Abstract
Objective: Despite some potential advantages, tissue-engineered constructs have low therapeutic efficacy after transplantation into bone defect sites due to uneven distribution of seed cells, which presents a major obstacle in a clinical setting. The aim of this study was to improve cell distribution within the scaffold, to increase seeding efficiency, to facilitate tissue ingrowth and vascularization of the resultant grafts, and finally to boost the successful rate of transplantation.
Approach: A syringe-aided inoculation method was designed to anchor bone marrow stromal cells (BMSCs) onto the central pores of the chitosan scaffold by repeat positive aspiration. The cell distribution, cell number, vascularization and mineralization at the central region of the tissue-engineered constructs treated by syringe-aided and regular two-side inoculation methods were evaluated side by side using the WST-8, immunofluorescence staining of CD31 and von Kossa staining.
Results: The tissue-engineered constructs treated by the syringe-aided inoculation methods exhibited a larger number of BMSCs with uniform distribution, as well as more robust vascularization and mineralization in the central regions when compared with those treated by the two-side inoculation method both in vitro and in vivo.
Innovation: This is the first time that the influence of a syringe-aided inoculation method has been evaluated with respect to the vascularization and mineralization of implanted grafts.
Conclusion: The syringe-aided inoculation methods used in this study might provide an approach through which to improve cell distribution within the scaffold, facilitate the subsequent tissue ingrowth into the scaffold, and promote the vascularization and mineralization of tissue-engineered grafts.

Zubing Li, PhD
Introduction
The management of bone defects caused by accidents, violence, and tumors remains one of the most urgent issues faced by orthopedic surgeons.1 As a promising technology for jawbone repair, bone tissue engineering has been receiving more and more attention and is widely used in craniofacial research.2,3 Seed cells and scaffolds comprise two of the three basic elements involved in bone tissue engineering.4 The combination of seed cells with a scaffold, that is, the inoculation of seed cells into a scaffold is the first and most critical step in creating a tissue-engineered bone graft and it plays a vital role in determining the formation of bone tissue.5 A large amount of research has focused on scaffolds and seed cells; however, few studies have investigated the interconnection between them.
Regular inoculation methods have been widely used in previous studies. With this method, a high concentration of seed cells is first suspended by a limited amount of liquid medium (culture medium or saline). Then, the seeded cell suspensions are added into the synthetic scaffolds; following that, the scaffold is sucked or squeezed out of water to facilitate the infiltration of cell suspensions.
Unfortunately, the regular inoculation methods noted above present a series of problems. One of them is the uneven distribution of seed cells within the scaffold.6,7 We recently published that the inoculation of bone marrow stromal cells (BMSCs) into the synthetic scaffold by the abovementioned methods resulted in a poor distribution of BMSCs within the scaffold. Especially, in the central areas of the scaffold, there were almost no cells located on it.8 Only through two-side inoculation of the cells could we obtain constructs with a limited amount of BMSCs anchored in the central pores of the scaffolds.8
The resulting low initial seeding density is the second disadvantage of the regular inoculation methods. Holy's study demonstrated that only 25% of initially seeded BMSCs had adhered to the polymer scaffolds by the regular inoculation method, irrespective of which initial BMSC seeding densities (0.5–10×106 cells/cm3) were used.9 The large amount of culture medium used to suspend the seed cells may have accounted for the low inoculation efficiency, as the culture medium with the seed cells was about to ooze out of the scaffold during one-time inoculation process.
Therefore, the abovementioned disadvantages highlight the need to modify the regular inoculation methods, with the aim of improving the cell distribution within the scaffold and increasing the seeding efficiency. Matthias inoculated mesenchymal stem cells (MSCs) spheroids into the polyurethane scaffold using a novel inoculation method.10 In addition, Laschke et al. also seeded adipose tissue-derived microvascular fragments in the porous scaffolds using the syringe-aided inoculation method.11 With the help of the negative and positive aspiration of a syringe, the spheroids or microvascular fragments could pass the scaffold from both sides and they were subsequently anchored in the central areas of the scaffold. However, to date, no one has inoculated individual cells into the synthetic scaffold using the syringe-aided inoculation method, nor have they evaluated the influence of repeat aspiration on the cell viability and the initial seeding efficiency of seed cells. Furthermore, tissue ingrowth and vascularization of the constructs treated by the syringe-aided inoculation method have also not been quantified and analytically documented.
In this study, we modified the syringe-aided inoculation method to anchor individual cells into the porous scaffolds using the abovementioned inoculation method. Subsequently, we compared the viability and spatial distribution of the seed cells in the center regions of the scaffolds, and we also evaluated the influence of syringe-aided or a regular two-side inoculation method on the vascularization and mineralization of constructs following transplantation into nude mice for 2, 4, and 6 weeks in vivo. When compared with the cell spheroids or microvascular fragments, individual cells with a smaller volume were easier to penetrate through the entire thickness of the porous scaffolds and they failed to anchor into the pores of the scaffolds. The reduced diameter of pore size and the increased pumping times of the syringe could promote the seeding efficiency. Given that the reduced diameter of pore size may have unfavorable effects on the osteogenesis and vascularization of tissue-engineered constructs,12 we hypothesized that increasing the times of aspiration would ultimately promote the percentage of cells that could anchor to the central pores of the scaffolds. Therefore, this prompted us to modify the syringe-aided inoculation method described above by repeat pumping of the individual seed cells through the scaffold. In addition, we also evaluated the effect of aspiration times on the seeding efficiency and distribution of seed cells in the inner region of the scaffolds.
Clinical Problem Addressed
The usage of tissue-engineered grafts has gained much interest for the repair of bone tissue damage caused by accidents, violence, and tumors. However, this technology has not worked well for large-sized bone defects. Cell necrosis frequently occurs in the inner region of bone tissue substitutes due to the low seeding efficiency and uneven distribution of seed cells, which would ultimately lead to the failure of transplantation.7 Static seeding may also be partially attributed to this phenomenon.13 Therefore, the objective of this study is, therefore, to develop a new inoculation strategy to facilitate the translation of laboratory research results into clinical application.
Materials and Methods
Fabrication of the chitosan scaffold
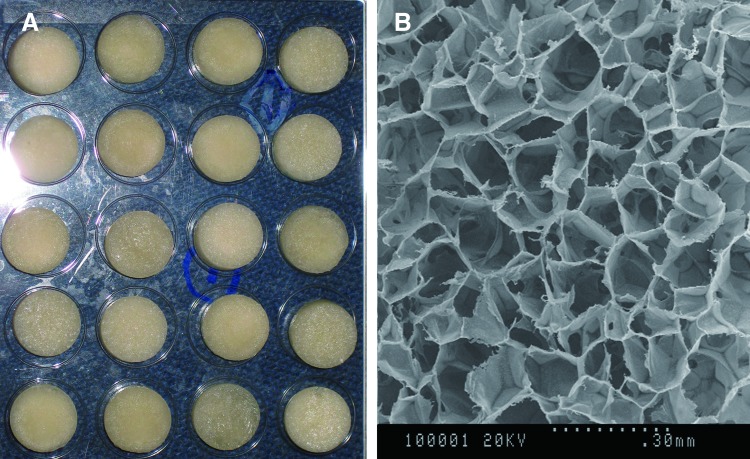
The 5 mm-high cylinder chitosan (CS) scaffolds with a diameter of 1.2 mm were fabricated according to the methods detailed in our previous study.8 The scaffolds had a mean porosity of 87.5% with pore sizes ranging from 80 to 130 μm (Fig. 1).
Figure 1.
(A) Gross observation of the fabricated CS scaffolds. (B) Interconnected pores were observed through a scanning electron microscope image of the porous scaffold. CS, chitosan. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Isolation and culture of rabbit BMSCs
All animal experiments were carried out in accordance with the protocols authorized by Wuhan University's Animal Care and Use Committee. The BMSCs of mice were collected from green fluorescent protein (GFP) transgenic mice (C57BL/6-Tg[UBC-GFP]0Scha/J) at 1 month of age. Following euthanasia by brief exposure in a CO2 chamber, both the tibia and femurs of the mice were carefully exposed and cut at both metaphyseal ends with a sterile bone ribbing rongeur. Then, the bone marrow was flushed out with high-glucose Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, NY) containing 10% fetal bovine serum, 100 U/mL of streptomycin and penicillin (Sigma-Aldrich Co., St. Louis, MO) using a 5-mL syringe. After being broken up by repeated pipetting, the resulting bone marrow solution was centrifuged at 1,000 g for 7 min, resuspended in fresh complete medium, seeded on culture flasks, and then incubated at 37°C, 5% CO2. After being cultured for 2 days in vitro, nonadherent cells were removed through the change of culture media. After reaching 80–100% confluence, the cells were detached with 0.25% (w/v) Trypsin-EDTA (Life Technologies), and the cells were subsequently subcultured into new culture flasks. Only passage 1 cells were used in the following experiments of this study.14
Seeding of BMSCs into the scaffold
All CS scaffolds were sterilized in 70% ethanol for 24 h, washed in sterile phosphate buffered saline (PBS), and incubated in complete culture medium for 48 h before seeding.
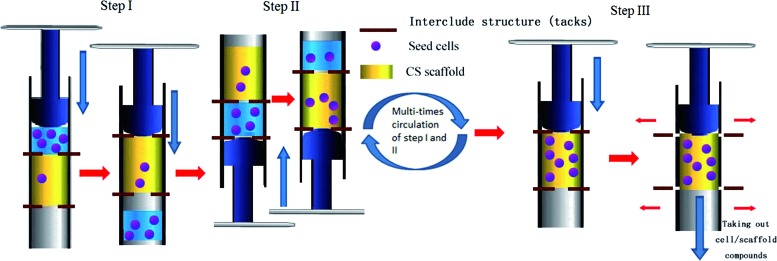
For the syringe-aided seeding procedures, we modified the recently established seeding procedure as previously described.10,11 As shown in Fig. 2, the scaffolds were shaped into a 1-cm-high cylinder with the same diameter to the lumen of a 2-mL syringe (Shandong Weigao Group Medical Polymer Co., Ltd., Shandong, People's Republic of China); the scaffolds were then fixed in the lumen of the syringe by inserting of interclude structure (tacks) into the top and bottom of the scaffolds. The bottom of the syringe was filled with 2 mL of BMSCs suspension (0.785×106 cells/mL). Positive pressure produced by the plunger of the syringe was performed to make the BMSC suspension penetrate through the entire thickness of the scaffold. Then, the syringe was rotated 180°, followed by one more time of positive aspiration from the other side of the syringe. In this way, positive aspiration was induced 10 (Group 10S), 20 (Group 20S), 40 (Group 40S), and 60 (Group 60S) times in the syringe so that the BMSCs could penetrate into both sides of the scaffold and they were finally trapped in the pores at the center of the scaffold (Fig. 2). After pulling out the interclude tacks from the syringe, the BMSC/scaffold compounds were carefully taken out of the syringe with a pair of tweezers, and the residual culture medium was collected for the evaluation of initial seeding efficiency. All cell/scaffold compounds were incubated at 37°C, 5% CO2 once 1 mL of the complete culture medium was added.
Figure 2.
Schematic diagram of the syringe-aided inoculation method representing the three steps used to seed BMSCs into the scaffold. After fixation of scaffold into the lumen of a 2-mL syringe with the tacks, positive pressure produced by the plunger of the syringe was performed from one side of the syringe to make the BMSC suspension penetrate through the scaffold. Then, the syringe was rotated 180°, followed by positive aspiration from the other side of the syringe. In this way, positive aspiration was induced 10 (Group 10S), 20 (Group 20S), 40 (Group 40S), and 60 (Group 60S) times. Finally, the BMSC/scaffold compounds were carefully taken out of the syringe with a pair of tweezers, following pulling out of the tacks. BMSCs, bone marrow stromal cells. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
For seeding of the scaffolds with BMSCs through the two-side inoculation method (Group T),8,15 water was squeezed from the scaffolds using the temporary compression of forceps, and the scaffolds were individually placed into the wells of a new six-well culture plate; a 100 μL aliquot of cell suspension (7.85×106 cells/mL) was then pipetted into one side of the scaffold. After the flexible scaffolds had restored their original shape, the cell suspension was sucked into the scaffolds. After 1.5 h of incubation at 37°C, 5% CO2, a 100 μL aliquot of the cell suspensions was added into the other side of the scaffolds. After another 1.5 h of incubation, 800 μL of the complete culture medium was supplied.
Measurement of cell viability and initial cell seeding efficacy
To analyze the influence of the positive pressure produced by the syringe on the viability of BMSCs, various times (10, 20, 40, and 60 times) of positive aspiration were performed to seed the cells into the scaffold using a 2-mL syringe. At 1 h, and 3, 5, 8, and 12 days after inoculation, the cell viability in all groups was analyzed by the WST-8 (water-soluble tetrazolium salts, 2-[2-methoxy-4-nitorophenyl]-3-[4-nitrophenyl]-5-[2, 4-disulfophenyl]-2H) (Cell Counting Kit-8; Dojindo, Kumamoto, Japan) assay, according to a previous study.16 Briefly, the cell/scaffold constructs of each group (n=5) were moved into a nontreated six-well plate and washed with PBS for at least three times. Then, 300 μL of the WST-8 reagent and 2.7 mL of complete medium were added to each well. After incubation for 1.5 h at 37°C, 5% CO2, a 200 μL aliquot of the solution from each well was transferred into a 96-well culture plate. Finally, the OD value was determined at 490 nm using a microplate reader (Biotek-mQuant; Bio-Tek Instruments, Inc., Winooski, VT).
To clarify whether BMSCs were lost during the process of inoculation, the initial seeding efficacy in all groups was determined according to a previous study.14 After seeding the BMSCs into the scaffold, the cell/scaffold compounds were rinsed with PBS for a total of three times. Then, the unattached BMSCs in the residual culture medium and collected PBS were counted using the Trypan blue method. The initial cell seeding efficacy was calculated as [1−(number of unattached cells/6.0×106)]×100%.
By evaluating the viability and seeding efficiency of the seed cells, the syringe-aided inoculation method with the most suitable times of positive aspiration was chosen and used in the following experiments.
Confocal laser scanning microscopic analysis
To visualize the spatial distribution of the BMSCs within the center of the seeded scaffolds, a confocal laser scanning microscope was used to observe Propidium Iodide (PI)-stained BMSCs cultured in vitro. At days 7 and 14 in culture, five BMSC/scaffold compounds per group were washed with sterile PBS to remove unattached cells. After being fixed with 10% formalin, 5 μm-thick consecutive sections cut from the center area of the cell/scaffold compounds were incubated with the PI solution (100 μg/mL) with 0.1% Triton X-100 for 10 min at 37°C. Finally, the slides containing the BMSC/scaffold compounds were observed using a confocal laser scanning electron microscope (Leica TCS Sp2 AOBS; Leica Microsystems GmbH, Wetzlar, Germany). The number of BMSCs in each slide was analyzed by counting the number of nuclei using the Image-Pro Plus 6.0.
Due to the low densities of the cells at the inner region of the constructs treated with the regular inoculation methods, we aimed to improve the cells distribution within the scaffolds using the syringe-aided inoculation method. Thus, only the central region of the scaffold was investigated in this study.
Transplantation of the BMSC/scaffold compounds into the athymic mice
Before transplantation of the cell/scaffold compounds into the dorsal skin of nude mice, osteogenic differentiation of the BMSCs was usually carried out for several days in the previous studies on bone tissue engineering.17 However, recent literature reports have shown that undifferentiated adipose-derived mesenchymal stem cell (adMSC) spheroids serve as individual vascularization units and promote the simultaneous formation of microvessels inside the implanted synthetic scaffolds10,18; conversely, the osteogenic differentiation of adMSCs significantly impaired the cells' in vivo vascularization capacity.19 Due to this reason, the osteogenic differentiation of BMSCs has not been performed before the ectopic implantation in this study.
After being cultured in vitro for 1 week, the BMSC/scaffold compounds (n=8) treated by the syringe-aided (Group 20S) and two-side inoculation methods (Group T) were transplanted into the subcutaneous pockets on the dorsal region of 4-week-old athymic mice (purchased from Wuhan University) for the in vivo analysis. Furthermore, the CS scaffold without inoculation of BMSCs was also transplanted into the dorsal skin of the nude mice as a blank control (Group C). After all animals were anesthetized by intraperitoneal injection of pentobarbital sodium at a dose of 40 mg/kg of body weight, 4 subcutaneous pockets were created on each of the 18 animals by dissecting subcutaneous facial tissue (between the skin and muscle layers) using dissecting scissors. Finally, BMSC/scaffold compounds were randomly delivered into the pockets and wounds were closed using sutures.20 All mice survived the operation procedure without complications.
Histological and immunofluorescence examination
Mice were sacrificed by CO2 asphyxiation. Immediately after death, the specimens were retrieved together with the surrounding soft tissue and they were cut into two parts under sterile conditions. The retrieved samples were embedded in an optimum cutting temperature compound (Sakura Finetek, Torrance, CA) and 5 μm-thick consecutive sections cut from the center area of the cell/scaffold constructs were first fixed in 10% formalin at 4°C for 10 min, and they were then incubated in a 1:1 mixture of methanol/acetone (Tianjin Kermel Laboratory Equipment Co., Ltd., Tianjin, China) at −20°C for 10 min. After this fixation procedure, the frozen slides were rinsed in PBS for 5 min. The sections were immersed in a methanol solution with 3% H2O2 for 10 min to quench the endogenous peroxidase activity, and they were then preincubated with a serum blocking solution for 20 min to block nonspecific binding. The sections were incubated overnight with anti-CD31 antibody (Abcam, Cambridge, United Kingdom) at 4°C and washed with PBS three times. Subsequently, the slides were incubated with anti-IgG (Invitrogen Alexa Fluor, 488; Thermo Fisher Scientific, Waltham, MA) at room temperature for 1 h in the dark, followed by rinsing with PBS three times. The nuclei were then counterstained by 4,6-diamidino-2-phenylindole (DAPI; 100 ng/mL; Sigma-Aldrich Co.).
To quantify the extent of neovascularization, the mean vessel density of the constructs was analyzed according to the methods of a previous study19: the total numbers of vessels on each slide were counted. To correct for differences generated by different BMSCs densities the vascularization percent was normalized to the total area of the scaffold in the same field of view.
Results
Initial seeding efficiency of BMSCs
Confirming the results of our previous study, the initial seeding number of 1.57×106 cells in the two-side inoculation group resulted in a 79.42%±9.87% initial seeding efficiency. Fewer BMSCs anchored in the cell/scaffold constructs of the 10-time syringe-aided inoculation group, resulting in a much lower percent attachment (53.74%±9.96%) when compared with the percentages of the two-side group (p<0.05). However, the 20-, 40-, and 60-time syringe-aided inoculation methods led to 92.63%±3.17%, 95.26%±4.78%, and 97.14%±1.30% seeding efficiencies, respectively.
Cell viability
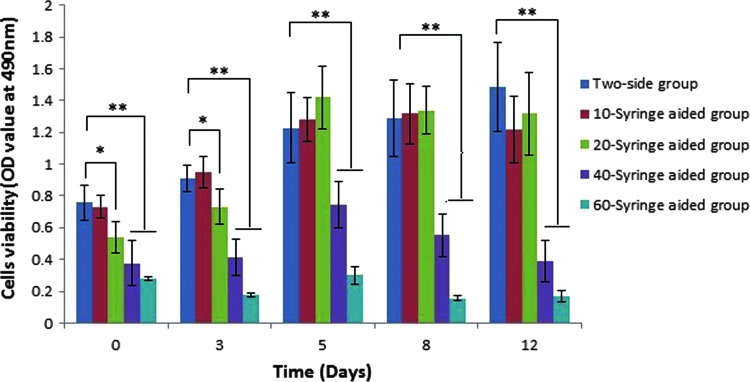
The WTS-8 assay of all groups was performed to evaluate the influence of different inoculation methods on BMSC viability. As shown in Fig. 3, the cell viability in the 10S group was the same as that of the two-side group at all time points (p>0.05), which demonstrated that the 10-time positive aspiration of BMSCs by a 2-mL syringe did not alter the viability of the BMSCs. At 1 h and 3 days of culture in vitro, the 20-time positive aspiration of BMSCs resulted in a relatively lower viability as compared with that of the two-side inoculation method (p<0.05). The cell viability of the 20S group was rising at 5 days of culture. At a later period of culture, the 20S group showed a similar cell viability with that of the two-side inoculation group (p>0.05). Cell viability of the 40S or 60S groups were significantly lower than that of the two-side group at all time points (p<0.001) (Fig. 3).
Figure 3.
Cell viability was determined 1 h, as well as 3, 5, 8, and 12 days after inoculation using a WST-8 assay. The data are presented as the mean±SD of the three experimental groups with a statistical significance of *p<0.05 or **p<0.01. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The spatial distribution of BMSCs cultured in vitro
The influence of the different inoculation methods on the cell proliferation and distribution of BMSCs was evaluated by viewing the cells within the scaffolds using fluorescence microscopy.
One hour after inoculation, a few of the cells were anchored into the pores at the center of the scaffolds in Group T (Fig. 4A), whereas a larger amount of cells was uniformly distributed within the center pores of the scaffolds treated with the syringe-aided inoculation method (Fig. 4D). After 7 days of in vitro culture, the 20S group showed an increase in cell number from 468±106 cells/cm2 up to 1,150±169 cells/cm2 (p<0.001; Fig. 4D, 4E, 4G). A similar trend was also observed from days 7 to 14 in terms of the number of BMSC in Group 20S (p<0.001; Fig. 4E, 4F, 4G). With respect to Group T, the number of BMSCs at 7 days was statistically greater than that observed at 1 h (p<0.001; Fig. 4A, 4B). Up until day 14, there was no statistically significant difference in the number of BMSCs at 7 and 14 days of culture (p>0.05; Fig. 4B, 4C, 4G). At all time points, Group 20S expressed a larger number and a more uniform distribution of BMSCs than did Group T (p<0.05; Fig. 4A–F).
Figure 4.
BMSC distribution (blue) at the center of the scaffolds in the experimental and control groups at 1 h (A, D), 7 days (B, E), and 14 days (C, F) of culture in vitro. Histological sections were stained with PI to identify the cell nuclei (red). Scale bars: 150 μm. (G) Shows the statistical analysis of the total number of BMSCs at the center of the scaffolds in all groups (mean±SD, *p<0.05, **P<0.001). PI, propidium iodide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The spatial distribution of BMSCs cultured in vivo
Although a limited number of cells at both surfaces gradually proliferated and penetrated into the scaffolds of Group T, cells at the center of the scaffolds were still rare after 2 weeks' culture in vivo (Fig. 5B). The penetration of the host cells in Group C was similar with that in Group T (Fig. 5C). In contrast, cells at the center of the scaffold in Group 20S were significantly larger than those in Groups T and C (Fig. 5A–C).
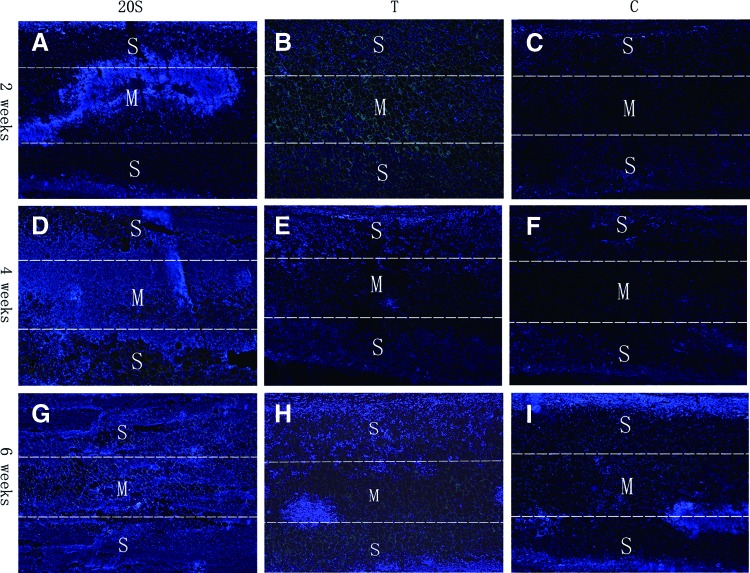
Figure 5.
BMSCs located in the center pores of the cells/scaffold compounds in the Group 20S (A, D, G), T (B, E, H), and C (C, F, I) at weeks 2 (A–C), 4 (D–F), and 6 (G–I) after implantation into the dorsal skin of the nude mice. In addition, 5 μm histological sections were stained with DAPI to identify the cell nuclei (blue). Scale bars: 200 μm. DAPI, 4,6-diamidino-2-phenylindole. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
At 4 weeks of implantation, there was no improvement in terms of cell distribution within the scaffold in the Groups T and C after another 2 weeks of culture in vivo (Fig. 5E, F). However, cells at the center of the scaffold in Group 20S proliferated and almost reached both surfaces of the scaffolds (Fig. 5D).
At 6 weeks of implantation, there was a slight increase in the number of BMSCs at the center of the scaffolds in Group T, which was probably due to penetration of the host cells and proliferation of the transplanted BMSCs at both surfaces of the scaffolds (Fig. 5H). There were still no cells located within the central region of the scaffolds in Group C (Fig. 5I); however, it appears that the proliferation of BMSCs was favored in the construct that was treated by the syringe-aided inoculation method, and at 6 weeks, the number of cells was obviously higher in Group 20S as compared with that in Groups T and C (Fig. 5G–I).
Colonization of the host cells
In this study, GFP-positive cells were visible by fluorescence microscopy (Fig. 6), but host migrating cells that were GFP-negative were invisible.
Figure 6.
The vascular-like structures in the cell/scaffold compounds of Groups 20S (A–C) and T (D–F) expressed CD31 at 2 weeks (A, D), 4 weeks (B, E), and 6 weeks (C, F) of culture in vivo. The new vessels resulting from the transplanted BMSCs could easily be detected by fluorescence microscopy due to their GFP-positive signal. The typical capillary-like tubules was formed in the constructs of Group 20S (yellow arrow). The nuclei were stained with DAPI (blue) (mean±SD, *p<0.05). Scale bar: 100 μm. Quantitative analysis of the microvessels in cells/scaffold compounds was shown in (G). GFP, green fluorescent protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Two weeks after implantation, cells within the cell/scaffold compound were mainly composed of the GFP-positive cells.
At 4 weeks after implantation, newly formed microvessels were composed by both GFP-positive and -negative cells, which indicated that the transplanted and migrating cells were all involved in microvessel formation of the cell/scaffold compounds.
By week 6, the GFP-negative cells of host origin colonized the entire thickness of the cell/scaffold compound.
Immunofluorescence staining of CD31
To evaluate the influence of the two inoculation methods mentioned above on the host's vascularization, immunofluorescence of CD31 was undertaken. Microvessels formed in the central pores of implants were positively stained with the anti-CD31 antibody. Since no CD31-positive cells were observed in the inner regions of the scaffolds in the control group, Group C was not included in the present analyses.
It can be clearly seen that, after being implanted into the dorsal skin of the nude mice for 2 weeks, the microvessel tissue has not yet infiltrated into the pores of the cell/scaffold implants, which was highlighted by a comparably low immunofluorescence density of CD31 in both 20S and T groups (Fig. 6A, D).
At 4 weeks after implantation, this ingrowth of microtissue was more pronounced in the constructs seeded with the syringe-aided inoculation method when compared with that in the constructs treated with the two-side inoculation method (Fig. 6B, E). Moreover, a few typical capillary-like tubules were formed in the constructs of Group 20S (Fig. 6B, yellow arrow), which was in contrast to what was observed in the constructs in Group T (Fig. 6E).
At 6 weeks, CD31 immunofluorescence staining revealed that the cell/scaffold constructs treated with the syringe-aided inoculation method exhibited a significantly enhanced vessel density in the center regions when compared with the constructs treated with the two-side inoculation method (Fig. 6C, F). Furthermore, it was observed that the well-developed capillary-like tubules were formed through the porous architecture of the scaffolds in Group 20S (Fig. 6C, yellow arrow). Constructs treated using the two-side inoculation method in Group T, on the other hand, generated premature vessel structures (Fig. 6F).
Von Kossa staining
Areas of mineralized matrix in constructs were indicated by von Kossa staining (black regions in Fig. 7). It can be seen in Fig. 7 that the mineralization of the cell/scaffold constructs in all groups had increased with implantation time. Among all groups, the constructs in Group 20S obviously showed the strongest mineralization in the pillars of the scaffold or matrix at all time points (Fig. 7).
Figure 7.
Areas of mineralized matrix in different implants as indicated by von Kossa staining (black regions, asterisks). Obviously, the cells/scaffolds treated with the syringe-aided inoculation method (A, D, G) induced more mineralized matrix than did those of the other groups at all time points (B, C, E, F, H, I, J) (mean±SD, *p<0.05). At 2 weeks of implantation, more mineralized nodes were formed in the constructs of Group T when compared with those of Group C, although it was not statistically significant (B, C, J) (p>0.05). After cultured for 4 to 6 weeks, the constructs treated with the two-sided inoculation method showed more mineralization than did those in Group C, with statistics significance (E, F, H , I, J) (mean − SD, *p<0.05). Scale bar 100 μm. Mean optical density of the mineralization nodules of the cells/scaffold compounds was shown in (J). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
At 2 weeks of implantation, the mineralized nodes in the constructs of Group T had formed around the pillars of the scaffold (Fig. 7B, asterisk), although to a lesser extent than those of Group 20S; however, black nodules were not formed in the matrix (Fig. 7B). In terms of the constructs in Group C, no mineralization was found (Fig. 7C). Whether they were cultured for 4 or 6 weeks, the constructs treated with the two-side inoculation method (Group T) showed more mineralization than did those in Group C (Fig. 7E, F, H, I).
Discussion
It is widely accepted that a high cell density was observed in the outer regions of the tissue-engineered constructs, whereas a low cell density was found in the inner regions.6,7 In our previous study, there were nearly no cells anchored in the central pores of the scaffold treated by the regular one-side inoculation method. Although more cells within the construct were obtained through the two-side inoculation of cells, an ideal number of BMSCs at the inner region of the constructs still could not be achieved.8 Thus, it is urgent for us to design a new inoculation approach where seed cells can be efficiently inoculated into the scaffold and subsequently distributed within the scaffold in a uniform manner.
Referring to the syringe-aided inoculation method described in the previous study, we trimmed the CS scaffold to conform to the inner lumen of a2-mL syringe.11 After fixation of the scaffold in the inner lumen of the syringe, which was filled with a limited amount of cell suspension in the tip, positive pressure was alternately induced 10–60 times. By alternative positive aspiration of cells by syringe, the maximum number of seed cells could infiltrate the scaffold at a faster rate and subsequently anchor into the central areas of the scaffold.
From the results of the initial seeding efficiency and cell viability analysis, it was found that positive aspiration more than 20 times resulted in a high initial seeding efficiency of BMSCs; however, it significantly impaired the viability of BMSCs. Therefore, the initial seeding efficiency analysis in this study had measured the seeding efficiency of the total number of BMSCs, including both living and dead cells. When choosing the suitable times for positive aspiration, one should consider both seeding efficiency and cell viability. Specifically, we found that the 10-times positive aspiration did not significantly alter the viability of BMSCs, but it did result in a low initial seeding efficiency of BMSCs; moreover, the 20-times positive aspiration led to a lower BMSCs viability at the early period of culture. Up until the later period of in vitro culture, however, the cell viability in the 10S group returned to a similar level as that of the two-side inoculation group. Furthermore, a relatively higher initial seeding efficiency was also obtained following 20 times of positive aspiration. It also took a shorter amount of time to perform positive aspiration for a total of 20 times (average: 4.6 min) than it did to perform in the 40S (6.0 min) and 60S (7.3 min) groups. Therefore, we easily drew the conclusion that the syringe-aided inoculation method with positive aspiration for a total of 20 times improved the number and distribution of BMSCs within the scaffold. On the contrary, the viability of BMSCs in the 40S or 60S groups was significantly lower than that of the two-side inoculation group at all time points, which indicated that too much positive aspiration might reduce the viability of BMSCs. Consequently, we standardized the positive aspiration time as 20 in the subsequent experiments in vivo.
However, it is worth mentioning that the abovementioned results concerning the initial seeding efficiency and cell viability only works for scaffolds with a porosity of 87.5% and pore sizes ranging from 80 to 130 μm. As for the scaffolds with larger pore sizes or smaller thicknesses, however, decreasing times of positive aspiration is helpful for cell retention, as the cells may penetrate the entire thickness of the scaffold and finally fail to load into the scaffold. Conversely, if the scaffold has a smaller pore size or a larger thickness, the infiltration rate of the seed cells can be low, resulting in an uneven distribution of cells within the scaffold. Therefore, the number of positive aspirations should be increased to transplant the cells into the central regions of the scaffold.
One hour after inoculation, a larger number of BMSCs with a more uniform distribution could be found in the cell/scaffold compounds treated by the syringe-aided inoculation method (Group 20S) when compared with that in the compounds treated with the regular two-side inoculation method. This indicated that a uniform distribution of BMSCs within the scaffold could be achieved by the syringe-aided inoculation method. After 7 days of in vitro culture, few BMSCs were observed in the central areas of the scaffold in Group T, which demonstrated that the low cell density slowed the proliferation of BMSCs. In contrast, the BMSCs in Group 20S expressed a large number and uniform distribution within the central areas of the scaffolds, with the aid of the repeated positive aspiration of the syringe. Up until 14 days, the number of BMSCs at the center of the scaffold in Group 20S continued to increase, whereas Group T showed a reduced number of BMSCs at the central areas of the scaffolds when compared to 7 days of in vitro culture.
In accordance with the in vitro culture findings, the in vivo culture results showed that the BMSCs in the central areas of the scaffold aggregated into the cluster (Fig. 5A, yellow arrow), whereas the T and C groups demonstrated that no cells anchored to the central areas of scaffolds at 2 weeks of culture in vivo (Fig. 5B, C). Before the establishment of the microvessels system, the penetration of the host cells into the central parts of the implant was limited. Therefore, the increase in seed cells at the central regions of the scaffold in Group 20S may have been a consequence of the proliferation of the preinoculated BMSCs, rather than the mobilization of the host cells at 2 weeks of culture in vivo. To further investigate whether the implanted BMSCs contributed directly to the increased number of cells within the scaffold, we took advantage of the fluorescence microscopy results, which recognized GFP-positive cells specifically, but not the recipient cells. The results of fluorescence microscopy analysis showed that most of the cells within the scaffold in both the 20S and T groups were GFP-positive BMSCs. Based on these findings, one can draw a conclusion that the preinoculated BMSCs in Group 20S resulted in a larger number of cells at the central areas of the scaffold within the first 2 weeks of implantation when compared with that of Group T (Figs. 5 and 6). Up until 4 weeks of in vivo culture, the cell clusters at the inner region of the scaffolds in Group 20S proliferated and almost reached both surfaces of the scaffolds (Fig. 5D). It is reasonable to hypothesize that the presence of the exogenous BMSCs plays a fundamental role in tissue development at the early stages of implantation, since there were still very few cells at the central pores of the scaffold in Groups T and C at 4 weeks of implantation (although both surfaces of the scaffold were covered by cells) (Fig. 5E, F). Furthermore, both groups showed a homogeneous mixture of GFP-positive and -negative cells within the constructs, indicating the contribution of both the preinoculated BMSCs and host-derived cells to the increased number of cells at the central areas of the scaffold (Fig. 6). This was in agreement with previous studies, which found that the presence of exogenous BMSCs resulted in the recruitment of host cells and was helpful in facilitating positive outcomes for bone tissue engineering.21,22 At week 6, there was a slight increase in the number of BMSCs at the center of the scaffolds in Group T, which was probably due to the penetration of the host cells and the proliferation of transplanted BMSCs at both surfaces of the scaffolds. However, the cell proliferation and tissue ingrowth were still more pronounced in cell/scaffold compounds that was treated by the syringe-aided inoculation method (Group 20S). Nearly the entire thickness of the scaffold was covered by the blue-stained cell nuclei, which represented the newly formed tissue (Fig. 5G). These results indicated the superior incorporation of the scaffolds into the host tissue when BMSCs were inoculated with the syringe-aided method.
With the exception of mobilization and recruitment of the host cells, exogenous BMSCs also had a positive effect on the angiogenesis of the cell/scaffold constructs. The intensity of immune-stained CD31 in the central area of the cell/scaffold compounds was stronger in Group 20S than in Group T (Fig. 6), which demonstrated that a large number of BMSCs with a more uniform distribution induced an angiogenic response with the formation of microvessels. Specifically, branching of the functional microvessels (Fig. 6B, yellow arrow) to the host vasculature was observed in the constructs of Group 20S, rather than in the constructs of Group T, at 4 weeks of ectopic implantation. Taken together, these results demonstrate that the syringe-aided inoculation method facilitated the proliferation and distribution of BMSCs within the central areas of the scaffold; the resulting well distribution of the BMSCs subsequently enhanced vessel formation and produced well-developed, vessel-like structures. This confirmed the results of previous studies, in so far as MSCs had promoted vessel formation by stimulating angiogenesis and cell differentiation.23,24 It has also been shown that MSCs attracted the CD31-enriched cell population into the graft and facilitated the vascularization of the tissue-engineered grafts.25 One possibility is that the BMSCs present in the grafts differentiated into endothelial lineage cells and subsequently generated vessels in vivo.
The influence of different inoculation methods on the mineralization was determined by von Kossa staining of the mineralized matrix within the constructs. At all time points, more mineralized matrices were formed in the constructs of Group 20S than in the constructs of Groups T and C. There may be two reasons for these findings. One is that there may have been an osteogenesis effect of the transplanted BMSCs; it is broadly accepted that the implantation of BMSCs can lead to bone formation in different animal models.26,27 Another possibility is that earlier vascularization of the constructs occurred in Group 20S. Apart from the removal of metabolic products and the supply of oxygen and nutrients—which are both necessary for the survival of the engrafted osteogenic cells—newly formed vessels could enable the recruitment of hematopoietic stem cells, osteoprogenitors, or monocytes, which may contribute to bone tissue regeneration and remodeling.28,29 In addition, with a sufficient vascular network, osteoblasts could also produce osteoid tissues, differentiate into osteocytes, and ultimately form bone tissue. It is well known that angiogenesis and osteogenesis are two intimately coupled processes that occur during bone healing30,31; this was also proven by our results, as the vascularization and mineralization of the constructs had all increased with time.
Taken together, these results demonstrate that the syringe-aided inoculation method resulted in increased graft vascularization and mineral tissue formation over the course of 6 weeks of healing, as compared with the grafts that were treated with regular inoculation method. Furthermore, positive aspiration of BMSCs for a total of 20 times produced relatively high seeding efficiency and cell viability.
Innovation
The technological innovation in this study lies in the designation of the simple, as well as in the rapid and novel inoculation method that was capable of anchoring seed cells into the synthetic graft, which featured a uniform distribution and a high seeding efficiency. The treatment of bone defects using the tissue-engineered grafts might benefit from the results of this study. It is also the first article to evaluate the influence of a syringe-aided inoculation method on the vascularization and mineralization of implanted grafts.
KEY FINDINGS.
• With increases in the number of times of positive aspiration, the initial seeding efficiency had also increased.
• Specifically, positive aspiration for 40 and 60 times had significantly reduced the viability of BMSCs, whereas positive aspiration for a total of 20 times slightly reduced the BMSC viability.
• The syringe-aided inoculation method used in this study resulted in increased graft vascularization and mineral tissue formation when compared with the regular two-side inoculation method.
Abbreviations and Acronyms
- adMSC
adipose-derived mesenchymal stem cell
- BMSCs
bone marrow stromal cells
- CS
chitosan
- DAPI
4,6-diamidino-2-phenylindole
- EDTA
ethylenediaminetetraacetic acid
- GFP
green fluorescent protein
- PBS
phosphate buffered saline
- PI
propidium iodide
- WST-8
water-soluble tetrazolium salts
Acknowledgments and Funding Sources
This work was supported by the National Natural Science Foundation of China (grant No. 81271107). English language editing of this article was provided by Journal Prep.
Author Disclosure and Ghostwriting
No competing financial interests exist in this study. The content of this article was expressly written by the authors listed and no ghostwriters were used.
About the Authors
Gu Cheng, MD, is a PhD candidate in the Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, China. Zhi Li, PhD, is an associate professor in the Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Wuhan University, Wuhan, China. Qilong Wan, PhD, is an associate professor in the Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Wuhan University, Wuhan, China. Rongtao Yang, PhD, is a surgeon in the Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Wuhan University, Wuhan, China. Kun Lv, PhD, is the Chief Resident in the Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Wuhan University, Wuhan, China. Zubing Li, PhD, is the Professor and director in the Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei Province.
References
- 1.Bran GM, Stern-Straeter J, Hormann K, Riedel F, Goessler UR. Apoptosis in bone for tissue engineering. Arch Med Res 2008;39:467–482 [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Jia W, Gu Y, Xiao W, Liu X, Wang D, et al. Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials 2010;31:5865–5874 [DOI] [PubMed] [Google Scholar]
- 3.Jang JH, Castano O, Kim HW. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev 2009;61:1065–1083 [DOI] [PubMed] [Google Scholar]
- 4.Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920–926 [DOI] [PubMed] [Google Scholar]
- 5.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol 2004;22:80–86 [DOI] [PubMed] [Google Scholar]
- 6.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol 2008;26:434–441 [DOI] [PubMed] [Google Scholar]
- 7.Cai L, Wang Q, Gu C, Wu J, Wang J, Kang N, et al. Vascular and micro-environmental influences on MSC-coral hydroxyapatite construct-based bone tissue engineering. Biomaterials 2011;32:8497–8505 [DOI] [PubMed] [Google Scholar]
- 8.Cheng G, Chen X, Li Z, Lu H, Davide O. Comparison of three inoculation methods for bone tissue engineering. Int J Oral Maxillofac Implants 2012;27:1340–1350 [PubMed] [Google Scholar]
- 9.Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. J Biomed Mater Res 2000;51:376–382 [DOI] [PubMed] [Google Scholar]
- 10.Laschke MW, Schank TE, Scheuer C, Kleer S, Schuler S, Metzger W, et al. Three-dimensional spheroids of adipose-derived mesenchymal stem cells are potent initiators of blood vessel formation in porous polyurethane scaffolds. Acta Biomater 2013;9:6876–6884 [DOI] [PubMed] [Google Scholar]
- 11.Laschke MW, Kleer S, Scheuer C, Schuler S, Garcia P, Eglin D, et al. Vascularisation of porous scaffolds is improved by incorporation of adipose tissue-derived microvascular fragments. Eur Cell Mater 2012;24:266–277 [DOI] [PubMed] [Google Scholar]
- 12.Lee YM, Park YJ, Lee SJ, Ku Y, Han SB, Choi SM, et al. Tissue engineered bone formation using chitosan/tricalcium phosphate sponges. J Periodontol 2000;71:410–417 [DOI] [PubMed] [Google Scholar]
- 13.Solchaga LA, Tognana E, Penick K, Baskaran H, Goldberg VM, Caplan AI, et al. A rapid seeding technique for the assembly of large cell/scaffold composite constructs. Tissue Eng 2006;12:1851–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu K, Ito A, Honda H. Mag-seeding of rat bone marrow stromal cells into porous hydroxyapatite scaffolds for bone tissue engineering. J Biosci Bioeng 2007;104:171–177 [DOI] [PubMed] [Google Scholar]
- 15.Stolzel K, Schulze-Tanzil G, Olze H, Schwarz S, Feldmann EM, Rotter N. Immortalised human mesenchymal stem cells undergo chondrogenic differentiation in alginate and PGA/PLLA scaffolds. Cell Tissue Bank 2015;16:159–170 [DOI] [PubMed] [Google Scholar]
- 16.Irie T, Takahata M, Majima T, Abe Y, Komatsu M, Iwasaki N, et al. Effect of selective estrogen receptor modulator/raloxifene analogue on proliferation and collagen metabolism of tendon fibroblast. Connect Tissue Res 2010;51:179–187 [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Liao X, Luo E, Chen W, Bao C, Xu HH. Mesenchymal stem cells systemically injected into femoral marrow of dogs home to mandibular defects to enhance new bone formation. Tissue Eng Part A 2014;20:883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutton DL, Moore EM, Gimble JM, Grayson WL. Platelet-derived growth factor and spatiotemporal cues induce development of vascularized bone tissue by adipose-derived stem cells. Tissue Eng Part A 2013;19:2076–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laschke MW, Schank TE, Scheuer C, Kleer S, Shadmanov T, Eglin D, et al. In vitro osteogenic differentiation of adipose-derived mesenchymal stem cell spheroids impairs their in vivo vascularization capacity inside implanted porous polyurethane scaffolds. Acta Biomater 2014;10:4226–4235 [DOI] [PubMed] [Google Scholar]
- 20.Li H, Xue K, Kong N, Liu K, Chang J. Silicate bioceramics enhanced vascularization and osteogenesis through stimulating interactions between endothelia cells and bone marrow stromal cells. Biomaterials 2014;35:3803–3818 [DOI] [PubMed] [Google Scholar]
- 21.Tasso R, Augello A, Boccardo S, Salvi S, Carida M, Postiglione F, et al. Recruitment of a host's osteoprogenitor cells using exogenous mesenchymal stem cells seeded on porous ceramic. Tissue Eng Part A 2009;15:2203–2212 [DOI] [PubMed] [Google Scholar]
- 22.Muraglia A, Martin I, Cancedda R, Quarto R. A nude mouse model for human bone formation in unloaded conditions. Bone 1998;22(5 Suppl):131S–134S [DOI] [PubMed] [Google Scholar]
- 23.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004;94:678–685 [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen 2007;15(Suppl 1):S18–S26 [DOI] [PubMed] [Google Scholar]
- 25.Tasso R, Fais F, Reverberi D, Tortelli F, Cancedda R. The recruitment of two consecutive and different waves of host stem/progenitor cells during the development of tissue-engineered bone in a murine model. Biomaterials 2010;31:2121–2129 [DOI] [PubMed] [Google Scholar]
- 26.Kretlow JD, Spicer PP, Jansen JA, Vacanti CA, Kasper FK, Mikos AG. Uncultured marrow mononuclear cells delivered within fibrin glue hydrogels to porous scaffolds enhance bone regeneration within critical-sized rat cranial defects. Tissue Eng Part A 2010;16:3555–3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang SC, Tai CL, Chung HY, Lin TM, Jeng LB. Bone marrow mesenchymal stem cells form ectopic woven bone in vivo through endochondral bone formation. Artif Organs 2009;33:301–308 [DOI] [PubMed] [Google Scholar]
- 28.Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med 2000;10:223–228 [DOI] [PubMed] [Google Scholar]
- 29.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med 2005;352:1959–1966 [DOI] [PubMed] [Google Scholar]
- 30.Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today 2003;8:980–989 [DOI] [PubMed] [Google Scholar]
- 31.Segar CE, Ogle ME, Botchwey EA. Regulation of angiogenesis and bone regeneration with natural and synthetic small molecules. Curr Pharm Des 2013;19:3403–3419 [DOI] [PubMed] [Google Scholar]