Abstract
Background
The colony stimulating factor 1 receptor (CSF1R) ligands, CSF1 and interleukin-34, and the KIT ligand, stem cell factor, are expressed in glioblastoma (GB). Microglia, macrophages, blood vessels, and tumor cells also express CSF1R, and depletion of the microglia reduces tumor burden and invasive capacity. PLX3397 is an oral, small molecule that selectively inhibits CSF1R and KIT, penetrates the blood–brain barrier in model systems, and represents a novel approach for clinical development.
Methods
We conducted a phase II study in patients with recurrent GB. The primary endpoint was 6-month progression-free survival (PFS6). Secondary endpoints included overall survival response rate, safety, and plasma/tumor tissue pharmacokinetics. Exploratory endpoints included pharmacodynamic measures of drug effect in blood and tumor tissue.
Results
A total of 37 patients were enrolled, with 13 treated prior to a planned surgical resection (Cohort 1) and 24 treated without surgery (Cohort 2). PLX3397 was given at an oral dose of 1000 mg daily and was well tolerated. The primary efficacy endpoint of PFS6 was only 8.6%, with no objective responses. Pharmacokinetic endpoints revealed a median maximal concentration (Cmax) of 8090 ng/mL, with a time to attain Cmax of 2 hour in plasma. Tumor tissue obtained after 7 days of drug exposure revealed a median drug level of 5500 ng/g. Pharmacodynamic changes included an increase in colony stimulating factor 1 and reduced CD14dim/CD16+ monocytes in plasma compared with pretreatment baseline values.
Conclusion
PLX3397 was well tolerated and readily crossed the blood–tumor barrier but showed no efficacy. Additional studies are ongoing, testing combination strategies and potential biomarkers to identify patients with greater likelihood of response.
Keywords: CSF1R, glioblastoma, glioma, microglia, PLX3397
Standard treatment of glioblastoma (GB) consists of surgical resection followed by radiation therapy (RT) in combination with temozolomide (TMZ) chemotherapy followed by adjuvant TMZ, which results in a median survival of 14.6 months.1 Other types of therapy appear to add some survival benefit, but the gain is limited at best.2,3 A novel agent that may improve overall survival (OS) or prolong time to progression is PLX3397, an oral, potent, and selective inhibitor of CSF1R, the receptor for colony stimulating factor 1 (CSF1, also known as macrophage-colony stimulating factor, M-CSF or FMS); KIT (the receptor for stem cell factor [SCF]); and oncogenic Flt3 (the receptor for the Flt3 ligand).
CSF1R and KIT are regulators of key components of the tumor microenvironment, namely microglia, macrophages, osteoclasts, and mast cells. A number of lines of evidence suggest that microenvironment influences the biology and behavior of GB. The rationale for testing the CSF1R/KIT inhibitor PLX3397 in GB is as follows: (i) high levels of infiltrating microglia are associated with reduced survival in patients with GB4; (ii) a mesenchymal signature, including well-described microglial markers such as CD68, protein tyrosine phosphatase receptor type C, tumor necrosis factor, and neurofibromatosis type 1 (NF1) mutations, also predicts reduced survival in GB5,6; (iii) a microglial-weakening polymorphism (V249I) is associated with increased survival in GB7; (iv) many glioma cell lines established from patient samples express high concentrations of ligands for CSF1R (CSF1 and interleukin-34) or KIT (SCF), which attracts and activates surrounding microglia, thereby facilitating tumor invasion via proteases such as membrane type 1 matrix metalloprotease and matrix metalloprotease 28,9; and (v) depletion of microglia in orthotopic GB models reduces tumor burden and spread (on file at Plexxikon). In pharmacology studies, PLX3397 (1 µM) has been shown to markedly reduce the invasion of GL261 GB cells when cocultured with microglia in a Matrigel invasion assay.10 Finally, PLX3397 avidly crosses into the CNS of rats and is not a substrate for active transport, suggesting that PLX3397 will reach GB tumors and surrounding stromal tissue within the CNS even with an intact blood-brain barrier (on file at Plexxikon). Based upon these promising data, we conducted a phase II study to determine the efficacy of PLX3397 in patients with recurrent GB.
Materials and Methods
Study Design
This was a phase II, open-label, single-agent trial of continuous 1000 mg daily oral dosing of PLX3397 in patients with recurrent GB (NCT01349036). The trial was conducted at 6 sites via the Ivy Foundation Early Phase Clinical Trials Consortium. There were 2 cohorts which were conducted in sequential order. Cohort 1, at least 10 patients with recurrent GB who required reoperation as part of their standard clinical care, were treated with PLX3397 for 7 days prior to surgery. Tumor tissue from the resection was evaluated for drug/tumor pharmacokinetic (PK) levels and pharmacodynamic (PD) effects. Cohort 2, a planned 30 patients, would be initiated only after the tissue PK analysis from patients in Cohort 1 confirmed that a PLX3397 concentration of ≥100 nM was achieved in at least 5 of 10 tumor tissue specimens. This degree of tumor tissue drug concentration was felt to be sufficient to result in CSF1R pathway inhibition based on preclinical PK/PD models.11 The patients in Cohort 2 did not require recurrent surgical resection to be eligible for enrollment.
The primary endpoint of therapeutic efficacy of PLX3397 was measured by 6-month progression-free survival (PFS6). Secondary endpoints included safety, PK in blood, OS, and median duration of radiographic response. Exploratory objectives included PK results and PD effects in resected tumor tissue. Safety evaluations were performed using the Common Terminology Criteria for Adverse Events (CTCAE, version 4). This study complied with the principles of Good Clinical Practice and the Declaration of Helsinki. Institutional review boards approved the protocol, and all patients provided written informed consent prior to enrollment.
Patient Eligibility
Patients 18 years or older were eligible if they had a diagnosis of GB, a KPS of ≥60, an estimated survival of >8 weeks, and a radiographically proven recurrence (at least first relapse) ≥3 months after radiation based upon the Response Assessment in Neuro-Oncology (RANO) criteria.12 Central pathology review was done retrospectively by a neuropathologist (K.L.L.). All patients were required to have previously received treatment with external beam radiation and TMZ. At least 10 unstained slides were required for exploratory analysis from any previous GB surgery (termed here “prestudy tissue”). For Cohort 1 patients, resected recurrent tumor on drug needed to yield at least 200 mg of tissue for PK and PD analysis (termed “on-study tissue”). Standard hematological, renal, and hepatic functions were needed for enrollment. All patients had to practice adequate contraception during treatment. Patients were excluded if they had a history of any other cancer unless in complete remission and off all therapy for that disease for a minimum of 3 years. Patients were not allowed use of enzyme-inducing antiepileptic drugs. For patients previously treated with enzyme-inducing antiepileptic drugs, use of the agent had to have been stopped at least 2 weeks prior to study treatment.
Pharmacokinetics and Patient Evaluations
Cohort 1 patients were enrolled first and began dosing of PLX3397 at 1000 mg daily, beginning 7 days prior to scheduled surgical resection of their recurrent GB. Daily dosing continued through the morning of surgery. A plasma PK sample was collected in the operating room in order to provide a reference for comparison with tissue PK measurement. One section of the surgical specimen was immediately formalin fixed for evaluation of PD biomarkers, while another was saline rinsed and flash frozen for measurement of tissue PK. Note, tissue in most cases was obtained from enhancing regions of the tumor, but imaging was not specifically utilized to guide surgery to enhancing/nonenhancing regions. Tissue PK was quantified by liquid chromatography–tandem mass spectrometry after extracting the sample homogenates with acetonitrile (containing internal standards) and diluting with 5× volume of 0.2% formic acid. Cohort 1 patients restarted PLX3397 following recovery from surgery (up to a maximum of 5 wk allowed). Beginning with the first cycle following surgery for Cohort 1, and for all Cohort 2 patients, PLX3397 blood PK samples were obtained predose and 1, 2, 4, and 6 h postdose. An additional sample was obtained on day 15 of this first cycle only. Each cycle consisted of 28 days. Blood was obtained for steady-state plasma PK prior to dosing on the first day of each cycle after cycle 1.
Patients were evaluated clinically at the start of each cycle. If PLX3397 was well tolerated and there was no clinical evidence of disease progression, continued dosing was permitted. Patients were monitored for response and disease progression with MRI brain scans every 2 cycles.
Clinical laboratory assessments were performed at screening; cycle 1, days 1 and 15; at the beginning of each subsequent 28-day cycle for those patients who continued on therapy; and at study completion or upon early withdrawal. Blood pressure, respiratory rate, pulse rate, and temperature were measured at each study visit. A standard 12-lead electrocardiogram was performed for each patient at screening, the first cycle, and study completion. A complete physical examination was performed for each patient at screening, at each subsequent visit, and at study completion or upon early withdrawal.
Pharmacodynamics
Blood samples for fluorescence activated cell sorting (FACS) analysis of blood monocyte subset CD14dim/CD16+ and for enzyme-linked immunosorbent assay analysis of circulating plasma CSF1, both shown to be reproducible PD markers of CSF1R kinase inhibition by PLX3397 in earlier phase I studies, were obtained prior to any dosing in Cohort 1 and Cohort 2, and then again prior to the next cycle only.
Tumor tissue PD response was measured in surgical specimens removed from Cohort 1 patients and compared with prestudy treatment-naïve “archival” tumor tissue from the same patient. Immunohistochemistry (IHC) for markers of tumor macrophages (IBA1, CD68, and CD163) were analyzed to determine whether macrophages were detectably affected in on-study tumor samples versus prestudy samples. IHC markers of tumor cell proliferation (mouse intestinal bacteria 1 [MIB1]), apoptosis (cleaved caspase-3), and activation of mitogen-activated protein kinase [MAPK] pathway (phosphorylated extracellular signal-regulated kinase [pERK]) were also analyzed to assess changes in tumor cells after PLX3397 treatment. An additional control cohort of 4 matching pairs of archival and post-RT/TMZ treatment GB samples were also studied (cohort unrelated to this study).
Study samples were stained and quantified for significant reductions or elevations in staining by automated (ImageJ software) and manual scoring by a neuropathologist (K.L.L.) for intensity and percentage of positive cells. Percentage of positive cells per field (2 × 40× fields in each sample) was calculated using Immunoratio software for ImageJ. Final sample percentage was calculated by average of individual field percentages.
Genomic Characterization
Whole exome sequencing was performed at the Dana-Farber Cancer Institute Center for Cancer Genome Discovery using formalin-fixed paraffin-embedded (FFPE) tumor samples from 2 subjects with extended survival (subject 03-003: prestudy, on-study, and off-study samples; subject 04-007: prestudy sample). Whole exome v5 enrichment was performed using Agilent SureSelect and a HiSeq 2500 in Rapid Run Mode (Illumina). Analysis was performed without matched normal blood, but results were informatically filtered for a targeted panel of cancer-causing genes (CCGD OncoPanel, version 3). The mutation status of isocitrate dehydrogenase (1IDH1-R132H) was assessed in all evaluable patients by IHC and scored as negative (<2% of cells positive) or positive (>2% of cells positive).
Fluorescence in situ hybridization (FISH) was performed for platelet-derived growth factor receptor alpha (PDGFRA) according to previously published methods.13 FISH was scored as follows:
High amplification >10% of cells with >12 or innumerable signals.
Low amplification <10% of cells with >12 signals or >40% with 6–12 signals.
Polysomy >10% of cells with 2–6 signals.
Normal no increase or <10% of cells with >6 PDGFRA signals.
Nanostring RNA Expression Profiling
To assess transcriptional subclass, patient tissue was subjected to a Nanostring-based analysis (Brennan, C. W., and Huse, J. T., unpublished results).14 The Nanostring nCounter platform directly quantifies up to 800 mRNA transcripts in a multiplexed fashion using specifically designed color-coded probe pairs (Geiss, G., et al, Nature Biotechnology, 2008). Raw transcript counts are normalized to a set of endogenous controls, in this case B2 M, B4GALT1, CLTC, E2F4, GAPDH, POLR2A, SDHA, TBP, and TCL1A.
For this particular analysis, 81 robustly performing genes were selected from the initial publication from The Cancer Genome Atlas (TCGA) on GB.6 This collection was then supplemented with genes whose expression levels were known to correlate with DNA copy number gains (EGFR, PDGFRA, SOX2, MDM4, MDM2, MET, CDK6, HGF, and MYCN) or losses (NF1, CDKN2C, CDKN2A, MGMT, PTEN, RB1) described in GB. Probes targeting fusion transcripts like epidermal growth factor receptor variant III (EGFRvIII) and PDGFRA were also included, for a final list of 152 unique probes. The method was then applied to 192 samples from TCGA with established subgroup assignments to generate expression space centroids.
Total mRNA samples (150–300 ng; RNeasy FFPE kit, Qiagen), derived from FFPE tissue obtained for each study patient, were then run on the Nanostring assay. Following normalization, data were mean-centered and correlated with the transcriptional subclass centroids described above. Final subclass assignments were based on strongest centroid correlation. In addition to transcriptional subclass, we directly assessed expression levels of receptor tyrosine kinase genes known to undergo genomic amplification in GB, comparing them with those of a spectrum of samples with known amplification status.
Statistics
The protocol consisted of a phase II, multicenter, single-agent, open-label study with the primary objective to estimate the PFS6 rate of adults with recurrent GB when treated with orally administered PLX3397. Approximately 40 patients were to be enrolled. A sample size of at least 10 participants was planned for Cohort 1, which was considered adequate to provide a reasonable assessment of PK and PD effects of PLX3397 in the absence of an immediate pretreatment baseline sample. In order to proceed to Cohort 2, at least 5 of the 10 samples from Cohort 1 had to demonstrate a tumor tissue PK value for PLX3397 of ≥100 nM. If the true success rate of adequate PLX3397 tissue penetration was 75%, there was a 92% chance of meeting this target.
For the primary endpoint of PFS6, we utilized historical comparison data from a pooled analysis of phase II experience in recurrent grade IV gliomas which documented a PFS6 rate of 9%.15 Using a one-sided alpha of 10%, with a sample size of 40 patients, there is 90% power to detect an improvement over the historical PFS rate of 10% for ineffective agents if the true PFS6 rate was 25%. The sample size of ∼40 patients was to be achieved by pooling the ∼10 patients from Cohort 1 with the ∼30 patients from Cohort 2. This was acceptable because surgical resection at the time of recurrence is known to not substantially impact PFS6.16
Efficacy and safety analyses were conducted on the modified intent-to-treat (ITT) population, which included data from all patients who received at least one dose of the study drug, met eligibility requirements, and had follow-up data. PFS and OS were estimated using the Kaplan–Meier method. All eligible patients for efficacy analyses were evaluable for PFS, calculated for each subject as the number of days from the first day of treatment to the date of the first documented disease progression or date of death, whichever occurred first. For Cohort 1 patients, the date of first treatment was considered the first treatment after recovery from surgery. Response to treatment was evaluated using the RANO criteria.12 For each subject with a response to therapy, duration of response was calculated. The duration of response was defined as number of days from the date of initial response to the date of first documented disease progression or death, whichever occurred first. The Kaplan–Meier method was used to estimate median duration of response.
A noncompartmental method of analysis was used to analyze the plasma concentrations of PLX3397. Maximum concentration (Cmax) and the time to attain the Cmax (Tmax) were determined directly from the observed data. A partial area under the curve (AUC0–6) was also calculated. In addition, relationships among antitumor activity, PD markers, and drug exposure levels were explored. No formal statistical analysis of PD endpoints was performed relative to outcome measures.
Results
Patient Characteristics
A total of 38 patients were enrolled from January to November 2012. Enrollment was stopped early because the PFS6 endpoint would not be met by enrolling additional patients. There were 14 patients enrolled in Cohort 1. This was more than the planned 10 patients due to concurrent enrollment. In addition, on-study resection of recurrent tumor in one Cohort 1 patient demonstrated completely necrotic tissue without evidence of viable tumor on pathologic review, which could not be utilized for planned PK or PD analysis and therefore was dropped from study and not treated with additional PLX3397. Thus, 13 patients from Cohort 1 were included in the modified ITT analysis. There were 24 patients in Cohort 2, bringing the total number of patients in the modified ITT analysis to 37. Table 1 shows the baseline characteristics for both cohorts. Median age for both cohorts was 58.5 years; median KPS was 90 (range, 60–100).
Table 1.
Patient characteristics of the modified ITT analysis
| Surgical, Cohort 1 | Nonsurgical, Cohort 2 | Total | |
|---|---|---|---|
| Age, y | |||
| n | 13 | 24 | 37 |
| Mean | 56.1 | 54.1 | 54.8 |
| Gender | |||
| Male | 8 (62%) | 17 (71%) | 25 (67%) |
| Female | 5 (38%) | 7 (29%) | 12 (33%) |
| Race | |||
| n | 13 | 24 | 37 |
| American Indian or Alaska Native | 0 | 0 | 0 |
| Asian | 0 | 1 (4%) | 1 (3%) |
| Black or African American | 0 | 1 (4%) | 1 (3%) |
| Native, Hawaiian, or other | 0 | 0 | 0 |
| White | 13 (100%) | 21 (88%) | 34 (92%) |
| Other | 0 | 1 (4%) | 1 (3%) |
Efficacy and Toxicity
Efficacy results are shown in Table 2. The primary endpoint of PFS6 was 8.8% (90% CI, 3.5%–21.6%), with no complete or partial imaging responses. Median OS was 9.4 months (90% CI, 6.67 mo–NA). The longest PFS was noted in patients 03-003 and 04-007: 03-003 had a PFS of 358 days and an OS of 371 days and then was censored; 04-007 had a PFS of 390 days and an OS of 393 days and then was censored. PLX3397 was generally well tolerated, as shown in Table 3. Hair color change was expected and is the result of the inhibition of KIT kinase. This effect is therefore considered a PD biomarker for the target activities of PLX3397. The mechanisms of elevated aspartate aminotransferase and alanine aminotransferase likely included a reduction in Kupffer cells as a consequence of the inhibition of CSF1R. Thirty-five patients discontinued treatment due to disease progression. One patient discontinued due to adverse events (AEs) of neutropenia and headache. After stopping the drug due to tumor progression, one other patient died within 3 days of study drug discontinuation, and death was attributed to seizure from tumor progression. All but 2 patients experienced at least 1 treatment-related AE. There were 18 patients who experienced at least 1 CTCAE grade 3–4 AE and 12 patients who experienced a CTCAE grade 3–4 AE that was considered by the investigator to be possibly related to study drug. The most common grade 3–4 AE was elevated alkaline phosphatase (n = 4). No deaths were reported while on therapy, though as stated above, one death was reported within 30 days of therapy discontinuation and attributed to tumor progression.
Table 2.
Efficacy endpoints
| Total (N = 37) | |
|---|---|
| PFS6 survival and 2-sided | 8.8% |
| 90% CIa | (3.5%, 21.6%) |
| Median OS and 90% CI | 9.4 mo (6.67 mo–NA) |
| OS 6-mo and 90% CI | 88.3 |
| (75%, 100%) |
PFS is defined as the number of days from cycle 1/day 1 to the date of the first documented disease progression or date of death, whichever occurs first. Point estimate and CIs are based on the Kaplan-Meier method.
Table 3.
Common (≥10%) treatment-related adverse events
| Event | Surgical, Cohort 1 (N = 13) | Nonsurgical, Cohort 2 (N = 24) | Total (N = 37) |
|---|---|---|---|
| Fatigue | 6 (46%) | 14 (58%) | 20 (55%) |
| Hair color change | 2 (15%) | 4 (17%) | 6 (16%) |
| Anorexia | 3 (23%) | 3 (13%) | 6 (16%) |
| Elevated AST | 3 (23%) | 3 (13%) | 6 (16%) |
| Elevated ALT | 2 (15%) | 3 (13%) | 5 (13%) |
| Headache | 1 (7%) | 3 (13%) | 4 (10%) |
| Nausea | 1 (7%) | 3 (13%) | 4 (10%) |
| Constipation | 3 (23%) | 1 (4%) | 4 (10%) |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Biomarker Evaluation of PLX3397-treated Patients Related to Efficacy
Analysis across the cohort of patients was performed to determine possible correlation with outcomes data. IHC for IDH1 (R132H) showed that only 3 of 36 patients (8%) were positive for the mutation. We performed FISH for the PDGFRA locus and identified 4/36 patients (11%) with amplification and 8/36 (22%) with gain at the locus. These amplifications and gains did not correlate significantly with PFS6 or other parameters.
Genomic and expression analyses of 2 patients with extended PFS in the study (03-003 and 04-007) were performed to explore features of possible relation to efficacy. IHC for IDH1 (R132H) was noted to be negative in both patients. Whole exome sequencing of FFPE samples demonstrated likely complete NF1 tumor suppressor inactivation in patient 03-003 (prestudy and poststudy samples). Poststudy changes were identified (single nucleotide variation: PIK3CG; somatic copy-number alteration: 5q loss) but were of no clear predicted interaction with PLX3397 responsiveness. The other patient (04-007) was noted to have novel variants in neurotrophic tyrosine kinase receptor type 1 and cyclin D2 but was of insufficient read depth overall to allow analysis of somatic copy-number alteration. Preclinical studies suggest that PDGF-driven gliomas, possibly of TCGA proneural expression subclass, may be particularly responsive to CSF1R inhibition, and recently partial inhibition of PDGFRB by PLX3397 has been reported.9,17 However, expression subclassification using a novel Nanostring-based GB custom assay showed closest correlation with the mesenchymal TCGA subclass for these 2 patients, and no evidence for EGFRvIII fusion or PDGFRA fusion.
Pharmacokinetics of PLX3397 in Blood and GB Tumor Tissue
In order to evaluate potential effects of PLX3397 on blood and tumor, PK and PD were analyzed in paired prestudy (archival) and on-study (surgical) samples from Cohort 1 patients. Tissue PK analysis confirmed that a PLX3397 concentration of ≥100 nM (0.1 µM) was achieved in at least 5 of 10 tumor tissue specimens. This criterion was met in all 11 of the Cohort 1 tissues submitted for PK bioanalysis (2 of the 13 patient tissues were not analyzed due to extensive necrosis in one patient and a shipping error for the second; see Table 4). The 11 Cohort 1 patient samples had a mean value of 29.6 µM ± 46.2 with a median value of 13 µM and range of 3–166 µM. Comparison with PK bioanalysis of plasma taken at the time of surgery revealed a median ratio of tumor/plasma PLX3397 concentrations of ∼70%.
Table 4.
Mean plasma pharmacokinetic parameters of PLX3397 after oral administration of 1000 mg/day
| Tmax (h) | Cmax (ng/mL) | ||
|---|---|---|---|
| Cohort 1 Cycle 1, day 15 |
N | 11.0 | 11.0 |
| Mean | 2.09 | 7760 | |
| SD | 1.04 | 2330 | |
| Min | 1.00 | 4750 | |
| Median | 2.00 | 7570 | |
| Max | 4.00 | 12200 | |
| CV% | 50.0 | 30.1 | |
| Cohort 2 Cycle 1, day 15 |
N | 21.0 | 21.0 |
| Mean | 1.71 | 8030 | |
| SD | 0.902 | 2790 | |
| Min | 1.00 | 3100 | |
| Median | 2.00 | 8520 | |
| Max | 4.00 | 13400 | |
| CV% | 52.6 | 34.7 |
Abbreviation: CV, coefficient variation.
The PK bioanalysis of plasma PLX3397 was generally consistent with the values expected from prior phase I-recommended phase II dose selection analysis. Thus, as anticipated, the 1000-mg dose of PLX3397 increased the plasma levels of CSF1 when comparing the first- and second-cycle predose levels, from a mean level of 665 ± 309 to a mean level of 5078 ± 805 (pg/mL). Similarly as expected, the FACS analysis of the CD14dim/CD16+ monocyte subset was decreased from a mean of 2.1 ± 0.3 to a mean of 1.1 ± 0.2 (% of total monocytes).
PLX3397 Inhibits Microglia Response in GB Tissue
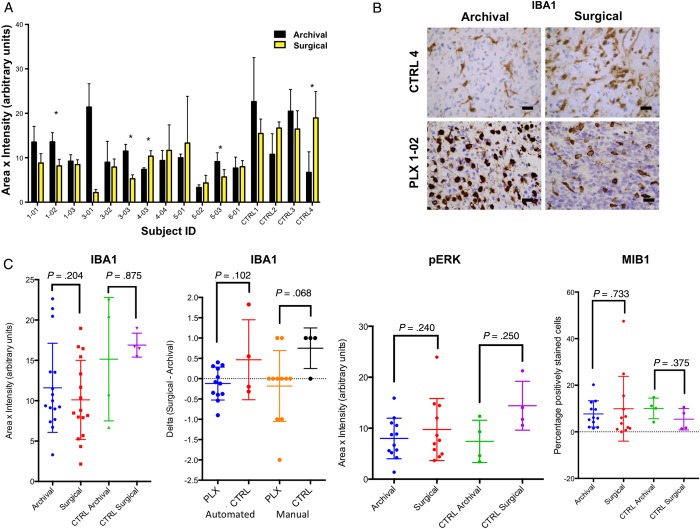
Given the excellent penetration of PLX3397 into tumor tissue, we sought to evaluate whether PLX3397 may affect the microglial populations within the tumor, as has been reported in normal mouse brain.11 Twelve Cohort 1 patients had evaluable paired tissue samples, and IHC for IBA1, a marker of microglia, was performed and quantified using automated and manual methods to evaluate the number of cells and intensity of staining within tumor tissue. No statisically significant difference was seen in the IBA1+ microglia from prestudy (archival) to on-study (surgical) samples as a group with either PLX3397 or following standard of care with RT + TMZ, using historical control samples (Fig. 1A and B).
Fig. 1.
Pharmacodynamic effects of PLX3397 in recurrent GB. (A) Automated quantification of IHC changes in IBA1 staining in paired archival and surgical specimens from study subjects on PLX3397 and nonstudy control subjects treated with RT + TMZ. (B) Representative IBA1 IHC staining for control (post RT + TMZ) and study subject PLX-102 (RT + TMZ followed by PLX3397). The size bar is 30 μm. (C) Group comparison of IBA1 vs the change seen in individual patients (second panel, delta) as assessed by automated and manual review. Automated quantification of IHC for pERK and MIB1.
Analysis of change in IBA1 scores in matched patient samples showed that 7 of 12 patients (58%) had lower IBA1 scores while on PLX3397 compared with their matching prestudy sample, a level simliar to that seen in control samples treated with only RT + TMZ. However, there was a trend (P = .068, manual review) for increased IBA1 microglia following RT + TMZ that appeared to be blunted in PLX3397-treated patients consistent with weak inhibitory effects of PLX3397 on microglia within GB tissue. No definitive effects of PLX3397 on microglial morphology were noted relative to RT + TMZ controls. Evaluation of PLX3397 effects on tumor cells was performed by IHC within Cohort 1 and the effects compared with the historically controlled paired samples with RT + TMZ. PLX3397 has effects on pERK and activation of MAPK signaling, and while average levels were lower in the PLX3397-treated samples as a group, this difference was not statistically significant (Fig. 1C).
Additionally, no statistically significant effects on reduced proliferation index (MIB1) (Fig. 1C) or increased apoptosis (CC3, data not shown) normally seen in RT + TMZ-treated samples was identified with PLX3397.
Discussion
As a single agent, PLX3397 showed no significant improvement in PFS compared with historical controls. However, the 1000 mg dose of PLX3397 showed both the expected peripheral PD changes of elevated CSF1 ligand and decreased CD14dim/CD16+ monocyte subset. Importantly, this study demonstrated that PLX3397 can achieve exposures in tumor tissue that are known to be sufficient for CSF1R kinase inhibition, and there is evidence that this agent does impact the number of tumor-associated macrophages in this patient population. However, additional work is required to refine our understanding of the relative permeability of PLX3397 within both enhancing and adjacent nonenhancing, infiltrative margins of disease.
While PLX3397 drug concentrations in tumor tissue were significant, PD effects, while detectable, were lower than expected compared with preclinical studies in mice. Reductions in IBA1 microglia appeared to be the most noteworthy effect of PLX3397, revealed in a subset of patients when their paired sample was analyzed. It is noteworthy that although there were no responses seen, one of the extended PFS patients (03-003) showed a statistically significant reduction in IBA1+ microglia. One possible explanation for differences in effects on microglia in mice and in patients on this study could be the duration of PLX3397 administration that may not have been sufficient to have maximal PD effects at time selected for resection of tissue samples. Future studies of the differences in effects in species may be needed. Furthermore, our results did not examine potential effects of PLX3397 on microglia function; however, such assays may require analysis of fresh tissue or isolated macrophage populations in future studies.
Preclinical rodent studies suggest that tumors of the proneural subtype respond well to CSF1R inhibition.9 However, other preclinical and bioinformatics investigations suggest that microglia may be particularly important in the mesenchymal subtype of GB.18–20 Nanostring RNA profiling of a subset of tumors from the current trial indicated that the 2 patients who had extended PFS were found to both represent the mesenchymal subtype, and the mutations seen in these tumors did not offer an explanation for their relative best PFS. The small sample size precludes any clear conclusions, but the good outcomes in these mesenchymal GBs with an agent with activity against microglia may serve as hypothesis generation for future studies.
In sum, PLX3397 is a potent inhibitor of tumor-associated macrophages and microglia, readily entered GB tumor tissue, and demonstrated PD effects in some patients. However, there was no significant improvement in efficacy in the present study compared with historical controls. PLX3397 is well tolerated and its safety profile is conducive to testing in combination with other treatments, should such combinations be thought to be more efficacious. Of note, a phase II trial is ongoing combining PLX3397 with RT and temodar in newly diagnosed GB.
Funding
This work was supported by Plexxikon and conducted with support from the Ivy Foundation Early Phase Clinical Trials Consortium.
Acknowledgments
The above information was partially presented at the Annual ASCO meeting 2014. We thank M. Ducar and members of the Center for Cancer Genome Discovery for assistance with sequencing analysis.
Conflict of interest statement. There are no conflicts of interest to report for any author.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2.Butowski N, Wong ET, Mehta MP, et al. A roundtable discussion on the clinical challenges and options for the treatment of glioblastoma: introducing a novel modality, TTFields. Semin Oncol. 2013;40(6):S2–S4. [DOI] [PubMed] [Google Scholar]
- 3.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. [DOI] [PubMed] [Google Scholar]
- 4.Komohara Y, Ohnishi K, Kuratsu J, et al. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. [DOI] [PubMed] [Google Scholar]
- 5.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 6.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodero M, Marie Y, Coudert M, et al. Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients with glioblastoma. J Clin Oncol. 2008;26(36):5957–5964. [DOI] [PubMed] [Google Scholar]
- 8.Sielska M, Przanowski P, Wylot B, et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230(3):310–321. [DOI] [PubMed] [Google Scholar]
- 9.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coniglio SJ, Eugenin E, Dobrenis K, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmore MR, Najafi AR, Koike MA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82(2):380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 13.Phillips JJ, Aranda D, Ellison DW, et al. PDGFRA amplification is common in pediatric and adult high-grade astrocytomas and identifies a poor prognostic group in IDH1 mutant glioblastoma. Brain Pathol. 2013;23(5):565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omuro A, Beal K, Gutin P, et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20(19):5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke JL, Ennis MM, Yung WK, et al. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol. 2011;13(10):1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patwardhan PP, Surriga O, Beckman MJ, et al. Sustained inhibition of receptor tyrosine kinases and macrophage depletion by PLX3397 and rapamycin as a potential new approach for the treatment of MPNSTs. Clin Cancer Res. 2014;20(12):3146–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler JR, Robinson AE, Smirnov I, et al. Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PloS one. 2012;7(8):e43339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Senbabaoglu Y, Peng W, et al. Genomic estimates of aneuploid content in glioblastoma multiforme and improved classification. Clin Cancer Res. 2012;18(20):5595–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhat KP, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]