Abstract
Background
With advances in the understanding of histopathology on outcome, accurate meningioma grading becomes critical and drives treatment selection. The 2000 and 2007 WHO schema greatly increased the proportion of grade II meningiomas. Although associations with progression-free survival (PFS) and overall survival (OS) have been independently validated, interobserver concordance has not been formally assessed.
Methods
Once mature, NRG Oncology RTOG-0539 will report PFS and OS in variably treated low-, intermediate-, and high-risk cohorts. We address concordance of histopathologic assessment between enrolling institutions and central review, performed by a single pathologist (AP), who is also involved in developing current WHO criteria.
Results
The trial included 170 evaluable patients, 2 of whom had 2 eligible pathology reviews from different surgeries, resulting in 172 cases for analysis. Upon central review, 76 cases were categorized as WHO grade I, 71 as grade II, and 25 as grade III. Concordance for tumor grade was 87.2%. Among patients with WHO grades I, II, and III meningioma, respective concordance rates were 93.0%, 87.8%, and 93.6% (P values < .0001). Moderate to substantial agreement was encountered for individual grading criteria and were highest for brain invasion, ≥20 mitoses/10 high-powered field [HPF], and spontaneous necrosis, and lowest for small cells, sheeting, and ≥4 mitoses/10 HPF. In comparison, published concordance for gliomas in clinical trials have ranged from 8%–74%.
Conclusion
Our data suggest that current meningioma classification and grading are at least as objective and reproducible as for gliomas. Nevertheless, reproducibility remains suboptimal. Further improvements may be anticipated with education and clarification of subjective criteria, although development of biomarkers may be the most promising strategy.
Keywords: concordance, cooperative group trial, meningioma, pathology, tumor grade
According to recent Central Brain Tumor Registry of the United States (CBTRUS) publications, meningioma is the most commonly reported central nervous system (CNS) neoplasm in the US, comprising 36.1% of all primary CNS tumors by histology, while gliomas combined account for 28.4%.1 In addition to increased reporting of meningioma, there has been widespread adoption of the 2000 and 2007 WHO nomenclature and grading standards, with a notable increase in the diagnosis of atypical meningioma. Before 2000, ∼90% of meningiomas were considered benign (WHO grade I), ∼5% atypical (grade II), and <5% anaplastic or malignant (grade III).2 There has since been a substantial increase in the diagnosis of grade II meningiomas, an attendant decrease in grade I, and a slight reduction in the frequency of grade III meningiomas. In one series, the fraction of grade II meningiomas reached as high as 35%, although this is not typical of most centers.3 Tight statistical associations between 2000/2007 WHO-grade designations and patient outcomes have been independently validated in a number of published meningioma series.4–8
Given a change of practice of this magnitude and its important implications for patient management, the NRG Oncology Radiation Therapy Oncology Group (RTOG) embarked on a phase II trial (0539) of observation for low-risk, and postoperative radiation therapy (RT) for intermediate- and high-risk meningiomas (risk group criteria defined in the Materials and Methods section). The trial opened in 2009 and completed accrual to all risk groups in 2012. An important secondary endpoint in the protocol was the evaluation of histopathologic concordance between enrolling institution and central review. For this report, we determined interobserver concordance and considered how this might direct future endeavors to improve the diagnosis and treatment of patients with meningioma.
Material and Methods
NRG Oncology RTOG 0539 was a trial entitled “Phase II trial of observation for low-risk meningioma, and of radiotherapy for intermediate- and high-risk meningioma.” Low-risk (group 1) was defined as a WHO grade I following either gross total resection (GTR, Simpson grade I-III) or subtotal resection (STR, Simpson grade IV, V), and these patients were prospectively observed, with tissue collected for future molecular analysis. Intermediate-risk (group 2) included recurrent WHO grade I irrespective of resection extent or WHO grade II with GTR, and these patients received postoperative fractionated external beam radiation therapy (EBRT), 54 Gy in 30 fractions. The high-risk (group 3) category included new WHO grade II with STR, any WHO grade III, or any recurrent WHO grade II, and these patients received EBRT, 60 Gy in 30 fractions.
The primary study endpoint was to estimate the rates of progression-free survival (PFS) at 3 years in each of the patient risk groups. The leading secondary endpoint was to study the concordance, or lack thereof, between central and parent institution histopathologic diagnosis, grading, and subtyping. The remaining secondary objectives were to appraise microscopic, immunohistochemical, microarray (mRNA expression profiling), molecular, and imaging correlates; to estimate the incidence of RTOG grade 2 or more acute and late effects; to evaluate adherence to protocol-specific target and normal tissue EBRT parameters; and to report the rates of overall survival (OS) at 3 years.
For each patient, a P4 form (Fig. 1) was completed by both the enrolling institution pathologist and the central review pathologist. Pathology specimens were evaluated for each patient initially by the enrolling institutions' neuropathologists. Form P4 included subtyping and assignment of grade. Please refer to the footer of the P4 form (Fig. 1) for details about the instructions provided for histopathologic review. Slides were then sent for central review to a single expert neuropathologist (AP), who performed the same functions prior to final protocol enrollment. Central review was independent of the institutional assessment. Final study group assignment and treatment were based upon central pathology review. It was a strict requirement for study eligibility that all H&E slides from the enrolling institution be submitted for central pathology review and that these include at least 1 H&E section from each block.
Fig. 1.
RTOG P4 Form for Parent Institution Pathology and Central Pathology Review.
Concordance rates for overall tumor grade, histopathologic subtype, high-grade variant histology, and individual grading criteria were assessed using the kappa (κ) coefficient for each evaluable P4 input.9 Kappa values from 0.01 to 0.20 are considered representative of slight agreement, 0.21 to 0.40 fair agreement, 0.41 to 0.60 moderate agreement, 0.61 to 0.80 substantial agreement, and >0.80 almost perfect agreement.9
This cooperative group protocol was approved by the institutional review boards at each participating study site, and documentation was received at the RTOG (now NRG Oncology) central office. Each patient signed an approved informed consent prior to trial enrollment.
Results
A total of 244 participants were initially enrolled to NRG Oncology RTOG-0539. The trial closed after reaching its accrual goals: group 1 (low-risk) September 29, 2010; group 2 (intermediate-risk) May 12, 2011; and group 3 (high-risk) August 24, 2012. Of the 244 participants who entered step I enrollment, 223 Pathology Review Forms (P4, Fig. 1) on 221 patients were submitted (2 patients had 2 P4 forms, each from 2 separate operative procedures). One hundred seventy-eight participants continued to step 2 with central pathology review. There were thus 66 participants enrolled on step 1 but not registered to step 2. This occurred due to patient refusal (n = 18), study closure prior to step 2 registration (n = 11), issues relating to pathology or tissue submission (n = 9), problems with eligibility (n = 7), timeline constraints (n = 6), physician preference not otherwise specified (NOS, n = 4), treatment started before central pathology review (n = 2), disease progression (n = 1), difficulty with comorbid illness (n = 1), and other factor NOS (n = 7). Eight of these 178 participants were ultimately deemed protocol ineligible, owing to no protocol treatment (n = 2), registration to the incorrect arm (n = 2), central pathology (although indeed completed) not done prior to step 2 registration (n = 1), extracranial meningioma present (n = 1), multiple meningiomas present (n = 1), and treatment started prior to central review (n = 1). This resulted in 170 participants with 172 completed pathology reviews, who were otherwise fully protocol eligible. Pretreatment patient characteristics of the 170 eligible participants are shown in Supplementary material, Table S1.
The diagnosis of meningioma, confirmed on central review, was required for protocol eligibility. In order to determine the concordance for the diagnosis of meningioma, all submitted P4 forms were examined (n = 223). Forms with incomplete site review (n = 5) were excluded. The 2 patients with tumor other than meningioma after central review did not proceed to step 2. This resulted in 216 participants and 218 P4 forms for concordance measures regardless of eligibility status.
Concordance between reviewers was measured using completed P4 forms (Fig. 1). Concordance for the diagnosis of meningioma itself was 99.1% (216 of 218 P4 forms), with 2 cases excluded on central review for alternate diagnostic considerations of gliosarcoma and an inflammatory process. Overall concordance for tumor grade was 87.2%. Each instance of grade discordance was by a single WHO grade, except for one case in which a meningioma graded as WHO I at the enrolling institution was diagnosed as WHO III upon central review. This discordance owed largely to mitotic index.
Table 1 documents the rates of concordance and discordance for individual WHO grades and by the 9 defined aggressive features defined in the P4 pathology form (Fig. 1). With benign meningioma, we encountered 93.0% concordance, atypical 87.8%, and anaplastic 93.6%. Table 2 examines this further for rates of agreement and disagreement between parent and central pathology grading, with the applicable kappa statistic. These fit into the “substantial agreement” range for WHO grades II and III and “almost perfect agreement” for benign meningioma. Twenty-two cases were reclassified based on central review. Most (40.9%) were reported by the institution as WHO grade I and reclassified as WHO grade II. There was one case in which the institutional and central reviewers differed by more than one WHO grade. In this case, an institution reported WHO grade I meningioma that was reclassified as grade III. Ten participants classified as WHO grade II by the institution were reclassified: 2 to WHO grade I and 8 to WHO grade III. Two participants graded as WHO III by the enrolling institution were reclassified to WHO II.
Table 1.
World Health Organization grades
| Concordance Rate | Discordance Rate | |
|---|---|---|
| WHO grade | ||
| Benign (WHO I) | 93.0% | 7.0% |
| Atypical (WHO II) | 87.8% | 12.2% |
| Anaplastic (WHO III) | 93.6% | 6.4% |
| Aggressive features | ||
| ≥4 mitoses/10 HPF | 79.1% | 20.9% |
| ≥20 mitoses/10 HPF | 95.3% | 4.7% |
| Brain invasion | 92.4% | 7.6% |
| Sheeting | 74.4% | 25.6% |
| Small cells | 79.1% | 20.9% |
| Macronucleoli | 76.7% | 23.3% |
| Hypercellularity | 73.3% | 26.7% |
| Spontaneous necrosis | 85.5% | 14.5% |
| Anaplasia | 93.6% | 6.4% |
Abbreviation: HPF, high-powered field.
Percentage concordance and discordance by WHO grade and by predefined aggressive features (WHO 2007 criteria).
Table 2.
World Health Organization meningioma grade concordance
| Parent Institution WHO Grade | Central Review WHO Grade |
Kappa Statistic | |
|---|---|---|---|
| Yes | No | ||
| Benign (WHO I) | n = 76 | n = 96 | 0.84a |
| Yes (n = 84) | 74 (88.1%) | 10 (11.9%) | (CI: 0.76–0.92) |
| No (n = 88) | 2 (2.3%) | 86 (97.7%) | |
| Atypical (WHO II) | n = 71 | n = 101 | 0.72a |
| Yes (n = 70) | 60 (85.7%) | 10 (14.3%) | (CI: 0.62–0.83) |
| No (n = 102) | 11 (10.8%) | 91 (89.2%) | |
| Anaplastic (WHO III) | n = 25 | n = 147 | 0.71a |
| Yes (n = 18) | 16 (88.9%) | 2 (11.1%) | (CI: 0.55–0.87) |
| No (n = 154) | 9 (5.8%) | 145 (94.2%) | |
Abbreviation: CI, confidence interval.
Numeric and percentage agreement in WHO grade between institution and central review among 172 pathology specimens in 170 patients, with the applicable kappa value and confidence interval. The emboldened percentages emphasize agreement between institution and central grading.
P value < .0001.
In 22 cases, the WHO grade was reclassified following central review. The most common discordance involved grade II meningioma and related to either the identification of mitoses or atypical features. Nine cases were reclassified from WHO grade I to II after central review. In 4 of the 9 cases, the site identified fewer than 4 mitoses per 10 high power fields (HPFs), whereas central review found >4. In 3 cases the site pathologist identified no atypia, while central review scored 3 atypical features. The most common of these was necrosis (found in all 3 cases), followed by sheeting or small cells (found in 2 cases each); macronucleoli and hypercellularity were each recognized in one case. For one patient, the site noted focal chordoid features, whereas diffuse chordoid was found upon central review. In the final grade I to grade II reclassification case, both site and central review identified brain invasion, although the site nevertheless finalized the meningioma as WHO grade I.
Eight cases were reclassified from WHO grade II to III following central review. Six of these were related to the central-review recognition of anaplasia, 2 to number of mitoses (≥20 per 10 HPFs), and one with both findings upon central review. Two cases read as WHO grade II after site review were finalized as grade I centrally, and 2 recorded as WHO grade III by the site were changed to grade II centrally. An additional single case read as grade I was reclassified as WHO grade III centrally.
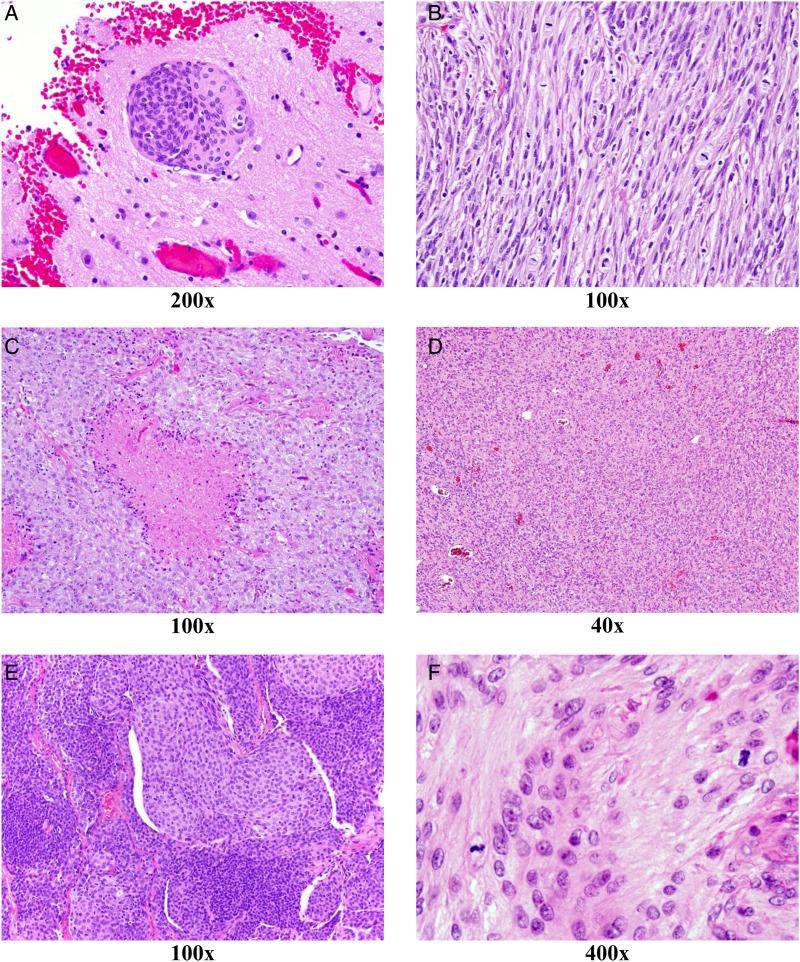
Table 3 describes numeric and percentage agreement between institution and central pathology, with κ values and confidence intervals for aggressive features and histologic variants. Kappa values were, in descending order, within the substantial agreement range for brain invasion, >20 mitoses per 10 high power fields (HPF), and spontaneous necrosis. Agreement was moderate for (in descending order) anaplasia, ≥4 mitoses per 10 HPF, macronucleoli, hypercellularity, and sheeting, while small cells had fair agreement. The lowest levels of agreement (kappa < 0.50) were encountered for macronucleoli, hypercellularity, sheeting, and small cells. Representative light microscopic images showing the 3 aggressive features with the highest kappa values, as well as the 3 with the lowest kappa values, are displayed in Fig. 2.
Table 3.
Aggressive features and histologic variants
| Parent Institution Review | Central Review |
Kappa Statistic (95% CI) | |
|---|---|---|---|
| Yes | No | ||
| >=4 mitoses/10 HPF | (n = 64) | (n = 108) | 0.51* |
| Yes | 33 (86.8%) | 5 (13.2%) | (0.38–0.64) |
| No | 31 (23.1%) | 103 (76.9%) | |
| >=20 mitoses/10 HPF | (n = 17) | (n = 155) | 0.67* |
| Yes | 9 (100.0%) | 0 (0.0%) | (0.46–0.88) |
| No | 8 (4.9%) | 155 (95.1%) | |
| Brain invasion | (n = 36) | (n = 136) | 0.76* |
| Yes | 27 (87.1%) | 4 (12.9%) | (0.64–0.88) |
| No | 9 (6.4%) | 132 (93.6%) | |
| Sheeting | (n = 72) | (n = 100) | 0.44* |
| Yes | 36 (81.8%) | 8 (18.2%) | (0.31–0.58) |
| No | 36 (28.1%) | 92 (71.9%) | |
| Small cells | (n = 48) | (n = 124) | 0.39* |
| Yes | 18 (75.0%) | 6 (25.0%) | (0.23–0.54) |
| No | 30 (20.3%) | 118 (79.7%) | |
| Macronucleoli | (n = 71) | (n = 101) | 0.49* |
| Yes | 36 (87.8%) | 5 (12.2%) | (0.36–0.62) |
| No | 35 (26.7%) | 96 (73.3%) | |
| Hypercellularity | (n = 82) | (n = 90) | 0.45* |
| Yes | 41 (89.1%) | 5 (10.9%) | (0.33–0.58) |
| No | 41 (32.5%) | 85 (67.5%) | |
| Spontaneous necrosis | (n = 59) | (n = 113) | 0.66* |
| Yes | 41 (85.4%) | 7 (14.6%) | (0.54–0.78) |
| No | 18 (14.5%) | 106 (85.5%) | |
| Anaplasia | (n = 16) | (n = 156) | 0.53* |
| Yes | 7 (77.8%) | 2 (22.2%) | (0.29–0.77) |
| No | 9 (5.5%) | 154 (94.5%) | |
| Chordoid - focal & diffuse | (n = 16) | (n = 156) | 0.70* |
| Yes | 9 (100.0%) | 0 (0.0%) | (0.49–0.91) |
| No | 7 (4.3%) | 156 (95.7%) | |
| Chordoid - focal | (n = 12) | (n = 160) | 0.39* |
| Yes | 4 (57.1%) | 3 (42.9%) | (0.10–0.68) |
| No | 8 (4.8%) | 157 (95.2%) | |
| Chordoid - diffuse | (n = 4) | (n = 168) | 0.32* |
| Yes | 1 (50.0%) | 1 (50.0%) | (0.00–0.81) |
| No | 3 (1.8%) | 167 (98.2%) | |
| Clear cell - focal & diffuse | (n = 9) | (n = 163) | 0.55* |
| Yes | 4 (80.0%) | 1 (20.0%) | (0.24–0.87) |
| No | 5 (3.0%) | 162 (97.0%) | |
| Clear cell - focal | (n = 9) | (n = 163) | 0.17 |
| Yes | 1 (50.0%) | 1 (50.0%) | (0.00–0.47) |
| No | 8 (4.7%) | 162 (95.3%) | |
| Clear cell - diffuse | (n = 0) | (n = 172) | N/A |
| Yes | 0 (0.0%) | 3 (100.0%) | |
| No | 0 (0.0%) | 169 (100.0%) | |
| Papillary - focal & diffuse | (n = 13) | (n = 159) | 0.36* |
| Yes | 3 (100.0%) | 0 (0.0%) | (0.15–0.65) |
| No | 10 (5.9%) | 159 (94.1%) | |
| Papillary - focal | (n = 11) | (n = 161) | 0.16 |
| Yes | 1 (100.0%) | 0 (0.0%) | (0.00–0.43) |
| No | 10 (5.8%) | 161 (94.2%) | |
| Papillary - diffuse | (n = 2) | (n = 170) | 0.49* |
| Yes | 1 (50.0%) | 1 (50.0%) | (0.00–1.00) |
| No | 1 (0.6%) | 169 (99.4%) | |
| Rhabdoid - focal & diffuse | (n = 13) | (n = 159) | 0.36* |
| Yes | 3 (100.0%) | 0 (0.0%) | (0.06–0.65) |
| No | 10 (5.9%) | 159 (94.1%) | |
| Rhabdoid - focal | (n = 11) | (n = 161) | 0.29* |
| Yes | 2 (100.0%) | 0 (0.0%) | (0.00–0.61) |
| No | 9 (5.3%) | 161 (94.7%) | |
| Rhabdoid - diffuse | (n = 2) | (n = 170) | 0.66* |
| Yes | 1 (100.0%) | 0 (0.0%) | (0.05–1.00) |
| No | 1 (0.6%) | 170 (99.4%) | |
Abbreviations: CI, confidence interval; HPF, high-powered fields.
Numeric and percentage agreement, with the applicable kappa value and confidence interval, according to WHO aggressive features and histologic variants from institution versus central pathology review.
P-value < .0001.
Fig. 2.
Representative light microscopy images showing the 3 aggressive features with the highest kappa values as well as the 3 with the lowest kappa values. The number below each image expresses the magnification factor. Grading criteria with highest agreement (κ > 0.68) between local and central pathology reviews included brain invasion (A), markedly elevated mitotic index (>20/10 HPF; B), and spontaneous necrosis (C). Grading criteria with the lowest agreement (κ < 0.50) between local and central pathology reviews included sheeting (D), small cells/hypercellularity (E), and focal increase in mitoses (≥4/10 HPF; F).
A breakdown by aggressive variant histology is also found in Table 3. This documents less concordance than found with the assignment of WHO grade or the determination of aggressive features. Diffuse rhabdoid histology was identified within the substantial agreement range, whereas diffuse papillary features fell within the moderate agreement kappa range. Focal chordoid, diffuse chordoid, and focal rhabdoid found fair agreement. Focal clear cell and focal papillary histology found slight agreement between parent and central pathology review, and there was no agreement for the 3 cases (1.4%) assigned diffuse clear cell features at parent institution review.
Discussion
Harvey Cushing, describing tumors that originate from the meningeal coverings of the brain and spinal cord, first coined the term “meningioma” in a 1922 edition of the journal Brain.10 His description has proved clinically useful and durable, although we have since made considerable progress in the characterization of meningioma grading and histology. These improvements are of pivotal importance for patients and clinicians since histopathologic findings, and in particular tumor grade, play key roles in therapeutic decision-making.
Since the initial descriptions of meningioma, it has been appreciated that these tumors display a wide range of histologic appearance and clinical behavior. The cell of origin for meningioma is presumed to be the arachnoidal cap cell, which has a complex embryological origin with mixed mesenchymal and epithelial features originating from both mesoderm and neural crest.11 Meningiomas thus exhibit a broad range of histologic patterns and cellular features. Reflecting this, there are multiple variants of meningioma, many of which have no known prognostic impact beyond WHO grade, but others of which portend more aggressive behavior. Chordoid and clear-cell variants are more aggressive and are classified as WHO grade II, even in the absence of atypical histologic features. Papillary and rhabdoid meningiomas are WHO grade III.
Many attempts, most notably by the WHO, have been made to systematically categorize meningiomas and to characterize the predominant microscopic appearance, the likelihood of progression or recurrence, and the need for initial or adjuvant treatment. Notable strides have been made in these regards with the current (2007) WHO grading system, and inroads toward improved understanding of molecular genetics and individualized outcome predictors are ongoing. However, at present we rely heavily upon light microscopic findings, extent of resection, and recurrence status to guide patient management.
In 1979, the WHO first categorized meningiomas into meningotheliomatous, fibrous, transitional, psammomatous, angiomatous, hemangioblastic, hemangiopericytotic, papillary, and anaplastic subtypes.12 This simple system recognized anaplastic meningioma but did not distinguish an intermediate prognosis atypical variant. The WHO updated its nomenclature and histologic subtyping in 1993, with the assignment of hemangiopericytoma to a nonmeningothelial category and the introduction of atypical meningioma as an intermediate grade. These were important steps conceptually, but the 1993 version offered only vague grading criteria and, as was also the case with the 1979 scheme, was not widely implemented. However, in 2000 the WHO fashioned guidelines based upon clinicopathologic correlation from two Mayo Clinic series, which were more objective and reproducible, and these gradually became the standard. In 2007 the WHO revised the 2000 criteria only slightly, defining brain invasion as a criterion for grade II. While seemingly a modest change, this has resulted in a further increase of meningiomas assigned to the grade II category.11,13
Perry et al, whose work became the impetus for the WHO 2000 and 2007 systems, reported 643 meningioma cases and identified a benign group with 5-year PFS of 88%, an atypical group with 5-year PFS of 59%, and an anaplastic cohort with 5-year PFS of 28%.14,15 In these large series, patients with benign (WHO grade I) meningioma comprised 72.1% of the total, atypical (WHO grade II) 24.3%, and anaplastic (WHO grade III) 3.6%. This increase in identification of grade II histology and the increased risk of recurrence and/or death with higher-grade meningioma have been confirmed in other single institution series.3,11,16–18 Willis et al from Scotland in 2005 regraded 314 cases according to WHO 2000 criteria and identified 20.4% grade II. They also reported that 38% of grade I patients diagnosed per WHO 1993 were reclassified as grade II.16 Pearson et al in 2008 found that the incidence of grade II histology rose from 4.4% between 1994 and 1999 up to 35% in 2006.3 Similarly, Backer-Grøndahl et al reviewed 196 consecutively treated patients in Norway and found that 18% were WHO grade II according to pre-2000 WHO, 26% per WHO 2000, and 30% per 2007 criteria. The increase from 2000 to 2007 occurred exclusively due to 9 otherwise grade I patients who had brain invasion. WHO grade III meningiomas remained relatively stable at 1%–2%.11
Using 2000 and 2007 WHO grading, significant differences in both disease-free survival and OS have been identified.19 Compared with benign meningioma, atypical and anaplastic tumors carry a 5- to 10-fold increased risk of recurrence at 5 years. Additionally, rates of OS in patients with atypical or anaplastic meningioma are, respectively, 3-fold and 10-fold inferior to their benign counterparts, and patients with WHO grade III tumors rarely survive 10 years.19 With changes of this magnitude in both grading and outcome, correct characterization has far-reaching consequences. However, it has not been clear to what extent the new WHO standards have been implemented. With this understanding, NRG Oncology RTOG 0539 was designed, among other important endpoints, to assess the interobserver and interinstitutional concordance of meningioma classification and grading by comparing light microscopic assessments of grade, variant histology, and aggressive features between parent-institution and central-review pathology.
We found a concordance rate of 93.0% for WHO grade I, 87.8% for WHO grade II, and 93.6% for WHO grade III. This compares favorably with published concordance rates for sarcoma, a soft tissue neoplasm for which subtyping and grading play critical roles. Lurkin et al20 completed a prospective study of 366 sarcoma patients from the Rhône-Alpes region of France, systematically comparing initial histologic type and grade with second-opinion findings by a regional or national expert. They identified 54% full concordance (histologic type and grade), 27% partial concordance (different histologic type or grade), and 19% discordance. Most discrepancies were with grade (19%). Ray-Coquard et al21 published a similar study within 3 European regions and analyzed 1463 patients. Full concordance was found in 56%, partial concordance in 35%, and discordance in 8%. The most common discrepancies were with histologic grade (43%).
This also compares favorably with studies assessing interobserver concordance with gliomas (Table 4). A single study by Scott et al, which also reported findings from an RTOG trial (8302), described 96% concordance for glioblastoma.22 This is similar to the current study's concordance of 99% for the diagnosis of meningioma in general. Among the remaining studies with more than one glioma grade represented, however, concordance has been considerably less impressive and covered a wide range from 8% to 88%, depending in part upon subspecialization among reviewing pathologists, academic versus community hospitals, and the complexity of microscopic findings (e.g. oligoastrocytoma vs astrocytoma) but tended to be inferior to the concordance figures found in the present trial with meningioma.
Table 4.
Glioma concordance studies compared with current meningioma trial (NRG Oncology RTOG-0539)
| Reference | Tumor Cohort | Reviewers | Classification Scheme(s) | Concordance | Kappa |
|---|---|---|---|---|---|
| Scott 1995 RTOG 8302 | 680 AA and GBM | North American SP/NP vs central NP review | WHO 1993 | 96% for GBM, 66% for AA | 0.67 |
| Coons 199726 | 244 (mostly) gliomas reviewed over 4 sessionsa | 4 NP | WHO 1979; St. Anne-Mayob | 1st set: 52% for 4/4 or 60% for 3/4 NP; 4th set: 69% for 4/4 or 75% for 3/4 NP | 1st: 0.66 2nd: 0.71 3rd: 0.76 4th: 0.82 |
| Aldape 2000 San Francisco (SF) Adult Glioma Study27 | 457 (mostly) diffuse gliomas | Local SP/NP (SF Bay area) vs UCSF central NP review | WHO 1993 | 77% overall; 88% with academic centers vs 74% with community hospitals (P = 0.004) | NR |
| Prayson 200028 | 24 A, AA, and GBM; 6 nonneoplastic | 5 NP and 5 SP | Modified Ringertz | 86.7% for 4/5 NP; 43% for 4/5 SP |
0.63 (NP); 0.36 (SP) |
| Kros 2007 EORTC 2695129 |
150 AO and AOA | European SP/NP vs consensus (6 of 9) central NP review | WHO 2000 | 52% for AO; 8% for AOA; 86% for AO among NP panel | Dx of AO: 0.46–0.72 (NP 1–9); 0.18 (SP) |
| Hildebrand 2008 EORTC 2688230 |
176 AA pre-CR; 61 AA post-CR | European SP/NP vs EORTC NP reviewer | WHO (presumably 1993 and 2000) | 35% | NR |
| Giannini 2008 RTOG 940231 |
247 AO and AOA | 2 central NP reviewers | WHO (presumably 2000 and 2007) | 78% | 0.55 |
| Wick 2009 NOA-0432 |
318 AA, AO, and AOA | German NP vs NOA central reviewers | WHO (1993 and 2000) | NR | 0.70 |
| Current Study RTOG 0539 | 218 total patients 172 path reviews (see results section) |
North American SP/NP vs central NP review | WHO 2007 | 99.1% for mening diagnosis; 86.0% for WHO grade | 0.69 (grade) |
Abbreviations: AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; CR, central review; Dx, diagnosis; GBM, glioblastoma; mening, meningioma; NP, neuropathologist; NR, not reported; path, pathology; SP, general surgical pathologist or local pathologist in a clinical trial; vs, versus.
Study revealed improved concordance after each set following group discussions of grading criteria.
The St. Anne-Mayo grading scheme formed the basis for the WHO grading of diffuse astrocytomas in 1993 and later versions of the WHO.
Although we have found the diagnosis of meningioma itself to be reliable (99.1% concordance with central review), this became somewhat less impressive when stratifying by WHO grade or individual histologic features and variants. Concordance rates by grade were 93.0% for WHO grade I, 87.8% for WHO grade II, and 93.6% for grade III. The lower rate for grade II was anticipated, given the complexities of the diagnostic criteria including chordoid or clear cell subtype, 4–19 mitoses per 10 HPFs, brain infiltration, or 3 or more of the following features: sheeting, small cells, macronucleoli, hypercellularity, and spontaneous necrosis.
Kappa statistics for various chordoid and rhabdoid findings revealed that agreement was moderate, fair, or slight. There was substantial agreement with brain invasion, >20 mitoses per 10 HPF, and spontaneous necrosis, but kappa values for all other atypical histologic features were either fair or moderate. A report from Trondheim University in Norway reached similar conclusions. They found that, although many of the criteria for ≥ grade II are mutually correlated, many are also subjective, including the number of mitotic figures that can be difficult to detect due to the instability of mitotic figures during fixation, pyknotic cells, regional variation in mitoses, and cellularity within meningioma specimens.11 Additionally, it is well known that there is great variability among pathologists regarding diligence and time spent searching for mitotic figures. The authors also found sheeting to be a challenging criterion, on occasion coexisting with other processes such as inflammatory infiltrates, blood vessels, or regions with syncytial architecture mimicking sheet-like growth. Small cell areas were considered difficult to interpret in whorled and hypercellular areas. Macronucleoli, according to the WHO criteria alone, were felt to be ambiguous, although a subsequent description by Perry and Brat, counting only nucleoli easily observed at 10x, was found helpful.23
The WHO 2000 and 2007 criteria are more objective, reliably applicable, and clinically predictive than previous schemes,6,11 but there remains considerable interobserver variability and subjectivity, for which repeated assessments and redefinitions may be required. At present, meningioma-grading criteria are based upon light microscopy alone. However, biologic behavior is incompletely accounted for by routine histopathology. It is expected that biochemical, molecular, and chromosomal correlates will be identified to provide more powerful and reproducible prognostic markers.24,25 Further molecular work is needed in these respects and is being undertaken by many investigators. Biomarker studies were also incorporated into the present trial (NRG Oncology RTOG 0539) with analyses planned for future publication.
Conclusion
Although limited by modest numbers and scant community hospital involvement, our data suggest that the current meningioma classification and grading criteria are at least as objective, if not more objective, than that utilized for gliomas. Despite the substantial agreement achieved for overall meningioma grade, the utility of the current scheme is limited by subjectivities in the component elements of grading.
Further improvements in interobserver concordance will likely require educational efforts, clarification of subjective morphologic criteria, and identification of objective molecular biomarkers that more accurately predict biology. The identification of such markers is a secondary endpoint in this RTOG phase II trial, and we expect a report of this once the data have matured.
Funding
This project was supported by grants U10CA21661, U10CA180868, U10CA180822 from the National Cancer Institute (NCI), and by a grant from the 2009 American Recovery and Reinvestment Act (ARRA) economic stimulus plan, administered through the CTEP Accelerating Clinical Trials of Novel Oncologic PathWays (ACTNOW) initiative.
Conflict of interest statement. Igor Barani has received speaking honoraria and research funding from BrainLab, Inc. and has received research funding from Elekta, Inc., and NIH outside the submitted work. Samuel T. Chao has received speaking honoraria from Varian Medical Systems outside the submitted work. Minesh Mehta has a leadership role with Pharmacyclics, stock or ownership interest in Pharmacyclics, consulting or advisory roles with Cavion, Elekta, Novartis, and Novocure and has received research funding from Novocure and Novellos outside the submitted work.
Supplementary Material
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16: iv1–iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–1095. [DOI] [PubMed] [Google Scholar]
- 3.Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: Evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24(5):E3. [DOI] [PubMed] [Google Scholar]
- 4.Ho DM, Hsu CY, Ting LT, Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002;94(5):1538–1547. [DOI] [PubMed] [Google Scholar]
- 5.Korshunov A, Shishkina L, Golanov A. Immunohistochemical analysis of p16INK4a, p14ARF, p18INK4c, p21CIP1, p27KIP1 and p73 expression in 271 meningiomas correlation with tumor grade and clinical outcome. Int J Cancer. 2003;104(6):728–734. [DOI] [PubMed] [Google Scholar]
- 6.Combs SE, Schulz-Ertner D, Debus J, von Deimling A, Hartmann C. Improved correlation of the neuropathologic classification according to adapted World Health Organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int J Radiat Oncol Biol Phys. 2011;81(5):1415–1421. [DOI] [PubMed] [Google Scholar]
- 7.Domingues PH, Sousa P, Otero Á, et al. Proposal for a new risk stratification classification for meningioma based on patient age, WHO tumor grade, size, localization, and karyotype. Neuro Oncol. 2014;16(5):735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olar A, Wani KM, Sulman EP, et al. Mitotic Index is an Independent Predictor of Recurrence-Free Survival in Meningioma. Brain Pathol. 2015;25(3):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 10.Cushing H. The meningiomas (dural endotheliomas). Their source and favoured seats of origin. Brain. 1922;45:282–316. [Google Scholar]
- 11.Backer-Grøndahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012;5(3):231–242. [PMC free article] [PubMed] [Google Scholar]
- 12.Scheithauer BW. Development of the WHO classification of tumors of the central nervous system: a historical perspective. Brain Pathol. 2009;19(4):551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanft S, Canoll P, Bruce JN. A review of malignant meningiomas: diagnosis, characteristics, and treatment. J Neurooncol. 2010;99:433–443. [DOI] [PubMed] [Google Scholar]
- 14.Perry A, Stafford SL, Scheithauer BW, et al. Meningioma grading: an analysis of histologic parameters. Amer J Surg Pathol. 1997;21:1455–1465. [DOI] [PubMed] [Google Scholar]
- 15.Perry A, Scheithauer BW, Stafford SL, et al. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients. Cancer. 1999;85:2046–2056. [DOI] [PubMed] [Google Scholar]
- 16.Willis J, Smith C, Ironside JW, Erridge S, Whittle IR, Everington D. The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141–149. [DOI] [PubMed] [Google Scholar]
- 17.Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53–60. [DOI] [PubMed] [Google Scholar]
- 18.Kaur G, Sayegh ET, Larson A, et al. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol. 2014;16(5):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry A. Meningiomas. In: McLendon R, Rosenblum M, Bigner DD, eds. Russell & Rubinstein's Pathology of Tumors of the Nervous System. 7th ed London, England: Hodder Arnold; 2006:427–474. [Google Scholar]
- 20.Lurkin A, Ducimetiére F, Vince DR, et al. Epidemiological evaluation of concordance between initial diagnosis and central pathology review in a comprehensive and prospective series of sarcoma patients in the Rhone-Alpres region. BMC Cancer. 2010;10:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray-Coquard I, Montesco MC, Coindre JM, et al. Sarcoma: concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann Oncol. 2012;23(9):2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott CB, Nelson JS, Farnan NC, et al. Central pathology review in clinical trials for patients with malignant glioma. A Report of Radiation Therapy Oncology Group 83–02. Cancer. 1995;76:307–313. [DOI] [PubMed] [Google Scholar]
- 23.Perry A, Brat DJ. Meningiomas. In: Perry A, Brat DJ, eds. Practical Surgical Neuropathology: A Diagnostic Approach. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. [Google Scholar]
- 24.Lee Y, Liu J, Patel S, et al. Genomic landscape of meningiomas. Brain Pathol. 2010;20(4):751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen R, Lee J. Predicting outcomes of patients with intracranial meningiomas using molecular markers of hypoxia, vascularity, and proliferation. Neurosurgery. 2012;71(1):146–156. [DOI] [PubMed] [Google Scholar]
- 26.Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. [DOI] [PubMed] [Google Scholar]
- 27.Aldape K, Simmons ML, Davis RL, et al. Discrepancies in diagnoses of neuroepithelial neoplasms: the San Francisco Bay Area Adult Glioma Study. Cancer. 2000;88:2342–2349. [PubMed] [Google Scholar]
- 28.Prayson RA, Agamanolis DP, Cohen ML, et al. Interobserver reproducibility among neuropathologists and surgical pathologists in fibrillary astrocytoma grading. J Neurol Sci. 2000;175:33–39. [DOI] [PubMed] [Google Scholar]
- 29.Kros JM, Gorlia T, Kouwenhoven MC, et al. Panel review of anaplastic oligodendroglioma from European Organization For Research and Treatment of Cancer Trial 26951: assessment of consensus in diagnosis, influence of 1p/19q loss, and correlations with outcome. J Neuropathol Exp Neurol. 2007;66:545–551. [DOI] [PubMed] [Google Scholar]
- 30.Hildebrand J, Gorlia T, Kros JM, et al. Adjuvant dibromodulcitol and BCNU chemotherapy in anaplastic astrocytoma: results of a randomised European Organisation for Research and Treatment of Cancer phase III study (EORTC study 26882). Eur J Cancer. 2008;44:1210–1216. [DOI] [PubMed] [Google Scholar]
- 31.Giannini C, Burger PC, Berkey BA, et al. Anaplastic oligodendroglial tumors: refining the correlation among histopathology, 1p 19q deletion and clinical outcome in Intergroup Radiation Therapy Oncology Group Trial 9402. Brain Pathol. 2008;18:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.