell-wide protein phosphorylation assessment, cytokine release, and FACS for activation markers further supported this. Low EV concentrations sensitized T cell signaling, resulting in enhanced signaling cascades related to proliferation, differentiation, and migration. High EV concentrations did not significantly alter intracellular phosphorylation of these same pathways but selectively enhanced proliferation cascades. Cytokine release revealed a similar alteration in predicted functional capacities at low EV concentrations but demonstrated a more immunosuppressive cytokine output at high EV concentrations. T cells were purified to study intracellular signaling changes, but the cytokine output was from total PBMCs as we presume this creates a more realistic image of what occurs in vivo where PBMCs work in concert. FACS for intracellular T cell IFNγ after treatment with high or low EV concentrations paralleled cytokine array data, confirming the T cell contribution to that readout. The changes in activation marker status (CD69 and IFNγ) also confirmed the predictions from the array data, and we obtained very similar expression profiles using GBM patient-derived EVs. Note that, in our hands, EV exposure did not induce a Treg phenotype (Supplementary material, Fig. S2). Treg production is a known feature of HGGs via tumor cytokine release or from other immunosuppressive cell types,10,37,38 and EVs from other tumors induce Tregs.39 Our data here do not support Treg induction, but this is admittedly is a limited evaluation.
We have previously27 shown that tumor EVs effectively drive brain tumor cell migration. In contrast, following functional analysis prediction based on cytokine release, PMBCs exposed to high glioma EV concentrations demonstrated reduced migration in Boyden chamber assays with EVs from both UPN933 and patient plasma. This suggests tumor-derived EVs attract infiltrating cancer cells while essentially repelling immune cells along the course. Tumor-derived EVs can remodel the extracellular matrix (driving stromal cells to do the same), allowing tumor cell migration,40,41 and this is likely true for glioma EVs and subsequent glioma invasion.42 However, tumor-EV effects on PBMC migration appear unstudied, and the dichotomy of response (attractive for tumor cells, preventative for lymphocytes) may be a novel form of immune suppression, particularly in light of the differential activation status of the PBMCs/T cells. EV-induced migratory suppression before other functions at low EV concentrations may be critical for a growing tumor, as it releases greater amounts of EVs to evade immune detection before actively suppressing other T cell functions. These consequences may be pertinent to autologous T cell transfer and vaccine-related studies, as migratory blocking could prevent even the best-educated cells from extravasation into the tumor. There are implications for T cell-based immunotherapies such as autologous T-cell transfer, in which patient plasma/sera are frequently used as part of the cell culture protocol.43,44 Tumor EVs in these biofluids may unwittingly inhibit T-cell activity ex vivo.
Since EVs are produced from all cell types and are utilized to maintain homeostasis, prevention of release may not be feasible. However, extracorporeal hemofiltration is proposed as a means of reducing tumor EV burden in blood45 and may improve PBMC responsiveness. Additionally, some of the signaling pathways affected (CD3/ZAP70, LCK, PLCG1, RAS/RAF, AP-1, NFAT/NFkB)46 suggest that if removal of EVs is not possible, stimulation of such pathways may resurrect T cell immune responses. Finally, outright blocking of EV/recipient cell interactions (eg, with heparin or other highly charged macromolecules47) may also be a viable means of diminishing tumor EV effects.
Despite EV-induced suppression, PBMCs can still produce immune responses when challenged in an environment with EVs removed. PBMCs with previous EV exposure challenged in MLR with the EVs washed out reveal a sensitization in IFNγ release. However, if EVs remain present during challenge, IFNγ release is significantly reduced. Thus, continued EV presence is necessary to maintain suppression. The ubiquitous presence of EVs in the tumor microenvironment may be a major obstacle preventing the full potential of immunotherapy. Our data reveal that EV-treated PBMCs are suppressed, but perhaps not permanently, and removal of EVs from the environment may be necessary to produce an efficient immune response. Our additional data imply that stimulation of other pathways may impact lymphocyte activation.46 The quantity of EVs in the tumor microenvironment remains an issue. We chose our EV concentrations to potentially mimic tumor EV levels in blood versus the tumor microenvironment (Supplementary material, Table S4; Supplemental material, Methods). Thus, we believe our EV concentrations utilized are not unrealistic physiologically but may actually be low, relative to the tumor microenvironment. Further work in this area is needed.
Clearly, glioma-derived EVs impact recipient immune cells, but the nature of those interactions and the molecular drivers remain unknown. Better characterization of glioma-derived EV contents and mechanisms of immune cell interactions are necessary to reverse EV-induced dysfunction; many of these putative pathways are noted in Supplementary material, Table S1. Further exploration of induced signaling pathways in recipient lymphocytes may offer interventional targets and correct tumor EV-driven suppressive phenotypes.46
Conclusion
Our work here suggests that high glioma-derived EV concentrations can deactivate T cells and hinder lymphocyte migration; the more EVs a tumor produces or the closer a T cell gets to a tumor EV gradient, the greater suppression likely exists. Understanding the intracellular/extracellular pathways implicated in alteration from active to suppressed (and relatively immobile) is essential for developing effective treatments aimed to restore function in immune-suppressed cells.
Supplementary Material
Funding
This work was supported by the University of Colorado Neurosurgery Department, University of Colorado Cancer Center, Cancer League of Colorado, and National Institutes of Health 5-R01-EB016378-0.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1.Smoll NR, Schaller K, Gautschi OP. Long-term survival of patients with glioblastoma multiforme (GBM). J Clin Neurosci. 2013;20(5):670–675. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3.Lee EQ, Nayak L, Wen PY, Reardon DA. Treatment options in newly diagnosed glioblastoma. Curr Treat Options Neurol. 2013;15(3):281–288. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. [DOI] [PubMed] [Google Scholar]
- 5.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7(12):1152–1160. [DOI] [PubMed] [Google Scholar]
- 6.Dixit S. Immunotherapy for high-grade glioma. Future Oncol. 2014;10(6):911–915. [DOI] [PubMed] [Google Scholar]
- 7.Marshall D, Mitchell DA, Graner MW, Bigner DD. Immunotherapy of brain tumors. Handb Clin Neurol. 2012;104:309–330. [DOI] [PubMed] [Google Scholar]
- 8.Reardon DA, Freeman G, Wu C, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16(11):1441–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gedeon PC, Riccione KA, Fecci PE, Sampson JH. Antibody-based immunotherapy for malignant glioma. Semin Oncol. 2014;41(4):496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vega EA, Graner MW, Sampson JH. Combating immunosuppression in glioma. Future Oncol. 2008;4(3):433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy BC, Maier LM, D'Amico R, et al. Dynamics of central and peripheral immunomodulation in a murine glioma model. BMC Immunol. 2009;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin N Am. 2010;21(1):31–42. [DOI] [PubMed] [Google Scholar]
- 13.Dolo V, Ginestra A, Cassara D, Ghersi G, Nagase H, Vittorelli ML. Shed membrane vesicles and selective localization of gelatinases and MMP-9/TIMP-1 complexes. Ann N Y Acad Sci. 1999;878:497–499. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Yu S, Zinn K, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375–1385. [DOI] [PubMed] [Google Scholar]
- 15.Battke C, Ruiss R, Welsch U, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60(5):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36(1–3):247–254. [DOI] [PubMed] [Google Scholar]
- 17.Ung TH, Madsen HJ, Hellwinkel JE, Lencioni AM, Graner MW. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014;105(11):1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu ZM, Wang YB, Yuan XH. Exosomes from murine-derived GL26 cells promote glioblastoma tumor growth by reducing number and function of CD8+T cells. Asian Pac J Cancer Prev. 2013;14(1):309–314. [DOI] [PubMed] [Google Scholar]
- 19.Redzic JS, Ung TH, Graner MW. Glioblastoma extracellular vesicles: reservoirs of potential biomarkers. Pharmgenomics Person Med. 2014;7:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10(1):42–46. [DOI] [PubMed] [Google Scholar]
- 21.Andre F, Schartz NE, Movassagh M, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21. [DOI] [PubMed] [Google Scholar]
- 23.Di Noto G, Paolini L, Zendrini A, Radeghieri A, Caimi L, Ricotta D. C-src enriched serum microvesicles are generated in malignant plasma cell dyscrasia. PLoS One. 2013;8(8):e70811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan S, Bennit HF, Turay D, et al. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer. 2014;14:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epple LM, Dodd RD, Merz AL, et al. Induction of the unfolded protein response drives enhanced metabolism and chemoresistance in glioma cells. PLoS One. 2013;8(8):e73267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Zeng Y, Chen X, et al. Human ovarian tumour-derived chaperone-rich cell lysate (CRCL) elicits T cell responses in vitro. Clin Exp Immunol. 2007;148(1):136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epple LM, Griffiths SG, Dechkovskaia AM, et al. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLoS One. 2012;7(7):e42064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smyth TJ, Redzic JS, Graner MW, Anchordoquy TJ. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim Biophys Acta. 2014;1838(11):2954–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadiu I, Narayanasamy P, Dash PK, Zhang W, Gendelman HE. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol. 2012;189(2):744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathan M, Keerthikumar S, Ang CS, et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15(15):2597–2601. [DOI] [PubMed] [Google Scholar]
- 31.Fryauff DJ, Church LW, Richards AL, et al. Lymphocyte response to tetanus toxoid among Indonesian men immunized with tetanus-diphtheria during extended chloroquine or primaquine prophylaxis. J Infect Dis. 1997;176(6):1644–1648. [DOI] [PubMed] [Google Scholar]
- 32.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans. 2013;41(1):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33(5):441–454. [DOI] [PubMed] [Google Scholar]
- 35.Wurdinger T, Deumelandt K, van der Vliet HJ, Wesseling P, de Gruijl TD. Mechanisms of intimate and long-distance cross-talk between glioma and myeloid cells: how to break a vicious cycle. Biochim Biophys Acta. 2014;1846(2):560–575. [DOI] [PubMed] [Google Scholar]
- 36.Ren Y, Yang J, Xie R, et al. Exosomal-like vesicles with immune-modulatory features are present in human plasma and can induce CD4+ T-cell apoptosis in vitro. Transfusion. 2011;51(5):1002–1011. [DOI] [PubMed] [Google Scholar]
- 37.Sonabend AM, Rolle CE, Lesniak MS. The role of regulatory T cells in malignant glioma. Anticancer Res. 2008;28(2B):1143–1150. [PubMed] [Google Scholar]
- 38.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. [DOI] [PubMed] [Google Scholar]
- 39.Pucci F, Pittet MJ. Molecular pathways: tumor-derived microvesicles and their interactions with immune cells in vivo. Clin Cancer Res. 2013;19(10):2598–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci USA. 2014;111(2):711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mu W, Rana S, Zoller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15(8):875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kucharzewska P, Christianson HC, Welch JE, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013;110(18):7312–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poschke I, Lovgren T, Adamson L, et al. A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma. Cancer Immunol Immunother. 2014;63(10):1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamers CH, Willemsen RA, Luider BA, Debets R, Bolhuis RL. Protocol for gene transduction and expansion of human T lymphocytes for clinical immunogene therapy of cancer. Cancer Gene Ther. 2002;9(7):613–623. [DOI] [PubMed] [Google Scholar]
- 45.Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellwinkel JE, Madsen H, Graner MW. Immune Modulation by Tumor-Derived Extracellular Vesicles in Glioblastoma. In: Lichtor T, ed. Molecular Considerations and Evolving Surgical Management Issues in the Treatment of Patients with a Brain Tumor. Rijeka, Croatia: InTech; 2015:329–354. [Google Scholar]
- 47.Atai NA, Balaj L, van Veen H, et al. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol. 2013;115(3):343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.