Abstract
The zebrafish (Danio rerio) is a valuable vertebrate model for human hearing disorders because of many advantages in genetics, embryology, and in vivo visualization. In this study, we investigated auditory function of zebrafish during the first week postfertilization using microphonic potential recording. Extracellular microphonic potentials were recorded from hair cells in the inner ear of wild-type AB and transgenic Et(krt4:GFP)sqet4 zebrafish at 3, 5, and 7 days postfertilization in response to 20, 50, 100, 200, 300, and 400-Hz acoustic stimulation. We found that microphonic threshold significantly decreased with age in zebrafish. However, there was no significant difference of microphonic responses between wild-type and transgenic zebrafish, indicating that the transgenic zebrafish have normal hearing like wild-type zebrafish. In addition, we observed that microphonic threshold did not change with the recording electrode location. Furthermore, microphonic threshold increased significantly at all tested stimulus frequencies after displacement of the saccular otolith but only increased at low frequencies after displacement of the utricular otolith, showing that the saccule rather than the utricle plays the major role in larval zebrafish hearing. These results enhance our knowledge of early development of auditory function in zebrafish and the factors affecting hearing assessment with microphonic potential recording.
Introduction
The zebrafish (Danio rerio) has become an increasingly important model for studying the development and function of the inner ear because it is genetically tractable and manageable, the majority of human genes are conserved in zebrafish, and genetic manipulations such as morpholino gene knockdown and CRISPR/Cas9 gene knockout/knockin can be easily achieved.1–7 In addition, the lateral line of zebrafish is externally exposed, which makes lateral line hair cells good candidates for experimental manipulations. Furthermore, external fertilization and optically transparent embryos allow the inner ear of zebrafish embryos to be easily accessible for live imaging and physiological recording.1,2,8–11 Overall, these advantages of the zebrafish make it an ideal and convenient model for research on inner ear hair cell death and regeneration, drug screens for ototoxins and otoprotectants, and discovery of new genes for sensorineural deafness.3–5,12–16
Several studies have reported important developmental stages of morphology of the zebrafish inner ear. At 16 hours postfertilization (hpf), the otic vesicle of zebrafish starts to form, and at about 19 hpf, two tiny otoliths become visible at the anterior and posterior ends of the otic vesicle.11 At 24 hpf, zebrafish have two hair-cell packed sensory maculae, each of which is attached to an otolith. The anterior macula and its associated otolith develop into the utricle, whereas the posterior macula and otolith become the saccule.17 The lagena is not yet formed within the first week after fertilization.17–19 Although early morphological development of the otic vesicle of zebrafish is well known, few studies have been conducted on the hearing ability of zebrafish during the first week of fertilization.20,21 Our previous study primarily focused on auditory responses of zebrafish larvae at one stimulus frequency, 200 Hz.20 In this study, we reported microphonic audiograms in a range from 20 to 400 Hz for 1-week-old and younger larvae.
Transgenic Et(krt4:GFP)sqet4 zebrafish have been widely used for hearing research because their hair cells in the inner ear as well as in the lateral line express the green fluorescent protein (GFP).4,5,20,22–25 The GFP fluorescent marker facilitates morphological observations of live hair cells and quantification of hair cells in zebrafish embryos/larvae. To further establish this transgenic zebrafish as a model for hearing disorders, there is a need to determine if this transgenic zebrafish has normal hearing like wild-type zebrafish. Thus, we compared audiograms of Et(krt4:GFP)sqet4 fish with those of wild-type AB fish in this study.
Behavioral and physiological approaches have often been used to assess auditory sensitivity of fishes. Behavioral methods such as classical conditioning and food reward measure the overall hearing ability of animals.26 However, it is time-consuming to train fish in response to sound using these behavioral methods. Using auditory evoked potential recording, the development of auditory function has been studied in several fish species that are juveniles and adults.27–29 However, the auditory evoked potential recording is hard to apply to larval zebrafish due to their small body size. Instead, in this study, we chose an electrophysiological method—microphonic potential recording that can quickly and reliably assess function of hair cells in the otic vesicle of larval zebrafish.30–33
Microphonic potential recording is used to assess function of sensory hair cells. In humans, cochlear microphonics are used to evaluate the functioning of the cochlea and its hair cells, which differs from the auditory brainstem recording assessment that is used to evaluate neural sensitivity to sound.34 Adrien used the term “cochlear microphonics” in 1931 to describe recordings that Wever and Bray originally reported as neural responses from a cat's ear the year before.35,36 Microphonic potentials are not limited to mammals and can be recorded from the inner ear of other vertebrates that do not possess the cochlea.37,38 Fishes as well as some amphibians have hair cells not only in the inner ear but also the lateral line neuromasts. Microphonic potential recordings have been made from the lateral line and inner ear (particularly the saccule, the major hearing organ for most fishes) of several teleost fish species.37,39–44
In contrast to mammalian cochlear microphonic waveforms that simply follow the stimulus frequency, fish microphonic waveforms are twice the stimulus frequency, which was already well explained as resulting from two opposing or opposite groups of hair cells that each responds to positive or negative deflection of each cycle of sinusoidal stimulation to yield the double-frequency effect in summation.40–42 In 1998, Nicolson et al. first showed microphonic potential responses from lateral line neuromasts of larval zebrafish in response to water puff stimulation.10 In 2004, Corey and Hudspeth laboratories first demonstrated microphonic potential recordings from the otic vesicle of zebrafish larvae in response to a vibratory stimulus probe.45–47 Since then, the ear microphonic potential recording has been a useful tool to assess auditory function of larval zebrafish, which has emerged as a powerful model for hearing research.8,20,48
Larval zebrafish younger than 1-week old have been used to investigate target genes that play important roles in hearing and balance because of the ease of using them for live imaging, gene knockdown, and genome editing.1–3,6,7 Although some studies indicate the utricle of zebrafish larvae functions in balance,48,49 it is still unclear whether or not sound is transduced predominantly by the saccule in zebrafish larvae. In this study, we determined the contributions of individual otolith organs of larval zebrafish to hearing, showing that the saccule rather than the utricle plays a predominant role in hearing.
Materials and Methods
Zebrafish preparation
Reproductive wild-type AB zebrafish were originally purchased from the Zebrafish International Resource Center, Eugene, Oregon, and then raised in the zebrafish facility at the University of Miami. Transgenic zebrafish, Et(krt4:GFP)sqet4 (ET4) with GFP expression in hair cells, were also used in this study.22,23 Fish care and egg production were conducted as previously described.20,24
Microphonic potential recording
An electrophysiology rig for microphonic potential recording was reported in detail in our previous study.20 Briefly, it consists of a Zeiss Axioskop 2 FS plus fluorescence microscope equipped with an EpiPlan 10× lens, a Plan Apochromat 40× water immersion lens (NA = 1.0, working distance = 3.0 mm), GFP and differential interference contrast filters, and a Gibraltar stage. The electrophysiology rig was enclosed by a Faraday cage and rested on a 63–500 antivibration table (Technical Manufacturing Corporation, Peabody, MA). The whole recording setup was placed in a custom-built, electrically and acoustically shielded audiometric booth (Accutone2 800A series; Industrial Acoustic Company, Bronx, NY).
Microphonic potentials were recorded from the otic vesicles of AB and Et(krt4:GFP)sqet4 zebrafish at 3, 5, and 7 days postfertilization (dpf) at 25°C room temperature. Zebrafish larvae were embedded dorsal up in agarose with 0.009% buffered MS-222 in MatTek Petri dishes and then covered with standard fish saline. The stimulus microprobe and microphonic recording electrode were prepared as reported.20 The displacement of the microprobe driven by a piezoelectric actuator (Piezosystem Jena, Inc., Hopedale, MA) was first calibrated at the selected frequencies under a 40× lens of the Zeiss Axioskop Microscope with a high-speed camera and then was set at 5.8 μm for initial experiments. The microprobe tip was placed against the skin at the posterior end of the otic vesicle (pointing to the saccular otolith) and provided linear oscillatory motion along an axis 20° off the longitudinal axis of the fish body.20,45,46 The size and angle of the microprobe affecting microphonic responses were kept constant for all recordings reported herein.
A system III auditory evoked potential setup was composed of two RP2.1 processors, two PA-5 programmable attenuators, and SigGen and BioSig software (Tucker-Davis Technologies [TDT], Alachua, FL).18 Pure tones of ±10 V (peak-to-trough) at 20, 50, 100, 200, 300, and 400 Hz were synthesized using SigGen software. Each tone at different frequencies had four stimulus cycles with pre- and post-tone periods of 20 ms, which yielded stimulus durations of 240 ms for 20 Hz, 120 ms for 50 Hz, and 80 ms for 100, 200, 300, and 400 Hz. The amplitude of tones was set at desired levels with the PA-5 programmable attenuators. Tones synthesized by SigGen were loaded into the TDT BioSig software, sent out to a PA-5 programmable attenuator, led to a piezoelectric actuator amplifier, and passed to an actuator to drive the stimulus microprobe to directly stimulate the otic vesicle of zebrafish.
The recording electrode, a sharp micropipette, was made from WPI glass capillaries (OD = 1.50 mm, ID = 1.12 mm) with the Sutter P97 electrode puller. The recording electrode was filled with standard fish saline (129.6 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, and pH = 7.2), and the electrode tip was beveled until the electrode resistance dropped to about 4–6 MΩ with the Sutter BV-10-E beveler. The recording electrode was mounted in a Warner half-cell holder secured by a Narishige micromanipulator. The beveled recording electrode was inserted into the fluid of the otic vesicle through the otic vesicle wall, and the electrode tip was positioned about 5 μm away from the edge of the saccular otolith of zebrafish (Fig. 1A, B). The half-cell electrode holder was connected to a Grass P55C preamplifier (Gain = 1000× and bandpass filter between 0.1 and 3000 Hz) through a Grass HZP high-impedance headstage. An Ag/AgCl reference electrode was placed into the fish saline in the recording chamber.
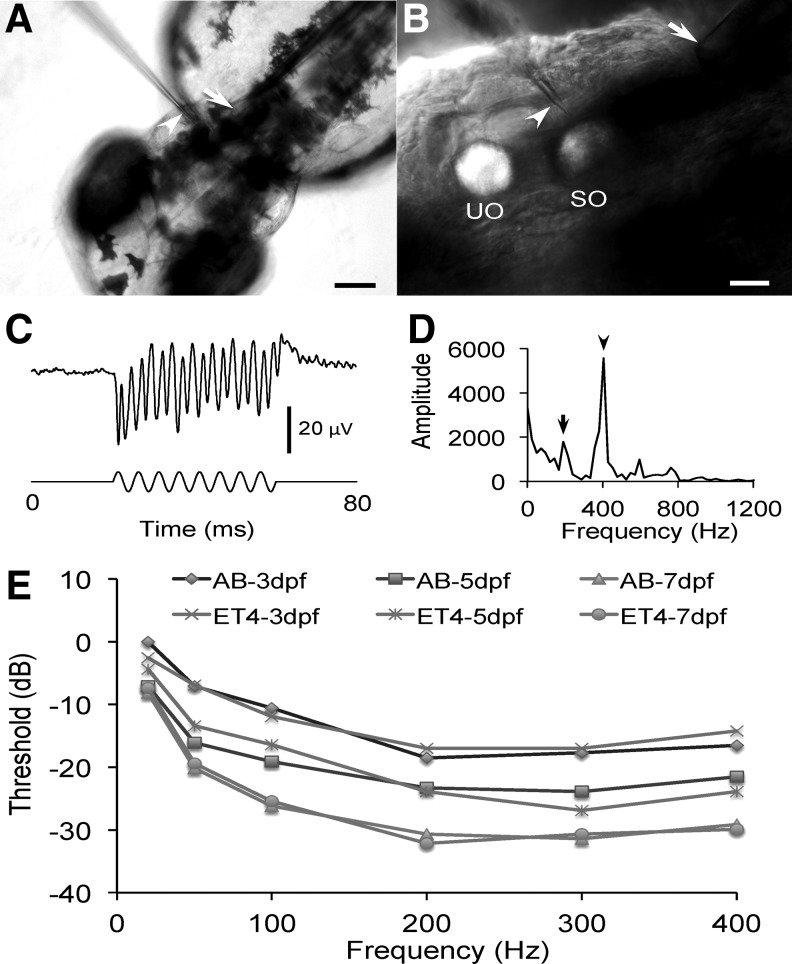
FIG. 1.
Microphonic potential recording and microphonic audiograms of wild-type AB and Et(krt4:GFP)sqet4 zebrafish at different ages. (A) Image of a 3-dpf, wild-type AB zebrafish under microphonic potential recording, showing the stimulus microprobe (arrow) and the beveled recording micropipette tip (arrowhead). Scale bar = 100 μm. (B) Enlarged image of the right otic vesicle shown in (A) with the saccular otolith (SO) and utricular otolith (UO), the stimulus probe (arrow), and the beveled recording electrode tip (arrowhead). Scale bar = 20 μm. (C) Microphonic potential waveform (top trace) recorded from hair cells of a larval zebrafish in response to 200-Hz stimuli at 1.4-μm displacement (bottom trace). (D) Fast Fourier transform plot of the microphonic waveform displayed in (C), showing that the microphonic response frequency (arrowhead) is twice the stimulus frequency (arrow). (E) Microphonic threshold versus stimulus frequency functions for wild-type AB and transgenic Et(krt4:GFP)sqet4 (ET4) zebrafish at 3, 5, and 7 dpf. The microphonic threshold obtained at 20 Hz from 3-dpf, wild-type AB zebrafish was defined as 0 dB. N = 10 for each zebrafish group.
After microphonic potential responses were amplified and filtered by the Grass P55C preamplifier, they were recorded at a sampling rate of 25 kHz and averaged 200 times with the TDT BioSig software. The amplitude of microphonic responses in root mean square was first measured, and the dominant frequency of microphonic response, that is, doubling of the stimulus frequency (2F), was then determined in fast Fourier transform plots. At each stimulus frequency, microphonic responses were recorded, as stimulus microprobe displacement gradually decreased at a step of 3 dB until a threshold was reached. Threshold was defined as the lowest microprobe displacement that yielded a microphonic response with the peak at 2F, which was just higher than the background noise level at 2F. Threshold was determined at each stimulus frequency using the TDT BioSig software.
Recording electrode locations
Microphonic potentials were recorded from hair cells in the otic vesicles of 3-dpf, wild-type AB zebrafish with the recording electrode tip pointing to the center of the saccular otolith or utricular otolith. In both cases, the recording electrode tip was kept about 5 μm away from the edge of the otolith (Fig. 2A, B). Microphonic audiograms obtained from the two electrode locations were compared to determine effects of the recording electrode location on microphonic responses.
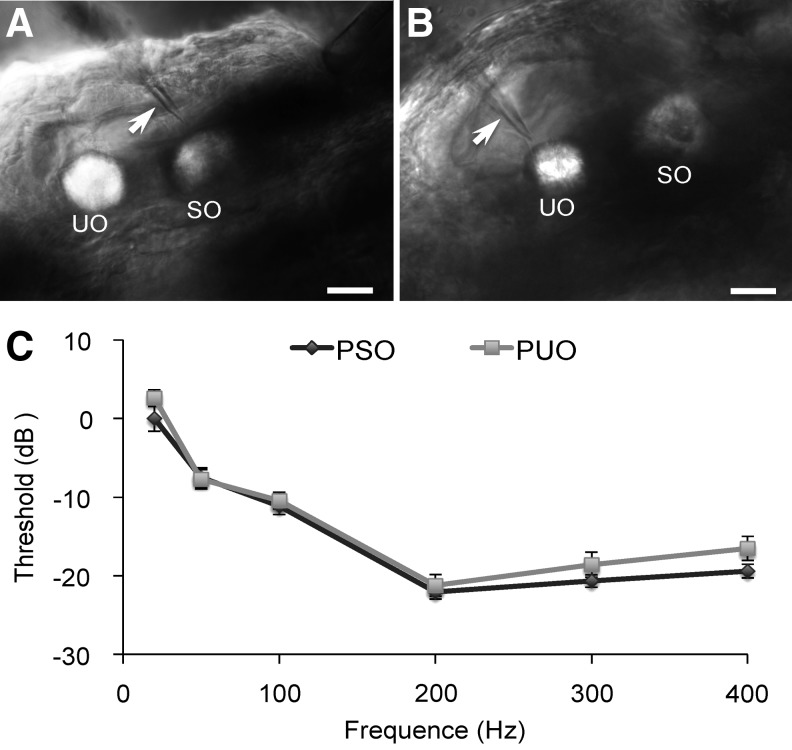
FIG. 2.
Effects of electrode location on microphonic threshold. (A) Image of the otic vesicle of a 3-dpf, wild-type AB zebrafish, showing the recording electrode tip (arrow) pointing to the center of SO (PSO). (B) Image of the otic vesicle of a 3-dpf, wild-type AB zebrafish, showing the recording electrode tip (arrow) pointing to the center of UO (PUO). In (A, B), scale bars = 20 μm. (C) Microphonic threshold versus stimulus frequency functions of 3-dpf, wild-type AB zebrafish with the two different recording electrode locations (PSO and PUO). The microphonic threshold obtained at 20 Hz with PSO was defined as 0 dB. N = 7 for each group.
Otolith displacement
We displaced individual otoliths to disrupt their function to assess their auditory roles. After displacing the saccular otolith, the utricular otolith, or both otoliths, microphonic responses at various stimulus frequencies were recorded from 3-dpf, wild-type AB zebrafish and then compared to those of zebrafish with intact otoliths. The recording electrode tip was used to push the otoliths away from their original positions, and the electrode tip was then moved back to its original recording position, that is, the recording electrode tip was pointed to the center of the saccular otolith and about 5 μm away from the edge of the otolith (Fig. 3A–C).
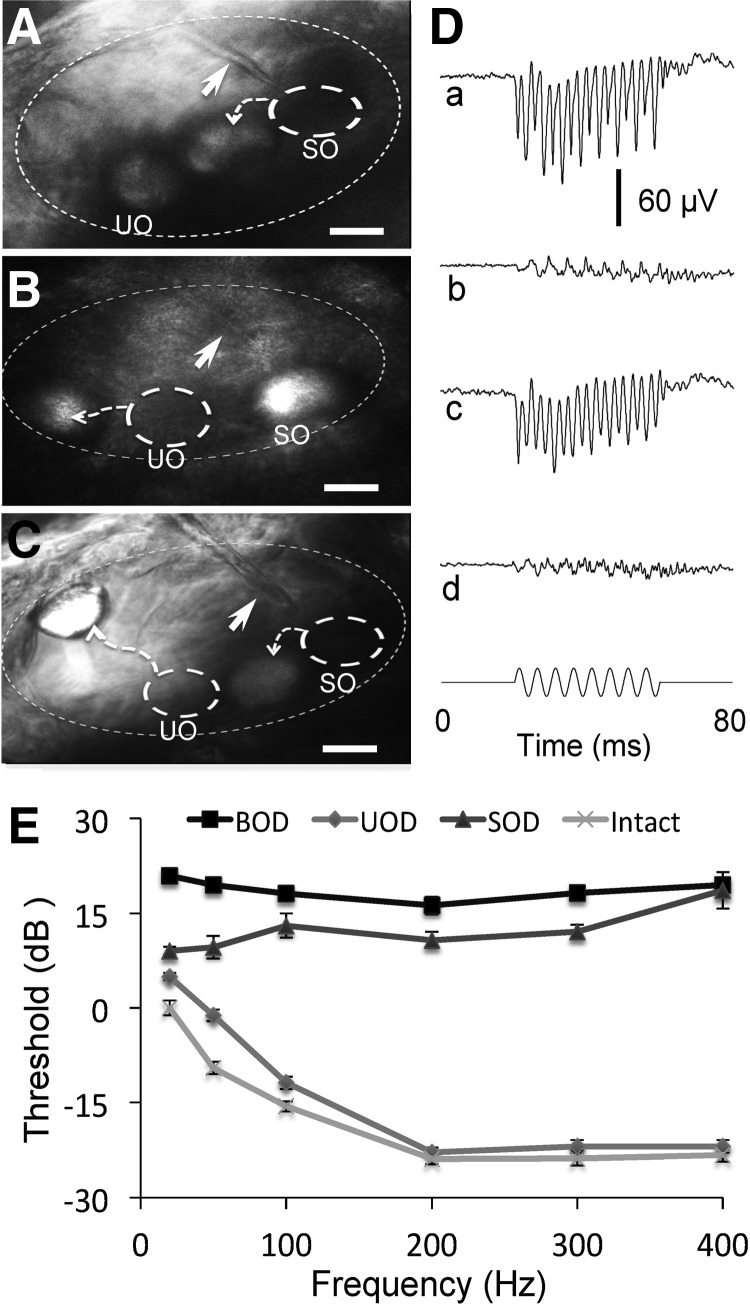
FIG. 3.
Effects of otolith displacement on microphonic response and threshold. (A) Image of the otic vesicle of a 3-dpf, wild-type AB zebrafish showing the displaced SO and the recording electrode tip (arrow) pointing to the original location of SO. (B) Image of the otic vesicle of a 3-dpf, wild-type AB zebrafish showing the displaced UO and the recording electrode tip (arrow) pointing to the location of SO. (C) Image of the otic vesicle of a 3-dpf, wild-type AB zebrafish showing displaced SO and UO, and the recording electrode tip (arrow) pointing to the original location of SO. In (A–C), the dashed circles indicate the original locations of the SO and UO before displacement, scale bars = 20 μm. (D) Microphonic responses of 3-dpf, wild-type zebrafish with intact otoliths (Intact, a), SO displacement alone (SOD, b), UO displacement alone (UOD, c), and both otolith displacement (BOD, d). Stimulus displacement: 200 Hz and 2.9 μm. (E) Microphonic threshold versus stimulus frequency functions for 3-dpf, wild-type AB zebrafish with Intact, SOD, UOD, and BOD. The microphonic threshold of zebrafish with intact otoliths at 20 Hz was defined as 0 dB. N = 7 for each group.
Imaging of hair cells in the otic vesicle
Imaging of hair cells in the inner ear has been described in detail in our previous studies.20,24 Both AB and Et(krt4:GFP)sqet4 zebrafish at 3, 5, and 7 dpf were fixed in 4% fresh paraformaldehyde at 4°C for 2 h and then rinsed in phosphate-buffered saline three times for 10 min each. The saccular otolith in embryos was dissolved in 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for up to 6 h (2 h for younger fish) at room temperature to visualize the entire saccular epithelium. AB zebrafish were kept in a 1:50 dilution of Alexa 546 phalloidin (Molecular Probes, Eugene, OR) solution with 0.5% Triton X-100 overnight to label ciliary bundles of hair cells. AB and Et(krt4:GFP)sqet4 zebrafish were subsequently positioned laterally in the VECTASHIELD antifading mounting medium (Vector Laboratories, Burlingame, CA) in 35-mm MatTek dishes. Z-stacks of images of saccular hair cells in AB zebrafish labeled with Alexa 546 phalloidin and saccular hair cells in Et(krt4:GFP)sqet4 doubly labeled with Alexa 546 and GFP were taken and then 3D reconstructed using a Nikon EZ C-1 confocal unit with 488 and 543 nm laser lines and a TE-2000 inverted microscope. From each 3D reconstructed saccular epithelium, hair cells were quantified by counting phalloidin-labeled hair cell ciliary bundles using Nikon NIS-Elements imaging software.
Statistics
Analysis of variance (ANOVA, StatPlus for Mac) and post hoc Tukey's honest significant difference (HSD) tests were used to determine if there were any significant differences in microphonic responses between zebrafish strains, at various fish ages, at stimulus frequencies, with different electrode locations, and before and after otolith displacement. In this study, the significance level was set at 0.05, and data were represented as mean ±1 standard error of the mean.
Results
Microphonic potentials from wild-type and transgenic zebrafish
Microphonic potentials were recorded from the otic vesicles of wild-type AB and Et(krt4:GFP)sqet4 zebrafish at 3, 5, and 7 dpf. Figure 1 shows the right otic vesicle of a 3-dpf zebrafish embedded in agarose with a stimulus microprobe tip positioned against the body adjacent to the posterior end of the otic vesicle and a beveled recording micropipette tip impaled into the otic vesicle, pointing to the saccular otolith, for microphonic potential recording. Iso-level frequency responses were recorded at 20, 50, 100, 200, 300, and 400 Hz. Microphonic responses were robustly recorded at these stimulus frequencies for both types of zebrafish at different ages. Microphonic response waveforms recorded from the otic vesicle of zebrafish have a characteristic feature of doubling the stimulus frequency (Fig. 1C, D). There was no significant difference in the microphonic response between AB and Et(krt4:GFP)sqet4 zebrafish (Fig. 1E, one-way ANOVA, Fstrain = 0.199, p > 0.05). Microphonic responses tended to increase with stimulus frequencies in the range of frequencies tested (Fig. 1E, one-way ANOVA, Ffrequency = 88.761, p < 0.001; Tukey HSD tests, Threshold20Hz ≠ Threshold50Hz ≠ Threshold100Hz ≠ Threshold400Hz = Threshold200Hz = Threshold300Hz, p < 0.001, p < 0.005, p < 0.005, p > 0.05, and p > 0.05, respectively). Microphonic threshold gradually and significantly decreased with age (Fig. 1E, one-way ANOVA, Fage = 39.803, p < 0.001; Tukey HSD tests, Threshold3 dpf ≠Threshold5 dpf ≠ Threshold7 dpf, p < 0.001 and p < 0.001).
Effects of the recording electrode location on microphonic responses
To determine if the location of the recording electrode tip affected the amplitude of recorded microphonic waveforms, we compared microphonic thresholds with the electrode tip positioned near the saccular otolith to those with the electrode tip near the utricular otolith (Fig. 2A, B). We observed no significant difference in microphonic threshold between these two electrode tip locations (Fig. 2C, two-way ANOVA, Felectrode position = 2.690, p > 0.05).
Effects of otolith displacement on microphonic responses
To determine which otolith organ (the saccule or utricle) plays the major role in hearing, we compared microphonic responses before and after the displacement of each or both otoliths. Figure 3A–C shows the displacement of the saccular otolith, the utricular otolith, and both otoliths, respectively. A huge reduction in microphonic amplitude was observed after the displacement of the saccular otolith alone, compared with both intact otoliths (Fig. 3D-a, b). However, only a small reduction in microphonic amplitude was observed after the displacement of the utricular otolith alone, compared with intact otoliths (Fig. 3D-a, c). The displacement of both saccular and utricular otoliths together remarkably reduced the microphonic response (Fig. 3D-a, d), which was slightly smaller than that with the saccular otolith displacement alone (Fig. 3D-b, d).
We then measured microphonic thresholds to determine if the otolith displacement affected microphonic thresholds. Microphonic thresholds increased significantly at all stimulus frequencies, particularly high frequencies after the saccular otolith alone was displaced (Fig. 3E, two-way ANOVA, Fotolith position = 1198.262, p < 0.001; Tukey HSD tests, ThresholdIntact ≠ ThresholdSOD, p < 0.001). Microphonic threshold also significantly increased, particularly at low frequencies, when the utricular otolith alone was displaced (Fig. 3E, Tukey HSD tests, ThresholdIntact ≠ ThresholdUOD, p < 0.001). Microphonic threshold robustly increased at all frequencies, especially high frequencies when both saccular and utricular otoliths were displaced, compared with the displacement of the utricular otolith alone (Fig. 3E, Tukey HSD tests, ThresholdBOD ≠ ThresholdUOD, p < 0.001). Microphonic threshold of zebrafish with both otolith displacement also increased significantly compared to those of zebrafish with the displacement of the saccular otolith alone (Fig. 3E, Tukey HSD tests, ThresholdBOD ≠ ThresholdSOD, p < 0.001).
Inner ear hair cells
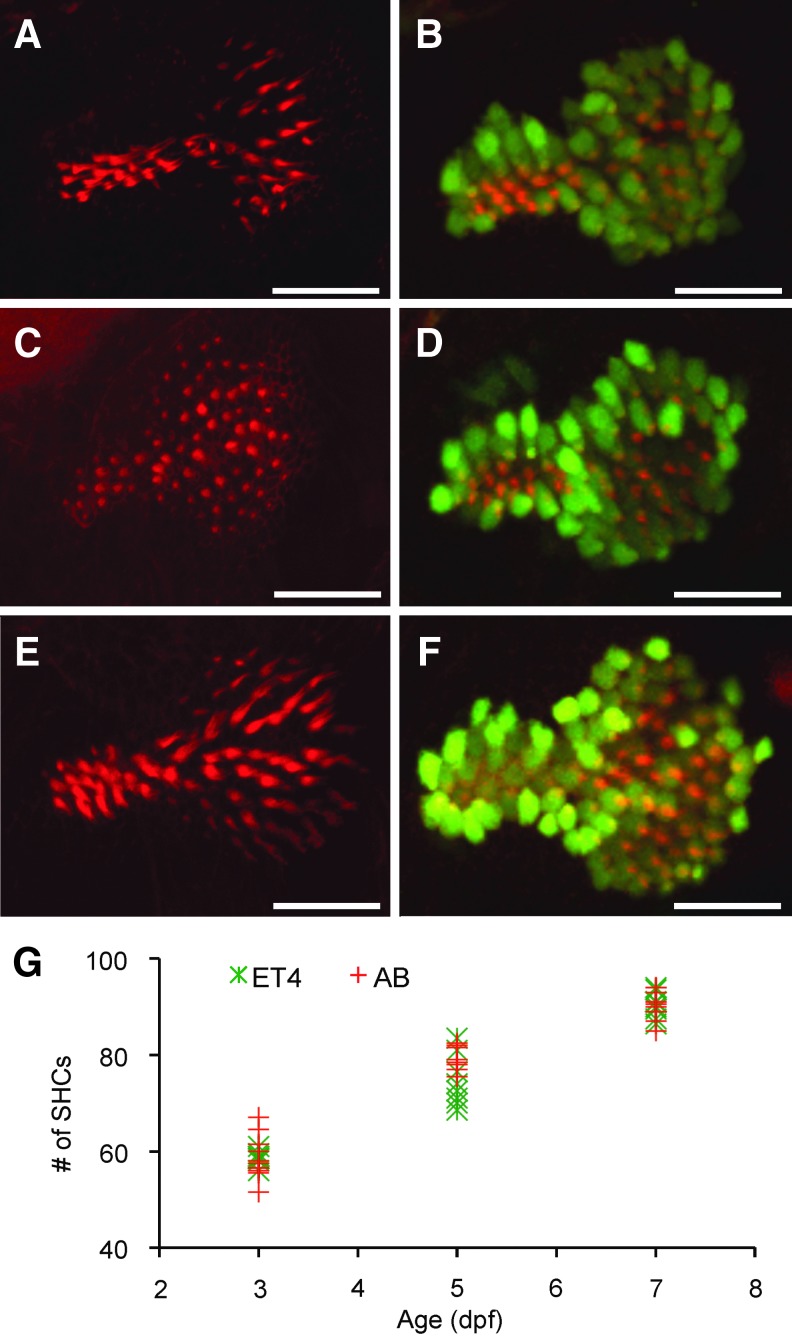
To determine if there is any difference in hair cell number between AB and Et(krt4:GFP)sqet4 fish, we quantified the total number of Alexa 546 phalloidin-labeled hair bundles of hair cells in the saccule, the major hearing organ for zebrafish larvae. We found no significant differences in the number of saccular hair cells between wild-type and transgenic fish (two-way ANOVA, p > 0.05), but a significant difference among three age groups for both types of fish (p < 0.001) (Fig. 4).
FIG. 4.
Comparison of number of saccular hair cells between wild-type AB and transgenic Et(krt4:GFP)sqet4 zebrafish. (A, B) Examples of saccular epithelia from 3-dpf, AB (A) and Et(krt4:GFP)sqet4 (B) fish. (C, D) Examples of saccular epithelia from 5-dpf, AB fish (C) and Et(krt4:GFP)sqet4 fish (D). (E, F) Examples of saccular epithelia from 7-dpf, AB (E) and Et(krt4:GFP)sqet4 (F) fish. Both AB and Et(krt4:GFP)sqet4 fish were stained with Alexa 546 phalloidin to label hair cell ciliary bundles (red). Green areas in saccular epithelia in (B, D, F) are green fluorescent protein (GFP)-labeled hair cell bodies. Scale bars = 25 μm. (G) Scatter plot comparison in number of saccular hair cells (no. of SHCs) between AB (red crosses) and ET4 (green asterisks) fish at 3, 5, and 7 dpf. NAB3d = 8, NET43d = 11, NAB5d = 8, NET45d = 8, NAB7d = 9, and NET47d = 11.
Discussion
In this study, we used microphonic potential recording to assess the auditory function of hair cells in the inner ear of zebrafish larvae. Mechanical shakers were previously used to provide acoustic stimulation to fish in hearing research.31,49,50–53 Instead of accelerating the entire fish body with a shaker apparatus, we used a stimulus microprobe to provide focal stimulation directly to the inner ear of zebrafish larvae,45,46 to simulate acoustic particle motion of sound. The linear push–pull motion of the microprobe tip causes vibration of the posterior wall of the otic vesicle, leading to auditory transduction of hair cells in the inner ear. Since the microprobe displacement is easy to measure and adjust, the displacement rather than velocity or acceleration was chosen as the acoustic parameter to interpret microphonic responses.
In addition, hair cells in otolith organs of larval zebrafish are oriented opposing to or opposite each other,49,54 which yields the characteristic feature of microphonic waveforms, that is, microphonic response frequency is twice the stimulus frequency20,38,44 (Fig. 1C, D). Based on this microphonic response property, we were able to easily distinguish a microphonic response from stimulus artifact or background noise and then determine microphonic threshold. The microphonic potential method has been successfully applied to wild-type, transgenic, and mutant/morphant zebrafish embryos and young larvae and has become a quick powerful tool to assess hair cell function in the zebrafish model.3–5,20,45,46 Moreover, we chose low stimulus frequencies from 20 to 400 Hz because the inner ears of zebrafish of 1-week old or younger are only “motion sensitive” through a direct stimulus pathway55 and are not yet pressure sensitive until the initial formation of the Weberian ossicles at about 20 dpf.56 Weberian ossicles are auditory accessories known to increase the auditory sensitivity at high frequencies. Hence, zebrafish larvae that we recorded from were likely more sensitive to lower frequencies due to the fact that they had not yet developed the Weberian ossicles.
For larval zebrafish, the saccular epithelium is positioned vertically, whereas the utricular epithelium lies in the horizontal plane. Hair cells in these otolith organs are oriented with their morphological and functional polarizations toward various directions. These hair cells are directional sensors, having the maximal responses along their polarization directions.57–59 The saccular macula of larval zebrafish has the standard hair cell orientation pattern,49,54,60 that is, the majority of the hair cells in the anterior saccular epithelium are oriented along the longitudinal axis of the fish, and most of the hair cells in the posterior saccular epithelium along the dorsoventral axis. Since the direction of vibration of the stimulus probe affects responses of the hair cells in the inner ear, the axis of the vibrating microprobe was kept constant to ensure the consistency of microphonic recordings from them.
In this study, we varied the recording electrode locations by positioning the electrode tip right by the saccular or utricular otolith. Although the microphonic amplitude at suprathreshold with the electrode tip pointing to the utricular otolith was slightly smaller than that with the electrode tip pointing to the saccular otolith (data not shown), we found that the microphonic threshold did not change with the recording electrode location, which is consistent with a previous study of inner ear microphonic responses from younger zebrafish.8 Therefore, the microphonic recording from the otic vesicle of zebrafish is quite repeatable because it is independent of electrode location.
In our previous study, we reported morphological and functional development of the inner ear of transgenic Et(krt4:GFP)sqet4 larvae during the first week postfertilization and showed no difference in the number of hair cells per neuromast between wild-type AB and Et(krt4:GFP)sqet4 fish.20 In this study, we further showed that Et(krt4:GFP)sqet4 larvae had the same hearing sensitivity as wild-type AB zebrafish (Fig. 1E), indicating that the GFP transgene has no effect on hearing function of Et(krt4:GFP)sqet4 larvae. Thus, our results have provided further evidence to confirm the validity of the use of transgenic Et(krt4:GFP)sqet4 zebrafish in hearing research.
Zebrafish have only two otolith organs, the saccule and utricle, in the otic vesicle during the first week postfertilization. These two otolith organs have a very similar structure, each of which is composed of a cell-packed sensory epithelium coupled with an otolith. Otolith organs have dual functions—hearing and balance.48,49,52,55 It has been demonstrated that the utricle of larval zebrafish is the key vestibular organ, whereas the saccule does not appear to be necessary for balance.49 However, the roles of different otolith organs in hearing of zebrafish are still unclear. In other words, is the saccule the major hearing organ in larval zebrafish? Does the utricle contribute to hearing of larval zebrafish at all? A previous study reported that the otolith size determines the function (hearing or balance) of otolith organs.48 The motion of the microprobe used in this study can evoke relative displacement between hair cells and their associated otolith, which presumably results in opening mechanically gated ion channels in the plasma membrane of hair cells in the inner ear, leading to auditory transduction. To have this transdunction occur, the otolith must be physically coupled with hair cells in the sensory epithelium. The complete displacement of an otolith from its macula shown in Figure 3A–C broke the coupling between hair cells and the otolith to make individual otolith organs dysfunctional.
We determined relative roles of the saccule and utricle in hearing by observing microphonic threshold upshifts after one or both otoliths were completely separated from its/their maculae. When we displaced the saccular otolith alone, microphonic amplitude dropped dramatically at all tested stimulus frequencies and threshold upshifted up to about 40 dB at high frequencies (Fig. 3D). However, when we displaced the utricular otolith alone, microphonic amplitude only reduced a little bit and threshold upshifts were small and only observed at low frequencies (Fig. 3D). Furthermore, after we displaced both utricular and saccular otoliths, the thresholds at low frequencies further increased. These results revealed that, in the first week of development, the saccule plays a crucial role in hearing, whereas the utricle just has a minor role in low-frequency hearing.
To investigate whether or not a transgene affects hair cells in the inner ear of zebrafish, we compared saccular hair cells between wild-type AB and transgenic zebrafish at different ages. We did not observe any difference in morphology of cell bodies or ciliary bundles. In general, the transgenic fish had the same number of saccular hair cells as wild-type AB fish during the first week of development, as shown in Figure 4. These hair cell data are consistent with functional data shown in Figure 1E for both AB and transgenic zebrafish.
In summary, microphonic potential recording is a fast, reliable, and powerful method that can assess hearing function of larval zebrafish. We observed that zebrafish increased auditory response and sensitivity during the first week of development. The transgenic Et(krt4:GFP)sqet4 zebrafish has the same auditory function as wild-type AB zebrafish, making it an excellent model for hearing research. Our results indicate that for larval zebrafish, the saccule is the key hearing organ, whereas the utricle plays a minor role in hearing at low frequencies.
Acknowledgments
This work was supported by the National Institutes of Health (R21DC009879 to Z.L., R01DC009645 to M.T., and R01DC012546 to X.L.), University of Miami Provost Research Awards, and College of Arts and Sciences Gabelli Fellowship to Z.L. The authors thank Bing Zou for his help in preparation of zebrafish for microphonic potential recordings.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet 2005;39:9–22 [DOI] [PubMed] [Google Scholar]
- 2.Whitfield TT. Zebrafish as a model for hearing and deafness. J Neurobiol 2002;53:157–171 [DOI] [PubMed] [Google Scholar]
- 3.Yariz KO, Duman D, Seco CZ, Dallman J, Huang M, Peters TA, et al. Mutations in OTOGL, encoding the inner ear protein otogelin-like, cause moderate sensorineural hearing loss. Am J Hum Genet 2012;91:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz-Horta O, Subasioglu-Uzak A, Grati M, DeSmidt A, Foster J II, Cao L, et al. FAM65B is a membrane-associated protein of hair cell stereocilia required for hearing. Proc Natl Acad Sci U S A 2014;111:9864–9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grati M, Chakchouk I, Ma Q, Bensaid M, Desmidt A, Turki N, et al. A missense mutation in DCDC2 causes human recessive deafness DFNB66, likely by interfering with sensory hair cell and supporting cell cilia length regulation. Hum Mol Genet 2015;24:2482–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol 2015;33:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis SP, Krey JF, Krystofiak ES, Cui R, Nanda S, Xu W, et al. A short splice form of Xin-actin binding repeat containing 2 (XIRP2) lacking the Xin repeats is required for maintenance of stereocilia morphology and hearing function. J Neurosci 2015;35:1999–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanimoto M, Ota Y, Inoue M, Oda Y. Origin of inner ear hair cells: morphological and functional differentiation from ciliary cells into hair cells in zebrafish inner ear. J Neurosci 2011;31:3784–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeddies DG, Fay RR. Development of the acoustically evoked behavioral response in zebrafish to pure tones. J Exp Biol 2005;208:1363–1372 [DOI] [PubMed] [Google Scholar]
- 10.Nicolson T, Rusch A, Friedrich RW, Granato M, Ruppersberg JP, Nusslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron 1998;20:271–283 [DOI] [PubMed] [Google Scholar]
- 11.Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol 1996;365:113–128 [DOI] [PubMed] [Google Scholar]
- 12.Chiu LL, Cunningham LL, Raible DW, Rubel EW, Ou HC. Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol 2008;9:178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Schier H, Starr CJ, Kappler JA, Kollmar R, Hudspeth AJ. Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the zebrafish. Dev Cell 2004;7:401–412 [DOI] [PubMed] [Google Scholar]
- 14.Owens KN, Coffin AB, Hong LS, Bennett KO, Rubel EW, Raible DW. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear Res 2009;253:32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffin AB, Ou H, Owens KN, Santos F, Simon JA, Rubel EW, et al. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish 2010;7:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buck LM, Winter MJ, Redfern WS, Whitfield TT. Ototoxin-induced cellular damage in neuromasts disrupts lateral line function in larval zebrafish. Hear Res 2012;284:67–81 [DOI] [PubMed] [Google Scholar]
- 17.Bever MM, Fekete DM. Atlas of the developing inner ear in zebrafish. Dev Dyn 2002;223:536–543 [DOI] [PubMed] [Google Scholar]
- 18.Platt C. Zebrafish inner ear sensory surfaces are similar to those in goldfish. Hear Res 1993;65:133–140 [DOI] [PubMed] [Google Scholar]
- 19.Bang PI, Sewell WF, Malicki JJ. Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio). J Comp Neurol 2001;438:173–190 [DOI] [PubMed] [Google Scholar]
- 20.Lu Z, DeSmidt AA. Early development of hearing in zebrafish. J Assoc Res Otolaryngol 2013;14:509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohmann KN, Tripp JA, Genova RM, Bass AH. Manipulation of BK channel expression is sufficient to alter auditory hair cell thresholds in larval zebrafish. J Exp Biol 2014;217:2531–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn 2004;231:449–459 [DOI] [PubMed] [Google Scholar]
- 23.Go W, Bessarab D, Korzh V. atp2b1a regulates Ca(2+) export during differentiation and regeneration of mechanosensory hair cells in zebrafish. Cell Calcium 2010;48:302–313 [DOI] [PubMed] [Google Scholar]
- 24.Zamora LY, Lu Z. Alcohol-induced morphological deficits in the development of octavolateral organs of the zebrafish (Danio rerio). Zebrafish 2013;10:52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleason MR, Nagiel A, Jamet S, Vologodskaia M, Lopez-Schier H, Hudspeth AJ. The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc Natl Acad Sci U S A 2009;106:21347–21352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cervi AL, Poling KR, Higgs DM. Behavioral measure of frequency detection and discrimination in the zebrafish, Danio rerio. Zebrafish 2012;9:1–7 [DOI] [PubMed] [Google Scholar]
- 27.Higgs DM, Souza MJ, Wilkins HR, Presson JC, Popper AN. Age- and size-related changes in the inner ear and hearing ability of the adult zebrafish (Danio rerio). J Assoc Res Otolaryngol 2002;3:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgs DM, Rollo AK, Souza MJ, Popper AN. Development of form and function in peripheral auditory structures of the zebrafish (Danio rerio). J Acoust Soc Am 2003;113:1145–1154 [DOI] [PubMed] [Google Scholar]
- 29.Alderks PW, Sisneros JA. Ontogeny of auditory saccular sensitivity in the plainfin midshipman fish, Porichthys notatus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2011;197:387–398 [DOI] [PubMed] [Google Scholar]
- 30.Kenyon TN, Ladich F, Yan HY. A comparative study of hearing ability in fishes: the auditory brainstem response approach. J Comp Physiol A Sens Neural Behav Physiol 1998;182:307–318 [DOI] [PubMed] [Google Scholar]
- 31.Lu Z, Tomchik SM. Effects of a red-tide toxin on fish hearing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2002;188:807–813 [DOI] [PubMed] [Google Scholar]
- 32.Lu Z, Xu Z. Effects of saccular otolith removal on hearing sensitivity of the sleeper goby (Dormitator latifrons). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2002;188:595–602 [DOI] [PubMed] [Google Scholar]
- 33.Sisneros JA, Bass AH. Ontogenetic changes in the response properties of individual, primary auditory afferents in the vocal plainfin midshipman fish Porichthys notatus Girard. J Exp Biol 2005;208:3121–3131 [DOI] [PubMed] [Google Scholar]
- 34.Zhang M. Using concha electrodes to measure cochlear microphonic waveforms and auditory brainstem recordings. Trends Amplif 2010;14:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wever EG, Bray CW. Action currents in the auditory nerve response to acoustic stimulation. Proc Natl Acad Sci U S A 1930;16:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adrien ED. The microphonic action of the cochlea: an interpretation of Wever and Bray's experiments. J Physiol 1931;71:28–30 [Google Scholar]
- 37.Adrien ED, Craik KJW, Sturdy RS. The electrical response of the auditory mechanism in cold blooded vertebrates. Proc R Soc London B Biol Sci 1938;125:435–455 [Google Scholar]
- 38.Corey DP, Hudspeth AJ. Analysis of the microphonic potential of the bullfrog's sacculus. J Neurosci 1983;3:942–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zotterman Y. The microphonic effect of teleost labyrinths and its biological significance. J Physiol 1943;102, 313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jielof R, Spoor A, de Vries HL. The microphonic activity of the lateral line. J Physiol 1952;116:137–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flock Å. Transducing mechanisms in the lateral line canal organ receptors. Cold Spring Harb Symp Quant Biol 1965;30:133–145 [DOI] [PubMed] [Google Scholar]
- 42.Furukawa T, Ishii Y. Neurophysiological studies on hearing in goldfish. J Neurophysiol 1967;30:1377–1403 [DOI] [PubMed] [Google Scholar]
- 43.Fay RR, Popper AN. Acoustic stimulation of the ear of the goldfsh (Carassius auratus). J Exp Biol 1974;61:243–260 [DOI] [PubMed] [Google Scholar]
- 44.Sisneros JA. Saccular potentials of the vocal plainfin midshipman fish, Porichthys notatus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2007;193:413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004;432:723–730 [DOI] [PubMed] [Google Scholar]
- 46.Starr CJ, Kappler JA, Chan DK, Kollmar R, Hudspeth AJ. Mutation of the zebrafish choroideremia gene encoding Rab escort protein 1 devastates hair cells. Proc Natl Acad Sci U S A 2004;101:2572–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kappler JA, Starr CJ, Chan DK, Kollmar R, Hudspeth AJ. A nonsense mutation in the gene encoding a zebrafish myosin VI isoform causes defects in hair-cell mechanotransduction. Proc Natl Acad Sci U S A 2004;101:13056–13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inoue M, Tanimoto M, Oda Y. The role of ear stone size in hair cell acoustic sensory transduction. Sci Rep 2013;3:2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riley BB, Moorman SJ. Development of utricular otoliths, but not saccular otoliths, is necessary for vestibular function and survival in zebrafish. J Neurobiol 2000;43:329–337 [DOI] [PubMed] [Google Scholar]
- 50.Fay RR. The goldfish ear codes the axis of acoustic particle motion in three dimensions. Science 1984;225:951–954 [DOI] [PubMed] [Google Scholar]
- 51.Lu Z, Popper AN, Fay RR. Behavioral detection of acoustic particle motion by a teleost fish (Astronotus ocellatus): sensitivity and directionality. J Comp Physiol A Sens Neural Behav Physiol 1996;179:227–233 [DOI] [PubMed] [Google Scholar]
- 52.Lu Z, Xu Z, Buchser WJ. Frequency coding of particle motion by saccular afferents of a teleost fish. J Exp Biol 2010;213:1591–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomchik SM, Lu Z. Modulation of auditory signal-to-noise ratios by efferent stimulation. J Neurophysiol 2006;95:3562–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haddon C, Mowbray C, Whitfield T, Jones D, Gschmeissner S, Lewis J. Hair cells without supporting cells: further studies in the ear of the zebrafish mind bomb mutant. J Neurocytol 1999;28:837–850 [DOI] [PubMed] [Google Scholar]
- 55.Popper AN, Fay RR. Rethinking sound detection by fishes. Hear Res 2011;273:25–36 [DOI] [PubMed] [Google Scholar]
- 56.Grande TYB. The ontogeny and homology of the Weberian apparatus in the zebrafish Danio rerio (Ostariophysi: Cypriniformes). Zool J Linn Soc 2004;140:241–254 [Google Scholar]
- 57.Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A 1977;74:2407–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Z, Popper AN. Morphological polarizations of sensory hair cells in the three otolithic organs of a teleost fish: fluorescent imaging of ciliary bundles. Hear Res 1998;126:47–57 [DOI] [PubMed] [Google Scholar]
- 59.Lu Z, Popper AN. Neural response directionality correlates of hair cell orientation in a teleost fish. J Comp Physiol A Sens Neural Behav Physiol 2001;187:453–465 [DOI] [PubMed] [Google Scholar]
- 60.Popper AN, Coombs S. The morphology and evolution of the ear in Actinopterygian fishes. Am Zool 1982;22:311–328 [Google Scholar]