Abstract
Objectives: The study aim was to compare the long-term effect of Western medicine and combined treatment with Traditional Chinese Medicine (TCM) and Western medicine on the prognosis (survival rate, symptom distress, physical function, and quality of life) of patients with lung cancer.
Design: Longitudinal study.
Setting/Location: Two medical centers, one each in Northern and Southern Taiwan.
Patients: Patients newly diagnosed with lung cancer and treated with Western medicine (n = 54) or TCM plus Western medicine (n = 30).
Outcome measures: Symptom distress, physical function, and quality of life were measured by using the Symptom Distress Scale, Eastern Cooperative Oncology Group-Performance Status Rating, and European Organization for Research and Treatment of Cancer Quality of Life Questionnaires (EORTC QLQ-C30 and EORTC QLQ-LC13), respectively. Data on these measures were collected at baseline (before treatment) and 1, 3, 6, and 12 months after starting treatment. Survival was estimated by Kaplan–Meier curves. Group differences in outcomes were analyzed by generalized estimating equations.
Results: Treatment groups did not differ significantly at baseline for demographic information; disease severity; symptom distress; or EORTC QLQ-C30 and QLQ-LC13 scores, except for pain and dyspnea. After adjustment for these baseline effects, the combined-treatment group had better physical function and role function than the Western medicine group at 6 months (p < 0.05). The combined treatment group had better cumulative survival, but this difference did not reach significance.
Conclusions: To more precisely estimate the long-term effectiveness of combined treatment on the prognosis of patients with lung cancer, future studies should standardize the number of TCM visits; increase the number of participants by continuous recruitment; and ask patients to complete daily logs with single-item measures of outcomes, such as symptom distress, quality of life, and physical function. Similar studies are suggested in patients with different cancers to develop a collaborative model using Western medicine and TCM.
Introduction
Worldwide 8.2 million people died of cancer in 2012,1 with lung cancer as the leading cause of death.2 Lung cancer can be divided into small-cell lung cancer and non–small-cell lung cancer (NSCLC). NSCLC accounts for 85%–88% of all lung cancers in Taiwan.3 Despite advances in medical science, the 5-year survival rate for lung cancer is <16.6%.4 This low survival rate may be due to few signs or symptoms in early-stage lung cancer, which delays a confirmed diagnosis until late-stage disease develops.
Given the poor prognosis for lung cancer with Western medical treatment, many patients use alternative treatments to increase therapeutic effect, reduce treatment adverse effects, and improve quality of life (QOL). In Chinese society, the most common alternative treatment is Traditional Chinese Medicine (TCM). Indeed, TCM has been a popular alternative treatment for lung cancer in China, especially in treating elderly patients with advanced NSCLC.5–11 TCM treatments are not standardized as in Western medicine but rather are individualized according to patients' syndromes.6,12 TCM also bases diagnosis and treatment on patients' pattern type or body constitution, which is classified into nine major types and accounts for patient sex and the season.13
In the past decade, the consensus treatment for Chinese patients with lung cancer has gradually become a combination of Western medicine and TCM.6,7 This combined treatment is considered more effective in improving patients' prognosis than Western medicine or pure TCM alone14 because it reduces chemotherapy adverse effects,8,15,16 enhances immunity,15 improves QOL,8,15,16 and prolongs survival.5 Treatment effects on prognosis of patients with lung cancer have been explored by using such indicators as survival rate,17–19 extent of invasive cancer,20 average survival time,20–24 cancer recurrence,25 QOL,20,23,26 physical function,23 pain intensity,23 overall health status,23 and discontinued treatment due to adverse effects.18
Therefore, the current study assessed the long-term effectiveness of combined TCM and Western medicine on the prognosis of patients with lung cancer by measuring survival time, symptom distress, physical function, and QOL.
Materials and Methods
Design, setting, and sample
For this 2-year nonexperimental observational, longitudinal study, patients were sampled concurrently and purposively from two medical centers in northern and southern Taiwan. Patients were included according to these criteria: (1) adults age >20 years, (2) new diagnosis of stage III–IV NSCLC, (3) no prior cancer treatment and plan to receive traditional chemotherapy, and (4) understanding of the research objectives and procedure and agreement to participate. Patients were excluded if they experienced brain metastasis, were unconscious, or could not communicate.
Study variables
The outcome variable, prognosis, was assessed by symptom distress, physical function, QOL, and emotional distress, measured by using the Symptom Distress Scale (SDS),27 Eastern Cooperative Oncology Group Performance Status Rating (ECOG-PSR),28 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire29 (EORTC QLQ-C30, version 3, EORTC QLQ-Lung Cancer-Specific [LC13]30), and Distress Thermometer (DT),31 respectively.
Symptom distress
Symptom distress was measured by using the Mandarin version of the SDS.32 Higher SDS scores indicate more symptom distress. The SDS has been widely used in cancer-related studies in Taiwan, with good reliability and validity.32,33 In this study, SDS internal consistency was 0.89.
Physical function
Functional status was measured by using the Mandarin version of the ECOG-PSR.34 Scores range from 0 (able to move freely) to 4 (unable to move by oneself; relying on others for self-care). Higher scores indicate poorer physical function.28 The original ECOG-PSR had good convergent validity; correlation between scores on the EOCG-PSR and Karnofsky performance status index was high (r = 0.87).28 This scale can be easily completed by patients or their families.
QOL
General QOL was measured by using the Mandarin version of the EORTC QLQ-C30.35 This instrument has five functional subscales (physical, role, cognitive, emotion, and social), eight common cancer symptoms (fatigue, pain, nausea and vomiting, dyspnea, loss of appetite, insomnia, constipation, and diarrhea), and two single-item indicators of global QOL and general health. In this study, internal consistencies for the overall EORTC QLQ-C30 and its various subscales were 0.88 and 0.77–0.93, respectively.
Lung cancer–specific QOL was measured by using the EORTC QLQ-LC13 with the EORTC QLQ-C30 to assess symptoms specific to lung cancer and its treatment.30 The EORTC QLQ-LC13 has 13 items rated as 1 (never), 2 (occasionally), 3 (frequently), or 4 (always). The reliability and validity of the Taiwanese versions of the EORTC QLQ-C30 and EORTC QLQ-LC13 were good.35 In this study, the internal consistency of the EORTC QLQ-LC13 was 0.81.
Emotional distress
Emotional distress was measured by the single-item DT,31 which assesses emotional distress in patients with cancer from 0 (no distress) to 10 (extreme distress).
Procedure
After the institutional review board approved this study (100-3236C), the principal investigator visited directors of the Division of Pulmonary Medicine at the study hospitals to explain the research objectives and gain support. Attending physicians at outpatient clinics explained the research objectives to patients who met the inclusion criteria. Interested patients were referred to the principal investigator, and a full-time research assistant explained the research objectives and methods to patients in detail. After patients signed informed consent, they were assigned to groups according to their preference.
The combined-treatment group received TCM from qualified TCM physicians. Unlike conventional treatments, which are administered according to treatment guidelines, TCM treatments are personalized and usually administered according to patients' pattern type and treatment principles. Furthermore, TCM physicians decided on the schedule of TCM treatments according to each patient's situation.
Most patients went to the TCM outpatient clinic every 2–4 weeks. Because appointment times at outpatient clinics for TCM and Western medicine were not on the same day, patients who did not benefit from TCM would not be willing to spend extra time and energy to receive TCM treatment during chemotherapy.
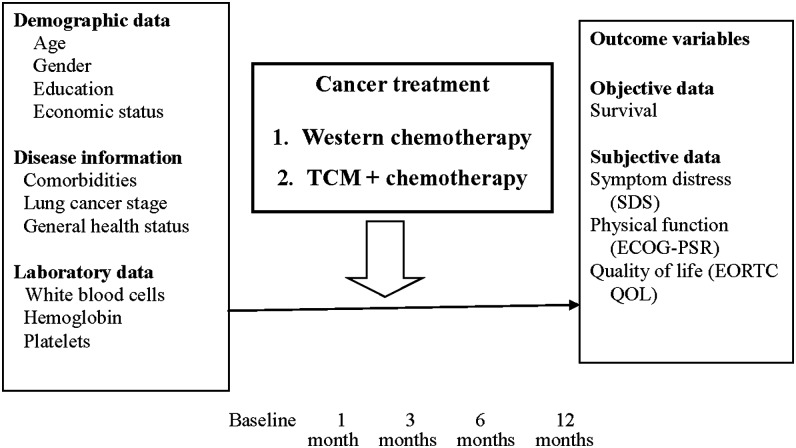
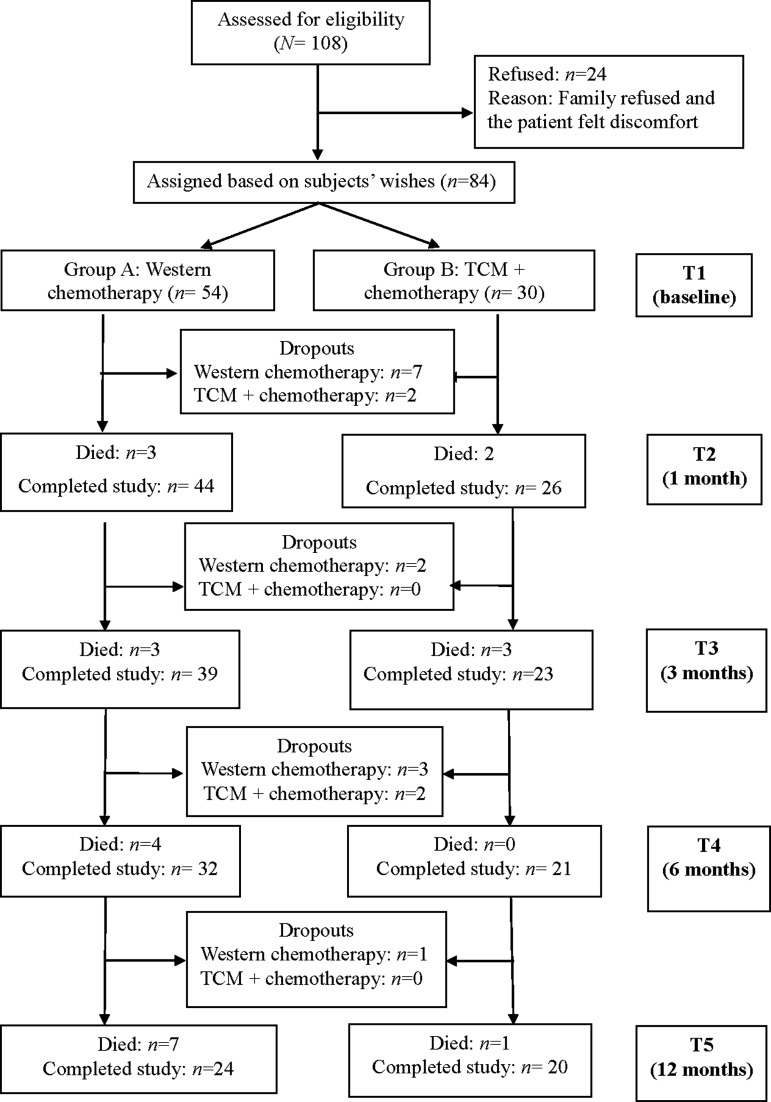
Data were collected at five times: baseline (before treatment), 1 month, 3 months, 6 months, and 12 months after starting treatment. Demographic data were collected only at baseline. At all other times, data were collected by using the SDS, EORTC QLQ-C30, EORTC QLQ-LC13, ECOG-PSR, DT, and relevant treatment data. The research framework and patient enrollment flowchart are shown in Figures 1 and 2. Most participants completed questionnaires by themselves. For illiterate patients, the research assistant read each questionnaire item aloud and recorded participants' answers.
FIG. 1.
Conceptual framework of the study. ECOG-PSR, Eastern Cooperative Oncology Group Performance Status Rating; EORTC QOL, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; SDS, Symptom Distress Scale; TCM, Traditional Chinese Medicine.
FIG. 2.
Study flowchart. TCM, Traditional Chinese Medicine.
Sample size estimate and statistical analysis
Sample size was estimated for α = 0.05, power = 0.80, a medium effect size, and the number of statistical comparisons in our study.36 A medium-effect size was based on combined treatment having a moderate effect on activities of daily living in patients with lung cancer.37 Thus, the study was estimated to have sufficient power with 65 patients in each group (130 patients in two groups).36 However, the authors took over this project from the Department of Health, which required that the study be completed within 2 years. Within this limited time, only 30 of 84 recruited patients were enrolled in the combined-treatment group.
Because participants in the two treatment groups did not differ significantly in baseline demographic and disease variables, differences in prognosis-related variables (SDS, EORTC QLQ-C30, and EORTC QLQ-LC13 scores) were compared by using generalized estimating equations without controlling for any variables. Group differences in physical function (ECOG-PSR scores), a categorical variable, were compared by using chi-square test. Data analyses were carried out with SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Of the 108 patients who met the inclusion criteria, 24 declined to participate (12 patients' families declined participation, 6 patients declined themselves, and 6 patients had participated in other clinical trials), leaving 84 participants, for a response rate of 77.8% (Fig. 2). Among the 84 participants, 54 received Western medicine, and their average age (± standard deviation) was 61.3 ± 11.12 years. Most were married (88.9%), male (64.8%), educated at the junior high school level or greater (72.2%), and unemployed (72.2%). Half of them perceived their economic status as moderate (50.0%), and more than half (57.4%) had comorbidities, such as hypertension or diabetes. Most participants were diagnosed with lung adenocarcinoma (80.8%), mainly at stage IV (71.7%) (Table 1).
Table 1.
Comparison of Baseline Demographic and Disease-Related Characteristics by Group (n = 84)
| Characteristic | Western medicine (n = 54) | Combined treatment (n = 30) | p-Value |
|---|---|---|---|
| Mean age ± SD (yr) | 61.33 ± 11.12 | 58.31 ± 10.79 | 0.236a |
| Sex | |||
| Male | 35 (64.8) | 22 (73.3) | 0.423 |
| Female | 19 (35.2) | 8 (26.7) | |
| Marital status | |||
| Single | 2 (3.7) | 2 (6.7) | 0.742b |
| Married | 48 (88.9) | 24 (80.0) | |
| Divorced | 1 (1.9) | 1 (3.3) | |
| Widow/widower | 3 (5.6) | 3 (10.0) | |
| Education | |||
| Junior high | 39 (72.2) | 14 (46.7) | 0.055 |
| High school | 10 (18.5) | 9 (30.0) | |
| College | 5 (9.3) | 7 (23.3) | |
| Employed | |||
| No | 39 (72.2) | 19 (63.3) | 0.550 |
| Yes | 15 (27.8) | 11 (36.7) | |
| Economic status | |||
| Insufficient | 22 (40.7) | 8 (26.7) | 0.189b |
| Moderate | 27 (50.0) | 20 (66.7) | |
| Sufficient | 5 (9.3) | 2 (6.7) | |
| Comorbidity | |||
| No | 23 (42.6) | 15 (50.0) | 0.671 |
| Yesc | 31 (57.4) | 15 (50.0) | |
| Lung cancer type | |||
| Adenocarcinoma | 42 (80.8) | 18 (60.0) | 0.214b |
| Large-cell carcinoma | 1 (1.9) | 0 (0.0) | |
| Squamous-cell carcinoma | 8 (15.4) | 10 (33.3) | |
| Non–small-cell carcinoma | 1 (1.9) | 2(6.7) | |
| Missing | 2 | 0 | |
| Stage of cancer | |||
| IIIA | 3 (5.7) | 4 (13.3) | 0.206b |
| IIIB | 12 (22.6) | 10 (33.3) | |
| IV | 38 (71.7) | 16 (53.3) | |
| Missing | 1 | 0 | |
| Western therapy | |||
| Chemotherapy | 27 (50.9) | 18 (60.0) | 0.690b |
| Chemotherapy + radiation therapy | 12 (22.6) | 7 (23.3) | |
| Targeted therapy | 8 (15.1) | 4 (13.3) | |
| Chemotherapy + targeted therapy | 3 (5.7) | 0 (0.0) | |
| Targeted therapy + radiation therapy | 3 (5.7) | 1 (3.3) | |
| Missing | 1 | 0 | |
| Chemotherapy | |||
| Docetaxel + cisplatin | 16 (43.2) | 15 (60.0) | 0.09b |
| Gemcitabine | 2 (5.4) | 2 (8.0) | |
| Pemetrexed disodium + cisplatin | 7 (18.9) | 2 (8.0) | |
| Oral vinorelbine + cisplatin | 4 (10.8) | 3 (12.0) | |
| Docetaxel | 8 (21.) | 0 (0.0) | |
| Gemcitabine + cisplatin | 0 (0.0) | 3 (12.0) | |
| Missing | 5 | 0 | |
Unless otherwise noted, values are number (percentage).
Independent t-test.
Fisher exact test.
Comorbidities include hypertension and diabetes mellitus.
SD, standard deviation.
The other 30 participants received combined treatment of TCM and Western medicine (combined treatment group) and had an average age of 58.31 ± 10.79 years. Most were married (80.0%) and male (73.3%). The largest proportion had at least a junior high school education (46.7%). The majority were unemployed (63.3%) and perceived their economic status as moderate (66.7%). About half of the patients (50.5%) had comorbidities, such as hypertension or diabetes, and a majority were diagnosed with lung adenocarcinoma (60.0%) at stage IV (53.3%) (Table 1).
At baseline, participants in the Western medicine group scored significantly higher for pain and dyspnea on the physical function dimension of the global QOL scale (EORTC QLQ-C30) than the combined treatment group (p < 0.05) (Table 2). Similarly, the Western-medicine group scored significantly higher on dyspnea in lung cancer–specific QOL (EORTC QLQ-LC13) than the combined treatment group (p < 0.05). Differences in other variables did not reach statistical significance (Table 2).
Table 2.
Baseline Comparison of Physical Function, Quality of Life, Symptom Distress, and Body Weight by Group (n = 84)
| Outcome measure | Western medicine (n = 54) | Combined treatment (n = 30) | p-Value |
|---|---|---|---|
| ECOG-PSR, n (%) | |||
| 0: Fully active, able to carry on all predisease performance without restriction | 25 (46.3) | 21 (70.0) | 0.167a |
| 1: Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work | 23 (42.6) | 6 (20.0) | |
| 2: Ambulatory and capable of all self-care but unable to carry out any work activities; up and about >50% of waking hours | 3 (5.6) | 1 (3.3) | |
| 3: Capable of only limited self-care, confined to bed or chair >50% of waking hours | 3 (5.6) | 2 (6.7) | |
| 4: Completely disabled; cannot carry on any self-care; totally confined to bed or chair | 0 (0.0) | 0 (0.0) | |
| EORTC QLQ-C30 (mean ± SD) | |||
| Physical functioning | 79.63 ± 21.49 | 86.00 ± 23.77 | 0.214 |
| Role functioning | 81.79 ± 28.27 | 90.00 ± 23.41 | 0.180 |
| Emotional functioning | 77.93 ± 23.52 | 81.94 ± 15.79 | 0.406 |
| Cognitive functioning | 82.41 ± 21.58 | 85.56 ± 20.87 | 0.519 |
| Social functioning | 73.77 ± 29.60 | 80.00 ± 20.72 | 0.263 |
| Global health status | 50.00 ± 26.50 | 59.20 ± 20.07 | 0.109 |
| Fatigue | 27.98 ± 26.85 | 20.00 ± 21.52 | 0.166 |
| Pain | 27.16 ± 27.34 | 15.56 ± 20.96 | 0.047 |
| Nausea and vomiting | 6.48 ± 16.32 | 10.56 ± 22.95 | 0.347 |
| Dyspnea | 29.01 ± 29.71 | 16.67 ± 24.37 | 0.056 |
| Insomnia | 28.40 ± 29.96 | 24.44 ± 26.16 | 0.547 |
| Appetite loss | 23.46 ± 28.69 | 17.78 ± 27.31 | 0.379 |
| Constipation | 9.88 ± 22.08 | 12.22 ± 20.50 | 0.634 |
| Diarrhea | 4.94 ± 11.95 | 4.44 ± 11.52 | 0.855 |
| Financial difficulties | 20.99 ± 26.93 | 24.44 ± 26.66 | 0.563 |
| EORTC QLQ-LC13 (mean ± SD) | |||
| Dyspnea | 27.78 ± 24.01 | 14.07 ± 17.73 | 0.008 |
| Coughing | 42.59 ± 27.79 | 35.56 ± 21.32 | 0.199 |
| Hemoptysis | 7.41 ± 15.41 | 5.56 ± 15.37 | 0.599 |
| Sore mouth | 6.17 ± 15.96 | 3.33 ± 13.42 | 0.412 |
| Dysphagia | 6.17 ± 15.96 | 3.33 ± 10.17 | 0.382 |
| Peripheral neuropathy | 6.79 ± 13.55 | 5.56 ± 12.63 | 0.683 |
| Alopecia | 6.79 ± 13.55 | 4.44 ± 11.52 | 0.426 |
| Pain in chest | 22.22 ± 22.43 | 22.22 ± 20.22 | 1.000 |
| Pain in arm or shoulder | 16.05 ± 24.00 | 15.56 ± 16.91 | 0.921 |
| Pain in other parts | 22.22 ± 27.47 | 13.33 ± 18.77 | 0.084 |
| Use painkiller | 12.35 ± 16.25 | 7.78 ± 14.34 | 0.187 |
| Symptom Distress Scale | 32.09 ± 8.69 | 30.20 ± 6.97 | 0.309 |
Fisher exact test.
ECOG-PSR, Eastern Cooperative Oncology Group Performance Status Rating; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-LC13, EORTC QLQ-Lung Cancer-Specific.
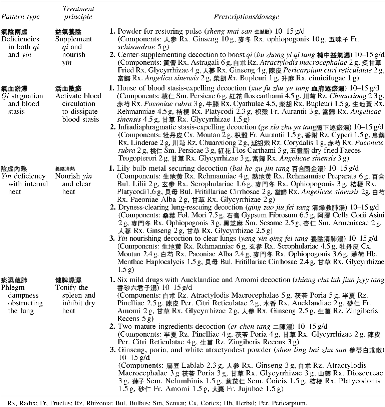
During the study, combined treatment group participants visited the TCM clinic on average 11.3 ± 8.3 (range, 2–32). The most common pattern types, treatment principles, and prescriptions are listed in Table 3.
Table 3.
Common Pattern Types, Treatment Principles, and Prescriptions for Combined Treatment Group (n = 30)
 |
Comparison of differences in physical function between the two groups at 1, 3, 6, and 12 months showed that at 3 months, significantly more patients in the combined-treatment group normally engaged in daily activities without any restriction (52.2%) than in the Western-medicine group (20.5%; p < 0.05) (Table 4). After adjustment for baseline differences, the combined treatment group also had significantly better general QOL than the Western -medicine group in terms of physical function, role function, pain, dyspnea, and emotional distress (p < 0.05) (Table 5). Although these symptoms were significantly lower for the combined treatment group than for the Western medicine group (Table 5), the Western medicine group had significantly higher pain and dyspnea at baseline (Table 2). Therefore, the analysis was adjusted first for baseline effects and performed with inclusion of group × time interactions in generalized estimating equation analysis to compare differences between the two groups at various time points. The combined treatment group had significantly higher dyspnea scores at 6 months than the Western-medicine group (Table 6). When assessed for general QOL (EORT QLQ-C30) and lung cancer–specific QOL (EORT QLQ-LC13), dyspnea scores increased 15.70 and 12.93 points, respectively (p = 0.019 and 0.014) (Table 6). The two groups did not differ significantly in any other dimensions at other times.
Table 4.
Between-Group Comparison of Physical Function 3 Months After Treatment (n = 62)
| Variable | Western medicine (n = 39) | Combined treatment (n = 23) | p-Value |
|---|---|---|---|
| ECOG-PSR, n (%) | |||
| 0: Fully active, able to carry on all predisease performance without restriction | 8 (20.5) | 12 (52.2) | 0.033 |
| 1: Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work | 23 (59.0) | 10 (43.5) | |
| 2: Ambulatory and capable of all self-care but unable to carry out any work activities; up and about >50% of waking hours | 6 (15.4) | 0 (0.0) | |
| 3: Capable of only limited self-care, confined to bed or chair >50% of waking hours | 2 (5.1) | 1 (4.3) | |
| 4: Completely disabled; cannot carry on any self-care; totally confined to bed or chair | 0 (0.0) | 0 (0.0) |
Table 5.
Generalized Estimating Equation Analyses of Quality of Life, Symptom Distress, and Body Weight for Combined Treatment Group Relative to Western Medicine Group (n = 84)
| Dependent variable | Estimate | Error | Chi-square | p-Value |
|---|---|---|---|---|
| EORTC QLQ-C30 | ||||
| Physical functioning | 8.19 | 3.78 | 4.700 | 0.030 |
| Role functioning | 9.22 | 4.19 | 4.843 | 0.028 |
| Emotional functioning | 0.10 | 2.97 | 0.001 | 0.973 |
| Cognitive functioning | 5.17 | 3.71 | 1.943 | 0.163 |
| Social functioning | 4.90 | 3.82 | 1.648 | 0.199 |
| Global health status | 6.41 | 3.38 | 3.589 | 0.058 |
| Fatigue | −5.76 | 3.87 | 2.210 | 0.137 |
| Pain | −10.61 | 3.94 | 7.268 | 0.007 |
| Nausea and vomiting | 1.40 | 2.43 | 0.331 | 0.565 |
| Dyspnea | −7.51 | 3.41 | 4.846 | 0.028 |
| Insomnia | −5.88 | 4.59 | 1.635 | 0.201 |
| Appetite loss | −3.75 | 3.92 | 0.914 | 0.339 |
| Constipation | −1.93 | 3.17 | 0.371 | 0.543 |
| Diarrhea | −0.02 | 2.82 | 0.064 | 0.994 |
| Financial difficulties | 5.29 | 4.41 | 1.444 | 0.230 |
| EORTC QLQ-LC13 | ||||
| Dyspnea | −9.92 | 3.19 | 9.685 | 0.002 |
| Coughing | −5.43 | 4.16 | 1.708 | 0.191 |
| Hemoptysis | −1.86 | 1.93 | 0.924 | 0.336 |
| Sore mouth | −3.25 | 2.39 | 1.848 | 0.174 |
| Dysphagia | −4.26 | 3.27 | 1.695 | 0.193 |
| Peripheral neuropathy | −2.34 | 2.72 | 0.739 | 0.390 |
| Alopecia | −5.36 | 3.35 | 2.558 | 0.110 |
| Pain in chest | −2.17 | 3.48 | 0.388 | 0.534 |
| Pain in arm or shoulder | −1.08 | 3.38 | 0.103 | 0.749 |
| Pain in other parts | −4.85 | 3.55 | 1.866 | 0.172 |
| Use painkiller | −3.59 | 2.34 | 2.345 | 0.126 |
| Symptom Distress Scale | −1.92 | 1.40 | 1.868 | 0.172 |
Reference group = Western chemotherapy.
Table 6.
Generalized Estimating Equation Analysis of Changes in Specific Symptoms After Adjusting to Baseline Data (n = 84)
| Dependent variable | Independent variable | Estimate | Error | Chi-square | p-Value |
|---|---|---|---|---|---|
| EORTC QLQ-C30 Pain | |||||
| Combined treatment × T5a | −5.53 | 6.12 | 0.816 | 0.366 | |
| Combined treatment × T4 | −4.42 | 5.92 | 0.559 | 0.455 | |
| Combined treatment × T3 | 6.63 | 5.59 | 1.406 | 0.236 | |
| Combined treatment × T2 | 5.38 | 5.90 | 0.833 | 0.362 | |
| EORTC QLQ-C30 | |||||
| Dyspnea | Combined treatment × T5 | 6.76 | 7.56 | 0.800 | 0.371 |
| Combined treatment × T4 | 15.70 | 6.69 | 5.541 | 0.019 | |
| Combined treatment × T3 | 3.17 | 6.70 | 0.225 | 0.635 | |
| Combined treatment × T2 | 7.58 | 6.84 | 1.231 | 0.267 | |
| EORTC QLQ-LC13 | |||||
| Dyspnea | Combined treatment × T5 | 2.84 | 5.68 | 0.248 | 0.618 |
| Combined treatment × T4 | 12.93 | 5.25 | 6.080 | 0.014 | |
| Combined treatment × T3 | 4.05 | 5.24 | 0.599 | 0.439 | |
| Combined treatment × T2 | 2.86 | 4.26 | 0.360 | 0.548 | |
Reference group = Western chemotherapy at T1.
T2, the first month after starting treatment; T3, the third month after starting treatment; T4, the sixth month after starting treatment; T5, the 12th month after starting treatment, all as representative variables.
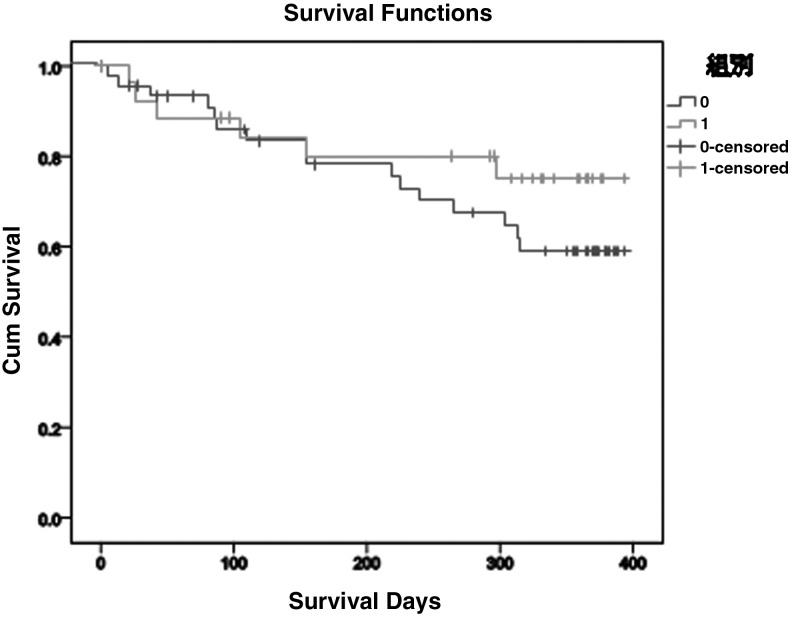
Finally, survival analysis showed that the Western medicine group lived on average 302.70 days and the combined-treatment group lived on average 324.21 days. The cumulative survival rate for the combined-treatment group >300 days' survival was 0.75, and that of the Western group was 0.68 (Fig. 3), but this difference was not significant (log rank, 1.136; p = 0.286).
FIG. 3.
Survival analysis for combined treatment (black line) and Western medicine treatment (gray line) groups.
Discussion
The physical and role functions of participants in the combined treatment group were significantly better than those of participants in the Western-medicine group, consistent with results for Chinese patients with NSCLC.8,38,39 The combined-treatment group in the current study had significantly higher dyspnea scores than the Western medicine group at 6 months for both general QOL (EORTC QLQ-C30) and disease-specific QOL (EORTC QLQ-LC13) (p < 0.05). However, the two groups did not differ significantly in respiratory symptoms at 1, 3, and 12 months. This lack of consistent effect might be due to TCM treatments being tailored to patients' body constitution and syndromes, unlike the standardized treatments emphasized in Western medicine. TCM practitioners request that patients visit the clinic only if they feel ill and their pulse is unstable. The 6-month data collection was just after chemotherapy terminated, when many patients feel very ill. However, only 4 patients in the combined treatment group went to TCM clinic, suggesting that other patients in the combined treatment group were too ill to leave home. Moreover, more patients in the Western medicine group withdrew from the study and died at 6 months. Thus, the Western-treatment group might have had better respiratory symptoms at 6 months than the combined medicine group because patients with worse respiratory symptoms had died.
The two treatment groups did not differ in other important variables (symptom distress, disease-related QOL, and survival time). The combined treatment group had a nonsignificantly higher cumulative survival rate for >300 days' survival than the Western medicine group. Similarly, when patients with NSCLC received combined treatment of TCM and Western medicine, their median survival time improved significantly more than that of patients treated only with Western medicine,6,10,40 but cumulative survival,10 5-year survival,6 and 1-year survival did not improve. These results might be due to the characteristics of TCM treatment, small sample size, and data collection times. In terms of TCM treatment characteristics, the efficacy of TCM treatment in patients with cancer cannot be observed in a short period;13,41 instead, its benefits are seen only after a long treatment period. Patients in the combined treatment group (n = 30) visited the TCM clinic on average 11.28 ± 8.3 times, but these times varied greatly. Thus, only a subset of this small sample of patients with patients may have received enough TCM treatments to verify the long-term effectiveness of TCM on their prognosis.
Moreover, the current sample might have been too small to permit comparison of between-group differences in important prognostic variables (e.g., symptom distress and disease-specific QOL). Retaining participants with NSCLC was difficult because of their poor prognosis and the challenges of their treatments. Indeed, 23 (27.4%) of the 84 participants died (17 in the Western medicine group and 6 in the combined treatment group), and 17 (20.2%) dropped out because of deteriorating condition. Samples in previous studies on the effectiveness of TCM or combined treatment ranged from 60 to 292.6,8,10,11,39,40,42,43 Therefore, increasing the sample size by continuous recruitment might increase the likelihood of detecting the long-term effectiveness of combined treatment on the prognosis of patients with lung cancer.
Data collection times were consistent with those of the hospitals' chemotherapy regimen (1–6 months), especially when treatment efficacy was assessed (at 3 and 6 months). However, these times were not when participants felt most ill after chemotherapy, which might have affected the comparison of important outcome variables between groups. Future studies are advised to include a daily log for patients to complete. For example, patients usually experience the most significant adverse effects of chemotherapy 2–4 days after each chemotherapy session.44 When patients feel extremely ill, they have great difficulty completing questionnaires. To reduce patients' questionnaire burden, using single-item indicators has been suggested.45 Thus, future studies could ask patients to complete daily logs with single-item indicators of relevant outcomes, such as symptom distress, QOL, and physical function. Thus, data could be collected at enough times to evaluate the long-term effectiveness of combined treatment on the prognosis of patients with NSCLC.
Patients in the combined treatment group mainly had pattern types that were deficiencies in both qi and yin, qi stagnation and blood stasis, yin deficiency with internal heat, and phlegm dampness obstructing the lung. These patients were mainly treated by therapeutic principles of supplementing qi and nourishing yin, activating blood circulation to dissipate blood stasis, nourishing yin and clearing heat, and tonifying the spleen and inhibiting dry heat. These pattern types are not completely consistent with the four commonly reported pattern types:6 lung-spleen qi deficiency; phlegm dampness obstructing the lung; deficiency of lung, stomach, and yang; and qi stagnation and blood stasis. This difference might be due to different times when TCM was included in cancer treatments. In the current study, the combined treatment group received chemotherapy in combination with TCM at the initial treatment. In the previous study, patients with lung cancer did not start using TCM until they completed chemotherapy or radiotherapy.6
Similarly, neither the current study nor the previous one used a fixed TCM prescription, and both studies relied on qualified TCM practitioners to determine treatment according to patients' pattern types. Although this approach seems to lack rigor from a scientific perspective, it is closest to real-world TCM practice. Indeed, experts in complementary and alternative medicine at the International Psycho-Oncology Society conference suggested not judging TCM-related studies from a Western scientific perspective to avoid limiting the development of knowledge on combined treatment with TCM and Western medicine.* Alternatively, the current results and those of Li et al.6 might differ because patients' pattern types and related therapeutic principles can be affected by their comorbidities and chemotherapy regimen. To recruit patients with more pattern types, use more therapeutic principles, and obtain more objective data on TCM prescriptions for lung cancer, future studies may consider a multinational or cross-institutional approach to recruit patients with similar lung cancer staging who used the same chemotherapy regimen.
Except for a few complaints about nausea caused by the TCM smell, most patients in the combined treatment group did not report adverse effects or toxicity, reflecting the mild and harmless characteristics of TCM. It was also possible that significant chemotherapy adverse effects overpowered any TCM side effects. However, patients in the combined treatment group did not experience more or more significant symptoms (as measured by the SDS) than the Western medicine group. Therefore, the results suggest that TCM can safely be used in patients with cancer during chemotherapy.
This study had several limitations. First, the findings cannot be generalized because of lack of information on participants' adherence to TCM. Research assistants accompanied patients to TCM clinic visits and recorded relevant data, but they did not know whether patients took the TCM as advised. Future studies should use a daily log to track patients' TCM adherence, thus increasing external validity of the results.
Second, the number of TCM clinic visits varied widely among patients. On average, most patients visited the TCM clinic once every 2–4 weeks. However, a few visited the TCM clinic weekly. This issue could be resolved by designing a joint TCM and Western medicine clinic. Such a joint clinic would help standardize the number of patients' TCM clinic visits; help intervention measures better meet scientific standards; and reduce the transportation burden, physical strength, and time for hospital visits among patients in the combined treatment group.
Third, a few participants experienced mild nausea due to the unique taste and smell of TCM. Future studies are advised to encapsulate TCM to reduce the discomfort caused by its taste and smell, facilitate patients' use of TCM, and decrease the withdrawal rate of patients in the combined treatment group.
Finally, the study design was observational, with a level of evidence lower than for an experimental design, because of TCM's distinctive smell; this compromised the ability to blind participants to their treatment. Moreover, patients in the combined treatment group knew their treatment because they spent time and energy going to the TCM clinic. This issue could be minimized in future studies by encapsulating TCM and designing a joint TCM and Western medicine clinic.
Conclusions
Patients with lung cancer in the combined treatment group had better physical and role function than patients in the Western medicine group. However, the two groups did not differ significantly in other important variables (e.g., symptom distress, disease-related QOL, and survival). To more precisely estimate the long-term effectiveness of combined treatment on these patients' prognosis, future studies should standardize the number of visits to the TCM clinic/practitioner; increase the number of participants by continuous recruitment; and increase the number of assessments by asking patients to complete daily logs with single-item indicators of outcomes, such as symptom distress, QOL, and physical function.
Acknowledgments
This work was supported by Department of Chinese Medicine and Pharmacy, Ministry of Health and Welfare, Executive Yuan in Taiwan (CCMP 101-RD-102). Special thanks to all the patients who participated in this project.
Author Disclosure Statement
No competing financial interests exist.
McGrath H, van Dam F. Use of Complementary/Alternative Interventions. Paper presented at IPOS 15th World Congress of Psycho-Oncology; November 4–8, 2013; Rotterdam, the Netherlands.
References
- 1.World Health Organization. GLOBOCAN 2012. Cancer incidence and mortality worldwide in 2012. 2012. Online document at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Accessed March30, 2015
- 2.World Health Organization. Cancer. 2015. Online document at: www.who.int/mediacentre/factsheets/fs297/en/ Accessed March30, 2015
- 3.National Health Research Institutes. Lung Cancer Practice Guideline. Taipei: Taiwan Cooperative Oncology Group; 2004 [Google Scholar]
- 4.National Cancer Institute. SEER Stat fact sheets: lung and bronchus cancer. 2012. Online document at: http://seer.cancer.gov/statfacts/html/lungb.html Accessed March30, 2015
- 5.Han MQ, Su JM, Huang HY, et al. Prognostic analysis of advanced non small cell lung cancer treated by sequential chemo-radiation therapy combined with traditional Chinese medicine: a report of 54 cases. Zhong Xi Yi Jie He Xue Bao 2003;1:195–198 [DOI] [PubMed] [Google Scholar]
- 6.Li JH. A clinical comparative study on traditional Chinese medicine serving as consolidation treatment in patients with advanced non-small cell lung cancer. Chin J Lung Cancer 2007;10:520–522 [DOI] [PubMed] [Google Scholar]
- 7.Ruan G, Zhou L, Liu J. Progress of traditional Chinese medicine and western treatment for elderly advanced lung cancer. Zhongguo Fei Ai Za Zhi 2008;11:805–810 [DOI] [PubMed] [Google Scholar]
- 8.Tian JH, Liu LS, Shi ZM, et al. A randomized controlled pilot trial of “Feiji Recipe” on quality of life of non-small cell lung cancer patients. Am J Chin Med 2010;38:15–25 [DOI] [PubMed] [Google Scholar]
- 9.Xu ZY, Jin CJ, Zhou CC, et al. Treatment of advanced non-small-cell lung cancer with Chinese herbal medicine by stages combined with chemotherapy. J Cancer Res Clin Oncol 2011;137:1117–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou DH, Lin LZ, Zhou YQ, et al. Prognostic analysis of stage III-IV non-small cell lung cancer patients treated by traditional chinese medicine. Ai Zheng 2005;24:1252–1256 [PubMed] [Google Scholar]
- 11.Jiang TH, Wu SY, Chen Y, et al. Influence of interventional chemotherapy combined with traditional Chinese medicine on the immune function of elderly patients with advanced lung cancer. J Intervent Radiol 2010;19:489–492 [Google Scholar]
- 12.Su SL. Medicine for the treatment of lung cancer. Taipei Res Trad Chin Med J 2006;9:24–33 [Google Scholar]
- 13.Xu W, Towers AD, Li P, Collet JP. Traditional Chinese medicine in cancer care: perspectives and experiences of patients and professionals in China. Eur J Cancer Care (Engl) 2006;15:397–403 [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Gao WY, Li KF. Chinese herbal medicine in the treatment of lung cancer. Asian J Trad Med 2008;3:1–11 [Google Scholar]
- 15.Qi F, Li A, Inagaki Y. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends 2010;4:297–307 [PubMed] [Google Scholar]
- 16.Guo L, Bai SP, Zhao L, Wang XH. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: effects on quality of life and survival. Med Oncol 2012;29:1656–1662 [DOI] [PubMed] [Google Scholar]
- 17.Wu YH, Qiao SL. Health Guide of Cancer. Taipei: Suncolor; 2006 [Google Scholar]
- 18.Herbst RS, O'Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007;25:4743–4750 [DOI] [PubMed] [Google Scholar]
- 19.Chen D, Xing K, Henson D, et al. Developing prognostic systems of cancer patients by ensemble clustering. J Biomed Biotechnol 2009;2009:632786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassileth BR, Lusk EJ, Guerry D, et al. Survival and quality of life among patients receiving unproven as compared with conventional cancer therapy. N Engl J Med 1991;324:1180–1185 [DOI] [PubMed] [Google Scholar]
- 21.Annakkaya AN, Arbak P, Balbay O, et al. Effect of symptom-to-treatment interval on prognosis in lung cancer. Tumori 2007;93:61–67 [DOI] [PubMed] [Google Scholar]
- 22.Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol 2008;26:3351–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang CH, Yu CJ, Shih JY, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol 2008;26:2745–2753 [DOI] [PubMed] [Google Scholar]
- 25.Pennathur A, Luketich JD, Heron DE, et al. Stereotactic radiosurgery for the treatment of lung neoplasm: experience in 100 consecutive patients. Ann Thorac Surg 2009;88:1594–1600; discussion 1600 [DOI] [PubMed] [Google Scholar]
- 26.Hlubocky FJ, Ratain MJ, Wen M, Daugherty CK. Complementary and alternative medicine among advanced cancer patients enrolled on phase I trials: a study of prognosis, quality of life, and preferences for decision making. J Clin Oncol 2007;25:548–554 [DOI] [PubMed] [Google Scholar]
- 27.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs 1978;1:373–378 [PubMed] [Google Scholar]
- 28.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer 1996;32a:1135–1141 [DOI] [PubMed] [Google Scholar]
- 29.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376 [DOI] [PubMed] [Google Scholar]
- 30.Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994;30a:635–642 [DOI] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network. NCCN distress management clinical practice guidelines in oncology. 2008. Online document at: http://www.nccn.org Accessed March30, 2015
- 32.Lai YH. Symptom distress and home care needs in patients receiving chemotherapy in an outpatient setting. J Nurs Res 1998;6:279–289 [Google Scholar]
- 33.Wu MH. The Effects of the Self-Regulation Protocol on Coping with Fatigue among Patients with Breast Cancer Receiving First Chemotherapy [Master's thesis]. Kaohsiung: School of Nursing, Kaohsiung Medical University; 2007 [Google Scholar]
- 34.Chen HC, Chen ML, Lotus Shyu YI, Tang WR. Development and testing of a scale to measure caregiving load in caregivers of cancer patients in Taiwan, the care task scale-cancer. Cancer Nurs. 2007;30:223–231 [DOI] [PubMed] [Google Scholar]
- 35.Ji WJ, Yang JS, Syu J, Lai JJ. Introduction of the EORTC Disease-Specific Quality of Life Questionnaires for cancer patients. Formos J Med 2002;6:220–227 [Google Scholar]
- 36.Rosenthal R, Rosnow RL. Essentials of Behavioral Research: Methods and Data Analysis. 2nd ed. New York: McGraw-Hill; 1991 [Google Scholar]
- 37.Yan GY, Xu ZY, Deng HB, et al. Effects of chemotherapy combined with Chinese herbal medicine Kangliu Zengxiao decoction on tumor markers of patients with advanced non-small-cell lung cancer: a randomized, controlled trial. Zhong Xi Yi Jie He Xue Bao. 2011;9:525–530 [DOI] [PubMed] [Google Scholar]
- 38.Lin HS, Li DR. Multi-center randomized clinical study on Shenqi-fuzheng injection combined with chemotherapy in the treatment for lung cancer. Zhonghua Zhong Liu Za Zhi 2007;29:931–934 [PubMed] [Google Scholar]
- 39.Yao YL. Effects of Feiji decoction for soothing the liver combined with psychotherapy on quality of life in primary lung cancer patients. Zhongguo Fei Ai Za Zhi. 2012;15:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YZ, Li ZD, Gao F, et al. Effects of combined Chinese drugs and chemotherapy in treating advanced non-small cell lung cancer. Chin J Integr Med. 2009;15:415–419 [DOI] [PubMed] [Google Scholar]
- 41.Liu CH, Tang WR, Wang HM, Lee KC. Cancer patients' experience of combined treatment with conventional and traditional Chinese medicine: a biopsychosocial phenomenon. Cancer Nurs. 2011;34:495–502 [DOI] [PubMed] [Google Scholar]
- 42.Zhang T, Ma SL, Yue JH. Clinical study on toxicity-attenuation effect of Yiguan Decoction in treatment of non-small cell lung cancer with NP protocol of chemotherapy. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007;27:396–399 [PubMed] [Google Scholar]
- 43.Wang XM, Xin H, Yang Z, et al. Clinical study on treatment of advanced stage non-small cell lung cancer by guben xiaoliu capsule. Zhongguo Zhong Xi Yi Jie He Za Zhi 2004;24:986–988 [PubMed] [Google Scholar]
- 44.Barsevick AM, Dudley W, Beck S, et al. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 2004;100:1302–1310 [DOI] [PubMed] [Google Scholar]
- 45.Youngblut JM, Casper GR. Single-item indicators in nursing research. Res Nurs Health 1993;16:459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]