Abstract
Background
Radiological characteristics may reflect the biological features of brain tumors and may be associated with genetic alterations that occur in tumorigenesis. This study aimed to investigate the relationship between radiological features and IDH1 status as well as their predictive value for survival of glioblastoma patients.
Methods
The clinical information and MR images of 280 patients with histologically confirmed glioblastoma were retrospectively reviewed. The radiological characteristics of tumors were examined on MR images, and the IDH1 status was determined using DNA sequencing for all cases. The Kaplan-Meier method and Cox regression model were used to identify prognostic factors for progression-free and overall survival.
Results
The IDH1 mutation was associated with longer progression-free survival (P = .022; hazard ratio, 0.602) and overall survival (P = .018; hazard ratio, 0.554). In patients with the IDH1 mutation, tumor contrast enhancement and peritumoral edema indicated worse progression-free survival (P = .015 and P = .024, respectively) and worse overall survival (P = .024 and P = .032, respectively). For tumors with contrast enhancement, multifocal contrast enhancement of the tumor lesion was associated with poor progression-free survival (P = .002) and poor overall survival (P = .010) in patients with wild-type IDH1 tumors.
Conclusions
Combining the radiological features and IDH1 status of a tumor allows more accurate prediction of survival outcomes in glioblastoma patients. The complementary roles of genetic changes and radiological features of tumors should be considered in future studies.
Keywords: glioblastoma, IDH1, radiology, survival outcome
Glioblastoma is the most common and aggressive type of malignant brain tumor in adults.1 Its clinical outcome varies substantially, with some patients succumbing to progressive disease within weeks while others survive for decades. The standard treatment is maximal surgical resection with radiation therapy and chemotherapy.2,3 Although various treatment strategies have been used, glioblastoma patients typically still have a poor prognosis with median progression-free survival (PFS) and overall survival (OS) of 6.9 months and 14.7 months, respectively.4 Clinical characteristics, including patient age, KPS, and the extent of resection have been previously investigated as prognostic factors for glioblastoma.5–8
IDH1 is a previously characterized biomarker in glioblastoma and is mutated in 70%–80% of secondary tumors and <10% of primary tumors.9–11 These mutations are considered important molecular events in gliomagenesis. As an independent prognostic indicator, IDH1 mutations are associated with a favorable outcome and longer survival in glioblastoma patients.12–15 Furthermore, tumors with an IDH1 mutation have markedly different clinical presentations, overall natural history, and concurrent molecular genetic alterations compared with their IDH1 wild-type counterparts.16
In addition to genetic signatures, radiological features of glioblastoma have also been identified as prognostic factors. It has been shown that tumor contrast enhancement,17,18 multifocality,19 tumor location,20,21 edema,17,19 and cysts22,23 are potentially associated with survival outcome in glioblastoma patients. Specifically, tumor-induced edema could be used to predict the survival outcomes of glioblastoma patients on the basis of MGMT promoter methylation but not the IDH1 status,15 which implies that there may be a relationship between genetic changes and radiological features. However, the relationship between IDH1 expression and certain MR imaging-derived features of glioblastoma have rarely been investigated. Therefore, we aimed to identify the association between the IDH1 tumor status and radiological features and to determine whether combining these factors could better predict the outcome for glioblastoma patients.
Materials and Methods
Patients
In total, 280 adult patients who were diagnosed with glioblastoma and who underwent surgical treatment at our institution between April 2007 and May 2010 were systematically reviewed. Cases were included if they met the following criteria: (i) aged ≥18 years; (ii) presurgical MR scans available (including T1-weighted, T2-weighted, and postcontrast T1-weighted); (iii) pathologically confirmed glioblastoma based on the WHO histological grading system; (iv) no previous diagnosis of any type of brain tumor; and (v) no previous adjuvant treatment. The histopathological diagnosis was evaluated and confirmed by 2 independent senior neuropathologists who were blinded to patients′ clinical and radiological information. Gross total resection (GTR) was defined as no visible contrast enhancement on postoperative MR images within 48 hours after surgery for contrast-enhanced tumors or the disappearance of all abnormal hyperintense changes on preoperative MR images for tumors not demonstrating contrast enhancement. In this study, resections that were not GTR were considered residual tumors (<GTR). The adjuvant treatment was radiation therapy alone or concomitant temozolomide administration with fractionated radiotherapy followed by up to 6 cycles of adjuvant temozolomide.4 The overall follow-up duration of the study was 74 months during the period between May 2007 and July 2013. This study was approved by our institutional review board, and written consent was obtained from all enrolled patients.
Image Acquisition
MR imaging was performed using a Siemens Trio 3T scanner (Siemens Healthcare). It typically included axial T1-weighted (repetition time [TR], 450 ms; echo time [TE], 15 ms; section thickness, 5 mm), T2-weighted fast spin-echo (TR, 6000 ms, TE, 140 ms; section thickness, 5 mm), and gadopentetate dimeglumine (DTPA-Gd Injection; (Beilu Pharma; 0.1 mmol/kg)-enhanced axial T1-weighted images (TR, 450 ms; TE, 15 ms; section thickness, 5 mm), with a 24 cm field of view and a matrix size of 256 × 256. Postcontrast images were acquired immediately after injection of the contrast agent. The interval between contrast injection and the start of contrast-enhanced T1-weighted image acquisition was always 75–85 seconds. Postoperative MR scans for determining the extent of resection were performed within 72 hours of this procedure, and the radiological parameters were maintained in accordance with the preoperative scans.
Identification of Imaging Features
Tumor contrast enhancement was assessed by 2 experienced neuroradiologists blinded to the patients’ clinical information. A third senior neuroradiologist re-examined the images and determined which should be used if the types of enhancement identified by the first 2 neuroradiologists were inconsistent. Briefly, a small (or no) region of edema (−) was defined as edema extending ≤1 cm from the margin of the tumor based on T2-weighted images; otherwise edema was graded as moderate to severe (+).15 Contrast enhancement was defined as a newly identified, unequivocal increase in signal intensity on a T1-weighted contrast image compared with a noncontrast T1-weighted image. Nonenhancement was defined as no apparent hyperintensity in the tumor-involved area on a postcontrast T1-weighted image. A multifocal-enhancing tumor was defined as more than one area of tumor enhancement located separately from each of the other enhanced areas on a postcontrast T1-weighted image.15 Patterns of tumor enhancement were identified based on the morphological feature of the largest enhanced tumor area on contrast-enhanced MR images, and ring-like enhancement was defined as cystic necrosis with peripheral enhancement, while any other pattern was defined as non-ring-like.
Detection of IDH1 Mutations and MGMT Promoter Methylation
IDH1 mutations were identified using DNA pyrosequencing, which we have described previously.24,25 Briefly, a QIAamp DNA Mini Kit (Qiagen) was used to isolate genomic DNA from frozen tumor tissue samples. The genomic region spanning the wild-type R132 of IDH1 was analyzed by amplifying a 75-base pair (bp) fragment with the following primers: 5′-GCTTGTGAGTGGATGGGTAAAAC-3′ and 5′-biotin-TTGCCAACATGACTTACTTGATC-3′. Duplicate PCR analyses were performed in 40 μL reaction volumes containing 1 μL each of 10 μM forward and reverse primers, 4 μL of 10× buffer, 3.2 μL of 2.5 mM dNTPs, 2.5 U HotStar Taq (Takara), and 2 μL of 10 μM DNA. The PCR conditions were as follows: 95°C for 3 minutes; 50 cycles of 95°C for 15 seconds, 56°C for 20 seconds, and 72°C for 30 seconds; and 72°C for 5 minutes (ABI PCR System 9700; Applied Biosystems). Single-stranded DNA was purified from the PCR products and pyrosequenced with a PyroMark Q96 ID System (Qiagen) using a 5′-TGGATGGGTAAAACCT-3′ primer and an EpiTect Bisulfite Kit (Qiagen). The methylation status of the MGMT promoter was determined by methylation-specific PCR after sodium bisulfite DNA modification, as described previously.25
Statistical Analysis
We used the chi-square test for categorical variables to compare each clinical and imaging feature between patients with IDH1-mutant and wild-type tumors. The agreement between judgments of the enhancement patterns was assessed by the 2 radiologists and was evaluated using the kappa consistency test. Kappa values ≥0.81, 0.61–0.80, and ≤0.60 were considered to reflect excellent, good, and poor agreement, respectively. Additionally, log-rank analyses of Kaplan–Meier survival curves were performed to compare the PFS and OS of the cohort. Factors that were significant (P < .05) in univariate analysis were entered into multivariate survival analysis based on the Cox proportional hazard ratio (HR) model. In addition, patients were further divided into subgroups according to their IDH1 status and radiological features in order to identify the prognostic values of these factors.
Results
Patient Characteristics
The clinical information and radiological data of 280 glioblastoma patients were systematically reviewed; the results are summarized in Table 1. The IDH1 mutation was detected in 45 tumors (16.1%). Age at diagnosis, contrast enhancement, and enhancing foci were significantly different between patients with mutant and wild-type IDH1 tumors (P < .001, chi-square test). A total of 145 patients (51.8%) underwent GTR, and 135 (48.2%) patients had residual tumors. Of the 280 patients in the study, 234 (83.6%) received the standard adjuvant therapy, 19 patients (6.8%) received only radiation treatment after surgery, and the other 27 patients (9.6%) did not receive any adjuvant therapy because of financial reasons. The chi-square test and Fisher exact test were performed in order to identify the clinical factors that contributed to patients not receiving adjuvant therapy in the mutant IDH1 group (Supplementary Table S1).
Table 1.
IDH1 mutation status of glioblastoma patients (n = 280)
| Characteristics |
IDH1 Status |
P Valuea | ||
|---|---|---|---|---|
| Total (n = 280) | Mutant (n = 45) | Wild-type (n = 235) | ||
| Age | ||||
| ≥50/<50 years | 139/141 | 6/39 | 133/102 | <.001 |
| Sex | ||||
| Male/female | 159/121 | 23/22 | 136/99 | .402 |
| KPS | ||||
| ≥80/<80 | 122/158 | 25/20 | 97/138 | .077 |
| Contrast enhancement | ||||
| Yes/no | 256/24 | 33/12 | 223/12 | <.001 |
| Enhancing focib | ||||
| Single/multiple foci | 199/57 | 19/14 | 180/43 | .003 |
| Pattern of enhancementb | ||||
| Ring-like/non-ring-like | 165/91 | 20/13 | 145/78 | .621 |
| Peritumoral edema | ||||
| ≤1 cm/>1 cm | 67/213 | 13/32 | 54/181 | .395 |
| MGMT promoter methylation | ||||
| Yes/no | 62/218 | 6/39 | 56/179 | .120 |
| Extent of resection | ||||
| GTR/<GTR | 145/135 | 28/17 | 117/118 | .126 |
| Adjuvant therapy | ||||
| Standard therapyc/radiation therapy/no therapy | 234/19/27 | 38/2/5 | 196/17/22 | .757 |
Abbeviation: GTR, gross-total resection.
Result obtained with the chi-square test.
Radiological features for tumors with contrast enhancement (n = 256).
Standard therapy includes concomitant temozolomide administration with fractionated radiotherapy followed by up to 6 cycles of adjuvant temozolomide.
Association Between the IDH1 Status and Radiological Features
Among the 280 patients, those with IDH1-mutant tumors were less likely to have contrast enhancement on MR images than patients with wild-type IDH1 tumors(73.3% vs 94.9%; P < .001; chi-square test) (see Supplementary Fig. S1). Of the 256 glioblastomas tumors with contrast enhancement (91.4%), multi-enhancing foci were more likely to be present in tumors with an IDH1 mutation compared with wild-type IDH1 tumors (42.4% vs 19.3%; P = .003). In addition, the pattern of tumor contrast enhancement was assessed in glioblastoma with enhancement. Tumor enhancement in a ring-like pattern was present in 165 patients (64.5%). The kappa value for the agreement of enhancement pattern judgments between the 2 evaluators was 0.98 (P = .08, kappa consistency test). Distribution of the tumor contrast enhancement patterns was not significantly different between patients with mutant and wild-type IDH1 tumors (P = .621, chi-square test).
Thirteen of 45 patients with an IDH1-mutant tumor (28.9%) and 54 of 235 patients (23.0%) with an IDH1 wild-type tumor did not show peritumoral edema. No significant difference in the incidence of edema was found between patients with mutant and wild-type IDH1 tumors (P = .395, chi-square test).
Prognostic Factors
Tumor occurrence was observed in 228 patients during the follow-up period, and the median PFS of patients enrolled in this study was 9.8 months (range, 2.3–73.1 mo). At the time of analysis, 66 patients (whose follow-up data were available) were still alive, with a median OS of 14.4 months (range, 1.0–86.8 mo). Univariate survival analysis in the entire cohort of patients showed that age at diagnosis (≥50 y vs <50 y, P = .008), preoperative KPS (≥80 vs <80, P = .019), contrast enhancement (P = .042), extent of resection (GTR vs <GTR, P = .029), IDH1 status (P = .022), and the administration of standard adjuvant therapy (P = .001) were significant prognostic factors for PFS. These were also predictive factors for OS (Supplementary Table S2).
Multivariate analysis revealed that age ≥50 years (P = .040; HR, 2.014), preoperative KPS <80 (P = .032; HR, 1.536), <GTR (P = .023; HR, 1.610), wild-type IDH1 (P = .029; HR, 1.372), and standard adjuvant therapy (P = .024; HR, 0.106) were significant prognostic factors for PFS. In addition, age ≥50 years (P = .046; HR, 1.725), preoperative KPS <80 (P = .030; HR, 1.668), <GTR (P = .035; HR, 1.506), and wild-type IDH1 (P = .026; HR, 1.851) predicted worse OS for glioblastoma patients, while the use of standard adjuvant therapy (P = .021; HR, 0.080) indicated a favorable OS (Supplementary Table S3). Univariate and step-wise multivariate analyses were performed for the mutant IDH1 tumor subgroup (Table 2) and for the wild-type IDH1 tumor subgroup (Table 3) in order to investigate specific prognostic factors according to the IDH1 status.
Table 2.
Univariate and multivariate analysis of survival outcomes for glioblastoma patients with an IDH1-mutant tumor (n = 45)
| PFS |
OS |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||||
| Characteristic | P Valueb | HR | 95% CI | P Valuec | HR | 95% CI | P Valueb | HR | 95% CI | P Valuec | HR | 95% CI |
| Age ≥ 50 y | .460 | 1.582 | 0.176–2.078 | .507 | 1.392 | 0.215–2.365 | ||||||
| Sex (male) | .219 | 1.472 | 0.743–2.933 | .761 | 1.007 | 0.470–2.042 | ||||||
| Preoperative KPS <80 | .926 | 1.073 | 0.431–3.552 | .691 | 1.463 | 0.173–2.983 | ||||||
| Contrast enhancement | .015 | 1.112 | 1.051–2.649 | .306 | 1.403 | 0.742–6.126 | .024 | 1.282 | 1.103–3.265 | .519 | 1.268 | .579–5.721 |
| Multifocala | .711 | 0.821 | 0.318–2.131 | .977 | 0.984 | 0.383–2.671 | ||||||
| Ring-likea | .745 | 1.138 | 0.522–2.489 | .461 | 1.322 | 0.278–1.861 | ||||||
| Edema (>1 cm) | .024 | 1.811 | 1.742–4.451 | .036 | 2.874 | 1.592–13.825 | .032 | 1.177 | 1.046–3.621 | .021 | 4.631 | 1.907–13.429 |
| <GTR | .016 | 2.123 | 1.273–5.520 | .028 | 3.966 | 1.465–12.587 | .037 | 2.179 | 1.156–4.281 | .033 | 2.164 | 1.203–9.315 |
| MGMT promoter methylation | .029 | 0.312 | 0.032–0.753 | .528 | 0.596 | 0.120–2.967 | .041 | 0.357 | 0.043–0.829 | .295 | 0.729 | 0.204–4.219 |
| Standard adjuvant therapy | .013 | 0.056 | 0.009–0.451 | .014 | 0.165 | 0.025–0.948 | .017 | 0.236 | 0.071–0.726 | .028 | 0.358 | 0.050–0.971 |
Abbreviations: CI, confidence interval; GTR, gross total resection; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; y, year.
In patients with tumor contrast enhancement (n = 33).
Log-rank analysis of Kaplan-Meier survival curves.
Cox proportional hazard regression analyses.
Table 3.
Univariate and multivariate analysis of survival outcomes for glioblastoma patients with an IDH1 wild-type tumor (n = 235)
| PFS |
OS |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||||
| Characteristic | P Valueb | HR | 95% CI | P Valuec | HR | 95% CI | P Valueb | HR | 95% CI | P Valuec | HR | 95% CI |
| Age ≥ 50 y | .025 | 1.480 | 1.045–2.179 | .034 | 6.531 | 1.392–33.896 | .006 | 1.602 | 1.125–2.328 | .039 | 2.708 | 1.771–27.342 |
| Sex (male) | .142 | 1.288 | 0.921–1.779 | .258 | 1.247 | 0.865–1.719 | ||||||
| Preoperative KPS <80 | .868 | 1.058 | 0.532–2.115 | .459 | 0.767 | 0.646–2.615 | ||||||
| Contrast enhancement | .098 | 2.643 | 0.837–8.335 | .073 | 3.621 | 0.889–14.633 | ||||||
| Multifocala | .002 | 2.136 | 1.317–3.462 | .469 | 2.555 | 0.411–7.998 | .010 | 1.908 | 1.168–3.115 | .583 | 2.310 | 0.512–6.624 |
| Ring-likea | .261 | 0.337 | 0.047–2.440 | .022 | 0.093 | 0.012–0.710 | .087 | 0.139 | 0.074–1.010 | |||
| Edema (>1 cm) | .242 | 1.309 | 0.833–2.057 | .191 | 1.372 | 0.855–2.193 | ||||||
| <GTR | .005 | 1.802 | 1.191–2.726 | .352 | 2.387 | 0.232–5.519 | .011 | 1.773 | 1.141–2.726 | .176 | 2.881 | 0.397–5.264 |
| MGMT promoter methylation | .481 | 0.785 | 0.400–1.539 | .868 | 0.926 | 0.469–1.894 | ||||||
| Standard adjuvant therapy | .005 | 0.239 | 0.095–0.593 | .027 | 0.038 | 0.016–0.828 | .001 | 0.203 | 0.073–0.491 | .035 | 0.064 | 0.031–0.902 |
Abbreviations: CI, confidence interval; GTR, gross total resection; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; y, year.
In patients with tumor contrast enhancement (n = 223).
Log-rank analysis of Kaplan–Meier survival curves.
Cox proportional hazard regression analyses.
Prognostic Role of Radiological Characteristics
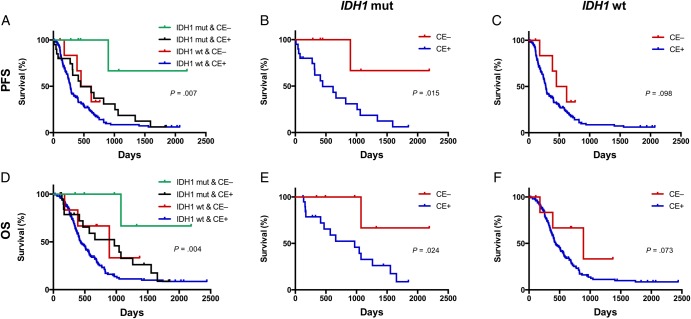
Among patients with IDH1-mutant tumors, those with nonenhanced lesions had a significantly longer median PFS and OS compared with those having contrast-enhanced lesions (for PFS, 11.4 vs 9.6 mo; P = .015; for OS, 18.5 vs 16.4 mo; P = .024) (Fig. 1). However, in patients with wild-type IDH1 tumors, contrast enhancement had no prognostic value for either PFS or OS (P = .098 and P = .073, respectively).
Fig. 1.
Kaplan–Meier analyses of survival among glioblastoma patients with respect to the tumor IDH1 status and contrast enhancement (CE). Mutant IDH1 tumors with no CE predicted better survival (progression-free survival [PFS], P = .007; overall survival [OS], P = .004, log-rank) (A and D). Furthermore, contrast enhancement was predictive of PFS (P = .015, log-rank) and OS (P = .024, log-rank) for tumors with mutant IDH1 (B and E), but not for wild-type (wt) IDH1 tumors (PFS, P = .098; OS, P = .073, log-rank) (C and F).
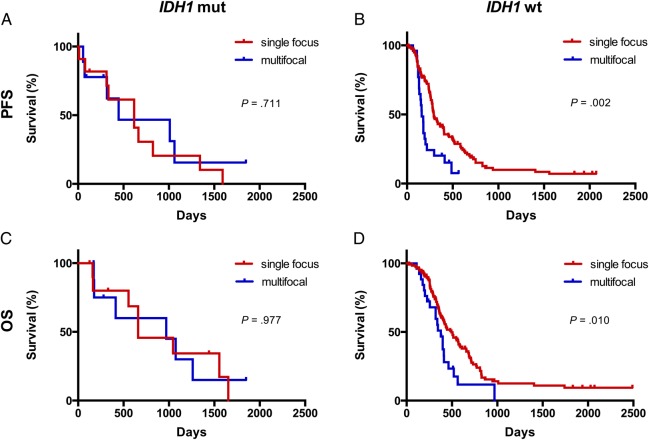
Notably, in 256 patients with contrast-enhanced tumors, the multifocality of enhancement combined with the IDH1 status improved the stratification of survival outcome (Fig. 2). In patients with a wild-type IDH1 tumor, a single enhancing focus was associated with a longer PFS (P = .002) and a longer OS (P = .010) than those with multifocal enhancement. However, among patients with a mutant IDH1 tumor, the number of enhancing foci had no prognostic value for either PFS or OS (P = .711 and P = .977, respectively).
Fig. 2.
Kaplan–Meier survival curves showing the prognostic value of multifocal enhancement. In patients with wild-type (wt) IDH1 tumors, lesions with multifocal enhancement were associated with shorter survival (P = .002 for progression-free survival [PFS]; P = .010 for overall survival [OS], log-rank) (B and D). However, multifocal enhancement was not a significant prognostic factor for patients with mutant (mut) IDH1 tumors (P = .711 for PFS; P = .977 for OS, log-rank) (A and C).
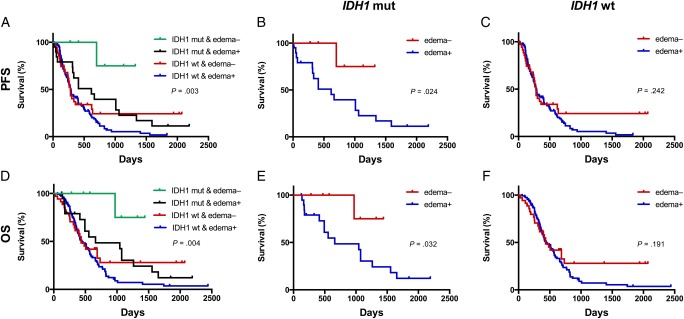
Kaplan–Meier analysis revealed that patients with mutant IDH1 tumors, but no apparent edema, lived significantly longer than all other patients (P = .003 for PFS, and P = .004 for OS) (Fig. 3). Specifically, in the mutant IDH1 group, the absence of peritumoral edema predicted longer PFS (P = .024) and longer OS (P = .032). However, peritumoral edema had no prognostic value in patients with IDH1 wild-type tumors with respect to PFS (P = .242) and OS (P = .191) (Fig. 3).
Fig. 3.
Kaplan–Meier plots for all 288 patients showing progression-free survival (PFS) and overall survival (OS) according to the combined IDH1 and tumor edema status. Mutant (mut) IDH1 tumors with no edema predicted better survival (PFS, P = .003; OS, P = .004, log-rank) (A and D). In patients with mutated IDH1, the absence of edema was also a significant prognostic factor for PFS (P = .024, log-rank) and OS (P = .032, log-rank) (B and E). However, the edema was not associated with survival in patients with wild-type (wt) IDH1 tumors (PFS, P = .242; OS, P = .191, log-rank) (C and F).
Discussion
In the present study, we combined clinical, radiological, and genetic characteristics to predict the prognosis of glioblastoma patients. Radiological features, including tumor contrast enhancement, multi-enhancing foci, and peritumoral edema, were found to be associated with the survival outcomes of glioblastoma patients stratified according to the IDH1 status.
As an outward manifestation of tumor-related genetic changes, radiological features may provide important information about the biological characteristics of glioblastoma. Tumor contrast enhancement is a key radiological feature of malignant gliomas. Previous studies found that the overexpression of genes including VEGF and NPTX2 was associated with edema, hypoxia, and angiogenesis in completely enhancing tumors26 and the upregulation of HIF1A, PGF, and VEGF was associated with angiogenesis within contrast-enhancement regions.27,28 Additionally, certain gene expression profiles (associated with microvascular expression, hypoxia, cellular mitosis, and overall cellularity) could be found in tumor regions with high blood volume and low apparent diffusion coefficient, which are related to angiogenesis and tumor aggressiveness.29 IDH1 mutations in glioblastoma and astrocytic neoplasms were also found to be associated with radiological characteristics including contrast enhancement, cysts, satellite lesions, frontal-lobe location, sharp tumor margins, and homogeneous signal intensity.15,30 In this study, we also found that IDH1-mutant glioblastomas were less likely to show contrast enhancement on MR images compared with their IDH1 wild-type counterparts. In addition, multifocal enhancement was more likely to be present on postcontrast T1-weighted images in IDH1-mutant tumors compared with wild-type IDH1 tumors. However, the frequency of edema did not vary with respect to the tumor IDH1 status.
In the current study, the IDH1 mutation was found in 16.1% of glioblastomas, consistent with the previously reported incidence (16.1%) among Chinese patients,25 and the standardized pyrosequencing protocol for IDH1 detection used in the current study was the same as that used in our previous studies.24,25,31,32 Thus, the higher incidence of IDH1 mutation may reflect ethnic differences as well as a different referral pattern at our institution. The IDH1 mutation has been shown to enable stratification of glioblastoma patients with respect to prognosis.14,16,33,34 Consistent with these findings, we also found that patients harboring IDH1-mutant tumors had significantly better survival than those with wild-type IDH1 tumors. The relationship between IDH1 mutations and other clinical factors in predicting prognosis should be considered further. Firstly, tumor IDH1 mutations were more frequent in younger patients,33 and age is widely regarded as a significant prognostic factor.35–37 Thus, it is likely that the age at diagnosis could be combined with the IDH1 status to determine survival outcomes. Secondly, IDH1 mutations occur frequently in low-grade gliomas but only rarely in primary glioblastomas.9,37,38 As recurrent glioblastomas were not included in this study, histopathology along with the IDH1 status may be useful for determining survival outcomes. In addition, mutations in the IDH1 gene have been shown to be an early genetic event in tumorigenesis and to drive other genetic changes in tumor cells.33 Tumors carrying an IDH1 mutation may consequently have specific genetic changes that lead to varied biological features.
We found that radiological characteristics combined with the IDH1 status better predicted the survival of glioblastoma patients. Notably, patients with tumors harboring IDH1 mutations who did not show enhancement on MR images survived significantly longer than other patients. This suggests that the combination of contrast enhancement and an IDH1 mutation may reflect a higher malignant potential of tumors. Interestingly, the multifocality of enhancement was identified as a prognostic indicator only in patients with wild-type, but not mutant, IDH1 tumors. Multifocality was suggested to predict a poor outcome for patients with high-grade gliomas.19 However, the multifocality of tumor enhancement has rarely been investigated in glioblastoma, especially combined with genetic changes in the tumor. During tumorigenesis, the IDH1 mutation may drive other genetic alterations that determine the biological features and radiological characteristics of tumors, which could be associated with the survival outcome of patients.33,39–41 The association between the IDH1 status and radiological characteristics in predicting the survival of glioblastoma patients remains to be investigated.
Tumor-induced edema is an inflammatory reaction that has also been found to be associated with a poor outcome.19,42 For example, edema was identified as a prognostic factor in patients with tumors carrying MGMT promoter hypermethylation.15 Interestingly, in this study, we found that tumor-related edema was associated with survival outcome in patients with mutant IDH1 tumors but not wild-type IDH1 tumors. Glioblastomas have been classified into four molecular subtypes (ie, proneural, neural, classical, and mesenchymal),39 and IDH1 mutations occur more frequently in the first of these, which is associated with a better prognosis in glioblastoma patients.39,41,43 It was hypothesized that tumors with little or no edema may be more likely to be categorized into the proneural subset.15 Therefore, the prognostic value of tumor-related edema may be attributed to the accompanying mutations that these tumors carry.
Previous studies showed that glioma patients with a methylated MGMT promoter generally survived longer,12,44,45 although another study failed to find any prognostic role for MGMT promoter methylation.46 A strong association was also found between MGMT promoter methylation, the IDH1 status, and age, whereby the prognostic significance of MGMT promoter methylation was lost in glioblastoma patients aged >50 years old.47 Moreover, MGMT promoter methylation is prognostic for patients with IDH1-mutant gliomas, while MGMT promoter methylation in patients with IDH1 wild-type tumors is associated with a better response to alkylating chemotherapy but does not predict survival.48
Several limitations of this study should be considered. First, the patients were enrolled from a single institution, and the data were analyzed retrospectively. Second, although the study was carefully controlled, a slight discrepancy in the interval duration between contrast injection and image acquisition could still exist between individuals. Third, the cohort may have included patients with secondary glioblastoma, which could account for the higher incidence of IDH1 mutations in the present study. In addition, the molecular subtype could not be determined in most glioblastomas because of the limited number of cases evaluated for other genetic alterations (eg, loss of ATRX and 1p19q codeletion). The prognostic role of radiological features associated with IDH1 mutations needs to be validated in future, prospectively designed investigations.
We found that radiological biomarkers, including contrast enhancement, multifocal enhancement, and tumor-related edema, were predictive factors in survival and could be combined with the tumor IDH1 status to provide a more accurate prediction of survival in glioblastoma patients. Further study is needed to elucidate the molecular basis of this association.
Supplementary Material
Funding
This work was supported by funds from the National 973 Program (2011CB707804 and 2015CB755500) and the National Natural Science Foundation of China (81271541).
Supplementary Material
Acknowledgments
We would like to thank Dr. Q. Chen and Dr. X. Chen for clinical data retrieval.
Conflict of interest statement. None declared.
References
- 1.Stancheva G, Goranova T, Laleva M, et al. IDH1/IDH2 but not TP53 mutations predict prognosis in Bulgarian glioblastoma patients. BioMed Research International. 2014;2014:654727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koca T, Basaran H, Sezen D, et al. Comparison of linear accelerator and helical tomotherapy plans for glioblastoma multiforme patients. Asian Pac J Cancer Prev. 2014;15(18):7811–7816. [DOI] [PubMed] [Google Scholar]
- 3.Sathornsumetee S, Rich JN, Reardon DA. Diagnosis and treatment of high-grade astrocytoma. Neurol Clin. 2007;25(4):1111–1139, x. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5.Field KM, Drummond KJ, Yilmaz M, et al. Clinical trial participation and outcome for patients with glioblastoma: multivariate analysis from a comprehensive dataset. J Clin Neurosci. 2013;20(6):783–789. [DOI] [PubMed] [Google Scholar]
- 6.Zhang GB, Cui XL, Sui DL, et al. Differential molecular genetic analysis in glioblastoma multiforme of long- and short-term survivors: a clinical study in Chinese patients. J Neurooncol. 2013;113(2):251–258. [DOI] [PubMed] [Google Scholar]
- 7.Kong J, Cooper LA, Wang F, et al. Integrative, multimodal analysis of glioblastoma using TCGA molecular data, pathology images, and clinical outcomes. IEEE Trans Biomed Eng. 2011;58(12):3469–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma X, Lv Y, Liu J, et al. Survival analysis of 205 patients with glioblastoma multiforme: clinical characteristics, treatment and prognosis in China. J Clin Neurosci. 2009;16(12):1595–1598. [DOI] [PubMed] [Google Scholar]
- 9.Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. [DOI] [PubMed] [Google Scholar]
- 10.De Carli E, Wang X, Puget S. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(21):2248, author reply 2249. [DOI] [PubMed] [Google Scholar]
- 11.Bleeker FE, Lamba S, Leenstra S, et al. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30(1):7–11. [DOI] [PubMed] [Google Scholar]
- 12.Molenaar RJ, Verbaan D, Lamba S, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 2014;16(9):1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv S, Teugels E, Sadones J, et al. Correlation of EGFR, IDH1 and PTEN status with the outcome of patients with recurrent glioblastoma treated in a phase II clinical trial with the EGFR-blocking monoclonal antibody cetuximab. Int J Oncol. 2012;41(3):1029–1035. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HB, Yue W, Xie C, et al. IDH1 mutation is associated with improved overall survival in patients with glioblastoma: a meta-analysis. Tumour Biol. 2013;34(6):3555–3559. [DOI] [PubMed] [Google Scholar]
- 15.Carrillo JA, Lai A, Nghiemphu PL, et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR. Am J Neuroradiol. 2012;33(7):1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. [DOI] [PubMed] [Google Scholar]
- 17.Hammoud MA, Sawaya R, Shi W, et al. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol. 1996;27(1):65–73. [DOI] [PubMed] [Google Scholar]
- 18.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 19.Pope WB, Sayre J, Perlina A, et al. MR imaging correlates of survival in patients with high-grade gliomas. AJNR. Am J Neuroradiol. 2005;26(10):2466–2474. [PMC free article] [PubMed] [Google Scholar]
- 20.Jafri NF, Clarke JL, Weinberg V, et al. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol. 2013;15(1):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappadakunnel M, Eskin A, Dong J, et al. Stem cell associated gene expression in glioblastoma multiforme: relationship to survival and the subventricular zone. J Neurooncol. 2010;96(3):359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maldaun MV, Suki D, Lang FF, et al. Cystic glioblastoma multiforme: survival outcomes in 22 cases. J Neurosurg. 2004;100(1):61–67. [DOI] [PubMed] [Google Scholar]
- 23.Utsuki S, Oka H, Suzuki S, et al. Pathological and clinical features of cystic and noncystic glioblastomas. Brain Tumor Pathol. 2006;23(1):29–34. [DOI] [PubMed] [Google Scholar]
- 24.Hu H, Wang Z, Liu Y, et al. Genome-wide transcriptional analyses of Chinese patients reveal cell migration is attenuated in IDH1-mutant glioblastomas. Cancer Lett. 2015;357(2):566–574. [DOI] [PubMed] [Google Scholar]
- 25.Yan W, Zhang W, You G, et al. Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China. PloS One. 2012;7(1):e30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope WB, Chen JH, Dong J, et al. Relationship between gene expression and enhancement in glioblastoma multiforme: exploratory DNA microarray analysis. Radiology. 2008;249(1):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diehn M, Nardini C, Wang DS, et al. Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci USA. 2008;105(13):5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Meter T, Dumur C, Hafez N, et al. Microarray analysis of MRI-defined tissue samples in glioblastoma reveals differences in regional expression of therapeutic targets. Diagn Mol Pathol. 2006;15(4):195–205. [DOI] [PubMed] [Google Scholar]
- 29.Barajas RF, Jr., Hodgson JG, Chang JS, et al. Glioblastoma multiforme regional genetic and cellular expression patterns: influence on anatomic and physiologic MR imaging. Radiology. 2010;254(2):564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi S, Yu L, Li H, et al. Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncology Letters. 2014;7(6):1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang CB, Bao ZS, Wang HJ, et al. Correlation of IDH1/2 mutation with clinicopathologic factors and prognosis in anaplastic gliomas: a report of 203 patients from China. J Cancer Res Clin Oncol. 2014;140(1):45–51. [DOI] [PubMed] [Google Scholar]
- 32.Zhong Z, Wang Z, Wang Y, et al. IDH1/2 mutation is associated with seizure as an initial symptom in low-grade glioma: A report of 311 Chinese adult glioma patients. Epilepsy Res. 2015;109:100–105. [DOI] [PubMed] [Google Scholar]
- 33.Dunn GP, Andronesi OC, Cahill DP. From genomics to the clinic: biological and translational insights of mutant IDH1/2 in glioma. Neurosurg Focus. 2013;34(2):E2. [DOI] [PubMed] [Google Scholar]
- 34.Sarmiento JM, Nuno M, Ortega A, et al. Cystic glioblastoma: an evaluation of IDH1 status and prognosis. Neurosurgery. 2014;74(1):71–75, discussion 75–76. [DOI] [PubMed] [Google Scholar]
- 35.Parkinson JF, Afaghi V, Payne CA, et al. The impact of molecular and clinical factors on patient outcome in oligodendroglioma from 20 years’ experience at a single centre. J Clin Neurosci. 2011;18(3):329–333. [DOI] [PubMed] [Google Scholar]
- 36.Reyes-Botero G, Dehais C, Idbaih A, et al. Contrast enhancement in 1p/19q-codeleted anaplastic oligodendrogliomas is associated with 9p loss, genomic instability, and angiogenic gene expression. Neuro Oncol. 2014;16(5):662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youland RS, Brown PD, Giannini C, et al. Adult low-grade glioma: 19-year experience at a single institution. Am J Clin Oncol. 2013;36(6):612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. [DOI] [PubMed] [Google Scholar]
- 39.Lee Y, Scheck AC, Cloughesy TF, et al. Gene expression analysis of glioblastomas identifies the major molecular basis for the prognostic benefit of younger age. BMC Med Genomics. 2008;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kyritsis AP, Levin VA, Yung WK, et al. Imaging patterns of multifocal gliomas. Eur J Radiol. 1993;16(3):163–170. [DOI] [PubMed] [Google Scholar]
- 43.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 44.Myung JK, Cho HJ, Kim H, et al. Prognosis of Glioblastoma With Oligodendroglioma Component is Associated With the IDH1 Mutation and MGMT Methylation Status. Transl Oncol. 2014;7(6):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogura R, Tsukamoto Y, Natsumeda M, et al. Immunohistochemical profiles of IDH1, MGMT and P53: Practical significance for prognostication of patients with diffuse gliomas. Neuropathology. 2015;35(4):324–335. [DOI] [PubMed] [Google Scholar]
- 46.Kalkan R, Atli EI, Ozdemir M, et al. IDH1 mutations is prognostic marker for primary glioblastoma multiforme but MGMT hypermethylation is not prognostic for primary glioblastoma multiforme. Gene. 2015;554(1):81–86. [DOI] [PubMed] [Google Scholar]
- 47.Boots-Sprenger SH, Sijben A, Rijntjes J, et al. Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: use with caution. Mod Pathol. 2013;26(7):922–929. [DOI] [PubMed] [Google Scholar]
- 48.Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology. 2013;81(17):1515–1522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.