Abstract
Background
The role of reoperation for recurrent glioblastoma (GBM) remains unclear. Prospective studies are lacking. Here, we studied the association of clinical outcome with extent of resection upon surgery for recurrent GBM in the patient cohort of DIRECTOR, a prospective randomized multicenter trial comparing 2 dose-intensified temozolomide regimens at recurrence of GBM.
Methods
We analyzed prospectively collected clinical and imaging data from the DIRECTOR cohort (N = 105). Volumetric analysis was performed on gadolinium contrast-enhanced MRI as well as fluid attenuated inversion recovery/T2 MRI and correlated with PFS after initial progression (PFS2) and post-recurrence survival (PRS). Quality of life was monitored by the EORTC QLQ-C30 and QLQ-BN20 questionnaires at 8-week intervals.
Results
Seventy-one patients received surgery at first recurrence. Prognostic factors, including age, MGMT promoter methylation, and Karnofsky performance score, were balanced between patients with and without reoperation. Outcome in patients with versus without surgery at recurrence was similar for PFS2 (2.0 mo vs 1.9 mo, P = .360) and PRS (11.4 mo vs 9.8 mo, P = .633). Among reoperated patients, post-surgery imaging was available in 59 cases. In these patients, complete resection of contrast-enhancing tumor (N = 40) versus residual detection of contrast enhancement (N = 19) was associated with improved PRS (12.9 mo [95% CI: 11.5–18.2] vs 6.5 mo [95% CI: 3.6–9.9], P < .001) and better quality of life. Incomplete tumor resection was associated with inferior PRS compared with patients who did not undergo surgery (6.5 vs 9.8 mo, P = .052). Quality of life was similar in these 2 groups.
Conclusion
Surgery at first recurrence of GBM improves outcome if complete resection of contrast-enhancing tumor is achieved.
Keywords: DIRECTOR, outcome, recurrent GBM, re-operation, volumetry
Standard of care for glioblastoma (GBM) consists of gross total resection whenever feasible followed by involved field radiotherapy (RT) with concomitant temozolomide (TMZ) chemotherapy and a subsequent 6 cycles of maintenance TMZ.1 Since introduction of this standard,2 overall survival has increased to ∼16 months in clinical trials, with superior outcome of 25–30 months in patients with MGMT promoter methylation.3–5 Microsurgical tumor resection as initial treatment contributes to prolonged overall survival, if complete resection of enhancing tumor (CRET) is achieved.6–9 While CRET is considered to be the gold standard whenever safely feasible at the time of initial diagnosis, its value at recurrence is still subject to debate.10 The skepticism about the value of surgical resection in recurrent GBM may be due to the poor prognosis of patients with recurrent GBM,11 a rather high incidence of surgical morbidity following reoperation,12 and the general debate on the value of microsurgical reduction of tumor burden in malignant glioma. The recently published prospective randomized multicenter DIRECTOR trial evaluated the efficacy and tolerability of 2 different regimens of temozolomide (TMZ) at first progression after adjuvant chemoradiotherapy (TMZ/RT→TMZ).13 Major inclusion criteria were progressive or recurrent GBM as documented with MRI no earlier than 180 days after first surgery and no earlier than 90 days after end of RT. Furthermore, information about MGMT methylation status and completion of concomitant RT/TMZ plus at least 2 cycles of maintenance TMZ were required. Patients with and without reoperation were included. KPS ≥50 was mandatory for inclusion. All patients received either regimen of a dose-intensified TMZ rechallenge—both arms showed similar outcome. Thus, this dataset provides a well-annotated patient cohort to analyze the association of extent of resection (EOR) on the basis of MRI volumetry with outcome in the framework of a well-controlled post-recurrence treatment setting.
Methods
Study Design
GBM patients enrolled in the DIRECTOR trial were analyzed for associations of surgery and EOR at first progression with outcome. Indication for surgery was commonly based on recommendations from multidisciplinary tumor boards. Outcome measures were progression-free survival (PFS) after initial progression (PFS2, as opposed to the time of diagnosis to first progression, PFS1) and post-recurrence survival (PRS). PFS2 was defined as the duration from the date of first study drug administration until further progression. PRS was defined as the duration from the date of the first study drug administration to the date of tumor-related death.
Disease status was monitored by MRI at 8-week intervals and assessed using Macdonald criteria.14 At the time of data analysis (April 17, 2015), tumor progression had been documented in 99 patients, and death in 95 of all 105 patients, all tumor related. Three patients were lost to follow-up before tumor progression. The DIRECTOR study was approved by the local ethical committees; all patients gave written informed consent prior to inclusion.
Neuroimaging Studies
Imaging data available from the original DIRECTOR trial was retrospectively analyzed for the present study, while all remaining data were based on prospective analyses. Postsurgical MRI was performed within 72 h following surgery.
Volumetric analyses of pre- and postsurgical MR images were performed by an experienced investigator (B.S.) blinded to patients' outcome. Manual segmentation of pre- and postsurgical contrast-enhanced (CE) T1 and T2/fluid attenuated inversion recovery (FLAIR) images was performed using the pencil-drawing tool of the Osirix software version 3.6.1 (Internet freeware). Volume calculation of CE-T1 and T2/FLAIR tumor portion was performed by multiplying the sum of the tumor areas outlined on each transverse slice by the corresponding slice thickness.15 In case of blood remnants along the borders of the resection cavity, pre-contrast T1 volume was subtracted from the CE-T1 volume.
Concerning the CE-T1 image sequences, we obtained: (i) volumes with necrotic or cystic areas including enhancing parts as well as (ii) volumes with solid enhancing parts only. Resection cavities resulting from surgery were not included, neither in the CE-T1 nor in the T2/FLAIR volume calculation.
In the surgery cohort, which did not include biopsies, CRET was defined as absence of any contrast-enhancing tumor volume on CE-T1 imaging, while incomplete resection referred to patients with remnant CE tumor after surgical procedure.
In addition, an analysis considering the functional relevance (motor/supplementary motor cortex, primary somatosensitive area, Broca and Wernicke areas) of the affected brain area was performed. Tumor location was classified as “eloquent” versus “non-eloquent,” accordingly.16
Quality of Life
Quality of life (QoL) was monitored by the questionnaires from the European Organisation for Research and Treatment of Cancer: the QLQ-Core (C)30 and the QLQ–Brain Neoplasm (BN)20 at 8-week intervals.13 The QLQ-C30 incorporates 5 functional scales: general physical symptoms, physical functioning, psychological distress, social functioning, and fatigue/malaise. The BN20 is a module developed in particular for patients with brain cancer and comprises 4 domain scores (future uncertainty, visual disorder, motor dysfunction, communication deficit) and 7 symptom items (headache, seizures, drowsiness, hair loss, itching, difficulty with bladder control, and weakness of the legs).17,18
Statistical Methods
The chi-square test was used to compare categorical variables, and the Wilcoxon test was applied to compare continuous variables. Events in survival models were defined as radiographic tumor progression for PFS2 and as death from any cause for PRS. The log-rank and bootstrap tests were used to compare median times and rates at fixed time points. Receiver operating characteristics (ROC) analysis was performed to establish MRI-based volume thresholds. Associations of surgery, EOR, and ROC-derived tumor volume thresholds with outcome were analyzed in a Cox proportional hazards model. Age, KPS, MGMT promoter methylation, and steroid intake were included in this Cox model. A Mann–Whitney U-test was performed for QoL data analysis, as the data were largely not normally distributed.
Results
Patient Characteristics
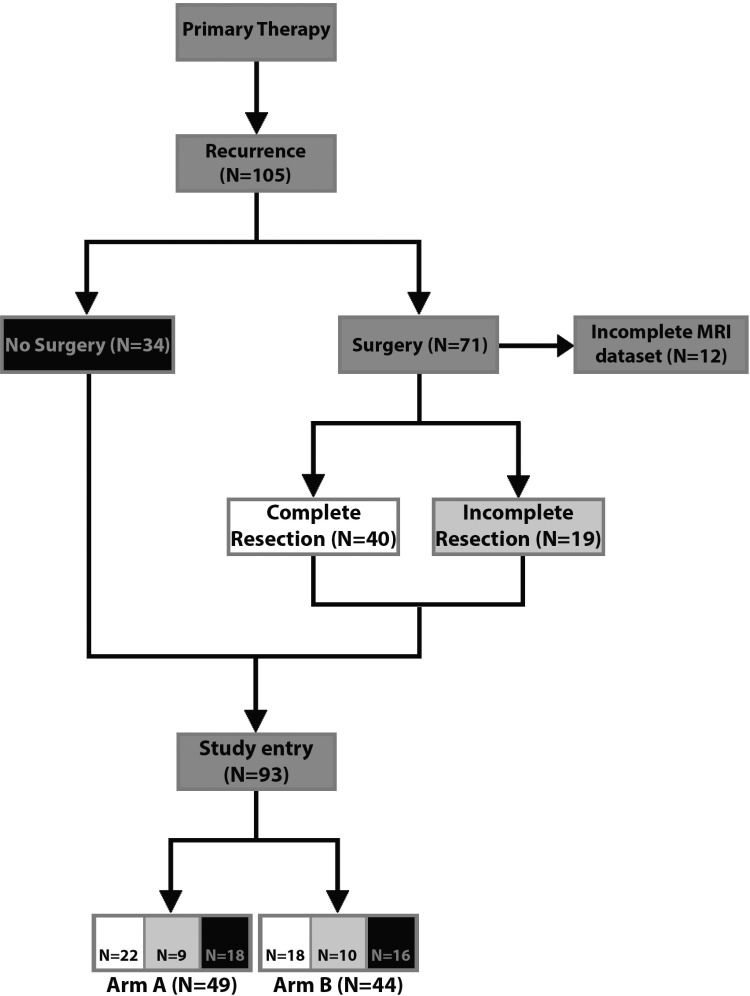
Of 105 patients enrolled, 71 underwent surgery prior to study entry (Fig. 1). Characteristics of patients who underwent surgery for recurrent disease versus patients who did not undergo surgery are summarized in Table 1. MGMT promoter methylation, first-line treatment, PFS from first diagnosis following first-line treatment (PFS1), as well as KPS and steroid intake at study entry were balanced between both groups.
Fig. 1.
Consort chart. Surgery and EOR prior to study entry in the DIRECTOR trial. Arm A (1 wk on [120 mg/m2 per day]/1 wk off TMZ); arm B (3 wk on [80 mg/m2 per day]/1 wk off TMZ). Black: patients who did not undergo surgery; white: patients with CRET; light gray: patients with incomplete resection.
Table 1.
Patient characteristics prior to enrollment
| Surgery for Recurrence |
P | ||
|---|---|---|---|
| Yes, n = 71 | No, n = 34 | ||
| Age at diagnosis, y | |||
| Median | 55 | 59.5 | .495 |
| Range | 25–77 | 21–72 | |
| Gender, n (%) | |||
| Male | 48 (67.6) | 21 (61.8) | .555 |
| Female | 23 (32.4) | 13 (38.2) | |
| MGMT promoter, n (%) | |||
| Methylated | 31 (43.7) | 15 (44.1) | .965 |
| Unmethylated | 40 (56.3) | 19 (55.9) | |
| First-line therapy, number of maintenance TMZ cycles | |||
| Median | 6.0 | 6.0 | .444 |
| Range | 2–12 | 2–12 | |
| Time to first progression, mo | |||
| Median | 11.5 | 10.7 | .366 |
| Range | 3.9–80.9 | 5.4–50.0 | |
| Tumor volume, cm3, at recurrence | |||
| Median | 9.5 | 5.1 | .234 |
| Range | 0.2–71.4 | 1.0–23.2 | |
| Tumor volume, cm3, at study entry | |||
| Median | 0.3 | 5.1 | <.001 |
| Range | 0–25.0 | 1.0–23.2 | |
| KPS at study entry, n (%) | |||
| 90–100 | 40 (56.3) | 20 (58.8) | .880 |
| 70–80 | 22 (31.0) | 9 (26.5) | |
| <70 | 9 (12.7) | 5 (14.7) | |
| Steroids at study entry, n (%) | |||
| Yes | 20 (31.3) | 8 (28.6) | .797 |
| No | 44 (68.7) | 20 (71.4) | |
| Study arm, n (%) | |||
| Arm A | 34 (47.9) | 18 (52.9) | .628 |
| Arm B | 37 (52.1) | 16 (47.1) | |
Abbreviation: MGMT, O6-methylguanine DNA methyltransferase.
Considers only patients with available imaging data.
Tumor volumes at recurrence were similar in patients who did and did not undergo surgery, but postoperative tumor volumes at study entry were smaller in the surgical cohort than in patients who did not undergo surgery. Radiographic CRET was achieved in 40 of 59 assessable patients (67.8%) who underwent surgery. Among patients who underwent surgery, MGMT promoter methylation, first-line treatment, PFS1, as well as KPS and steroid intake at study entry were balanced between patients with CRET versus incomplete resection. Preoperative tumor volumes at recurrence were larger in patients with incomplete resection (P = .004), and tumors with incomplete resection were more often localized in eloquent regions (P = .178) (Supplementary Table S1). One patient developed a postoperative wound infection within the first month after surgery and required discontinuation of TMZ, but no other severe complications from tumor resection were documented.
Outcome by Surgery
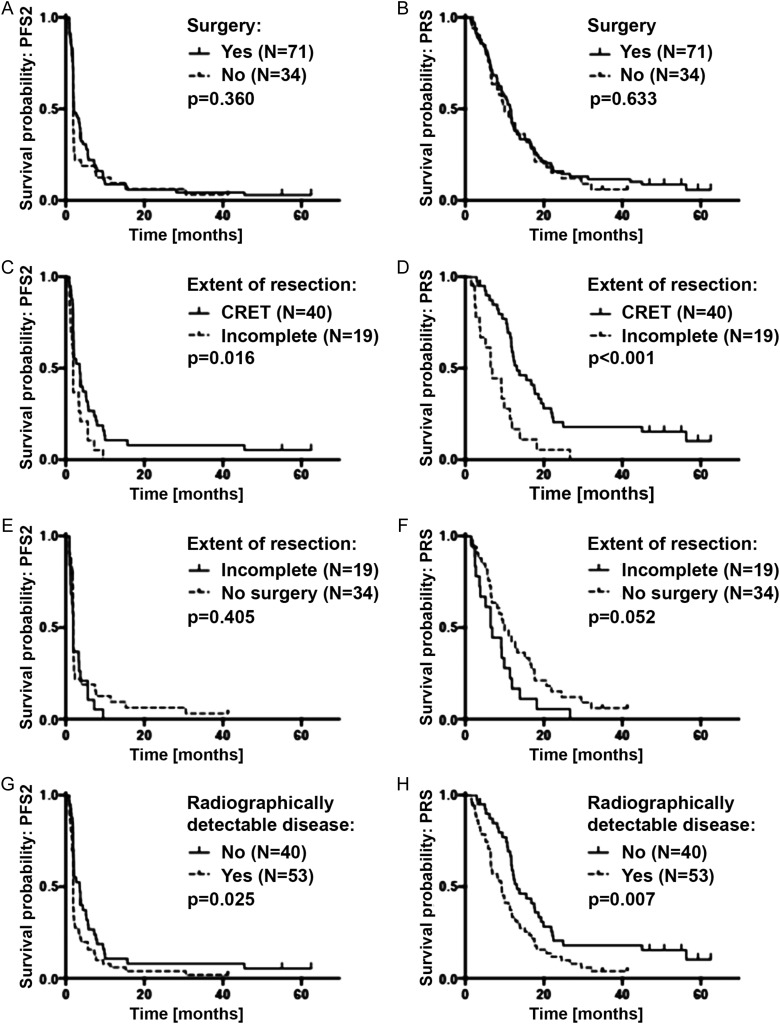
Clinical outcome parameters were comparable in patients who underwent surgery versus patients who did not undergo surgery for recurrent disease prior to study entry (Fig. 2A and B, Supplementary Table S2). PRS was 11.4 months (95% CI: 8.4–12.3) in patients who underwent surgery versus 9.8 months (95% CI: 6.6–15.1) in patients who did not undergo surgery (P = .633).
Fig. 2.
Outcome by surgery and residual disease on the baseline MRI. (A) PFS2 (time to next progression) and (B) PRS in all patients who underwent surgery for recurrent disease (n = 71) vs patients who did not undergo surgery (n = 34). (C) PFS2 and (D) PRS in patients who underwent surgery for recurrent disease prior to study entry with residual disease (n = 19) vs CRET (n = 40) on the baseline MRI. (E) PFS2 and (F) PRS in patients with incomplete tumor resection (n = 19) vs patients who did not undergo surgery (n = 34). (G) PFS2 and (H) PRS in patients with radiographically detectable disease on the baseline MRI (n = 53) vs patients with CRET (n = 40).
Next we explored a prognostic role for EOR. Among patients who underwent surgery at recurrence, PRS was longer in patients with CRET (12.9 mo [95% CI: 11.5–18.2]) than in patients with incomplete tumor resection (6.5 mo [95% CI: 3.6–9.9]) (P < .001) (Fig. 2C and D, Table 2). Incomplete tumor resection was even associated with a trend to inferior PRS compared with patients who did not undergo surgery (6.5 vs 9.8 mo, P = .052) (Fig. 2E and F, Supplementary Table S3). Comparing outcome between no surgery and CRET in the univariate analysis, there was an advantage for CRET as far as PFS2 is concerned: 1.87 months (95% CI: 1.84–1.9) versus 3.5 months (95% CI: 1.8–5.2), log-rank P = .05. The difference was pronounced but not statistically significant for PRS: 9.6 months (95% CI: 6.2–12.9) versus 13.2 months (95% CI: 9.1–17.3), log-rank P = .087. Multivariate analysis revealed CRET to be prognostic for PRS (P = .05) besides MGMT and KPS (Supplementary Table S7). Volumetric analysis across the entire study population of volumes prior to study entry revealed CE-T1 volumes with as well as without necrosis to be highly associated (P < .0001) with longer PFS2 and PRS.
Table 2.
Outcome by extent of resection
| Extent of Resection |
P | ||||||
|---|---|---|---|---|---|---|---|
| Gross Total Resection |
Incomplete |
||||||
| Patients | Events | Time, mo (95% CI) | Patients | Events | Time, mo (95% CI) | ||
| Median PFS2 | 40 | 37 | 3.5 (2.0–5.1) | 19 | 19 | 1.9 (1.3–3.5) | .016 |
| Median PRS | 40 | 34 | 12.9 (11.5–18.2) | 19 | 18 | 6.5 (3.6–9.9) | <.001 |
| Rate, % (95% CI) | Rate, % (95% CI) | ||||||
| PFS2 at 6 mo | 39 | 29 | 25.6 (11.3–40.0) | 18 | 16 | 11.1 (5.0–27.2) | .063 |
| Survival rate at 12 mo from first study drug administration | 39 | 16 | 59.0 (42.8–75.1) | 18 | 15 | 16.7 (2.4–35.7) | <.001 |
Of note, tumor volumes at study entry were similar in patients who underwent incomplete tumor resection (median 4.0 cm3 [range: 1.1–25.3]) versus patients who did not undergo surgery (median 5.1 cm3 [range: 1.0–25.2]) (P = .635). Tumor location regarding eloquent brain regions was not significant for outcome (11.08 mo [95% CI: 9.3–12.9] versus 11.5 mo [95% CI: 5.2–17.8]).
Finally, we analyzed the outcome of all patients with CE tumor at study entry (eg, patients without surgery and those with an incomplete resection) versus patients with CRET (Fig. 2G and H, Supplementary Table S4). Detection of CE tumor was associated with shorter PFS2 (P = .025) and PRS (P = .007). ROC analysis using the median PRS (10.5 mo) for determining the volume threshold for discrimination between inferior and superior post-recurrence survival was performed. Concerning postoperative CE-T1 with and without necrosis as well as T2/FLAIR volumes, a threshold could not be determined due to the large number of cases with complete resection.
Multivariate Modeling of Postoperative Outcome
We applied a Cox proportional hazards model to identify prognostic factors after surgery. Univariate analyses of the association of factors included in this Cox model with outcome are summarized in Supplementary Table S5. CRET was prognostic for PRS on multivariate analysis, in contrast to age, MGMT promoter methylation status, KPS, or steroid intake at study entry (Table 3). Gender or study arm was also not prognostic when tested in this model as additional single variables (data not shown). CRET was not prognostic for PRS when Cox proportional hazards modeling was applied to PFS2 (P = .061). When CRET was replaced with surgery “yes versus no” in the same Cox model, only MGMT promoter methylation status was prognostic for OS (Supplementary Table S6) and PFS2 (P = .003) in the log-rank test.
Table 3.
Multivariate analyses of predictors of inferior postoperative survivala
| Hazard Ratio (95% CI) | P | |
|---|---|---|
| Extent of resection: GTR vs incomplete | 0.42 (0.21–0.85) | .015 |
| Age at study entry: 18–54 vs 55+ y | 1.25 (0.65–2.41) | .508 |
| MGMT promoter: methylated vs unmethylated | 0.58 (0.30–1.11) | .100 |
| KPS at study entry: 90%–100% vs KPS 50%–80% | 0.82 (0.43–1.54) | .528 |
| Steroids at study entry: no vs yes | 0.82 (0.42–1.62) | .566 |
Abbreviations: GTR, gross total resection; MGMT, O6-methylguanine DNA methyltransferase.
Patients with complete clinical and imaging data (n = 52).
Quality of Life Results
Regarding the entire group at the first follow-up after 8 weeks, patients who received surgery had higher cognitive functioning values (P = .046). Constipation was also more common in this group (P = .039). Patients who underwent an incomplete resection were more likely to suffer from general motor dysfunction (P = .04) and to have a worse global health status (P = .008) compared with those who underwent CRET.
Discussion
In contrast to the existing standard of care in primary GBM, treatment of GBM progression after standard of care treatment remains poorly defined and is increasingly individualized, taking into consideration prior treatment, time of relapse, and pattern of tumor spread at relapse, as well as increasingly molecular marker profiles.1,11,19 Surgery with complete resection of solid, CE tumor has been shown to be associated with improved survival in newly diagnosed GBM.7,8,20–22 However, only a minority of patients are considered eligible for second surgery—KPS, tumor volume, and eloquent tumor location have recently been identified as selection criteria for patients to benefit from reoperation.23,24 Only 13%–30% of all recurrent GBM patients are considered candidates for a second surgery.25
Yet, the evidence for these recommendations is low and commonly based on retrospective case series. The DIRECTOR trial explored tolerability and efficacy of 2 different regimens of dose-intensified TMZ in patients with GBM at first progression.13 Since this trial cohort is clinically well annotated and both TMZ treatment arms had identical outcomes, it provides an excellent opportunity to explore the association of surgery at recurrence with outcome. The majority (68%) of patients had surgery for recurrent disease prior to being enrolled in this trial. This number is considerably higher than in previous reports on reoperation in recurrent GBM (13%–30%); however, the present data are based on a multicenter trial enrolling patients from 10 large neurosurgical centers.25,26 As reported in the primary report of the DIRECTOR trial, patients with versus without surgical intervention prior to study enrollment had a similar outcome.13 Recently, in a cohort study from a prospective registry in Italy with 764 patients over a period of 14 years, no survival benefit was detected for reoperation, similar to an analysis done by the North American Brain Tumor Consortium (NABTC) on 758 patients being enrolled over 11 years.25–27 However, none of the 3 reports considered the volumetric EOR but merely whether patients had undergone second surgery at all. In addition, reports in which extent of re-resection was considered were not controlled for additional therapies or had data prospectively collected according to a protocol.
Here we categorized the surgical intervention at recurrence in a simple binary mode: after reoperation, 40 patients had no residual tumor determined by volumetry of CE MRI, whereas 19 patients did. A comparison of these 2 cohorts demonstrated that extent of resection was prognostic for outcome. Further sensitivity analyses demonstrated that the presence versus absence of residual tumor at study entry remained prognostic irrespective of surgery. In fact, incomplete resection showed even a trend to inferior outcome compared with no resection at all prior to study entry. Of note, it has to be assumed that some patients undergoing second surgery in this setting had postoperative morbidity that prevented them from being eligible for the DIRECTOR trial according to the inclusion criteria. Hence, the cohort of this study has undergone selection by omitting patients with severe postoperative complications, and the present analysis can focus more specifically on the role of resection itself. In addition, the uniform treatment of all patients with dose-intensified TMZ rechallenge provides a much more homogeneous cohort to analyze the role of EOR, since the spectrum of additional therapy after re-resection was heterogeneous in previous studies.
A recent literature review performed by use of the PubMed and Ovid Medline databases for 1980 through 2013 revealed only 31 studies with data from single or multiple institutions. Twenty-nine proposed a survival benefit or improved functional status after reoperation for recurrent high-grade glioma.10 This was confirmed in a recent study analyzing 503 patients.28 However, selection criteria for and influence of additional post re-resection therapy remained unclear. Furthermore, the role of incomplete re-resection is yet vague, since no comparison with matched cohorts of non–re-resected patients had been performed. More aggressive resection bears the risk of increased surgical morbidity and has been found to be doubled once volumetric resection of more than 80% was achieved.12 The influence of procedure-related morbidity on timing and intensity of additional antitumor treatment after re-resection had never been analyzed, although one might assume that patients with surgical complications might be withheld from intensified oncological therapy in the context of recurrent GBM. These confounding factors do not interfere in our study because of the inclusion criteria and treatment design of the DIRECTOR trial, which led to a very homogeneous cohort.
Here we report that only those patients experience prolonged survival in whom a complete resection of the solid CE tumor mass was accomplished. Quality of life regarding cognitive and neurological function was superior in this cohort compared with patients with measurable disease on MRI after reoperation. Compared with the nonresected cohort, incomplete resection did not result in better outcome regarding either survival or QoL. Indeed, PRS was shorter by trend in patients with residual tumor after reoperation despite similar tumor volumes. These considerations allow the conclusion that surgery for recurrent GBM should be considered only if CRET can be safely achieved.
Future studies dealing with post-recurrence treatment have to analyze whether the study arms are balanced for CRET. Clarke et al26 concluded from their NABTC analysis that data from patients with and without reoperation might be combined for the assessment of new treatment options, since 6-month PFS and overall survival were similar in both cohorts. However, the simple discrimination solely between surgery yes/no revealed no difference in the past, in contrast to our study and former reports adjusting for extent of re-resection.12,13,25,26,29
Our study has inherent limitations. Although the data were collected prospectively, it is still a retrospective, exploratory analysis that was not prespecified. Furthermore, it still remains a possibility that tumors that were amenable to CRET were per se tumors with a better prognosis due to a different biology (eg, being less invasive). A randomized trial to prospectively assess the role of complete resection at GBM progression has recently started (NCT 02394626).
Conclusion
According to the data of this well-controlled study population, surgery at first recurrence of GBM improves outcome in terms of both survival and QoL only if complete resection of CE tumor is safely achieved.
Supplementary Material
Funding
The DIRECTOR trial (NCT 00941460) was supported by a grant from Merck Sharp and Dohme (MSD; formerly Schering Plough) and a matching-funds grant from University Hospital Zurich, Switzerland.
Conflict of interest statement. M.W. is an advisory board member for MSD, from which he reports receiving research grants. G.T. reports receiving a travel grant from MSD. U.H. reports receiving speakers bureau honoraria from Medac. U.S. is a consultant/advisory board member for Roche and reports receiving speakers bureau honoraria from GlaxoSmithKline and Medac. G.R. reports receiving commercial research grants from Roche and speakers bureau honoraria from Amgen and Roche. W.W. reports receiving speakers bureau honoraria from Roche, MSD, and Prime Oncology. J.C.T. is a consultant/advisory board member for Merck Serono and Roche and reports receiving speakers bureau honoraria from them both. All other authors report no disclosures.
Supplementary Material
References
- 1.Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–e403. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 5.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 6.Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576; discussion 564–576. [DOI] [PubMed] [Google Scholar]
- 7.Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 8.Kreth FW, Thon N, Simon M, et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013;24(12):3117–3123. [DOI] [PubMed] [Google Scholar]
- 9.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 10.Hervey-Jumper SL, Berger MS. Reoperation for recurrent high-grade glioma: a current perspective of the literature. Neurosurgery. 2014;75(5):491–499; discussion 498–499. [DOI] [PubMed] [Google Scholar]
- 11.Weller M, Cloughesy T, Perry JR, et al. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neurooncol. 2013;15(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oppenlander ME, Wolf AB, Snyder LA, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. [DOI] [PubMed] [Google Scholar]
- 13.Weller M, Tabatabai G, Kastner B, et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21(9):2057–2064. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald DR, Cascino TL, Schold SC, Jr., et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 15.Suchorska B, Jansen NL, Linn J, et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84(7):710–719. [DOI] [PubMed] [Google Scholar]
- 16.Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nature reviews. Neuroscience. 2002;3(8):606–616. [DOI] [PubMed] [Google Scholar]
- 17.Efficace F, Bottomley A. Health related quality of life assessment methodology and reported outcomes in randomised controlled trials of primary brain cancer patients. Eur J Cancer. 2002;38(14):1824–1831. [DOI] [PubMed] [Google Scholar]
- 18.Taphoorn MJ, Claassens L, Aaronson NK, et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 19.Niyazi M, Siefert A, Schwarz SB, et al. Therapeutic options for recurrent malignant glioma. Radiother Oncol. 2011;98(1):1–14. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 21.Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 22.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park CK, Kim JH, Nam DH, et al. A practical scoring system to determine whether to proceed with surgical resection in recurrent glioblastoma. Neuro Oncol. 2013;15(8):1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JK, Hodges T, Arko L, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28(24):3838–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nava F, Tramacere I, Fittipaldo A, et al. Survival effect of first- and second-line treatments for patients with primary glioblastoma: a cohort study from a prospective registry, 1997–2010. Neuro Oncol. 2014;16(5):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke JL, Ennis MM, Yung WK, et al. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol. 2011;13(10):1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorlia T, Stupp R, Brandes AA, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48(8):1176–1184. [DOI] [PubMed] [Google Scholar]
- 28.Ringel F, Pape H, Sabel M, et al. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117(6):1032–1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.