Abstract
Background
Primary intraocular lymphoma is a rare variant of primary CNS lymphoma for which the optimum treatment strategy remains unknown.
Methods
We performed a retrospective single-center study including patients who underwent uniform management from October 2007 in which patients were offered sequential rituximab, methotrexate, procarbazine, and vincristine (R-MPV) followed by binocular radiotherapy and consolidative high-dose cytarabine.
Results
Eleven patients with median age 66 years (range, 48–72) were included. All patients received binocular radiotherapy to a median dose of 36 Gy (range, 30.6–39.6) in 20 fractions. Grade 3+ anemia, thrombocytopenia, and neutropenia occurred in 1 (9%), 2 (18%), and 3 (27%) patients, respectively; raised creatinine and peripheral sensory neuropathy occurred in 4 (36%) and 3 (27%) patients, respectively. Grade 3+ ocular toxicities included cataract formation and keratitis in 6 (54%) and 3 (27%) patients, respectively. Ten patients (91%) achieved complete response and 1 (9%) partial response. After median follow-up of 4.2 years (range, 1.8–7.6), the median progression-free survival was 3.8 years and the estimated 4-year overall survival was 75.8% (95% CI: 30.5%–93.7%). The initial site of disease progression was the CNS in 4 of 7 patients (57%) and within the eye in 3 of 7 (43%). Five patients achieved responses to salvage therapies.
Conclusions
Combined modality treatment with R-MPV, binocular radiation, and high-dose cytarabine is effective with moderate toxicity. Both local and CNS relapses occur; however, the achievement of second and subsequent remissions is possible.
Keywords: methotrexate, primary central nervous system lymphoma, primary intraocular lymphoma, radiotherapy, treatment
Primary intraocular lymphoma (PIOL), or vitreoretinal lymphoma, is a rare variant of primary central nervous system lymphoma (PCNSL) in which there is lymphomatous involvement of the retinal, vitreous, or optic nerve.1 Similar to lymphomas arising in other immune privileged sites such as the testis, the typical histologic subtype is diffuse large B-cell lymphoma (DLBCL), and disease relapses are local and/or involve the brain parenchyma.2,3 Due to the rarity of this disease, optimal management remains undefined. From October 2007, at MD Anderson Cancer Center we adopted a uniform management policy in which newly diagnosed patients with PIOL were treated with rituximab, high-dose intravenous methotrexate, procarbazine, and vincristine (R-MPV), sequential binocular radiation, and high-dose ara-C, or cytarabine (HDAC).4 This policy was adopted in an attempt to provide local disease control and mitigate the risk of local and CNS relapse. The purpose of this report is to describe the toxicity and clinical outcomes of this uniform treatment strategy.
Methods
Inclusion Criteria and Diagnostic and Staging Procedures
We identified patients with PIOL treated at our institution between October 1, 2007 and April 1, 2015, using the databases of the Departments of Lymphoma, Radiation Oncology, and Ophthalmology at MD Anderson Cancer Center. Our institutional review board approved the research protocol. All patients underwent pars plana vitrectomy with cytologically confirmed DLBCL and staging procedures including bilateral ophthalmic examination, MRI of the brain,18 F-fluorodeoxyglucose PET-CT from the skull to mid-thighs, lumbar puncture with CSF analysis, and bone marrow biopsy. All patients with biopsy-proven PIOL, without evidence of disease outside the CNS, who were considered suitable for high-dose methotrexate and cytarabine were included in this study. Treatment-related adverse events were recorded by treating clinicians as part of standard of care at the time of occurrence. We retrospectively collected baseline characteristics, treatment details, and clinical outcomes by review of electronic medical records, with details verified by 2 investigators. Adverse events were graded using the Common Terminology Criteria for Adverse Events version 4.03.
Treatment
Patients were offered a treatment strategy consisting of R-MPV (rituximab 375 mg/m2 i.v. day 1, methotrexate 3.5 g/m2 i.v. over 2 h day 1 followed by leucovorin rescue, procarbazine 100 mg/m2/day orally for 7 days with odd cycles only and vincristine 1.4 mg/m2 capped at 2 mg i.v. on day 1) given every 2 weeks for 5 cycles. Binocular radiotherapy (RT) was given a minimum of 2 weeks after the last cycle of methotrexate to a total dose of 30.6–39.6 Gy in 17–22 fractions. At least 1 month after the completion of RT, 2 cycles of rituximab (375 mg/m2 i.v. day 1) and cytarabine (3 g/m2 i.v. over 3 h once daily on days 1 and 2) were administered on 2 occasions, 2 weeks apart, for a total of 7 cycles of chemotherapy. Intrathecal (i.t.) methotrexate was typically administered once per cycle, unless CSF was positive at diagnosis (in which case it was administered twice per week until negative, then weekly). Responses were defined according to an adaptation of published criteria for primary CNS lymphoma.5 The ophthalmic exams were standardized and comprised a slit lamp assessment, dilated ophthalmoscopy with baseline fundus photography, and ocular echography performed by the same ocular oncologist (D.S.G.). Angiography, ocular coherence tomography, and vitreal interleukin-10 levels were not routinely obtained. Complete response (CR) was defined by dilated ophthalmic assessment showing no evidence of vitreal or retinal disease and negative MRI of the orbit and brain, while partial response (PR) was defined by significant improvement in posttreatment ophthalmologic findings (decrease in vitreous cellular and retinal infiltrate, if present) and negative MRI.
Statistical Analysis
Continuous variables were expressed as median and range and compared using the Mann–Whitney U-test. Categorical variables were reported as percentages and compared using the chi-squared test. Progression-free survival (PFS), overall survival (OS), and time to relapse were determined from date of diagnosis using the Kaplan–Meier method. Time to CNS relapse was defined from the date of diagnosis to the date of brain biopsy or lumbar puncture demonstrating lymphoma. Median follow-up was based on observation time of patients alive at date of last data census (April 29, 2015). All statistical analyses were performed using Stata v12.1.
Results
We identified 11 patients with PIOL treated using the combined modality described above; an additional patient (age 90) was considered unsuitable for high-dose chemotherapy and was excluded. The median age was 66 years (range, 48–72), and 9 patients (81%) were female. The most common presenting symptoms (more than one possible per patient) were floaters (n = 8; 73%), blurred vision (n = 6; 55%), and/or visual loss (n = 6; 55%). Ophthalmologic examination findings included vitreous cells/opacity (n = 9; 82%) and/or retinal/choroidal infiltrate (n = 4; 36%). All patients were negative for HIV infection at time of diagnosis. One patient had evidence of leptomeningeal disease with CSF showing cytologic and immunophenotypic evidence of lymphoma; however, the MRI showed no leptomeningeal enhancement or parenchymal lesions. Flow cytometry was performed on the CSF of 8 patients (73%) at diagnosis—positive in 1, negative in 5, and indeterminate in 2.
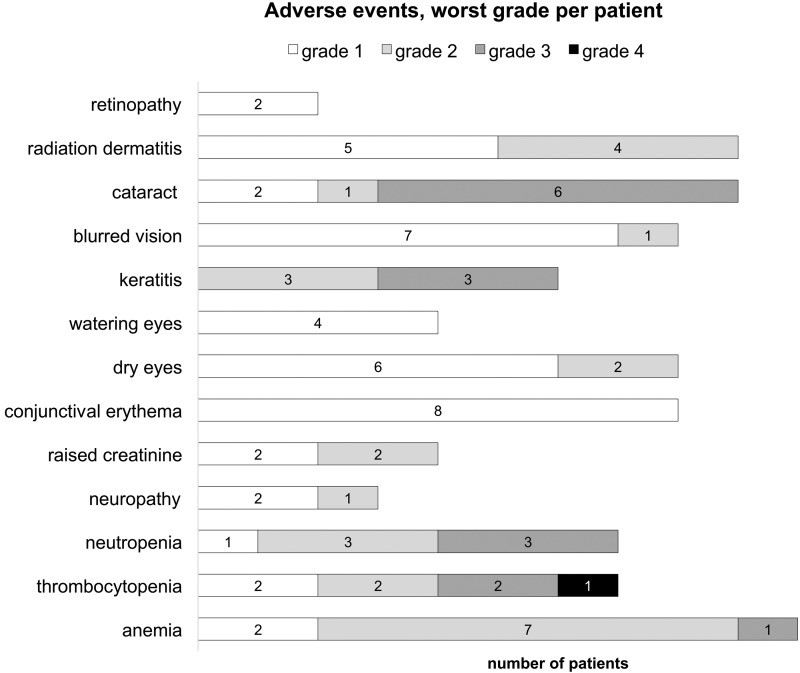
Eight patients (73%) completed all cycles of chemotherapy; 3 had chemotherapy abbreviated due to toxicities after 6, 5, and 4 cycles of chemotherapy. All patients received binocular RT to a median dose of 36 Gy in 20 fractions (range, 30.6–39.6 Gy in 17–22 fractions). RT targeted the bilateral orbits, including the globes and optic nerves. The technique for radiation delivery was opposed tangential 6 megavolt (MV) photon beams (n = 2; 18%), intensity-modulated RT with 6 MV photons (n = 3; 27%), or appositional 12–16 MeV electron beams with custom skin collimation (n = 6; 55%). The radiation dose and technique were selected based on the treating physician′s preference. Intrathecal methotrexate was administered to 10 (91%) patients, who received a median of 3 (range, 1–10) doses via lumbar puncture (dose, 12 mg) with the exception of the patient with positive CSF, in whom i.t. therapy was delivered via an Ommaya reservoir (dose, 6 mg). Two patients (18%) had dose reductions: methotrexate was reduced by 20% because of renal impairment from the preceding cycle in one patient, and vincristine was reduced due to grade 2 sensory neuropathy from cycle 4 onward in the other. Chemotherapy delays occurred in 6 patients (54%) due to methotrexate-related renal impairment (n = 4), thrombocytopenia (n = 1), and radiation keratopathy (n = 1). Early treatment discontinuation occurred in 3 patients. One patient developed renal impairment with cycle 1 of R-MPV and was switched to cytarabine for subsequent cycles. Cycle 3 of cytarabine was complicated by grade 3 facial cellulitis, and subsequent chemotherapy was declined. Another 2 patients developed methotrexate-related renal impairment after the second and fourth cycles, respectively, of therapy, and no further methotrexate was given. Grade 1–2 adverse events occurring in 2 or more patients and all grade ≥3 adverse events are displayed in Fig. 1. The acute, self-limiting side effects of RT were conjunctival erythema (n = 8; 73%), periorbital dermatitis (n = 9; 82%), and tearing (n = 4; 36%). Eight patients (73%) developed xerophthalmia, which typically began late during the course of RT and persisted beyond one year. Two patients (18%) developed retinopathy (both grade 1), and 6 (55%) keratopathy. Three patients underwent cataract surgery prior to their diagnoses; cataracts were diagnosed in 4 others before starting RT; and in the remaining 4, visually significant cataracts were diagnosed during ophthalmologic follow-up after the completion of RT. Among the 9 patients alive at last follow-up, corrected visual acuity had improved compared with baseline in 7 eyes, remained unchanged in 7 eyes, and had deteriorated in 4 eyes (all due to cataract formation, with reversal after cataract extraction).
Fig. 1.
Adverse events (grade 1–2 occurring in more than 2 patients, and all grade ≥3 events) according to Common Terminology Criteria for Adverse Events v4.03. Only the worst grade for each patient is reported.
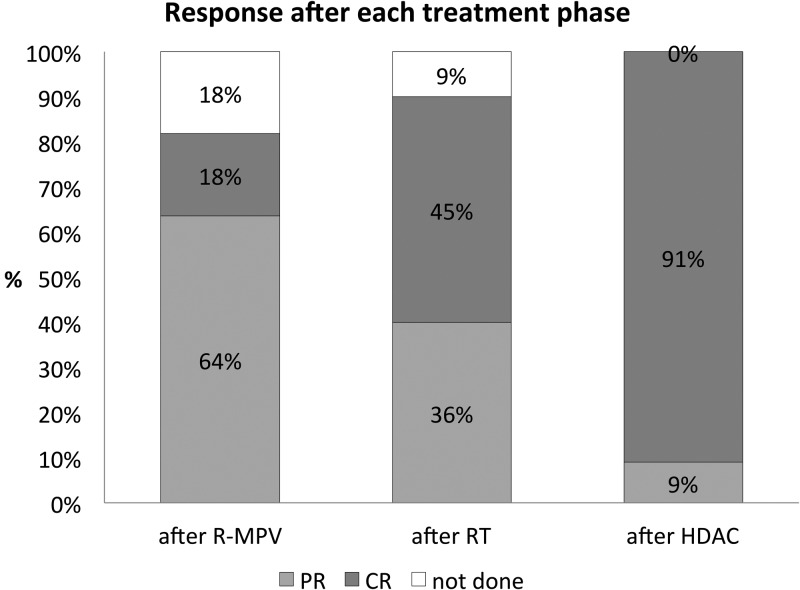
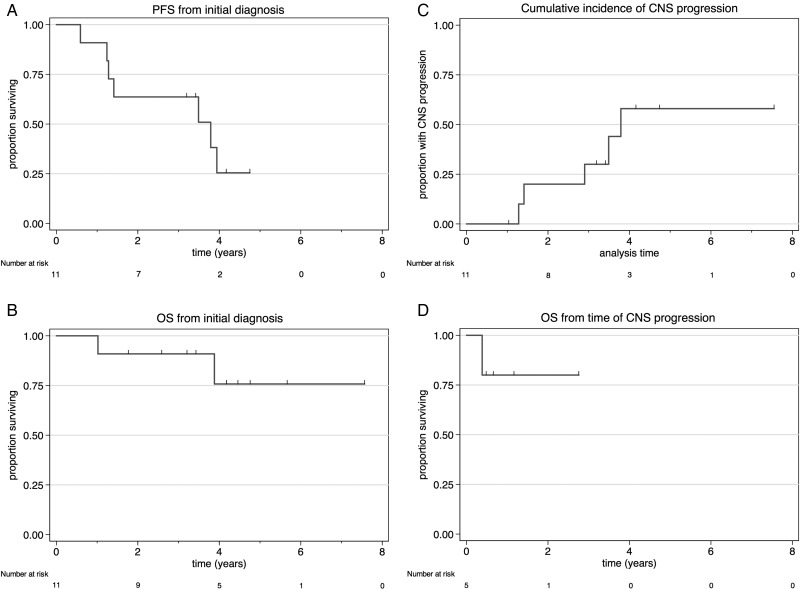
Response assessments were typically performed after R-MPV, after RT, and following completion of HDAC consolidation. All patients achieved a response, with a progressive increase in the CR rate with each stage of therapy (Fig. 2). Ten patients (91%) achieved a CR and one (9%) a PR at restaging assessment after the completion of cytarabine. After a median follow-up of 4.2 years (range, 1.8–7.6), 7 patients (64%) have experienced disease progression. The initial site of disease progression was the CNS in 4/7 (57%; brain parenchyma in 3, leptomeningeal in 1) and within the eye in a further 3/7 (43%), including the patient who achieved a PR. The patient with positive CSF at diagnosis achieved CR and remains free of disease at 4.7 years. The median PFS was 3.8 years (Fig. 3A) and the median OS was not reached, with the estimated 4-year OS of 75.8% (95% CI: 30.5%–93.7%; Fig. 3B). The 4-year cumulative incidence of CNS progression was 58% (95% CI: 28.5%–89.3%; Fig. 3C). Among the 5 patients who developed brain parenchymal or leptomeningeal progression, 4 (80%) remain alive, with a median follow-up of 10.8 months (range, 5.7–33.0) after CNS progression (Fig. 3D). The management of patients with relapsed disease was varied (subsequent therapeutic strategies, responses, and outcomes are summarized in Table 1). Most patients received retreatment with high-dose methotrexate or cytarabine. In aggregate, 3/7 patients (43%) achieved CR to second-line treatment, though 2/4 patients (50%) who did not respond initially achieved CR to third-line treatment. In 4/5 patients (80%) who achieved second CR, high-dose chemotherapy and autologous stem cell rescue was used. Two patients who experienced disease progression after transplant have achieved third CR with further therapies.
Fig. 2.
Objective response rates as assessed by ophthalmic examination after each treatment phase. Abbreviations: R, rituximab; MPV, methotrexate, procarbazine, vincristine; RT, radiotherapy; HDAC, high-dose cytarabine; PR, partial response; CR, complete response.
Fig. 3.
(A) PFS, (B) OS, (C) cumulative incidence of CNS progression, and (D) OS of patients who experienced CNS progression, from the time of event.
Table 1.
Management of patients with disease recurrence and subsequent outcomes
| Pt | CR1 Duration, mo | Site of First Progression | Second-line Rx | Resp | Third-line Rx | Resp | ASCT | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 41 | Vitreous | R-HD-MTX, i.t. MTX, intravitreal R | CR | – | – | R-BEAM | PD ASCT +8 mo (vitreous) treated with intravitreal R, i.t. R/MTX → CR in remission at 32 mo from 2nd relapse |
| 2 | 10 | Vitreous | Intravitreal MTX | PD | I.t. + intravitreal R | CR | GemBuMel | PD ASCT +11 mo (cerebellum) treated with R-i.t. AC + i.t. R → PD; WBRT 30.6 Gy; posterior fossa boost +9 Gy → CR in remission +33 mo from 2nd relapse |
| 3 | 5 | Vitreous, optic nerve | R-MPV ×3 | PD | – | – | – | Died from PD |
| 5 | 35 | Frontal lobe | R-HD-MTX ×3 | PD | – | – | – | Died from septic shock/multi-organ failure |
| 6 | 40 | Temporoparietal lobe | R-MPV × 5 | CR | – | – | Thiotepa, carmustine, R | In remission ASCT +4 mo |
| 10 | 10 | Leptomeningeal | R-HDAC ×2 + i.t. MTX | PD | R-temozolomide + i.t. MTX | CR | R-BEAM | In remission ASCT +6 mo |
| 11 | 9 | Cerebelluma | R-HD-MTX ×4 | CR | – | – | – | In remission HD-MTX +1 mo |
Abbreviations: Pt, patient; Rx, treatment; MTX, methotrexate; HD, high-dose; R, rituximab; resp, response; PD, progressive disease; BEAM, carmustine, etoposide, cytarabine, melphalan; GemBuMel, gemcitabine, busulfan, melphalan; AC, cytarabine; WBRT, whole brain radiotherapy.
Not biopsy proven, as the site of suspected involvement was deemed unsafe for stereotactic biopsy and CSF negative.
Discussion
Our data suggest that a multimodal treatment approach for PIOL consisting of R-MPV, binocular RT, and HDAC is effective at inducing remission but unable to prevent local and CNS relapse. Our results are comparable to those observed in a cohort of 6 patients treated with R-MPV by Stefanovic et al, who reported a 4-year PFS and OS of 83% with no local failures and one CNS relapse.6 The median PFS of 3.8 years compares favorably with results seen in other recent retrospective series, in which the reported median PFS is in the range 11–30 months (Table 2). However, despite the intensive strategy, a majority of patients in our cohort experienced treatment failure, consistent with published experience with this disease even when CNS-penetrating systemic therapy is used. The crude incidences of CNS progression in patients treated with high-dose methotrexate in the Memorial Sloan Kettering experience7 and a large multicenter European retrospective series8 was 7/14 (50%) and 14/30 (47%), respectively. It is probable that high-dose antimetabolite therapy does not achieve adequate concentration within the vitreous fluid to completely eradicate lymphoma and prevent recurrence.
Table 2.
Summary of selected recent studies detailing clinical outcomes in patients with primary intraocular lymphoma
| Lead Author | n, PIOL | Med FU | Treatment | Ocular Relapse | CNS Relapse | PFS | OS |
|---|---|---|---|---|---|---|---|
| Hormigo7 | 17 | 45 mo | HD-MTX or ara-C 4 HD-MTX or ara-C + RT 10 RT alone 3 |
3 (75%) 1 (10%) 1 (33%) |
1 (25%) 6 (60%) 2 (67%) |
Med 12 mo | Med 39 mo |
| Teckie20 | 18 | 25 mo | HD-MTX ± ara-C + RT 6 RT only 12 |
1 (17%) 3 (25%) |
2 (33%) 5 (42%) |
2 y 53% 2 y 48% |
2 y 83% 2 y 100% |
| Grimm21 | 83 | 32 mo | Ocular (RT ± intraocular Rx) 23 “extensive” systemic Rx 53 |
14/83 (17%)a | 22/83 (26%)a | Med 29.6 moc | Med 58 moc |
| Stefanovic6 | 6 | 44 mo | R-MPV 6 + binocular RT | 0 (0%) | 1 (17%) | 4 y 83% | 4 y 83% |
| Levasseur22 | 12 | NR | RT 4 HD-MTX 6 unknown 2 |
1 (25%) 0 (0%) – |
1 (25%) 1 (17%) – |
Med 11 moa | Med 33 moa |
| Riemens8 | 78 | 49 mo | None 3 ocular (RT ± intraocular Rx) 31 “extensive” systemic Rx 21 extensive + ocular Rx 23 |
0 (0%) 4 (13%) 6 (29%) 7 (30%) |
0 (0%) 10 (32%)c 9 (43%)c 9 (39%)c |
NRc | NR 4 y 58%b 4 y 84%b 4 y 40%b |
| Soussain17 | 11 | 17 mo | ESHAP 3 EHSAP + RT 2 ESHAP + HD-MTX 4 HD-MTX 2 |
5/11 (54%) | 1/11 (11%) | NR | NR |
| Current study | 11 | 50 mo | R-MPV + RT + ara-C | 3 (27%) | 5 (54%) | Med 45.6 mo | 4 y 75.6% |
Abbreviations: FU, follow-up; med, median; Rx, treatment; HD, high-dose; MTX, methotrexate; ara-C, cytarabine; R, rituximab; NR, not reported; ESHAP, etoposide, methylprednisone, cytarabine, cisplatinum.
Information for different treatment groups not provided.
Exact figure for each treatment group not provided in text, estimated from Kaplan–Meier curve. No significant difference between groups.
No significant difference reported between groups.
Unfortunately, RT provides insufficient local control in some cases. For the 3 patients in our series who experienced local relapse, the RT dose delivered was 30.6–39.6 Gy. Although data regarding the radiosensitivity of PIOL are scarce, we may extrapolate from the PCNSL literature. In Radiation Therapy Oncology Group trial 8315, in which patients with PCNSL received definitive whole brain RT to 40 Gy, followed by a 20-Gy boost to all radiographically apparent disease (total 60 Gy), 25/41 patients (61%) experienced local treatment failure, predominantly within the boost field.9 This suggests that some cases of PCNSL, and by extrapolation PIOL, may be inherently radioresistant. On the other hand, investigators have shown that 23.4 Gy is sufficient to address microscopic residual disease after a complete radiographic response to chemotherapy in PCNSL.4 Taken together, these results suggest that cases of PCNSL that are responsive to therapy require only low-dose RT for consolidation; on the other hand, unresponsive disease may be refractory to even high RT doses. We feel that further dose escalation in these refractory cases would increase toxicity without preventing local relapse. For these cases, another strategy appears necessary for improved disease eradication.
One of the noteworthy features of this cohort was the ability to successfully induce durable second and subsequent remissions in a majority of patients, even after CNS parenchymal relapse. Accordingly, there was marked disparity between the 4-year PFS of 25% and the 4-year OS of 75.8%. Although the median observation from time of CNS progression was brief, 4/5 patients were alive in remission at last follow-up. This clinical course is in stark contrast to secondary CNS relapse after systemic DLBCL, in which the median OS from time of CNS progression is 5.4 months.10 The means of achieving second remissions varied depending on the site of recurrence: CNS parenchymal disease was treated with repeated exposure to high-dose methotrexate or temozolomide, while ocular recurrence was treated with intravitreal rituximab. Other investigators have reported that re-induction with high-dose methotrexate results in an objective response rate (ORR) of 85%–91%,11,12 while recent data from a French study support the utility of temozolomide, which achieved an ORR of 75% (CR 63%) in a series of 16 patients with relapsed/refractory PIOL.13 Intravitreal administration of either rituximab or methotrexate was a useful means of treating recurrent disease in the vitreous compartment. Although intravitreal methotrexate is limited by corneal and retinal toxicity with prolonged administration,14 intravitreal rituximab was successful in 2 patients in our series who experienced ocular recurrence, suggesting a potential role in this setting. Other investigators have reported promising ocular response rates from intravitreal rituximab.15,16
Four of the 5 patients with disease relapse achieving a second CR underwent high-dose chemotherapy and autologous stem cell rescue, with a variety of conditioning regimens, including one patient who received CNS-penetrating agents (thiotepa and carmustine). The small sample size and short duration of follow-up post autologous stem cell transplant (ASCT) preclude robust analysis of the impact of ASCT in this study; however, other investigators have reported promising results using this approach. Soussain et al17 treated 5 patients with refractory PIOL with thiotepa, busulfan, and cyclophosphamide conditioned ASCT, with 3/5 (60%) remaining in remission a median of 15 months posttransplant.The same group also reported the largest experience of ASCT in patients with relapsed PIOL, a multicenter retrospective French study, in which patients with relapsed PCNSL received the same conditioning regimen with autologous stem cell support.18 The 11 patients with PIOL had an impressive median OS of 86 months. Investigators from Memorial Sloan Kettering recently reported the results of a phase II study of R-MPV followed by upfront thiotepa, busulfan, and cyclophosphamide conditioned ASCT in 32 patients with PCNSL, including 3 patients (9%) with intraocular involvement; the 2-year PFS and OS were 75% and 81%, respectively.19 Although the number of patients with ocular involvement was low, these data suggest enhancement of disease control using a CNS-penetrating conditioning regimen.
While the combined modality approach used in our study is effective at inducing remission, it is not without toxicity. Unfortunately our study did not include a control arm in which patients received local therapies alone, thus we were unable to determine the incremental benefit of chemotherapy. It is worth noting, however, that the proportion of patients achieving CR increased with each successive phase of treatment, suggesting that combined modality therapy may be needed for optimal initial disease control. Further, older series have found intraocular recurrence rates to be high if binocular RT is omitted,17 while the use of binocular RT alone has resulted in reasonable response rates (albeit with frequent disease recurrence).20 Importantly, the high proportion of patients who experienced subsequent disease progression in our study (and others) suggests that frontline consolidation with ASCT (using a CNS-penetrating regimen such as thiotepa/busulfan) may be needed to further improve outcomes. This hypothesis should ideally be evaluated in a prospective clinical trial.
The chemotherapy component was associated with moderate toxicity, with dose reductions and delays necessary in around one-third and one-half of patients, respectively. Renal impairment, a well-described complication of high-dose methotrexate, was the main cause of treatment delays and occurred in spite of adequate urinary alkalinization and leucovorin rescue. Hematologic toxicity was moderate and comparable to that observed in patients treated on the phase II protocol.4 The late side effects of ocular RT (dry eye, cataracts, retinopathy, and keratopathy) are expected sequelae. Despite these local effects, improvement or preservation of vision was achieved in all cases. The only cases of visual decline were due to cataracts, with visual acuity restored by cataract correction surgery in all cases.
This study has a number of limitations. Firstly, the small number of patients enrolled limits the strength of our conclusions. However, the rarity of PIOL makes the conduct of prospective clinical trials challenging, even for large referral centers and cooperative groups. Secondly, as an observational study, despite the intent of offering a standardized treatment approach to all patients, deviations occurred which were managed at investigator discretion, rather than being protocol directed, thus affecting the interpretation of adverse events and dose delays. The retrospective nature of the study means that referral and selection bias are difficult to exclude; however, all but one of the patients (aged 90) diagnosed with PIOL at our center during the specified time period underwent this treatment strategy. Although treating clinicians documented and graded adverse events at the time of treatment performed, it is likely that subjective adverse events (eg, fatigue) were underestimated. Finally, we included one patient with positive CSF in our cohort. Although such a patient may not be considered to have primary PIOL using the strictest definition, in a large series the presence of positive CSF without brain parenchymal lesions was not adversely prognostic for OS.21
Conclusion
Combined modality treatment with R-MPV, binocular radiation, and HDAC achieves a high ORR in patients with PIOL with manageable toxicity. Although ocular and CNS relapses are frequent, patients should be offered salvage therapies appropriate for the site of recurrence and consideration of ASCT with CNS-penetrating conditioning regimens in second remission. Given the limited PFS, prospective evaluation of frontline ASCT in PIOL is warranted.
Funding
No funding to disclose.
Conflict of interest statement. The authors declare no conflicts of interest in relation to this work.
References
- 1.Chan C-C, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: A report from an international primary central nervous system lymphoma collaborative group symposium. Oncologist. 2011;16(11):1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimm SA, McCannel CA, Omuro AM, et al. Primary CNS lymphoma with intraocular involvement: International PCNSL collaborative group report. Neurology. 2008;71(17):1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheah CY, Wirth A, Seymour JF. Primary testicular lymphoma. Blood. 2014;123(4):486–493. [DOI] [PubMed] [Google Scholar]
- 4.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25(30):4730–4735. [DOI] [PubMed] [Google Scholar]
- 5.Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. [DOI] [PubMed] [Google Scholar]
- 6.Stefanovic A, Davis J, Murray T, et al. Treatment of isolated primary intraocular lymphoma with high-dose methotrexate-based chemotherapy and binocular radiation therapy: a single-institution experience. Br J Haematol. 2010;151(1):103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hormigo A, Abrey L, Heinemann MH, et al. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol. 2004;126(2):202–208. [DOI] [PubMed] [Google Scholar]
- 8.Riemens A, Bromberg J, Touitou V, et al. Treatment strategies in primary vitreoretinal lymphoma: a 17-center European collaborative study. JAMA Ophthalmol. 2015;133(2):191–197. [DOI] [PubMed] [Google Scholar]
- 9.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23(1):9–17. [DOI] [PubMed] [Google Scholar]
- 10.Hollender A, Kvaloy S, Lote K, et al. Prognostic factors in 140 adult patients with non-hodgkin's lymphoma with systemic central nervous system (CNS) involvement. A single centre analysis. Eur J Cancer. 2000;36(14):1762–1768. [DOI] [PubMed] [Google Scholar]
- 11.Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol. 2014;117(1):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643–5646. [DOI] [PubMed] [Google Scholar]
- 13.Choquet S, Baron M, Soussain C, et al. Treatment of refractory/relapse vitreoretinal lymphoma: result of a multicenter retrospective study from the LOC network on temozolomide in monotherapy. J Clin Oncol. 2015;33:suppl; abstr 8546. [Google Scholar]
- 14.Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109(9):1709–1716. [DOI] [PubMed] [Google Scholar]
- 15.Larkin KL, Saboo US, Comer GM, et al. Use of intravitreal rituximab for treatment of vitreoretinal lymphoma. Br J Ophthalmol. 2014;98(1):99–103. [DOI] [PubMed] [Google Scholar]
- 16.Hashida N, Ohguro N, Nishida K. Efficacy and complications of intravitreal rituximab injection for treating primary vitreoretinal lymphoma. Transl Vis Sci Technol. 2012;1(3):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soussain C, Merle-Beral H, Reux I, et al. A single-center study of 11 patients with intraocular lymphoma treated with conventional chemotherapy followed by high-dose chemotherapy and autologous bone marrow transplantation in 5 cases. Leuk Lymphoma. 1996;23(3–4):339–345. [DOI] [PubMed] [Google Scholar]
- 18.Soussain C, Choquet S, Fourme E, et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica. 2012;97(11):1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teckie S, Yahalom J. Primary intraocular lymphoma: treatment outcomes with ocular radiation therapy alone. Leuk Lymphoma. 2014;55(4):795–801. [DOI] [PubMed] [Google Scholar]
- 21.Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an international primary central nervous system lymphoma collaborative group report. Ann Oncol. 2007;18(11):1851–1855. [DOI] [PubMed] [Google Scholar]
- 22.Levasseur SD, Wittenberg LA, White VA. Vitreoretinal lymphoma: a 20-year review of incidence, clinical and cytologic features, treatment, and outcomes. JAMA Ophthalmol. 2013;131:50–55. [DOI] [PubMed] [Google Scholar]