Abstract
Advancement of engineered ear in clinical practice is limited by several challenges. The complex, largely unsupported, three-dimensional auricular neocartilage structure is difficult to maintain. Neocartilage formation is challenging in an immunocompetent host due to active inflammatory and immunological responses. The large number of autologous chondrogenic cells required for engineering an adult human-sized ear presents an additional challenge because primary chondrocytes rapidly dedifferentiate during in vitro culture. The objective of this study was to engineer a stable, human ear-shaped cartilage in an immunocompetent animal model using expanded chondrocytes. The impact of basic fibroblast growth factor (bFGF) supplementation on achieving clinically relevant expansion of primary sheep chondrocytes by in vitro culture was determined. Chondrocytes expanded in standard medium were either combined with cryopreserved, primary passage 0 chondrocytes at the time of scaffold seeding or used alone as control. Disk and human ear-shaped scaffolds were made from porous collagen; ear scaffolds had an embedded, supporting titanium wire framework. Autologous chondrocyte-seeded scaffolds were implanted subcutaneously in sheep after 2 weeks of in vitro incubation. The quality of the resulting neocartilage and its stability and retention of the original ear size and shape were evaluated at 6, 12, and 20 weeks postimplantation. Neocartilage produced from chondrocytes that were expanded in the presence of bFGF was superior, and its quality improved with increased implantation time. In addition to characteristic morphological cartilage features, its glycosaminoglycan content was high and marked elastin fiber formation was present. The overall shape of engineered ears was preserved at 20 weeks postimplantation, and the dimensional changes did not exceed 10%. The wire frame within the engineered ear was able to withstand mechanical forces during wound healing and neocartilage maturation and prevented shrinkage and distortion. This is the first demonstration of a stable, ear-shaped elastic cartilage engineered from auricular chondrocytes that underwent clinical-scale expansion in an immunocompetent animal over an extended period of time.
Introduction
Engineered auricle is a promising alternative to current ear reconstructive options. However, several challenges limit the further advancement of engineered ear in clinical practice.1–4 The greatest challenge is to maintain the complex, largely unsupported, three-dimensional (3D) auricular neocartilage structure. After implantation, engineered auricular cartilage is subjected to strong mechanical forces during maturation and wound healing, which can result in shrinkage and distortion. To overcome these forces, internal supportive scaffolds made of various materials and additional external molds or stenting have been used with varying degrees of success.5–13
We proposed a composite ear-shaped scaffold composed of porous collagen, which supports chondrocyte attachment and matrix deposition, and an embedded titanium wire, which prevents shrinkage and distortion.14,15 The size and shape of engineered ears were maintained for up to 12 weeks in immunocompromised rodents. Moreover, the flexibility of engineered ears was preserved and the titanium framework had no negative effect on the quality of engineered cartilage. Here, we describe the performance of our composite ear-shaped scaffold in immunocompetent animals in longer-term studies.
The second challenge for advancing, engineered ear technology is to reproducibly generate high-quality elastic cartilage in immunocompetent animals. Active inflammatory and immunological responses in the subcutaneous environment of an immunocompetent host negatively affect chondrogenesis.16,17 Few publications describe the autologous auricular cartilage generation in immunocompetent animals; the reported neocartilage quality has been inconsistent, and its long-term stability has not been demonstrated. Improvement of neocartilage properties and alleviation of inflammation after implantation have been achieved after in vitro preimplantation culture.18–20 Following this approach, we successfully engineered autologous elastic cartilage in a disk shape, with demonstrated stability up to 12 weeks in sheep.21 In this study, engineered human ear-shaped, elastic cartilage was followed for up to 20 weeks in sheep.
Another limitation hindering the advancement of engineered ear is the lack of sufficient number of autologous chondrogenic cells. Autologous chondrocytes remain the most reliable and the only practical source for clinical applications of engineered cartilage while stem cells sources are being explored. A 300- to 500-fold increase in chondrocyte numbers is needed because of low cell yield from a limited size cartilage biopsy, low cellularity of cartilage tissue, and the large number of cells required to engineer replacement cartilanginous tissues. Dedifferentiation during chondrocyte expansion results in an irreversible loss of chondrogenic properties. Preservation of cartilage-forming ability of chondrocytes during expansion has been addressed in laboratory experiments22; scale-up and regulatory challenges must be considered for translation of experimental findings into healthcare practice.
Several types of procedures have been explored to produce large numbers of autologous chondrogenic cells using clinically appropriate methods. One promising approach involves the addition of either freshly isolated or cryopreserved primary passage 0 (P0) chondrocytes to expanded dedifferentiated chondrocytes. In vitro experiments published to date demonstrate potential but used only articular chondrocytes.23–27 In another approach, chondrocyte dedifferentiation during in vitro expansion was prevented by supplementing culture medium with basic fibroblast growth factor (bFGF).28,29 We successfully used these protocols to generate engineered elastic cartilage in immunocompromised mice using auricular chondrocytes that underwent clinically relevant expansion.30
Multiple efforts to engineer ear-shaped cartilage have been extensively reviewed by our group and others.1,2 To date, only a few reports described attempts to engineer autologous auricular cartilage in the shape of a human ear from extensively expanded chondrocytes in immunocompetent animals with varying results.8,10,31 To the best of our knowledge, stable contiguous auricular neocartilage has not been demonstrated with preserved size and shape of a human ear and engineered from extensively, expanded autologous chondrocytes.
Toward our ultimate goal of developing a living replacement ear for human auricular reconstruction, the objective of this exploratory study was to engineer stable human ear-shaped cartilage in an immunocompetent animal model using extensively expanded chondrocytes. Sheep auricular chondrocytes were proliferated in vitro to achieve clinically relevant expansion. Two approaches were evaluated to preserve chondrogenic ability, and one approach was selected to engineer ear-shaped cartilage. Human ear-shaped scaffolds for engineered auricles were made from porous collagen with an embedded titanium wire framework. Autologous chondrocyte-seeded scaffolds were implanted subcutaneously in sheep after 2 weeks in vitro incubation. The quality of the resulting neocartilage, its stability, and retention of the original ear size and shape were evaluated 6, 12, and 20 weeks postimplantation.
Materials and Methods
Experimental design
The study was conducted in two stages to engineer stable ear-shaped elastic cartilage in an immunocompetent animal model using extensively expanded chondrocytes (Table 1). First, two approaches were evaluated to obtain extensively expanded chondrocytes with preserved cartilage-forming ability. Based on the results of the first experiment, one of the approaches was selected to engineer ear-shaped cartilage. All procedures were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital and performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Table 1.
Experimental Design
| 6 Weeks | 12 Weeks | 20 Weeks | ||
|---|---|---|---|---|
| Experiment 1: Engineer cartilage from expanded chondrocytes | ||||
| Sheep, n = 2 | P2+P0 | Disk, n = 6 | Disk, n = 6 | |
| P1 | Disk, n = 4 | Disk, n = 4 | ||
| P2 | Disk, n = 4 | Disk, n = 4 | ||
| Sheep, n = 2 | P2+bFGF | Disk, n = 6 | Disk, n = 6 | |
| P1 | Disk, n = 4 | Disk, n = 4 | ||
| P2 | Disk, n = 4 | Disk, n = 4 | ||
| Experiment 2: Evaluate stability of cartilage and retention of ear shape | ||||
| Sheep, n = 2 | P1 | Disk, n = 6 | Disk, n = 6 | Disk, n = 6 |
| Ear, n = 2 | ||||
| Sheep, n = 2 | P2+bFGF | Disk, n = 6 | Disk, n = 6 | Disk, n = 6 |
| Ear, n = 2 | ||||
bFGF, basic fibroblast growth factor; P0, passage 0; P1, passage 1; P2, passage 2.
Experiment 1: Obtain sufficient number of extensively expanded chondrocytes with preserved cartilage-forming ability: impact of P0 chondrocytes versus exogenous bFGF
Auricular chondrocytes from two sheep were expanded in monolayer culture to passage 2 (P2) and mixed with cryopreserved P0 chondrocytes of the same origin (P2+P0) at an 80:20 ratio23 before seeding onto the disk-shaped scaffolds (n = 6 per sheep, three per time point). In an alternative approach, chondrocytes harvested from another two sheep were expanded to P2 with 5 ng/mL bFGF (R&D Systems, Minneapolis, MN) medium supplementation (P2+bFGF). These bFGF-expanded chondrocytes were seeded onto the disk-shaped scaffolds (n = 6, three per time point); the constructs were cultured in vitro in dynamic conditions for 2 weeks in medium without bFGF. Disk-shaped scaffolds seeded with moderately expanded P1 chondrocytes (n = 4 per sheep) and dedifferentiated P2 chondrocytes (n = 4 per sheep) served as controls in all animals. Implants were retrieved in a survival surgery at 6 weeks and after animal sacrifice at 12 weeks.
Experiment 2: Evaluate stability of cartilage engineered from extensively expanded chondrocytes and retention of the original ear size and shape
Chondrocytes harvested from two sheep were expanded to P2 in monolayer in the culture medium supplemented with 5 ng/mL of bFGF (P2+bFGF), as described above, and chondrocytes were seeded on ear-shaped scaffolds (n = 1 per animal) and disks (n = 9 per animal, three per time point). In two control sheep, chondrocytes expanded to P1 in standard culture medium were seeded on ear-shaped scaffolds (n = 1 per animal) and disks (n = 9 per animal, three per time point). Disk implants were retrieved during survival surgeries at 6 and 12 weeks to validate cartilage formation at intermediate time points, and the final disks were retrieved along with the ear-shaped constructs after animal sacrifice at 20 weeks.
Chondrocyte isolation and culture
Chondrocytes were isolated from sterilely harvested sheep auricular cartilage (n = 8, Polypay, female, 11.5 ± 0.8 months old) as previously described.14,21 Briefly, finely minced cartilage was digested with 0.1% collagenase type II (Worthington Biochemical Corp., Lakewood, NJ) at 37°C for 16 h. Freshly isolated chondrocytes were plated at 3 × 103 cells/cm2 into vented cell culture flasks (BD Falcon, Franklin Lakes, NJ) in Ham's F12 medium (Invitrogen Co., Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich Co., St. Louis, MO), 100 U/mL of penicillin, 100 μg/mL of streptomycin, 292 μg/mL of l-glutamine, and 0.1 mM nonessential amino acids (all from Invitrogen Co.).
Upon reaching confluency the chondrocytes were further passaged. The chondrocytes were detached with 0.05% trypsin-ethylenediaminetetraacetic acid (EDTA; Mediatech, Inc., Manassas, VA) and replated at the same density into vented cell culture flasks (BD Falcon). At each passage, a cell aliquot was frozen for RNA extraction. Population doublings (PD) were calculated using the equation PD = log10(N/N0) × 3.33, where N is the number of harvested and N0 the number of plated cells. Excess P0 chondrocytes were cryopreserved using standard techniques.
Scaffold preparation and cell seeding
Two scaffold geometries were utilized in the study: disk and ear-shaped scaffolds. Porous bovine dermis-derived type I collagen sheets, 2 mm thick, were provided by DSM Biomedical Corp. (Exton, PA). Ten-millimeter-diameter disks were made using dermal biopsy punches (Acuderm, Inc., Ft. Lauderdale, FL).
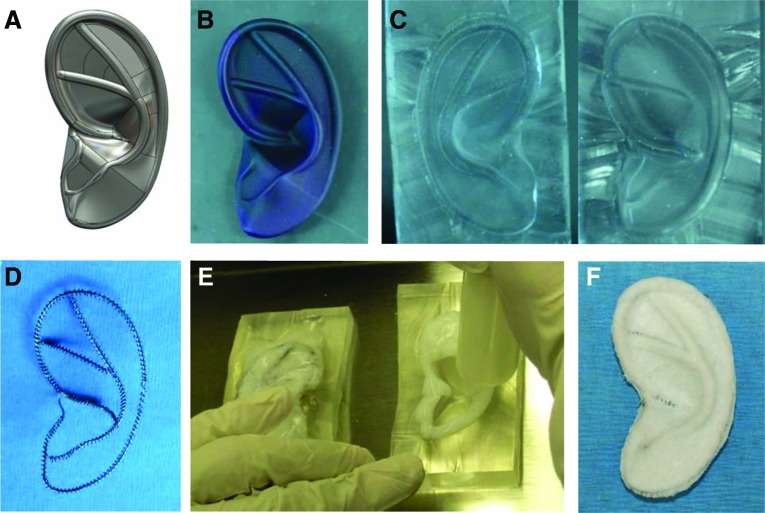
Composite ear-shaped scaffolds were designed and fabricated as previously described (Fig. 1).15 A digital image of an adult human outer ear (SolidWorks software; SolidWorks Corp., Waltham, MA) was modified based on input from a facial plastic surgeon, and a 3D plastic ear model was printed (Realize, Inc., Noblesville, IN). A two-piece clamshell mold was manufactured from poly(dimethylsiloxane) (PDMS; Dow Corning Corp., Midland, MI) using this 3D ear model. Titanium wire frameworks, manually produced to replicate the shape of the prominent ear contours, consisted of an inner core surrounded by an outer coil. To create ear-shaped scaffolds, these wire frameworks were embedded in fibrillar collagen slurry (bovine dermis type I collagen; DSM Biomedical Corp.) within the PDMS molds and subjected to lyophilization. Before cell seeding, all scaffolds were sterilized with cold ethylene oxide gas.
FIG. 1.
Composite ear-shaped scaffold fabrication. Digital image of an adult human ear was modified (A) and a three-dimensional plastic ear model printed (B). A two-piece clamshell mold (C) was manufactured using this model. Titanium wire framework (D) was inserted into the mold, and the collagen slurry was cast, embedding the framework (E). The resulting composite collagen scaffold with embedded wire framework (F). Color images available online at www.liebertpub.com/tea
Chondrocytes were suspended in the culture medium at 50 × 106 cells/mL; 100 × 106 cells were seeded on each ear-shaped scaffold and 12 × 106 cells on a disk-shaped scaffold. Half of the cell suspension was pipetted onto the each side of a scaffold in a 20-min interval; the scaffold was then flipped every 20 min for 3 h to facilitate a more uniform distribution of cells. Ear-shaped constructs were cultured in wide mouth, 500-mL polypropylene jars with screw-top lids (Fisher Scientific, Waltham, MA). For gas exchange, 4-mm-diameter holes were drilled into the lid and the bottom of the containers and covered with a silicone film, which was glued to the cap and bottom of the container as previously described.15
Disk-shaped constructs were cultured in ultralow attachment six-well plates (Dow Corning Corp.). Ear and disk-shaped construct culture was performed on a Talboys orbital shaker (Henry Troemner LLC, Thorofare, NJ) at 40 rpm for 2 weeks in standard incubator conditions. The culture medium supplemented with 50 μg/mL of ascorbic acid (Wako Pure Chemical Industries Ltd., Osaka, Japan) was changed twice a week. Five to seven days before implantation, FBS in the culture medium was replaced with autologous sheep serum. To obtain autologous serum, 100 mL of blood was drawn during ear cartilage harvest and allowed to clot at 37°C for 30–60 min; serum was decanted into a new centrifuge tube and centrifuged at 1800 g for 10 min. The supernatant containing the serum was collected and stored at −20°C until use.
Construct implantation
Autologous constructs were implanted subcutaneously in the dorsolateral aspect of the sheep neck. The animal was sedated with an intramuscular injection of 4.4 mg/kg of telazol and, after induction of endotracheal intubation, was maintained on 1–2% isoflurane in oxygen for the surgery duration. A preoperative dose of 0.3 mg/kg buprenorphine was administered intramuscularly for intraoperative analgesia. Incisions (10 cm) were made starting at the condyle of the mandible and extending caudally on both sides of the neck. Individual subcutaneous pockets were created through blunt dissection and one construct (disk or ear-shaped) was implanted into each pocket. Titanium rings (12 mm diameter) with surrounding disk constructs were implanted to facilitate identification at explant. The incisions were closed with absorbable sutures. Postoperative analgesia was achieved with 1 mg/kg of flunixin meglumine for 72 h postoperatively.
Gross evaluation
Surrounding connective tissue was carefully dissected from all retrieved implants. Digital images were obtained of ear-shaped constructs and length and width measured by three researchers individually using Adobe Photoshop tools.
Histological and immunochemical analyses
Full-thickness, 7-mm-diameter biopsies were punched at four locations in engineered ears; each biopsy and each disk-shaped implant were bisected. One-half of each sample was fixed in 10% buffered formalin for histology, and the other half was snap frozen and stored at −80°C. Paraffin sections were cut at 8 μm and stained with hematoxylin and eosin (H&E), safranin O, toluidine blue, and elastin (Verhoeff's Elastin Staining Kit; American MasterTech Scientific, Lodi, CA) using standard protocols. Collagen was detected with mouse anti-human collagen type II antibody (1:100, clone 6B3; EMD Millipore, Temecula, CA) and mouse anti-human collagen type I antibody (1:100, clone I-8H5; EMD Millipore) after pretreatment with 1 mg/mL of pepsin as described before.21
Biochemical assays
For the DNA content, frozen samples were weighed, and DNA extracted and purified with the DNeasy Kit (Qiagen, Inc., Valencia, CA). Total DNA content was determined using the PicoGreen dsDNA assay (Invitrogen Co.). For glycosaminoglycan (GAG) determination, samples were lyophilized for 24 h and digested overnight at 60°C in 125 μg/mL of type III papain (Sigma-Aldrich Co.) in phosphate buffer with EDTA, pH 6.5. GAG content was determined using the Blyscan Glycosaminoglycan Assay Kit (Biocolor Ltd., Carrickfergus, United Kingdom). Elastin content was determined using the Fastin Elastin Assay Kit (Biocolor Ltd.) after lyophilized samples were digested in 0.25 M oxalic acid at 95°C.
Gene expression analysis
Total RNA was extracted with the RNeasy Mini Kit (Qiagen Sciences, Gaithersburg, MD) and the concentration determined by absorbance. First-strand complementary DNA (cDNA) was synthesized using SuperScript III (Life Technologies, Carlsbad, CA). Relative COL1A1, COL2A1, COL3A1, and versican (VCAN) gene expression profiles were determined using end point polymerase chain reaction (PCR). Primer pairs were designed to span introns.
Each 25 μL of reaction contained template cDNA derived from 10 ng of input RNA and 0.5 units of Taq DNA polymerase in 1× ThermoPol Buffer (New England Biolabs, Ipswich, MA). Annealing temperatures are listed in Table 2. Ten microliters of each reaction product was then electrophoresed on a 2% agarose gel. Specificity of the product was confirmed by fragment length of the observed band. Relative expression levels were determined in ImageJ and normalized using GAPDH for each cDNA sample. For graphing, the mean expression level of COL2A1 in P0 chondrocytes was assigned a value of 1.
Table 2.
Oligonucleotide Primers Used for RT-PCR, Including Annealing Temperatures and Size of the Amplified Products
| Gene | Primer | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| Sheep COL1A1 | Forward: AGG GAC CCA AAG GAG ACA CT | 55 | 198 |
| Reverse: GCA CGG AAA TTC CTG TTG AT | |||
| Sheep COL2A1 | Forward: CGT CAC CTA CCA CTG CAA GA | 58 | 219 |
| Reverse: GGG AGA CGT GAG GTC TTC TG | |||
| Sheep COL3A1 | Forward: CTT TTC GCT CTG CTT CAT CC | 55 | 216 |
| Reverse: TTC TCC AAA CGG GAT TTC AG | |||
| Sheep VCAN | Forward: CCTGTTGTAGAAAATGCCAAGACC | 58 | 344 |
| Reverse: GTTAGGACTTTGGCTGAAATGATGA | |||
| GAPDH | Forward: CTC ACT GGC ATG GCC TTC CG | 55 | 293 |
| Reverse: ACC ACC CTG TTG CTG TAG CC |
COL1A1, collagen type I; COL2A1, collagen type II; COL3A1, collagen type III; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcription polymerase chain reaction; VCAN, versican.
Statistical analysis
Values are expressed as mean ± standard deviation. Statistical analysis was performed using Student's t-test; p < 0.05 was considered significant.
Results
To engineer stable ear-shaped elastic cartilage in an immunocompetent animal model, two approaches were evaluated to obtain extensively expanded chondrocytes with preserved cartilage-forming ability. The most promising approach was used to engineer ear-shaped cartilage.
Extensively expanded auricular chondrocytes produced neocartilage in vivo: impact of P0 chondrocytes versus exogenous bFGF
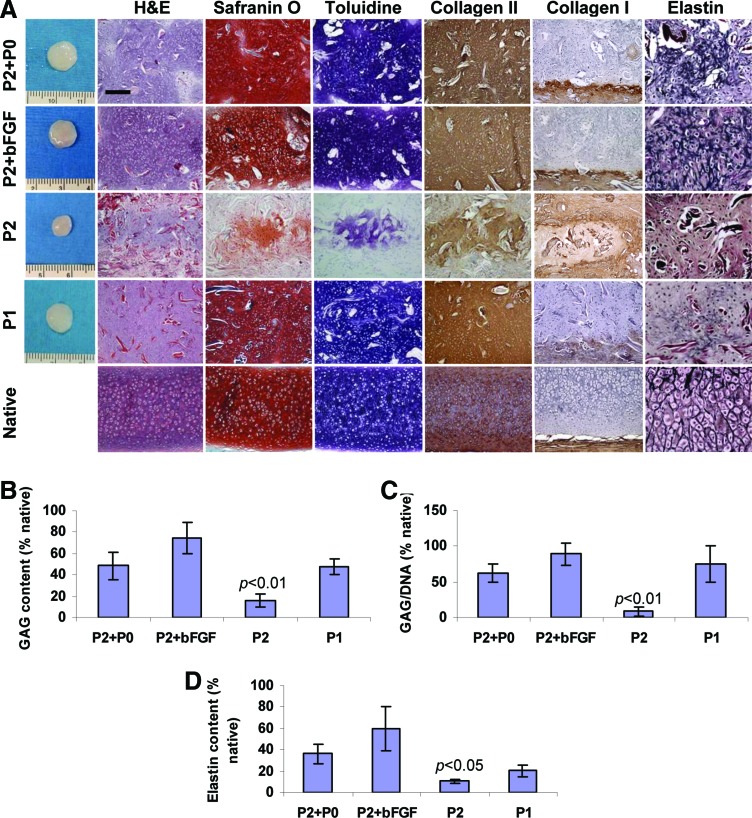
Both approaches to produce neocartilage from extensively expanded chondrocytes were successful in disk-shaped cartilaginous constructs (Fig. 2A). After 12 weeks implantation in autologous sheep, high-quality neocartilage formed from P2+P0 chondrocytes except for elastin expression. Histologically, this neocartilage looked similar to that produced from moderately expanded P1 chondrocytes. Cells were located in lacunae structures and were surrounded by cartilaginous matrix. The matrix stained intensely for GAG, based on toluidine blue and safranin O staining as well as for type II collagen. Type I collagen was expressed in the capsule surrounding engineered cartilage. Elastin fibers were sparse and stained faintly.
FIG. 2.
Histological and biochemical evaluation of neocartilage engineered from extensively (passage 2 [P2]) and moderately (P1 [passage 1]) expanded chondrocytes in fibrous collagen disks after 12 weeks implantation in sheep compared to native sheep auricular cartilage. High-quality neocartilage was engineered from P2+passage 0 (P0) and from P2+basic fibroblast growth factor (bFGF) chondrocytes (A). This cartilage was comparable to neocartilage engineered from P1 chondrocytes. Control P2 chondrocytes formed poor quality cartilage. Glycosaminoglycan (GAG) content and GAG/DNA ratio (B, C) and elastin content (D) confirmed histological findings (n = 4 for each group). Residual scaffold fibers stained red on hematoxylin and eosin (H&E)-stained sections. Scale bar: 200 μm except 100 μm for elastin. Color images available online at www.liebertpub.com/tea
Neocartilage produced from P2+bFGF chondrocytes demonstrated marked elastin fiber formation in addition to characteristic morphological cartilage features. The intensity of the elastin staining was similar to that in the native sheep auricular cartilage. Control samples produced from P2 chondrocytes expanded in standard medium were markedly smaller; histologically, neocartilage was sparse, noncontiguous, and staining intensity was low.
Quantitative extracellular matrix analysis (Fig. 2B–D) confirmed histological findings. The highest GAG content was produced from P2+bFGF chondrocytes (74.5% ± 14.1% of native) followed by P2+P0 and P1 groups. GAG content in the P2 group was significantly lower than in other groups (p < 0.01). Similar results were obtained for the GAG/DNA ratio; it was the highest in the P2+bFGF group at 88.9% ± 15.9% of native. The lowest GAG/DNA ratio was in the P2 group, relative to other groups (p < 0.01). P2+bFGF chondrocytes produced cartilage with the highest elastin content (120.7 ± 40.9 μg/mg dry weight). Elastin content in the P2 group was significantly lower than in other groups (p < 0.05). In native sheep auricular cartilage, GAG content measured 124.4 ± 27.6 μg/mg dry weight, DNA content measured 124.4 ± 41.7 ng/mg wet weight, and GAG/DNA ratio was 1.1 ± 0.3. Elastin content was 201.4 ± 43.3 μg/mg dry weight.
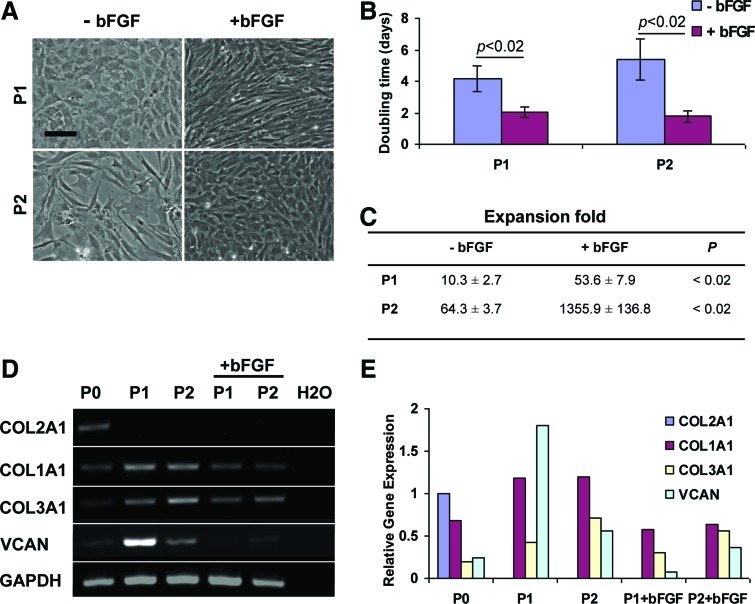
bFGF supplementation during chondrocyte expansion in monolayer culture had an impact on cell morphology, proliferation rate, and collagen gene expression
Morphology
Chondrocytes became smaller and more elongated within the first several days after culture medium was supplemented with bFGF (Fig. 3A). During the same period, chondrocytes cultured in standard medium preserved their size and rounded shape. By P2, chondrocytes cultured with bFGF supplementation regained a more rounded shape but remained small. Chondrocytes cultured in standard medium became larger and acquired a fibroblast-like morphology.
FIG. 3.
Effect of bFGF culture medium supplementation on sheep auricular chondrocytes. Chondrocyte morphology changed (A), doubling times were significantly reduced (B), and expansion fold significantly increased (C) in the presence of bFGF compared to standard conditions (n = 4 for each group). Detectable levels of COL2A1, COL1A1, COL3A1, and versican (VCAN) were present in P0 chondrocytes (D). COL1A1, COL3A1, and VCAN were upregulated in expanded chondrocytes. bFGF supplementation resulted in COL1A1, COL3A1, and VCAN downregulation but COL2A1 was not rescued (E). Scale bar: 100 μm. Color images available online at www.liebertpub.com/tea
Expansion
Compared to standard conditions, bFGF supplementation significantly reduced population doubling times (p < 0.02) and resulted in significantly greater cumulative cell expansion fold (p < 0.02) regardless of passage number (Fig. 3B, C). Only chondrocytes expanded with bFGF supplementation, not in standard medium, achieved the target, clinically relevant, cumulative expansion exceeding 500-fold at P2.
Gene expression
End point PCR demonstrated that P0 chondrocytes expressed the three collagen type genes COL2A1, COL1A1, and COL3A1, and VCAN (Fig. 3D, E). COL2A1 was downregulated past the threshold of detection, and COL1A1, COL3A1, and VCAN were upregulated in low (P1) and higher (P2) passage chondrocytes expanded in standard conditions confirming their dedifferentiation. Supplementation with bFGF resulted in COL1A1, COL3A1, and VCAN downregulation for both P1 and P2 chondrocytes compared to cells expanded in standard conditions; however, COL2A1 expression remained below the detection limit.
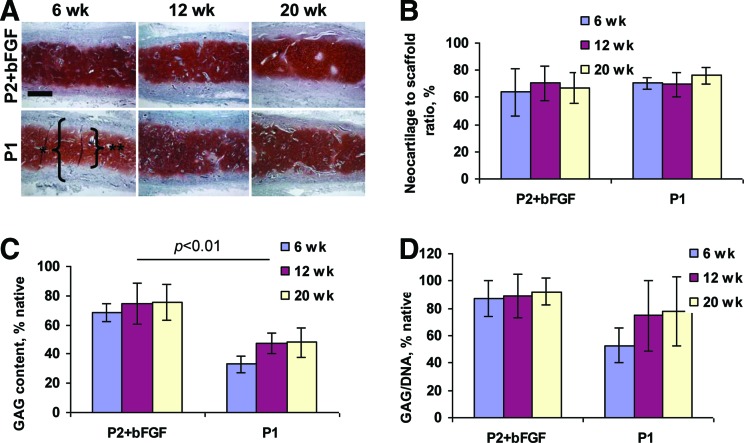
Elastic neocartilage produced from chondrocytes extensively expanded in the presence of bFGF was stable in long-term in vivo studies
Neocartilage was stable as demonstrated by histological cross sections of disk-shaped constructs explanted at 6, 12, and 20 weeks and stained with safranin O for GAG (Fig. 4A). Neocartilage was markedly thinner than the scaffold at 6 weeks. However, the neocartilage to scaffold thickness ratio remained unchanged with increased implantation time, both in constructs made with extensively expanded P2+bFGF and with control moderately expanded P1 chondrocytes (Fig. 4B).
FIG. 4.
Stability of neocartilage engineered from extensively expanded P2+bFGF and moderately expanded P1 chondrocytes. High-quality neocartilage formed from both P2+bFGF and P1 chondrocytes at all time points. * scaffold, ** neocartilage (A). Neocartilage to scaffold ratio remained unchanged throughout the experiment (B). GAG (C) and GAG/DNA ratio (D) remained stable in neocartilage engineered from P2+bFGF chondrocytes (n = 4 for each group). Safranin O stain, scale bar: 500 μm. Color images available online at www.liebertpub.com/tea
All constructs stained intensely with safranin O indicating high GAG content. This was confirmed by GAG quantification and GAG/DNA calculation (Fig. 4C, D); the GAG content remained unchanged during the observation period (p > 0.1) in both P2+bFGF and control P1 groups. However, GAG content was higher in P2+bFGF constructs than in control P1 constructs at all time points (p < 0.01) and approximated that of native sheep auricular cartilage. GAG/DNA ratio was also higher in the P2+bFGF group, at all time points and remained constant.
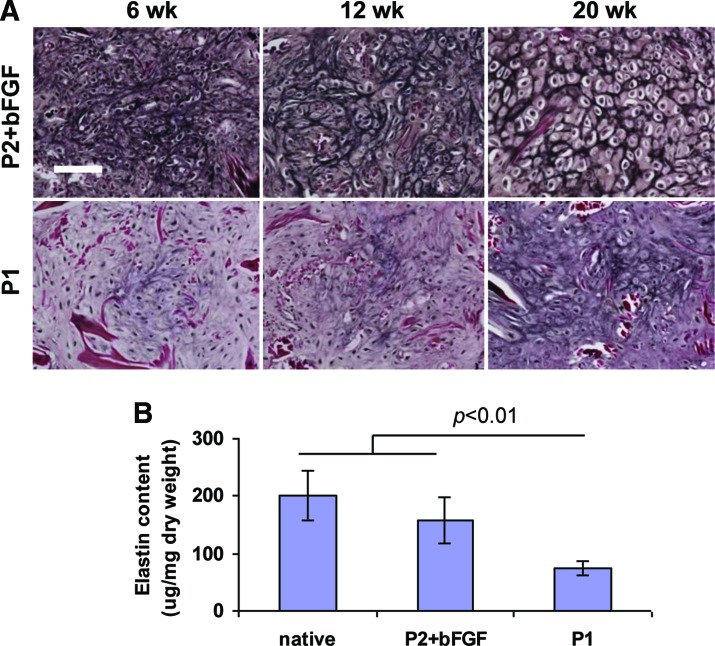
Elastin expression was more prominent in P2+bFGF constructs at all time points. First elastin expression was seen in P2+bFGF constructs at 6 weeks; in P1 constructs, it was delayed to 20 weeks (Fig. 5A). Elastin stained more intensely and more expansively with time. At 20 weeks implantation, elastin content reached 78.1% ± 19.5% of native sheep cartilage in constructs engineered from P2+bFGF chondrocytes (Fig. 5B). Elastin content was significantly less (p < 0.01) in P1 constructs. In native sheep auricular cartilage, elastin content was 201.4 ± 43.2 μg/mg dry weight.
FIG. 5.
Elastin expression in neocartilage engineered from extensively expanded P2+bFGF and moderately expanded P1 chondrocytes. Elastin staining was most prominent in P2+bFGF constructs at all time points; first elastin expression was seen in P2+bFGF constructs at 6 weeks and in P1 constructs it was delayed to 20 weeks (A). Elastin content approximated that of native cartilage in P2+bFGF constructs and was significantly lower in P1 constructs at 20 weeks postimplantation (n = 4 for each group) (B). Verhoeff's elastin stain, scale bar: 100 μm. Color images available online at www.liebertpub.com/tea
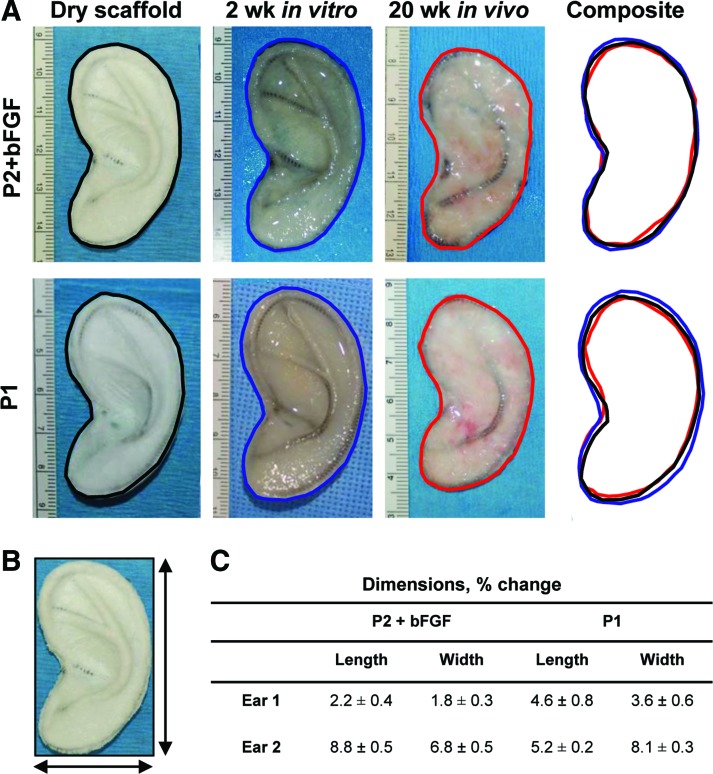
Ear-shaped constructs had minimal shrinkage and distortion after 20 weeks in vivo
The overall ear shape was maintained in all constructs after 20 weeks implantation in sheep (Fig. 6A). Slight swelling was observed during 2 weeks in vitro culture, regardless of the chondrocyte source. Some shrinkage occurred after 20 weeks implantation compared to the dry scaffold. The length and width of the ear construct were measured as shown in Figure 6B; the decrease in dimensions varied between constructs but did not exceed 10% at the 20-week time point (Fig. 6C).
FIG. 6.
Gross images of representative ear-shaped constructs and size comparison. Same ear scaffolds were imaged before chondrocyte seeding, after 2 weeks in vitro culture, and after 20 weeks in vivo (A). Length and width were measured (B). Decrease in dimensions did not exceed 10% at the 20-week time point (C). Color images available online at www.liebertpub.com/tea
High-quality elastic neocartilage was uniformly distributed throughout the ear scaffold after 20 weeks in vivo
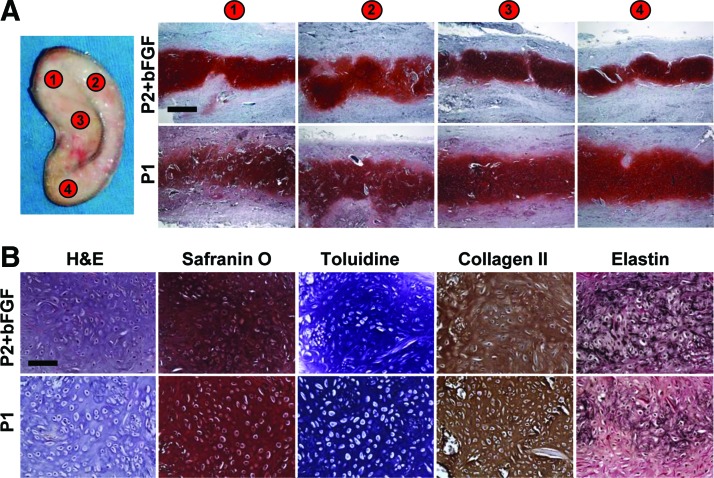
The neocartilage quality within ear constructs was evaluated in four full-thickness biopsies obtained throughout the construct (Fig. 7A). All sections stained intensely with safranin O indicating high GAG content. High-power images demonstrate neocartilage morphology similar to native cartilage and intensive additional toluidine, collagen type II, and elastin cartilage-specific stainings (Fig. 7B). More elastin was detected in P2+bFGF constructs.
FIG. 7.
Neocartilage quality in ear-shaped constructs after 20 weeks implantation. Neocartilage in all four biopsies taken throughout ear scaffold stained intensely with safranin O (A). Neocartilage from P2+bFGF and from P1 chondrocytes was of high quality as confirmed by cartilage-specific stains (B). The elastin was more prominent in P2+bFGF neocartilage. Scale bars: 500 μm (A) and 100 μm (B). Color images available online at www.liebertpub.com/tea
Discussion
In our earlier work, we systematically addressed several major challenges that have prevented the further advancement of engineered auricle in clinical practice.14,15,21,30 This manuscript describes our exploratory study to engineer stable human ear-shaped elastic cartilage in an immunocompetent animal model using chondrocytes that underwent clinically relevant expansion.
To obtain a sufficient population of chondrocytes with preserved cartilage-forming ability, sheep auricular chondrocytes were expanded with and without bFGF supplementation. Cells were plated at low density for two-dimensional (2D) culture to decrease auricular chondrocyte dedifferentiation and achieve greater cell numbers in shorter time.32 As expected, significantly higher expansion was achieved with bFGF supplementation.28,32 To reverse dedifferentiation of cells cultured without bFGF, we combined them with cryopreserved P0 chondrocytes at the time of scaffold seeding. Similarly to our previously reported studies in immunodeficient nude mice,30 better quality cartilage was engineered from P2+bFGF than from P2+P0 chondrocytes in sheep, based on histological findings and biochemical analysis.
The mechanism of expanded chondrocyte redifferentiation in the presence of P0 chondrocytes is poorly understood. The factors secreted by P0 chondrocytes that are responsible for chondrocyte phenotype recovery have not been identified. The ability of P0 chondrocytes to induce redifferentiation depends on donor age; P0 chondrocytes from older donors failed to induce redifferentiation.25 Most importantly, redifferentiation has not been demonstrated when human P0 chondrocytes were used.25,26,30 Therefore, the applicability of this approach for future clinical use remains questionable, warranting further investigation.
In contrast, bFGF has been successfully used to expand human autologous chondrocytes in several clinical applications outside the United States.33,34 The effect of bFGF on maintaining chondrocyte differentiation during standard 2D culture remains poorly understood despite significant research efforts.28,35,36 Chondrocyte morphological changes during expansion may be due to cytoskeleton reorganization in the presence of bFGF.28 Our gene expression analysis demonstrated that the dedifferentiation genes COL1, COL3, and VCAN were downregulated in auricular P2+bFGF chondrocytes compared to chondrocytes expanded in regular conditions. Other groups reported significant suppression of type II and III collagen gene expression in human auricular chondrocytes expanded with bFGF supplementation.37
In our experiment, the COL2 expression was not rescued by bFGF supplementation in auricular chondrocytes. However, the loss of COL2A1 expression is reversible at this time as chondrocytes are able to redifferentiate after being placed on a 3D scaffold and into an in vivo environment. Collagen type II was abundant in engineered cartilage after in vivo incubation demonstrating that cartilage-forming phenotype of auricular P2+bFGF chondrocytes was readily induced when chondrocytes were seeded on a 3D scaffold and implanted. This phenotypic rescue did not occur with P2 chondrocytes that had been cultured in standard conditions without bFGF supplementation.
Supplementation with bFGF has been reported to cause significant elastin gene downregulation in expanded chondrocytes.37 However, in our studies, we detected more robust elastin fibers in neocartilage engineered from bFGF-expanded chondrocytes than from control P1 chondrocytes after in vivo incubation. Strong collagen type II expression and elastin fiber formation confirms the overall high quality of cartilage engineered from extensively expanded chondrocytes. Elastin has been demonstrated in neocartilage engineered from freshly isolated P0 auricular chondrocytes in swine and canine models.6,38–40 To date, we know of no studies describing elastin formation in neocartilage engineered from extensively expanded chondrocytes in immunocompetent animals.
Neocartilage engineered from expanded P2+bFGF chondrocytes was stable and not subjected to resorption during 6–20 weeks observation as neocartilage to scaffold thickness ratio remained unchanged. Some resorption occurred before the 6-week time point, possibly due to inflammatory reaction. Complete resorption of auricular cartilage engineered from expanded autologous chondrocytes was accompanied by severe inflammation in other large animal models.8,41 Local environment, scaffold materials and their degradation products, and unprotected antigen-presenting chondrocyte surface have been implicated in the generation of inflammatory and immune responses, which negatively affect chondrogenesis and contribute to autologous neocartilage resorption.16,17,41–45
The overall shape of engineered ears was well preserved with minimal dimensional changes that were similar to those reported in our prior rodent studies.14,15 The wire frame within the engineered ear was able to withstand mechanical forces during wound healing and neocartilage maturation and prevented shrinkage and distortion.
Several limitations exist in our study. Chondrocyte expansion was performed in FBS-containing medium; autologous serum was used in the final stages of in vitro culture to minimize the inflammatory response to the implants. Defined serum-free culture medium or medium supplemented exclusively with autologous serum will need to be tested to confirm our findings. The quality of neocartilage in the engineered ears was assessed from punch biopsies. Because of technical challenges due to the titanium wire frame, we were unable to obtain full-size cross sections to demonstrate the contiguous nature of neocartilage throughout the engineered ear.
Although the results of this pilot study are encouraging, the quality of engineered ear-shaped cartilage could be further improved through the refinement of scaffold materials and the manufacturing process. More uniform cell seeding, possibly with commercially available sprayers,40 could improve the neocartilage quality. Size and shape of engineered ears were evaluated based on the image outlines in this study while we were developing techniques to assess the complex 3D shape of a human ear.15 Comprehensive, noninvasive, 3D shape analysis could lead to improved design and incorporation of alternative framework materials.15 Elevation of implanted engineered ear will need to be performed and its survival demonstrated in future studies to more fully recapitulate clinical methodology.
Conclusions
To the best of our knowledge, this is the first demonstration of ear-shaped elastic cartilage engineered from auricular chondrocytes that underwent clinical-scale expansion in an immunocompetent animal model. The stability of this neocartilage and of the resulting engineered auricle was evaluated over several months. The quality of engineered cartilage was confirmed by robust auricular cartilage-specific GAG, collagen type II, and elastin expression.
The present work made significant advances in the development of engineered elastic neocartilage for human auricular reconstruction.
Acknowledgments
This research was sponsored in part by the U.S. Army Medical Research and Materiel Command award No. W81XWH-12-1-0334 and by the Armed Forces Institute of Regenerative Medicine award No. W81XWH-08-2-0034. The content of this article does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. The fibrous collagen scaffolds were generously provided by the DSM Biomedical Corp. The authors would like to thank Drs. T. Hadlock and M. Cheney for their valuable input on ear scaffold design and M. Johnson and J. Beagle for excellent animal care assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bichara D.A., O'Sullivan N.A., Pomerantseva I., Zhao X., Sundback C.A., Vacanti J.P., and Randolph M.A. The tissue-engineered auricle: past, present, and future. Tissue Eng Part B Rev 18, 51, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Nayyer L., Patel K.H., Esmaeili A., Rippel R.A., Birchall M., O'toole G., Butler P.E., and Seifalian A.M. Tissue engineering: revolution and challenge in auricular cartilage reconstruction. Plast Reconstr Surg 129, 1123, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Huey D.J., Hu J.C., and Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science 338, 917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nimeskern L., van Osch G.J., Müller R., and Stok K.S. Quantitative evaluation of mechanical properties in tissue-engineered auricular cartilage. Tissue Eng Part B Rev 20, 17, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Cao Y., Vacanti J.P., Paige K.T., Upton J., and Vacanti C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg 100, 297, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Arévalo-Silva C.A., Eavey R.D., Cao Y., Vacanti M., Weng Y., and Vacanti C.A. Internal support of tissue-engineered cartilage. Arch Otolaryngol Head Neck Surg 126, 1448, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Kamil S.H., Vacanti M.P., Aminuddin B.S., Jackson M.J., Vacanti C.A., and Eavey R.D. Tissue engineering of a human sized and shaped auricle using a mold. Laryngoscope 114, 867, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Shieh S.J., Terada S., and Vacanti J.P. Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials 25, 1545, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Xu J.W., Johnson T.S., Motarjem P.M., Peretti G.M., Randolph M.A., and Yaremchuk M.J. Tissue-engineered flexible ear-shaped cartilage. Plast Reconstr Surg 115, 1633, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Neumeister M.W., Wu T., and Chambers C. Vascularized tissue-engineered ears. Plast Reconstr Surg 117, 116, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Xue J., Feng B., Zheng R., Lu Y., Zhou G., Liu W., Cao Y., Zhang Y., and Zhang W.J. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials 34, 2624, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Enjo M., Terada S., Uehara M., Itani Y., and Isogai N. Usefulness of polyglycolic acid-polypropylene composite scaffolds for three-dimensional cartilage regeneration in a large-animal autograft model. Plast Reconstr Surg 131, 335e, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Hwang C.M., Lee B.K., Green D., Jeong S.Y., Khang G., Jackson J.D., Atala A., Lee S.J., and Yoo J.J. Auricular reconstruction using tissue-engineered alloplastic implants for improved clinical outcomes. Plast Reconstr Surg 133, 360e, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Zhou L., Pomerantseva I., Bassett E.K., Bowley C.M., Zhao X., Bichara D.A., Kulig K.M., Vacanti J.P., Randolph M.A., and Sundback C.A. Engineering ear constructs with a composite scaffold to maintain dimensions. Tissue Eng Part A 17, 1573, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Cervantes T.M., Bassett E.K., Tseng A., Kimura A., Roscioli N., Randolph M.A., Vacanti J.P., Hadlock T.A., Gupta R., Pomerantseva I., and Sundback C.A. Design of composite scaffolds and three-dimensional shape analysis for tissue-engineered ear. J R Soc Interface 10, 413, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W., and Cao Y. Application of scaffold materials in tissue reconstruction in immunocompetent mammals: our experience and future requirements. Biomaterials 28, 5078, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Haisch A. Ear reconstruction through tissue engineering. Adv Otorhinolaryngol 68, 108, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Luo X., Zhou G., Liu W., Zhang W.J., Cen L., Cui L., and Cao Y. In vitro precultivation alleviates post-implantation inflammation and enhances development of tissue-engineered tubular cartilage. Biomed Mater 4, 025006, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Deponti D., Di Giancamillo A., Mangiavini L., Pozzi A., Fraschini G., Sosio C., Domeneghini C., and Peretti G.M. Fibrin-based model for cartilage regeneration: tissue maturation from in vitro to in vivo. Tissue Eng Part A 18, 1109, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Miot S., Brehm W., Dickinson S., Sims T., Wixmerten A., Longinotti C., Hollander A.P., Mainil-Varlet P., and Martin I. Influence of in vitro maturation of engineered cartilage on the outcome of osteochondral repair in a goat model. Eur Cell Mater 23, 222, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Bichara D.A., Pomerantseva I., Zhao X., Zhou L., Kulig K.M., Tseng A., Kimura A.M., Johnson M.A., Vacanti J.P., Randolph M.A., and Sundback C.A. Successful creation of tissue-engineered autologous auricular cartilage in an immunocompetent large animal model. Tissue Eng Part A 20, 303, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Bobick B.E., Chen F.H., Le A.M., and Tuan R.S. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today 87, 351, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Gan L., and Kandel R.A. In vitro cartilage tissue formation by co-culture of primary and passaged chondrocytes. Tissue Eng 13, 831, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ahmed N., Gan L., Nagy A., Zheng J., Wang C., and Kandel R.A. Cartilage tissue formation using redifferentiated passaged chondrocytes in vitro. Tissue Eng Part A 15, 665, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Taylor D.W., Ahmed N., Gan L., Gross A.E., and Kandel R.A. Proteoglycan and collagen accumulation by passaged chondrocytes can be enhanced through side-by-side culture with primary chondrocytes. Tissue Eng Part A 16, 643, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Ahmed N., Taylor D.W., Wunder J., Nagy A., Gross A.E., and Kandel R.A. Passaged human chondrocytes accumulate extracellular matrix when induced by bovine chondrocytes. J Tissue Eng Regen Med 4, 233, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Tan A.R., Dong E.Y., Andry J.P., Bulinski J.C., Ateshian G.A., and Hung C.T. Coculture of engineered cartilage with primary chondrocytes induces expedited growth. Clin Orthop Relat Res 469, 2735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin I., Vunjak-Novakovic G., Yang J., Langer R., and Freed L.E. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp Cell Res 253, 681, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Martin I., Suetterlin R., Baschong W., Heberer M., Vunjak-Novakovic G., and Freed L.E. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. J Cell Biochem 83, 121, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Tseng A., Pomerantseva I., Cronce M.J., Kimura A.M., Neville C.M., Randolph M.A., Vacanti J.P., and Sundback C.A. Extensively expanded auricular chondrocytes form neocartilage in vivo. Cartilage 5, 241, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Bomhard A., Veit J., Bermueller C., Rotter N., Staudenmaier R., Storck K., and The HN. Prefabrication of 3D cartilage contructs: towards a tissue engineered auricle – a model tested in rabbits. PLoS One 8, e71667, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandl E.W., van der Veen S.W., Verhaar J.A., and van Osch G.J. Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue Eng 10, 109, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Yanaga H., Imai K., Koga M., and Yanaga K. Cell-engineered human elastic chondrocytes regenerate natural scaffold in vitro and neocartilage with neoperichondrium in the human body post-transplantation. Tissue Eng Part A 18, 2020, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulco I., Miot S., Haug M.D., Barbero A., Wixmerten A., Feliciano S., Wolf F., Jundt G., Marsano A., Farhadi J., Heberer M., Jakob M., Schaefer D.J., and Martin I. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet 384, 337, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Ito T., Sawada R., Fujiwara Y., and Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology 56, 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narcisi R., Signorile L., Verhaar J.A., Giannoni P., and van Osch G.J. TGFβ inhibition during expansion phase increases the chondrogenic re-differentiation capacity of human articular chondrocytes. Osteoarthritis Cartilage 20, 1152, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Shasti M., Jacquet R., McClellan P., Yang J., Matsushima S., Isogai N., Murthy A., and Landis W.J. Effects of FGF-2 and OP-1 in vitro on donor source cartilage for auricular reconstruction tissue engineering. Int J Pediatr Otorhinolaryngol 78, 416, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Cao Y., Rodriguez A., Vacanti M., Ibarra C., Arevalo C., and Vacanti C.A. Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J Biomater Sci Polym Ed 9, 475, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Xia W., Liu W., Cui L., Liu Y., Zhong W., Liu D., Wu J., Chua K., and Cao Y. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater 71, 373, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Morotomi T., Wada M., Uehara M., Enjo M., and Isogai N. Effect of local environment, fibrin, and basic fibroblast growth factor incorporation on a canine autologous model of bioengineered cartilage tissue. Cells Tissues Organs 196, 398, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Kanazawa S., Fujihara Y., Sakamoto T., Asawa Y., Komura M., Nagata S., Takato T., and Hoshi K. Tissue responses against tissue-engineered cartilage consisting of chondrocytes encapsulated within non-absorbable hydrogel. J Tissue Eng Regen Med 7, 1, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Rotter N., Ung F., Roy A.K., Vacanti M., Eavey R.D., Vacanti C.A., and Bonassar L.J. Role for interleukin 1alpha in the inhibition of chondrogenesis in autologous implants using polyglycolic acid-polylactic acid scaffolds. Tissue Eng 11, 192, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Fujihara Y., Asawa Y., Takato T., and Hoshi K. Tissue reactions to engineered cartilage based on poly-L-lactic acid scaffolds. Tissue Eng Part A 15, 1565, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Lotz A.S., Havla J.B., Richter E., Frolich K., Staudenmaier R., Hagen R., and Kleinsasser N.H. Cytotoxic and genotoxic effects of matrices for cartilage tissue engineering. Toxicol Lett 190, 128, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Fujihara Y., Takato T., and Hoshi K. Immunological response to tissue-engineered cartilage derived from auricular chondrocytes and a PLLA scaffold in transgenic mice. Biomaterials 3, 1227, 2010 [DOI] [PubMed] [Google Scholar]