Abstract
The recent discovery of distinct, ultra-long, and highly functional membrane protrusions in gliomas, particularly in astrocytomas, extends our understanding of how these tumors progress in the brain and how they resist therapies. In this article, we will focus on ideas on how to target these membrane protrusions, for which we have suggested the term “tumor microtubes” (TMs), and the malignant multicellular network they form. First, we discuss TM-specific features and their differential biological functions known so far. Second, the connection between 1p/19q codeletion and the inability to form functional TMs via certain neurodevelopmental pathways is presented; this could provide an explanation for the distinct clinical features of oligodendrogliomas. Third, the role of TMs for primary and potentially also adaptive resistance to cytotoxic therapies is highlighted. Fourth, avenues for therapeutic approaches to inhibit TM formation and/or function are discussed, with a focus on disruption (or exploitation) of network functionality. Finally, we propose ideas on how to use TMs as a biomarker in glioma patients. An increasing understanding of TMs in clinical and preclinical settings will show us whether they really are a long-sought-after Achilles' heel of treatment-resistant gliomas.
Keywords: 1p/19q codeletion, astrocytoma, glioblastoma, resistance, oligodendroglioma, tumor microtubes
It has been known for more than a hundred years that the tumor cells of particularly aggressive and treatment-resistant primary brain tumors have a phenotype that is indicative of multiple cellular extensions. Precisely described by R. Virchow as “glioma durum” (1863–1865), and by T. Simon as “spider-cell glioma” (1874), it soon became clear that tumor cells have an “astrocytic” (i.e., star-shaped) morphology, leading to the term “astrocytomas.”1 These starlike processes in reactive glial and astrocytic tumor cells appeared broader and shorter in thin sections than the extensions of, for instance, neurons in the normal brain. However, “fibrillary” features in astrocytic tumors were reported from early on, first by Bailey and Cushing in 1926.1 The biological basis for these morphological phenomena of astrocytomas, however, had not gained much attention until recently.
By using in vivo 2-photon microscopy through a chronic cranial window in mice and following the growth of brain-implanted primary glioma cell lines that were derived from patients and strictly kept under nondifferentiating (stemlike) growth conditions, it was possible to increase our understanding of these tumors by 2 more dimensions.2 Seeing all 3 dimensions in space was instrumental to discover that a plethora of extremely long (up to >500 µm) and very thin (1–2 µm thick) membrane protrusions were formed by glioblastoma (astrocytoma grade IV) cells, partly invading the normal brain at the invasive edge, partly interconnecting single tumor cells to a multicellular network. The fourth dimension, time, was instrumental to further characterize these membrane protrusions. By following individual tumor cells in distinct tumor regions over minutes to months (up to one year), it became evident that these membrane protrusions were used to constantly scan the brain, to colonize it by travel of nuclei in these tubes to distant places, and to finally form a communicating multicellular network that helps glioma cells to resist the adverse effects of radiotherapy.2,3
In this review article, we will highlight our current concepts with respect to these membrane tubes and discuss clinical implications: how this knowledge helps us to understand some known differences in the clinical course of gliomas, how it might be used to develop novel therapies in the future, and how it could potentially help to stratify patients for established ones.
Features of Tumor Microtubes: Their Biological Roles and Their Relation to Known Cellular Extensions
Two fundamental biological roles have been discovered for tumor microtubes (TMs) so far: (i) as leading structures for glioma cell invasion and proliferation, allowing effective brain colonization, and (ii) as a means of interconnection of single glioma cells to one large syncytium, resulting in a functional and resistant network.3 At this moment in time, it is not clear whether only one “type” of TM exists or at least 2 different types of TMs are responsible for both fundamental biological roles. The neuronal growth-associated protein 43 (GAP-43) appeared to be important for all of those known functions of TMs. In contrast, the gap junction protein connexin 43 (Cx43; not to be confused with GAP-43), regularly located at TMs and their frequent crossing sites and thus connecting tumor cells to one functional syncytium, did not clearly influence the occurence and function of invasive TMs.2 This might also explain why different groups found divergent roles with respect to glioma progression for Cx43.4 We will learn in the future what molecular players drive the different biological functions of TMs in gliomas.
In principle, the discovery of TMs in gliomas supports the increasing perception of tumors as organism-like entities that hijack normal developmental programs to successfully grow, interact with the host, and withstand adverse events.5–7 In line with this, GAP-43 is highly expressed both in axonal growth cones8,9 and in the growth cone–like tips of growing TMs. Furthermore, it drives both neuronal progenitor cell10 and TM-dependent astrocytoma cell migrations. In the end, GAP-43 seems to be a master regulator of basic cellular programs leading to long membrane extensions: GAP-43 overexpression has been described before as being sufficient for the outgrowth of membrane tubes in neuronal11 and even in nonneuronal12 cells.
In development, invasive cell migration has been shown to depend on long cellular extensions that act as “pathfinders” in response to guidance cues.13 This seems to apply for neuronal migration during brain development, too.14 Remarkably, these findings are very similar to what we have seen for TMs during progression of astrocytomas in the mouse brain.2 Moreover, very long cellular extensions and their role in cell-to-cell communication processes have caught the attention of researchers before.15 In development, intercellular membrane tubes were first described in Drosophila,16 where they seem to be involved in stem cell signaling.17 Likewise, in adult cell types, they can play a role in functional cell-cell coupling,18–20 which includes tumor cells.21 These membrane tubes have received many names, including membrane nanotubes (MNs), tunneling nanotubes, and cytonemes. However, the definitive function(s) of membrane tube connections for mammalian tissues and in tumor biology remains an open question.22
Although TMs share many features with these previously described MNs, differences do exist (Table 1). These unique features led us to propose the new term “tumor microtubes” for the membrane extensions of glioma cells. This does not exclude that tumor cell MNs in vitro could be a model for TMs in vivo, where much longer time intervals are available for growth of size, extent of intercellular connectivity, and increase in functionality. More than that, future research will reveal whether these membrane microtubes can also be found in other tumor entities inside and outside the CNS, and enhance our knowledge about their distinct cytological characteristics, which will support the determination of the best terminology. For now, we regard “TM” as a working term.
Table 1.
Characteristics of TMs in comparison with known features of membrane nanotubes (MNs; also known as tunneling nanotubes)
| Feature | TMs | MNs |
|---|---|---|
| Width | Mean, 1.7 µm | <1 µm |
| Maximum length | >500 µm | ∼100 µm |
| Lifetime | Days, up to >200 | Minutes, up to 60 |
| Connection between cells | GJ separated | Open/GJ separated |
| Content (frequent) | ||
| Actin | + | + |
| Mitochondria | + | + |
| Endoplasmic reticulum | + | + |
| Microvesicles | + | + |
| Myosin IIa | + | ? |
| Myosin X | − | + |
| Microtubules | + | (+; only subsets) |
| Functions | ||
| Directed travel of nuclei | + | − |
| Directed travel of mitochondria | + | + |
| Propagation of ICWs | + | + (if GJ separated) |
| Active scanning of environment | + | (+) |
| Cell invasion | + | ? |
| Mediation of resistance to cytotoxic therapy | + | ? |
| Repair of damage in/to connected cells | + | + |
| Stem cell signaling | ? | + |
| Pathogen spread | ? | + |
Abbreviation: GJ, gap junctions.
A Possible Explanation of Why 1p/19q Codeleted Gliomas Respond Better to Treatment
It has been a long-standing question in neuro-oncology about why patients with 1p/19q codeleted gliomas, that is, oligodendrogliomas (according to the upcoming revision of the World Health Organization [WHO] classification), show such a different course of disease compared with patients with 1p/19q non-codeleted tumors, that is, astrocytomas. It was clear that not a single gene but most probably a combination of factors located on the 2 chromosomal parts have to be involved, since deletion of only 1p or 19q was not found to be associated with improved survival.23 This issue becomes even more interesting in light of the recent discovery that 1p/19q codeleted and non-codeleted gliomas share frequent molecular alterations, like mutations in the isocitrate dehydrogenase (IDH) 1 and 2 genes. However, the 1p/19q codeletion defines an own, clear molecular subgroup24–26 and is associated with a high responsiveness to combined radio- and chemotherapy27,28 and increased apoptosis of tumor cells.29 Of note, diffuse astrocytomas remain incurable neoplasms for the vast majority of patients, which includes those suffering from IDH-mutated tumors. This is indeed in contrast to 1p/19q codeleted, regularly IDH-mutated oligodendrogliomas, where long-term, secondary analyses of 2 phase III studies (with median follow-up times of 135–140 mo) showed a remarkably high number of long-term survivors, reaching 40%–50% when these patients received combined radiochemotherapy in the primary setting.27,28 Importantly, another analysis revealed that the 1p/19q codeletion still prolonged survival compared with the subgroup of IDH-mutated, non-codeleted gliomas (14.7 vs 5.5 y overall survival when treated with combined radiochemotherapy in the primary setting30). These data support the prognostic importance of the 1p/19q codeletion, independent of the also favorable IDH mutation.
In our recent work, we demonstrate that (i) 1p/19q non-codeleted patients' gliomas are rich in long and intercellular TMs, while 1p/19q codeleted ones are not; (ii) TM length increases with WHO grade; and (iii) the RNA-Seq gene expression data of 250 human gliomas of the database of The Cancer Genome Atlas can be used to detect crucial molecular pathways that are relatively higher expressed in 1p/19q non-codeleted gliomas, which includes the gap junction protein Cx43 (among the top 100 higher expressed genes in non-codeleted tumors), but also core pathways that drive neurite formation and the extension of neurite-like membrane protrusion.2 Core neurotrophic factors that drive GAP-43 expression are located on both chromosomal parts 1p and 19q.2
These data—together with the role of TMs in treatment resistance (see the next section)—provide a novel explanation for the differences associated with 1p/19q status in gliomas. It does not explain, however, why a relevant number of gliomas acquire the 1p/19q codeletion at all; one might consider unknown advantages in early tumorigenesis and/or a promotive role of the IDH mutation for the unbalanced translocation that leads to this codeletion. It is also not clear whether the strong connection of TM and 1p/19q status in patient tissue is restricted to untreated gliomas; only those have been analyzed so far. Finally, it remains to be seen whether TMs can be found in other glioma types without 1p/19q codeletion (eg, ependymoma, ganglioglioma, pilocytic astrocytoma).
Tumor Microtube Connectivity as a Cellular Factor of Primary and Adaptive Resistance
In the previous paragraphs, we have argued that TMs are characteristic for 1p/19q non-codeleted gliomas, where they connect single tumor cells with each other, but are strongly reduced in 1p/19q codeleted tumors, at least in the primary setting. This was a first indication that TMs might be involved in treatment resistance. To better understand the role of TMs for tumor integrity and resistance, we took advantage of the fact that individual astrocytoma cells showed a striking heterogeneity: While the proportion of TM-connected cells slightly increased with tumor progression, a substantial part of astrocytoma cells (40%–60%) remained unconnected, in both mouse and human. Indeed, while TM-connected astrocytoma cells were protected from cell death that followed radiotherapy in mice, neighboring unconnected tumor cells died in relevant numbers. Moreover, when formation of functional TMs was inhibited via GAP-43 knockdown, radioresistance of astrocytomas greatly decreased; this might, however, be also attributable to GAP-43 functions unrelated to TM formation. Finally, TM connectivity and function increased after radiotherapy in experimental astrocytomas in vivo. Remarkably, TM-connected individual tumor cells of the astrocytoma network were regularly replaced by a TM-dependent repair response after being depleted by a micro laser beam; this was only infrequently (25%) observed when tumor cells were not TM connected to others. Similar mechanisms can be observed when a larger brain region is photodamaged (Fig. 1). Together this speaks for an important role of TM-mediated network connectivity for tumor cell resistance and for maintaining the integrity of the entire tumor cell network. Future research should address the question of whether these “protect-and-repair” functions of TMs are also involved when other standard glioma therapies are applied.
Fig. 1.
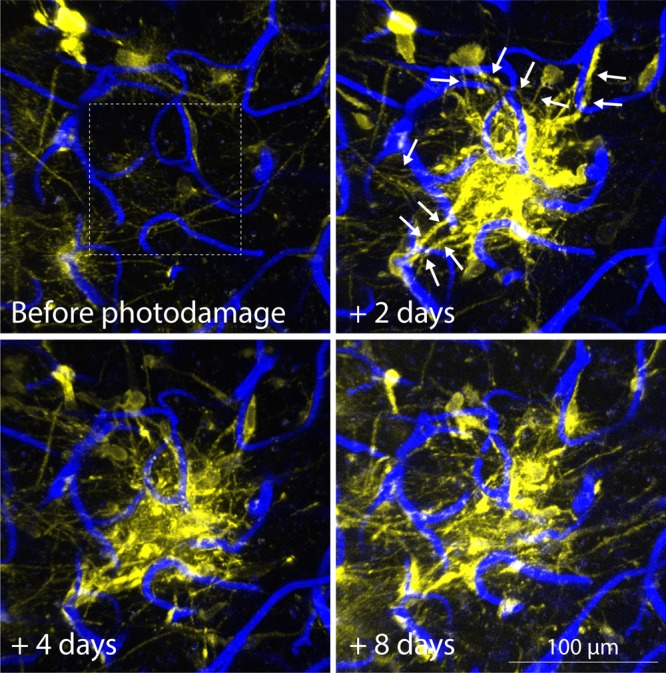
TMs in damage repair. Photodamage via high-intensity laser scanning of a mouse brain subregion of 100 × 100 µm (dashed box). The brain region is diffusely invaded by single glioblastoma cells, and long, very thin TMs are visible. After photodamage of the subregion that harbored TMs of neighboring glioblastoma cells, those extend new, long, actin-rich processes into the damaged area (arrows), and the area is rapidly colonized by densely packed tumor cells. Note also the overall increase in TMs over time. Yellow, LifeAct-YFP (an in vivo marker for actin), expressing primary glioblastoma cells. Blue, brain parenchyma microvessels labeled by i.v. injection of a fluorescent dextran. Images were acquired by in vivo 2-photon microscopy through a chronic cranial window.
To explore potential mechanisms of TM-mediated protection from cytotoxicity, we measured basal intracellular calcium levels in astrocytoma cells before and during radiotherapy, using a ratiometric calcium indicator. Basal calcium levels were very homogeneous in nonirradiated cells, as well as in TM-connected cells during radiotherapy, while unconnected cells developed a high variability of their intracellular calcium levels during irradiation.2 Increases of intracellular calcium levels are required for radiotherapy-induced cytotoxicity,31 and even small calcium increases are involved in intrinsic apoptotic cell death in glioma cells.32 Thus, one can speculate that intercellular TMs can serve as a means for an individual cell to distribute small molecules like calcium within the larger network, achieving nonlethal levels.
Of note, the gap junction protein Cx43 is also highly expressed in nonmalignant astrocytes, connecting them to one functional multicellular network. Remarkably, their connectivity by Cx43 gap junctions makes them resistant to oxidative stress,33 which resembles our astrocytoma data. Normal brain astrocytes can also develop MNs, which depends on p53 activation.34 Astrocytes are particularly resistant to chemotherapy,35 and it has been described before that they can even connect to melanoma cells and protect them from chemotherapy via gap junction connections, allowing for better cellular homeostasis when cytotoxic stress occurs,36 just as we have suggested for astrocytomas. Finally, a recent study found another potential mechanism of how astrocytes can protect brain metastatic tumor cells: by a phosphatase and tensin homolog (PTEN) loss conferred by PTEN downregulating miRNAs that are present in astrocytic exosomes.37 Together with the known role of PTEN loss in chemoresistance in glioma,38 and the fact that miRNAs can also use gap junctions to travel between glioma cells,39 the question emerges whether glioma cells themselves can use their TM-associated gap junctions to protect themselves by exchanging not only small inorganic molecules like calcium, but also miRNAs, thus orchestrating gene expression in connected cells.3
Potential Avenues for the Development of Therapies Targeting Tumor Microtubes
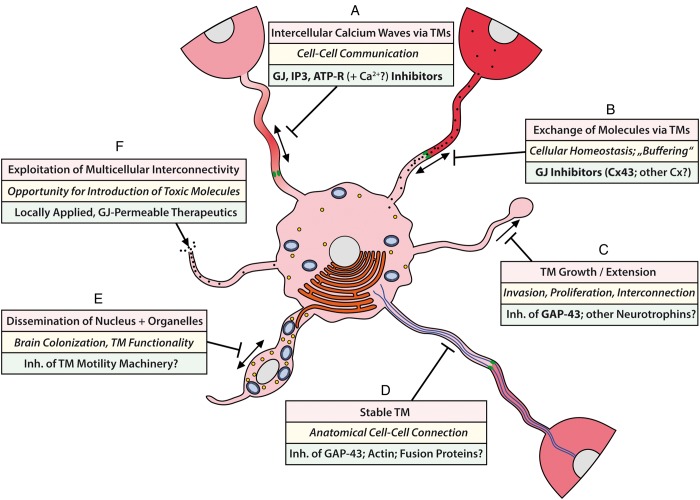
When considering the described morphology and functions of TMs, multiple avenues emerge that might be used to exploit these features for novel antitumor therapies in the future. Figure 2 gives an overview of 6 different options that we feel are promising strategies to target TM integrity and function. These include approaches with existing experimental data supporting principal effectiveness (Fig. 2A–C), but also more theoretical considerations that appear promising for future explorations (Fig. 2D–F).
We have demonstrated that astrocytoma cells use their TMs to communicate with each other over long distances, which includes cells in denser tumor areas, as well as cells invading deep into the normal brain parenchyma, thus connecting the more solid and diffuse areas of one glioma to a large functional syncytium. The tumor cells employ intercellular calcium waves (ICWs) that frequently run over large tumor areas to communicate with each other. ICWs can coordinate the activity of individual cells in a multicellular tissue, thus creating a communicating functional network.40 This includes astrocytes of the normal brain,41,42 neurons,40 and radial glial cells during CNS development.43 While astrocytoma cells used all of the main known strategies for TM-mediated ICW communication (inositol triphosphate [IP3] traveling through gap junctions, and extracellular ATP40), co-recorded normal brain astrocytes in these brain areas used primarily ATP; thus, an opportunity for specific tumor targeting might arise. However, the multiple physiological functions of gap junctions40 need to be considered. Next to gap junction–inhibiting drugs (including carbenoxolone, which is approved for gastric ulcer treatment in some countries and showed strong inhibitory effects on astrocytoma ICWs in our model), inhibitors of other ICW propagating molecules (IP3, ATP receptors) and of other classes of calcium antagonists might be interesting candidates. Nevertheless, a particularly effective inhibition of Cx43 will most likely result in side effects, given the important physiological role of this gap junction protein.3 Other connexins with a potentially higher relevance for the more hypoxic core of astrocytomas, such as Cx46,44 should also be considered—if specific inhibitors can be developed.
Inhibition of gap junctions might also prevent cellular homeostasis between the TM-connected cells of the tumor network, thereby making glioma cells more vulnerable. We have validated that different gap junction–permeable molecules can be exchanged between astrocytoma cells via their TMs, but large molecules that do not pass through gap junctions (such as green or red fluorescent jellyfish proteins) are not exchanged.2 Table 2 gives an overview of molecules and ions that can in principle pass through gap junctions. As outlined above, exchange of the gap junction–permeable calcium ion likely contributes to the radioresistant phenotype of TM-connected tumor cells. In accordance with this, inhibition of functional TMs by GAP-43 deficiency leads not only to a strong downregulation of the Cx43 gap junction protein, but also to a dramatic increase in radioresponse in our astrocytoma animal model.2 Thus, gap junction inhibition is an interesting option that should be particularly explored as a radiosensitizer, perhaps concomitant with other cytotoxic agents.45
Inhibition of the formation of functional TMs by GAP-43 deficiency decreased invasion, proliferation, interconnection, and markedly tumor radioresistance in our astrocytoma mouse model. As outlined above, 1p/19q non-codeleted astrocytomas show a distinct activation pattern of pathways that are involved in early neuronal development. In normal development, these pathways drive the outgrowth of neurites, which leads to neuronal axons and dendrites; they should be less important for the adult brain. This is true for GAP-43 (which, however, might still be involved in experience-dependent plasticity until adulthood46) and potentially also for other neurogenesis pathways, making them interesting principle targets for drug development. Next to screening approaches for small molecule inhibitors, gene therapy might be considered.
TMs have a distinct morphology, but many of their structural features are shared with normal cells in the body, such as high actin levels, and a potent cellular motility machinery. Thus, instead of attacking these structures directly, it appears more promising to study whether interference with GAP-43 (and potentially other proteins of neurite outgrowth) will inhibit not only the formation of TMs, but also their stability. This is unknown so far. Moreover, cell adhesion molecules that are crucial for TMs and support their stability and connectivity should be investigated; their extracellular position would make them feasible drug targets.
The high content of mitochondria and other cell organelles in TMs and the efficient and fast travel of nuclei after mitosis in TMs make one wonder whether the TM motility machinery has specific features, and thus can be targeted without compromising normal cellular functions. This is unknown so far.
Maybe a completely different approach should be considered: not attacking TMs, but exploiting them, thus “hijacking” the network connectivity to distribute local or CSF-injected toxic molecules to distant tumor cells. These molecules, however, need to be gap junction permeable. A list of theoretical options of gap junction–permeable agents is provided in Table 2.
Fig. 2.
Schematic of TM function in glioma cells and potential avenues of how to target them. For details, see text. Bold print, approaches where supportive experimental data regarding significant inhibition exist.2 Upper boxes: Features of TMs; middle boxes: biological functions mediated by TMs; lower boxes: therapeutics suggested to target or exploit TMs. GJ, gap junction(s); ATP-R, ATP receptors.
Table 2.
Molecules that have been found to be gap junction–permeable and thus can in principle be exchanged between TM-connected glioma cells
Gap junction–permeable molecules potentially beneficial for glioma cells
|
Gap junction–permeable molecules potentially detrimental for glioma cells
|
Tumor Microtube Status as a Predictive Biomarker?
While O6-methylguanine-DNA methyltransferase promoter methylation status has emerged as a good biomarker to predict the likelihood of response to temozolomide chemotherapy in gliomas,47,48 a clinically useful predictive biomarker for radiotherapy is lacking. Using the IDH1-R132H mutation specific antibody49 available in (neuro)pathology departments throughout the world, we could show that glioma cell–derived membrane extensions indicative of TMs can be readily detected in standard paraffin sections of resected glioma tissue from patients. Technically, the more invasive tumor parts allowed best measurement of the maximum length of TMs, and this value correlated clearly with 1p/19q status and tumor grade. However, there was a substantial overlap between 1p/19q codeleted and non-codeleted gliomas, particularly in the mid-range of 50–100 µm maximum TM length in standard thin sections.2 This makes it very interesting to explore whether maximum TM length in resected gliomas correlates with response to the subsequent radiotherapy and thus should be prospectively tested in clinical trials as a predictive biomarker.
Importantly, the ability to unequivocally detect tumor cell–derived filamentous structures in the brain parenchyma that is filament rich by itself has proved instrumental to reliably measure TM length in standard sections. Besides the antibody specific for IDH1-R132H, not many other established options exist to achieve this; the antibody specific for BRAF V600E mutation appears to be a feasible method,2 but this mutation is rare in diffuse gliomas. Therefore, new methods that allow unambiguous staining and measurement of tumor cell–derived TMs are warranted to extend these analyses to primary glioblastomas.
Conclusions
The recent discovery of brain-colonizing and interconnecting TMs in astrocytomas, including glioblastomas, has the potential to modify our understanding of the nature of these diseases. More than that, it inspires our imagination of how particularly resistant gliomas might be approached with classes of therapeutics that are different from those established today or currently under investigation. The future will show whether these hopes will materialize into a feasible, brain-penetrable, well-tolerated therapy that is effective in glioma patients. At this moment in time, a TM-targeting therapy given concomitantly with established treatment modalities such as irradiation appears most promising. To target TMs, inhibition of the neurodevelopmental pathways instrumental for TM formation but probably less important for the adult brain should be preferentially considered.
Conflict of interest statement. MO, WW, FW: Patent pending regarding the Cx43 and GAP-43 inhibition to inhibit TM functions and formation.
References
- 1.Zülch KJ. Brain Tumors: Their Biology and Pathology (English edition based on 2nd German edition; translated by A. B. Rothballer and Jerzy Olszewski) New York: Springer; 1957. [Google Scholar]
- 2.Osswald M, Jung E, Sahm F, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–98. [DOI] [PubMed] [Google Scholar]
- 3.Sontheimer H. Brain cancer: tumour cells on neighbourhood watch. Nature. 2015;528(7580):49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sin WC, Crespin S, Mesnil M. Opposing roles of connexin43 in glioma progression. Biochim Biophys Acta. 2012;1818(8):2058–2067. [DOI] [PubMed] [Google Scholar]
- 5.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 7.Cuddapah VA, Robel S, Watkins S, et al. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15(7):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goslin K, Schreyer DJ, Skene JH, et al. Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature. 1988;336(6200):672–674. [DOI] [PubMed] [Google Scholar]
- 9.Skene JH, Jacobson RD, Snipes GJ, et al. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986;233(4765):783–786. [DOI] [PubMed] [Google Scholar]
- 10.Haag D, Zipper P, Westrich V, et al. Nos2 inactivation promotes the development of medulloblastoma in Ptch1(+/–) mice by deregulation of Gap43-dependent granule cell precursor migration. PLoS Genet. 2012;8(3):e1002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aigner L, Arber S, Kapfhammer JP, et al. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83(2):269–278. [DOI] [PubMed] [Google Scholar]
- 12.Zuber MX, Goodman DW, Karns LR, et al. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science. 1989;244(4909):1193–1195. [DOI] [PubMed] [Google Scholar]
- 13.Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat Cell Biol. 2002;4(9):715–719. [DOI] [PubMed] [Google Scholar]
- 14.Yee KT, Simon HH, Tessier-Lavigne M, et al. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24(3):607–622. [DOI] [PubMed] [Google Scholar]
- 15.Rorth P. Communication by touch: role of cellular extensions in complex animals. Cell. 2003;112(5):595–598. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97(5):599–607. [DOI] [PubMed] [Google Scholar]
- 17.Inaba M, Buszczak M, Yamashita YM. Nanotubes mediate niche-stem-cell signalling in the Drosophila testis. Nature. 2015;523(7560):329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiung F, Ramirez-Weber FA, Iwaki DD, et al. Dependence of Drosophila wing imaginal disc cytonemes on decapentaplegic. Nature. 2005;437(7058):560–563. [DOI] [PubMed] [Google Scholar]
- 19.Smith IF, Shuai J, Parker I. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys J. 2011;100(8):L37–L39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Veruki ML, Bukoreshtliev NV, et al. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci U S A. 2010;107(40):17194–17199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou E, Fujisawa S, Morozov A, et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One. 2012;7(3):e33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherer NM. Long-distance relationships: do membrane nanotubes regulate cell-cell communication and disease progression? Mol Biol Cell. 2013;24(8):1095–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesseling P, van den Bent M, Perry A. Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):809–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiestler B, Capper D, Sill M, et al. Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128(4):561–571. [DOI] [PubMed] [Google Scholar]
- 25.Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 28.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wharton SB, Maltby E, Jellinek DA, et al. Subtypes of oligodendroglioma defined by 1p,19q deletions, differ in the proportion of apoptotic cells but not in replication-licensed non-proliferating cells. Acta Neuropathol. 2007;113(2):119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tombal B, Denmeade SR, Gillis JM, et al. A supramicromolar elevation of intracellular free calcium ([Ca(2+)](i)) is consistently required to induce the execution phase of apoptosis. Cell Death Differ. 2002;9(5):561–573. [DOI] [PubMed] [Google Scholar]
- 32.McFerrin MB, Turner KL, Cuddapah VA, et al. Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am J Physiol Cell Physiol. 2012;303(10):C1070–C1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le HT, Sin WC, Lozinsky S, et al. Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem. 2014;289(3):1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Cui J, Sun X, et al. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 2011;18(4):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wick A, Wick W, Hirrlinger J, et al. Chemotherapy-induced cell death in primary cerebellar granule neurons but not in astrocytes: in vitro paradigm of differential neurotoxicity. J Neurochem. 2004;91(5):1067–1074. [DOI] [PubMed] [Google Scholar]
- 36.Lin Q, Balasubramanian K, Fan D, et al. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia. 2010;12(9):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Zhang S, Yao J, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knobbe CB, Merlo A, Reifenberger G. PTEN signaling in gliomas. Neuro Oncol. 2002;4(3):196–211. [PMC free article] [PubMed] [Google Scholar]
- 39.Suzhi Z, Liang T, Yuexia P, et al. Gap junctions enhance the antiproliferative effect of microRNA-124-3p in glioblastoma cells. J Cell Physiol. 2015;230(10):2476–2488. [DOI] [PubMed] [Google Scholar]
- 40.Leybaert L, Sanderson MJ. Intercellular Ca(2+) waves: mechanisms and function. Physiol Rev. 2012;92(3):1359–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuchibhotla KV, Lattarulo CR, Hyman BT, et al. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323(5918):1211–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornell-Bell AH, Finkbeiner SM, Cooper MS, et al. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247(4941):470–473. [DOI] [PubMed] [Google Scholar]
- 43.Weissman TA, Riquelme PA, Ivic L, et al. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43(5):647–661. [DOI] [PubMed] [Google Scholar]
- 44.Hitomi M, Deleyrolle LP, Mulkearns-Hubert EE, et al. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep. 2015;11(7):1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy SF, Varghese RT, Lamouille S, et al. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res. 2016;761:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20(2):84–91. [DOI] [PubMed] [Google Scholar]
- 47.Wick W, Weller M, van den Bent M, et al. MGMT testing—the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372–385. [DOI] [PubMed] [Google Scholar]
- 48.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 49.Capper D, Zentgraf H, Balss J, et al. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118(5):599–601. [DOI] [PubMed] [Google Scholar]
- 50.Weber PA, Chang HC, Spaeth KE, et al. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87(2):958–973. [DOI] [PMC free article] [PubMed] [Google Scholar]